Abstract
Tight junction proteins are critical in maintaining homeostatic intestinal permeability. Multiple intestinal inflammatory diseases are correlated with reduced expression of tight junction proteins. We have recently reported that oral treatment of mice with Hyaluronan 35 kDa (HA35) increases colonic expression of tight junction protein zonula occludens-1 (ZO-1). Here, we investigate whether HA35 treatment enhances ZO-1 expression by direct interaction with intestinal epithelium in vitro and have identified the HA receptor responsible for HA35-mediated ZO-1 induction in colonic epithelium in vitro and in vivo. Our results reveal that HA35 treatment increases ZO-1 expression in mouse intestinal epithelial organoids, while large HA 2000 kDa is not internalized into the cells. Our immunofluorescence data indicate that layilin, but neither toll-like receptor-4 (TLR-4) nor CD44, mediate the HA35-induced ZO-1 expression in colonic epithelium in vitro and in vivo. Additionally, using layilin null mice we have determined that layilin mediates HA35 induction of ZO-1 in healthy mice and during dextran sulfate sodium (DSS)-induced colitis. Furthermore, we find that while ZO-1 expression levels are reduced, layilin expression levels are equivalent in inflammatory bowel disease (IBD) patients and non-IBD controls. Together, our data suggest that layilin is an important HA receptor, that mediates the effect of oral HA35 treatment on intestinal epithelium. HA35 holds promise as a simple dietary supplement to strengthen gut barrier defense.
Keywords: Hyaluronan, Tight junction protein, Layilin, Mouse intestinal organoids, Epithelial barrier
1. Introduction
The most apical junctional complex that forms the primary cellular barrier at mucosal surfaces is known as the tight junction. The tight junction is composed of transmembrane proteins including occluden, claudins and junctional adhesion molecules (JAMs) and scaffolding proteins zonula occludens [1]. The tight junction complex is critical for the regulation of gut permeability, which is essential for water and nutrition absorption and supports the epithelial integrity preventing the entrance of toxins and pathogens into the lamina propria. Disruptions of the tight junction complex are correlated with the down-regulation of tight junction proteins and have been observed in many intestinal diseases such as inflammatory bowel disease (IBD), celiac disease and necrotizing colitis [2]. The mechanisms that regulate tight junction protein expression are not clear. Several studies, including our recently published study, explored the role of hyaluronan (HA) and its effects on the expression of one of the tight junction proteins, zonula occludens-1 (ZO-1) in vitro and in vivo [3–5]. Understanding the role of HA in mediating ZO-1 expression may help to elucidate the mechanism of tight junction protein regulation.
HA is a linear glycosaminoglycan polymer, which consists of repeating disaccharides of β-glucuronic acid and N-acetylglucosamine. HA, which is synthesized by HA synthases as a high molecular weight polymer up to 10,000 kDa, is a major component in extracellular matrix in many tissues [6]. During inflammation, specific enzymes and non-specific pathways including reactive oxygen species (ROS)-mediated mechanisms, degrade large HA polymers into smaller fragments [7,8]. Size-specific functions of HA have been investigated and generally large sizes of HA inhibit inflammation, angiogenesis and cell proliferation, while small sizes of HA have opposite effects [6].
HA-mediated effects are transmitted through HA binding to its receptors. The best characterized of these receptors is CD44, a major cell membrane receptor expressed in many cell types including epithelial, mesenchymal, and endothelial cells [9]. CD44 glycosylation, density and arrangement on cell surface, as well as the conformational state of HA are suggested to contribute to the affinity and avidity of HA binding to CD44 [10]. Toll-like receptors (TLRs), especially TLR-2 and TLR-4, are thought to facilitate many of the HA-mediated effects, however, direct binding between HA and TLRs has not been shown [11]. In addition to CD44 and TLRs, the receptor for HA mediated motility receptor RHAMM, (also known as HMMR), HA receptor for endocytosis (HARE), lymphatic vessel endothelial HA receptor 1 (LYVE1), and layilin are additional receptors known to bind HA [6].
Layilin is an integral membrane protein and an under-investigated HA binding receptor expressed by many cell types and in many organs. HA binding to layilin was first discovered by Bono, et al.[12], and Forteza, et al.[13] have shown that the effects of small sizes of HA are mediated through layilin in lung epithelial cells via the activation of RhoA/ Rho-associated protein kinase (ROCK) pathway. Layilin does not contain a link domain (also known as HA binding domain), which is present in many other HA receptors including CD44, HARE, and LYVE-1 [14]. However, in place of a link domain, layilin contains a C-type lectin domain that forms a similar set of hydrophobic core residues that are required for binding of HA [15]. Furthermore, layilin, which is expressed on the cell surface of migratory cells, interacts with the cytoskeletal proteins talin, merlin and radixin [16]. The inhibition of layilin expression using RNA interference (RNAi) has a direct effect on cell invasion, as demonstrated using A549 cells in vitro and through the reduction of lymph metastasis of A549 cells in vivo [17].
Previous reports have demonstrated CD44 and TLR-4 play roles in mediating the exogenous HA effects in the intestine. Riehl, et al.[18] have shown that CD44 and TLR-4 mediate the large HA-induced normal intestinal and colonic growth. Our group has also shown that both CD44 and TLR-4 in vivo mediate the expression of the antimicrobial peptide murine beta defensin-3 (mBD-3, the mouse orthologue of human beta defensin-2) in vivo that is induced by HA isolated from human milk (milk HA) [19]. Interestingly, we have found that HA 35 kDa (HA35)-mediated defensin induction requires TLR-4 but not CD44 [20]. Additionally, we have recently reported that oral administration of HA35 increases expression of the tight junction protein zonula occluden-1 (ZO-1) in the distal colon of wild type mice under normal and challenge conditions [5]. ZO-1 is a scaffold tight junction protein that binds to transmembrane tight junction proteins and forms the complete tight junction complex [21]. In addition to forming tight junction complexes, other roles for ZO-1 also have been discovered. For example, ZO-1 directly binds to F-actin to establish the linkage between the tight junction and actin cytoskeleton [22]. Furthermore, nuclear localization of ZO-1 was observed primarily in subconfluent epithelial cells, whereas in confluent cells ZO-1 predominates at cell margins where cell-to-cell contacts occur [23].
Based on our earlier work, we hypothesize that HA receptors expressed by the intestinal epithelium mediate HA35 induced expression of ZO-1 in colonic epithelium. In this study, we sought to identify the HA receptor involved in HA35 mediated ZO-1 induction. We demonstrate that HA35 treatment increases ZO-1 expression directly in mouse intestinal epithelial cells in vitro. We find that HA35-mediated ZO-1 induction is TLR-4 independent in vivo and that CD44 is not detectable in mouse colonic epithelium of the distal colon. Importantly, we show that the induction of ZO-1 following HA35 treatment is layilin dependent in vitro and in vivo, using healthy mice and the DSS-induced colitis mouse model. Additionally, the inhibition of ROCK signaling, a known downstream mediator of layilin effects, reduces HA35 induction of ZO-1 and HA uptake in vitro. Moreover, we observe that ZO-1 expression levels are lower in the colonic epithelium of IBD patients as compared to non-IBD patients, while layilin expression levels remain the same. These data suggest the potential efficacy of HA35 as an oral supplement for IBD patients to strengthen their epithelial barrier function.
2. Results
2.1 HA35 treatment increases ZO-1 expression in mouse intestinal epithelial cells in vitro
We previously showed that HA35 treatment increases ZO-1 expression in vivo and that ZO-1 induction is highly dependent on HA size. Our earlier study indicated that HA35 is the most potent inducer of ZO-1, while treatment with other small sizes of HA (HA 4.7 kDa, 16 kDa, and 74 kDa) increase ZO-1 expression but to a lesser extent in the distal colon of mice[5]. Importantly, large size HA 2000 kDa (HA2000) did not induce ZO-1 expression in vivo.
In the present work, we investigated whether the ZO-1 increase observed post HA35 treatment is the result of the direct effect of HA35 on intestinal epithelial cells. To achieve this, we used an in vitro culture model of isolated intestinal epithelium established by Miyoshi, et al [24]. Isolated crypts from the mouse distal colon were cultured as primary mouse colonic epithelial organoids, and passaged as described in the methods section. For experiments, replicate samples of mouse colonic epithelial organoids were cultured in media containing no HA, HA35, or HA2000 for 30h and then fixed. The concentration of HA was selected based on our previous work and 30h was the time point when organoids showed the highest levels of intracellular HA35 based on our time point experiment (data not shown) [20]. Fluorescence histochemistry was performed to detect HA and ZO-1 expression in organoids. The results in Fig. 1A showed that HA35 was internalized by mouse intestinal epithelial cells and HA35 treated organoids had higher ZO-1 levels than those treated with water (Fig. 1B). Importantly, HA35 treated organoids had clustered ZO-1 proteins present at the cell junctional area (Fig. 1A). Strikingly, HA2000 was not internalized by mouse colonic epithelial cells and did not induce ZO-1 expression (Fig. 1). To further examine the internalization of HA35, we immunostained HA treated mouse intestinal epithelial oragnoids to detect a membrane protein, E-cadherin. The results showed that HA35 was clearly internalized while HA2000 was not (Fig. 1A). To confirm the histological findings, we performed Western blot analysis for ZO-1 in lysates of mouse intestinal epithelial organoids treated with no HA, HA35, or HA2000 for 30h. GAPDH was used to normalize ZO-1 expression levels. The ZO-1 protein expression was significantly increased in HA35-treated organoids as compared to the no HA control group (Fig. 1C).
Figure 1.
HA35, but not HA2000 treatment increases ZO-1 expression and HA35 but not HA2000 is internalized in mouse intestinal organoids in vitro. A. Sections of mouse intestinal organoids treated with no HA, HA35 (350µg/ml) or HA2000 (350µg/ml) for 30h were fixed and stained for ZO-1 or E-cadherin (red), HA (green), and nuclei (blue). Z-stack images were obtained using confocal microscopy. B. Quantification of stained images from 8 organoids per condition. ZO-1 staining intensity was measured as described in “Methods” and normalized to the number of epithelial cells by counting the number of nuclei. The unpaired t-test was used to test the statistical significance of differences between no HA and the HA35-treated groups (*, p<0.05.) Error bars = S.E.M.
Additionally, since Forteza. et al demonstrated that 40 kDa HA treatment decreases the expression of E-cadherin, an adherens junction protein in lung epithelial cells, we tested whether HA35 treatment modulates the expression of E-cadherin in intestinal epithelial cells. Western blot analysis showed E-cadherin expression does not change post HA35 treatment in mouse intestinal epithelial organoids (Fig. 1D). Together these data suggest that HA35 treatment specifically induces ZO-1 expression in mouse intestinal epithelial cells whereas HA2000 does not. Additionally, while HA35 is taken up into colonic epithelium, HA2000 is not.
2.2. HA35 induced ZO-1 induction appears to be independent of TLR-4 and CD44
HA can mediate its effects through multiple receptors, including toll like receptors (TLRs) and CD44 [18,20,25,26]. TLR-4 has been identified as a critical receptor for HA35 mediated mBD-3 induction in the mouse colon and therefore, we tested if TLR-4 was involved in HA35 mediated ZO-1 induction in mouse colonic epithelium [20]. Wild type and TLR-4 knockout mice were gavaged with 300µg of HA35 once daily for 5 days prior to being sacrificed. Immunofluorescent staining for ZO-1 in colon sections of the water and HA35 treated mice revealed that HA35 induces ZO-1 expression in intestinal epithelium of both wild type and TLR-4 knockout mice (Fig. 2A and 2B), indicating that HA-35 induction of ZO-1 in the distal colon was TLR-4 independent.
Figure 2.
HA35-mediated ZO-1 induction is TLR-4 independent and murine distal colon epithelium does not express detectable levels of CD44. A. Distal colons from wild type or tlr4−/− mice gavaged with HA35 (300µg per mouse daily) or water for 5 days were immunostained for ZO-1 (red) (n=5/group). Images were taken using fluorescence microscopy of a single plane. B. The quantification of ZO-1 intensity was performed on 2 stained sections per mouse, 5 mice per group and normalized to the number of epithelial cells. The statistical significance was tested with the unpaired t-test. (**, p<0.01, ***, p<0.0001). Error bars=S.E.M. C. Sections of proximal and distal colons from wild type mice were stained for CD44 (green) and nuclei (blue). Nonspecific (NS) indicates the secondary antibody staining control. D. Western blot analysis of CD44 protein in mouse colon tissue lysates from wild type mice and mouse colonic epithelial organoids cultured in 50% L-WRN conditioned media. Villin-1, an epithelial cell marker, and GAPDH were used as loading controls.
Next, since CD44 is the best characterized and frequently highly expressed HA receptor, we wanted to determine whether CD44 is being expressed in mouse intestinal epithelium. We previously reported that CD44 is an important receptor mediating epithelial responses to HA present in human milk [19]. To evaluate CD44 expression in colonic epithelium, we performed immunostaining of the proximal, transverse, and distal colon tissues from mice. We found that CD44 was expressed only in the epithelium within the crypt area of the proximal colon but was not detectable in distal colon (Fig.2C). As expected, in the distal colon we observed CD44 positive cells in the subepithelial lamina propria area, which were the most likely leukocytes and fibroblasts (Fig. 2C). We were somewhat surprised at this result, since CD44 is known to be expressed in most cell types including lung epithelial cells, so confirmation of the histological finding was obtained using immunoblotting of protein lysates prepared from mouse colonic epithelial organoids. Whole colon lysates were used as a positive control for CD44 detection since, as noted, CD44 is expressed by fibroblasts and endothelial cells [27]. Villin-1, which is an epithelial specific marker, and GAPDH were used as a loading controls. Western blot results (Fig. 2D) indicated that CD44 was present in whole colon lysate control but was not in the primary mouse epithelial organoids. Collectively, our data suggest that neither TLR-4 nor CD44 are involved in the induction of ZO-1 post HA35 treatment in mouse distal colons.
2.3. HA35 treatment increases epithelial ZO-1 expression via layilin in vitro
Next, we investigated which of the other known HA receptors, besides TLR-4 and CD44, may mediate HA35-induced ZO-1 expression. As reported on the publicly available database, the Human Protein Atlas (http://www.proteinatlas.org/) [28], one of the most highly expressed HA receptors in human colonic epithelium is layilin. Furthermore, the activation of RhoA, a known downstream signaling pathway of layilin was reported to increase the expression of ZO-1 in human dental pulp cells[29]. Additionally, specific sized HA had been shown to bind to layilin and signal through the RhoA/ROCK pathway in lung epithelial cells [13]. Together, these studies suggested that HA35-mediated induction of ZO-1 may be facilitated through layilin.
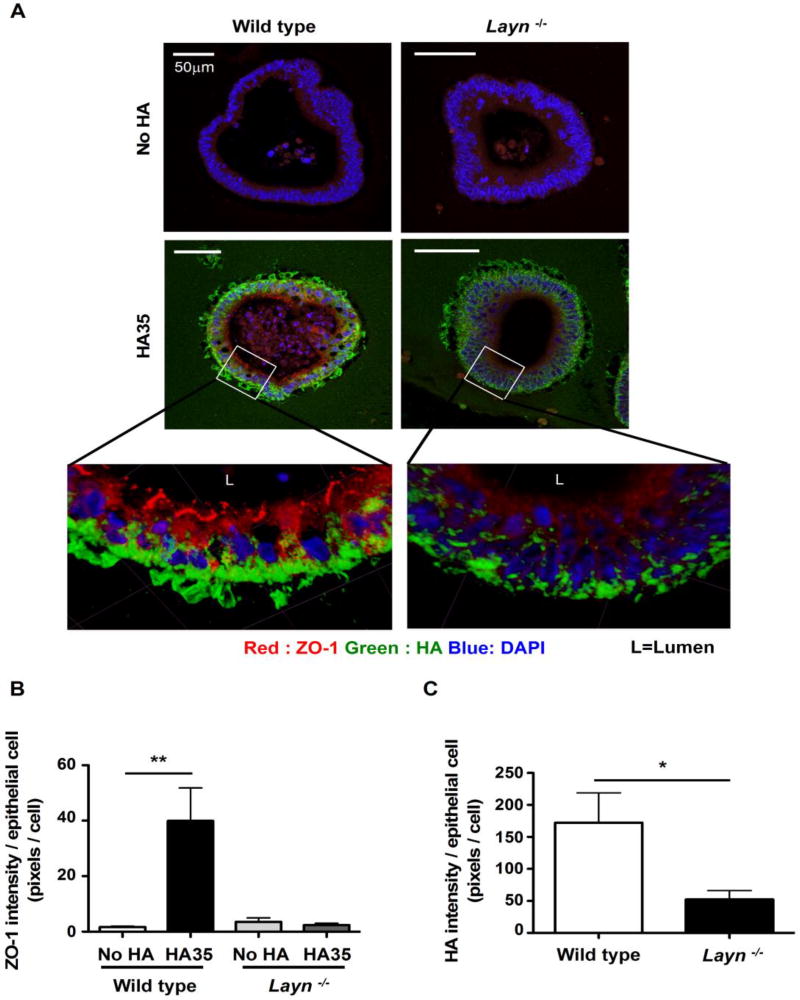
To determine the role of layilin in HA35-induced ZO-1 expression in mouse colonic epithelium, we used primary epithelial organoids from wild type and layilin (Layn) knockout mice. Layilin knockout mice were generated from the breeding of heterozygous mice and the complete knockout of the layilin gene was confirmed in both colon tissues and intestinal organoids by qRT-PCR (Supplemental figure 1). Furthermore, to examine the possibility that Layn−/−mouse colonic epithelial organoids express higher levels of CD44 to compensate in HA uptake, we performed Western blot analysis for CD44 in Layn−/− mouse colonic epithelial organoids lysates. We found that Layn−/− mouse colonic epithelial organoids do not express CD44 (Supplemental figure 2).To test whether layilin is involved in ZO-1 induction post HA35 treatment, cultured wild type or Layn−/− mouse colonic epithelial organoids were treated with medium containing HA35 or no HA for 30 hours and fixed. Organoids sections were fluorescently stained for ZO-1 and HA using specific antibody and HA-binding protein probes. The quantification of internalized HA detected in wild type and Layn−/− organoids indicated that there was less intracellular HA in Layn−/−organoids as compared to wild type organoids (Fig. 3C). The analysis of ZO-1 protein expression levels revealed that the overall ZO-1 levels were significantly increased post HA35 treatment in wild type organoids but not in Layn−/− organoids (Fig. 3B) Even when we evaluated individual epithelial cells from wild type and Layn−/− organoids that contained a comparable amount of HA, it was clear that ZO-1 expression was not induced in Layn−/− organoids, and additionally ZO-1 was not aligned at the cell junctional area, in contrast to what we observed in HA35 treated wild type epithelium (Fig. 3A). These results indicated that layilin participates in internalization of HA35 and mediates the induction of ZO-1 in mouse colonic epithelial cells.
Figure 3.
Layilin mediates HA35-induced ZO-1 increase in vitro. A. Sections of no HA and HA35 treated (350µg/ml for 30h) wild type and Layn−/− mouse intestinal organoids were stained for ZO-1 (red), HA (green) and nuclei (blue). Z-stacks images were obtained using confocal microscopy. B. ZO-1 intensity was quantified using 9 organoids per condition and normalized to the number of epithelial cells. The statistical analysis was done using the one-tailed unpaired t-test. (**, p<0.01). C. Intracellular HA intensity was measured using 9 organoids per condition and normalized to the number of epithelial cells. The one-tailed unpaired test was used to test the statistical significance. (*, p<0.05.) Error bars = S.E.M.
2.4. Layilin mediates HA35-induced ZO-1 expression in healthy and DSS treated mice
Our previous study showed that HA35 treatment increases ZO-1 expression in both healthy and DSS-treated wild type mice [5]. To investigate whether layilin is involved in the induction of ZO-1 post HA35 treatment in vivo, wild type and Layn −/− mice were gavaged with water or HA35 once daily for 5 days. We collected the distal colon from these mice and performed immunostaining for ZO-1. Analysis of ZO-1 expression levels revealed that HA35 treatment significantly increased ZO-1 expression in wild type mice but not in Layn −/− mice (Fig. 4A and 4B).
Figure 4.
Layilin mediates HA35 induced ZO-1 induction in healthy and DSS-treated mice. A. Sections of distal colon from wild type and Layn−/− mice gavaged once daily with water or HA35 (300µg per mouse) for five days were immunostained for ZO-1 (red). Single plane images were taken with a confocal microscope. B. ZO-1 staining intensity was quantified using 2 stained sections per mouse and 6 mice per group. Quantified ZO-1 intensity was normalized to the number of epithelial cells. C. Groups of wild type and Layn−/− mice were orally gavaged with water or HA35 (300µg per mouse, once daily) for 5 days before, as well as 3 days during 2.5% DSS treatment. After euthanasia, colons were collected for fixation and histologic examination. Transverse and distal colons were immunostained for ZO-1 (red). Images were taken using confocal microscopy of a single plane. D. The intensity of ZO-1 staining was quantified using 2 stained sections per mouse, with 7 mice per group. ZO-1 intensity was normalized to the number of epithelial cells and the statistical significance of difference between water-treated groups and HA35-treated groups was tested using unpaired t-test. (*, p<0.05, **, p<0.01) Error bars = S.E.M.
We previously observed that ZO-1 expression was increased post HA35 treatment not only in the distal colon of healthy mice but also in both transverse and distal colons of the DSS-induced colitis mouse model [5]. We next wanted to investigate layilin involvement in the induction of ZO-1 post HA35 treatment in DSS-treated mice. The treatment with DSS in this model damages the epithelium and initiates bacterial invasion and intestinal inflammation. We gavaged wild type and Layn−/− mice with water or HA35 once daily for 5 days and then 2.5% DSS was administered in drinking water for 3 days. HA35 and water gavage treatment were continued once per day during DSS treatment. Transverse and distal colons were collected from treated mice, fixed and stained for ZO-1. Fluorescent microscopy analysis of ZO-1 staining revealed a significant difference in ZO-1 in transverse and distal colons in wild type, but not in Layn−/− mice at the early stage of DSS-induced colitis (Fig. 4C and 4D). These results demonstrate that the ZO-1-induction post HA35 treatment, in both healthy and mice challenged with DSS, requires layilin.
2.5. ROCK signaling mediates HA35-mediated ZO-1 induction in vitro
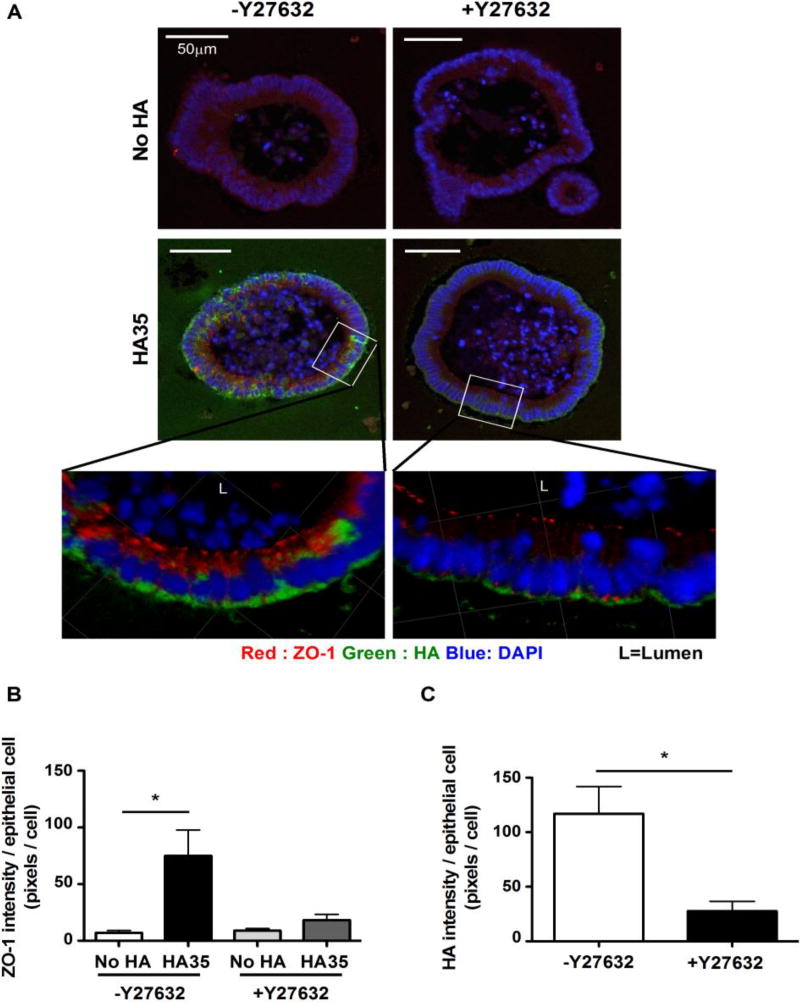
Layilin downstream signaling pathways have not been extensively studied. The only known downstream signaling mechanism of layilin is the RhoA/ROCK pathway [13]. Previous reports showed that HA signals through layilin via RhoA/ROCK in lung epithelial cells and RhoA/ROCK activation increases the ZO-1 expression in human dental pulp cells [13,29]. To test whether ROCK mediates the induction of ZO-1 upon HA35-layilin interaction, we used a highly specific ROCK inhibitor Y-27632 [13], previously shown to stimulate the growth of mouse intestinal epithelial organoids [24]. We used the same effective concentration of Y-27632 (10µM) known to have effects on our mouse intestinal epithelial organoids. Cultured wild type mouse colonic epithelial organoids were treated with medium containing HA35 or no HA for 30 hours with or without Y-27632 and fixed. Organoid sections were immunofluorescently stained to detect ZO-1 and HA. Quantification of ZO-1 protein expression levels indicated that inhibiting ROCK signaling diminishes the induction of ZO-1 post HA35 treatment (Fig. 5A and 5B). Unexpectedly, analysis of organoid-internalized HA revealed that the internalized amount of HA was also significantly reduced in the presence of ROCK inhibitor. These results indicate that ROCK signaling mediates the induction of ZO-1 and is involved in the internalization of HA35 in mouse colonic epithelial cells.
Figure 5.
The inhibition of ROCK kinase decreases HA35-induced ZO-1 levels and affects HA internalization in mouse intestinal organoids. A. Sections of mouse intestinal organoids treated with no HA or HA35 (350µg/ml, for 30h) in the presence or absence of ROCK inhibitor (Y-27632,10µM) were fixed and stained for ZO-1 (red), HA (green), and nuclei (blue). Confocal microscopy was performed to visualize and obtain Z-stack images. B. Quantification of fluorescence from images for multiple organoids (n=13) per condition. ZO-1 intensity was measured as described in “Methods” section and are normalized to the number of epithelial nuclei by manually counting each image. One-tailed unpaired t-test was used for statistical analysis between no HA and the HA35-treated groups (*, p<0.05). C. The intensity of intracellular HA was measured using 16 organoids per condition and normalized to the number of epithelial nuclei. The one-tailed unpaired test was used to test the statistical significance (*, p<0.05). Error bars = S.E.M.
2.6. ZO-1 expression levels are decreased in epithelium of IBD patients but layilin expression is equivalent
Previous studies have shown that ZO-1 protein levels to be lower in IBD patients as compared to non-IBD patients and the ZO-1 decreases with progressing inflammation [30,31]. This latter finding is consistent with the DSS mouse model [32]. In the present study, we investigated whether layilin levels are affected similarly to ZO-1 expression in the epithelium of IBD patients as compared to non-IBD patients. To measure these proteins, we isolated epithelial cells from newly resected human colon tissues using Ethylenediaminetetraacetic acid (EDTA) [33]. Because antibodies that specifically detect human layilin are available, additionally, we confirmed the results with immunostaining of human colonic tissue sections. As seen in Fig. 5A and 5B, Western blot and immunofluorescence analyses confirmed that ZO-1 protein levels are downregulated in colonic epithelium of both UC and CD patients as compared to non-IBD patients. The ‘Non-IBD patients’ included those with diverticulitis, adenocarcinoma, constipation, and fistula. Next, we assessed the layilin protein expression levels in epithelial cells among the same patient groups. Western blot analysis showed layilin to be statistically equivalent in the identical colonic epithelium patient samples used for ZO-1 analysis (Fig. 6C). This result suggested that the decreased ZO-1 protein levels observed in colonic epithelium of IBD patients were not due to deficiency in layilin expression.
Figure 6.
ZO-1 expression is decreased, yet layilin expression is similar, in the intestinal epithelium of IBD patients as compared to non-IBD controls. A. Western blot analysis of ZO-1 protein in epithelial cell lysates from non-IBD, ulcerative colitis (UC), and Crohn’s disease (CD) patients. The same amount of proteins was loaded per lane and the levels of ZO-1 were normalized to the levels of villin-1 (an epithelial cell marker). Representative blots show two different patient samples per group (1 and 2). The relative expression level of ZO-1 in epithelium of non-IBD, UC, and CD patients was calculated using the densitometric quantification (n=11 (non-IBD), n=8 (UC), n=7 (CD)). Unpaired t-test was used to test the statistical significance between groups. (*, p<0.05, ***, p<0.0001) Error bars = S.E.M. B. Sections of colon from non-IBD, UC, and CD patients were immunostained for ZO-1 (red) and nuclei were stained blue (DAPI). Images were obtained using confocal microscopy of a single plane C. Western blot analysis of layilin protein in epithelial cell lysates from non-IBD, UC, and CD patients was performed by loading the same amount of protein. The densitometric quantification of layilin and vilin-1 protein bands was used to calculate the relative expression level of layilin.
3. Discussion
Our previous study has demonstrated that HA35 treatment enhances the epithelial barrier function, in part via promoting expression of the tight junction protein ZO-1 in both healthy and Citrobacter rodentium infected mice[5]. Furthermore, we have shown that HA35 treatment diminishes intestinal permeability at the early stages of DSS-induced colitis in mice. In the present study, we have investigated whether HA35 treatment increases ZO-1 expression in colonic epithelium in vivo through the direct interaction with intestinal epithelial cells and we have identified the HA receptor that mediates HA35 mediated ZO-1 induction. Here we report that HA35 treatment directly enhances the expression of ZO-1 in mouse intestinal epithelial cells and using layilin knockout mice showed that layilin is the primary receptor for the HA induced effects in vitro and in vivo. Additionally, our data underscore that ZO-1 protein levels are lower in colonic epithelium of IBD patients than in non-IBD patients, yet layilin expression levels are similar between the two groups.
HA is a linear polymer, consisting of repeating disaccharides of N-acetyl glucosamine and glucuronic acid and is an important, normally very large, extracellular matrix component in the body that can be degraded under physiological conditions into various sized fragments [6]. To date, the mechanism of HA size specific effects remains unclear. Reports have suggested that a single binding site in traditional HA receptors recognizes 6–10 sugar residues of HA, independent of the length of HA [34]. However, studies including those from our group have indicated that the effects of HA can be very size dependent [5,7,20]. In our previous reports, we found that while HA35 induced the expression of ZO-1, and mBD-3 in mouse colonic epithelium, the large HA2000 did not[5,20]. Similarly HA35, but not larger or smaller HA, can modulate the activation of Kuppfer cells, the resident hepatic macrophages [35]. Forteza, et al.[13] also reported size-dependent effects of HA on lung epithelial cells.
We and others have previously hypothesized that part of the size specificity of HA the effects is due to physical exclusion of large HA from cell surfaces. Both the mucus layer covering the top of the intestinal epithelium in vivo and the cell glycocalyx in vitro may act as barriers limiting the access of large molecular weight HA to its receptors, whereas smaller fragments may penetrate more easily and prevent large HA from mediating its effects[5,36]. Our current study suggests that even though large HA2000 gets access to the surface of mouse intestinal epithelial cells, it is not internalized, while HA35 is taken up readily (Fig. 1A).
Most of the previous studies investigating the HA receptors involved in the recognition of exogenous HA have identified CD44 or TLRs as mediators of HA effects [18–20,25]. Specifically, in terms of the effect of HA on colonic epithelium, our group has shown HA35 treatment induces expression of human beta defensin-2 expression in human colonic epithelial cells in vitro and murine beta defensin-3 in vivo via TLR-4 [20]. Similarly, the Riehl, et al [18] has reported that intraperitoneal injection of HA 750 kDa stimulates stem cell growth in mouse colon through CD44 and TLR-4. In the present study, the observed HA35-dependent ZO-1 induction in mouse colonic epithelium was found to be mediated by an under-investigated HA receptor, layilin. Layilin is known to be localized in membrane ruffles of migratory cells and polarized cells [13,37]. Importantly migration and polarization are physiology features of intestinal epithelium as they move from the crypt base to the luminal surface.
Previous studies investigating layilin in epithelial cells by Forteza et al. have shown that low molecular weight (≤ 35 kDa) HA binding to layilin in lung epithelial cells decreases the expression of E-cadherin, which results in the increased tissue permeability [13]. Interestingly, this effect is not observed in our study, where we observe that HA treatment increases the expression of tight junction protein ZO-1, and leads to a subsequent decrease in the intestinal permeability [5]. Furthermore, we have found that E-cadherin expression levels do not change in mouse intestinal organoids post HA35 treatment. These findings point at a possible significant difference in HA response between the lung and the colonic epithelial cells. Although additional studies are required, one possibility is the presence of CD44 receptor in lung epithelial cells but not in colonic epithelial cells.
While downstream signaling pathways of layilin in general have not been widely studied, Forteza et al. has shown that HA binding to layilin activates RhoA/ROCK signaling and the inhibition of RhoA/ROCK signaling diminishes the HA mediated effects in lung epithelial cells [13]. Futhermore, Xu et al. has shown that the activation of RhoA/ROCK mediates the increase in the expression of ZO-1 in human dental pulp cells [29]. In the present study we show that the inhibition of ROCK signaling diminishes the HA35-meditaed ZO-1 induction (Fig. 5). Noticeably, HA35 uptake was also reduced with ROCK inhibitor treatment in mouse intestinal organoids. Cellular uptake of HA is frequently achieved by endocytosis, and the best understood mechanisms involve CD44 [38]. ROCK signaling is known to regulate actin cytoskeletal dynamics, which is involved in the process of endocytosis [39,40]. Furthermore, a study has shown that the inhibition of ROCK inhibits endocytosis in Drosophila epithelial cells derived from embryos [41]. Our data is consistent with a layilin mediated mechanism of endocytosis of selectively small molecular weight HA.
Until fairly recently, there has not been an in vitro system that represents normal colonic epithelial cells. However, both the Clevers and Stappenbeck laboratories have recently established methods for culture of primary colonic epithelial organoids from the isolated fresh human and mouse crypts derived from intestinal tissues [24,42]. We have successfully adapted the method to culture mouse colonic epithelial organoids which facilitated investigations of the effects of HA35 directly on intestinal epithelial cells [24]. In the future, once we have devised a consistent method for polarizing human intestinal organoids, we will test the effect of HA35 treatment on organoids derived from patients without IBD as well as from those with Crohn’s disease and ulcerative colitis.
Our study demonstrates a novel mechanism of HA35 control of tight junction protein ZO-1 expression in vitro and in vivo by showing the role of the receptor layilin. Furthermore, in the face of downregulated ZO-1 expression in human colonic epithelium of IBD patients, layilin levels are not changed. Therefore our data supports the concept that oral HA35 treatment may be a safe and effective prophylactic treatment for IBD patients to promote intestinal barrier function.
4. Methods
4.1. Animals
All experiments were conducted according to protocols approved by Lerner Research Institute's Institutional Animal Care and Use Committee (IACUC). Wild type C57/Bl6 mice and B6.B10ScN-Tlr4lps-del/JthJ mice (6 to 8 weeks old) were used in the experiments and all were purchased from Jackson Laboratory (Bar Harbor, ME). Heterozygous Layn+/− mice (B6N.129S5-Layntm1Lex/Mmcd) were purchased from the Mutant Mouse Resource & Research Centers (MMRCC) and homozygous Layn−/−mice were generated by breeding heterozygous mice. No obvious abnormalities were observed in Layn−/−mice. Mouse tissue was used to obtain genomic DNA using the protocol from Jackson Laboratory Briefly, tissue samples were incubated in 50µl of 25mM NAOH/0.2mM EDTA for 1 hr at 98°C. 50µl of 40mM Tris HCl (pH 5.5) was added to each sample and then the samples were centrifuged at 9400 g for 3 minutes. 90µl aliquots were used for PCR. The Epicentre Technologies PCR Kit (Madison, WI) was used and the samples processed in a Thermo Scientific HyBaid PCR Express machine (Wilmington, DE) with the following run parameters: 95°C for 30 sec, 53°C for 30 sec, and 72°C for 30 sec, 30 cycles. The amplified PCR products generated by each primer set are as follows: 271 bp Layn wild-type allele (for: 5’ tttggctaagcagaggagc 3’ and rev: 5’ cagactgccaagagaaagc 3’) and 572 bp Layn−/−allele (for: 5’ taggcaaactcagaatctccc 3’ and rev: 5’ ccctaggaatgctcgtcaaga 3’).
4.2. HA preparation
Highly purified, certified endotoxin free, HA fragments were purchased from Lifecore Biomedical, LLC and were used for all experiments. For in vivo studies, HA35 was dissolved at 2 mg/ml in the drinking water provided in our animal housing facility and dissolved with shaking at 4 °C overnight before being aliquoted and frozen in −80 °C until use. For in vitro studies, HA was dissolved in sterile Milli Q purified water (HA35 at 5 mg/ml and HA2000 was dissolved at 2.5 mg/ml, to prevent gel formation).
4.3. DSS-induced epithelial damage model
Dextran sulfate sodium (DSS) induced epithelial damage was achieved as previously described [5]. Briefly, treated mice were provided with water containing 2.5% dextran sodium sulfate (#160110, MP Biomedicals, Solon, OH), and controls given water alone. Mice were weighed and monitored daily for signs of colitis. Mice were sacrificed according to IACUC approved methods on day 3. Colons were removed then fixed overnight in ten times the tissue volume of molecular biology grade Histochoice (AMRESCO, Solon, OH) prior to paraffin embedding and tissue sectioning.
4.4. Isolation of mouse intestinal crypts
The protocol for isolation of mouse intestinal crypts was slightly modified from that reported by the Stappenbeck laboratory [24]. Briefly, mouse distal colons were isolated and rinsed with ice-cold Hank’s buffered saline solution (HBSS). The tissue was minced with fine scissors in 1ml of collagenase solution [(2mg/ml collagenase type I and gentamicin 50µg/ml in washing medium (DMEMF/12 containing 10% FBS, 1%Pen/Strep and 1% L-glut)] and resuspended, using a 1ml pipette, every 10 minutes. Once ~80% of epithelial units appeared digested from the larger tissue fragments, the digest was put into a 100µm cell strainer (Corning, Corning, NY) to separate the lager pieces form the dissociated epithelium. The strainer was rinsed with 9 ml of washing medium and the filtered cell solution was transferred to 15ml test tubes and cells collected (centrifuged at 100g for 5 min). The cell pellet was resuspended in 0.5ml of washing medium and the number of live crypts was counted using a hemocytometer. Crypt samples were centrifuged at 200 g for 5 min and resuspended in matrigel (354277, Corning) at a density of 6000 intact epithelial units/15 µl of matrigel, and then plated into 2 cm2 wells. The cells were cultured in 50% L-WRN cells conditioned media. This conditioned media were generated from L-WRN cells and used as described by Miyoshi, et al[24]. The cultures were incubated at 37°C in 5% CO2 in air atmosphere in a humidified incubator for up to 6 days.
4.5. Culture of mouse intestinal organoids
For epithelial expansion, sub-culture of organoids, and preparation for experiments the protocol reported by Miyoshi, et al[24] was adapted and slightly modified. Once epithelial spheroid cultures were observed in the primary culture, they were treated with dissociation buffer (50µl DTT (1mM) and 50µl EDTA (0.5M) in 50ml PBS) to remove matrigel (0.5ml of dissociation buffer per one well of 24 well plates). Up to 4 wells of dissociated organoids were collected into one 15 ml test tube, and pelleted by centrifugation (200 g for 5 minutes at room temperature). Organoids were resuspended in 1ml of PBS-EDTA (0.5mM EDTA), centrifuged again and then pellets were resuspended in 0.3ml of Trypsin-EDTA (0.25% trypsin in PBS-EDTA). Samples were incubated at 37°C for 2 min, after which 0.4ml of washing medium (DMEMF/12 containing 10% FBS, 1%Pen/Strep and 1% L-glut) was added and the sample vigorously mixed by pipetting 40 times. 5ml of washing medium was mixed with each sample and then centrifuged for 5 min at 200 g. The pellet was resuspended in 1ml and an aliquot was removed for viable cell counting on a hemocytometer. Again the cells were collected (centrifuged for 5 min at 200 g), kept on ice and resuspended in matrigel (354277, Corning, NY). 10,000 cells/15 µl of matrigel were plated into wells of a 24-wells plate (2 cm2 wells). Organoids were cultured in 50% L-WRN cell conditioned media. The cultures were incubated at 37°C in 5% CO2 in air atmosphere in a humidified incubator. For experiments, the medium was changed every other day and on day 6 after plating, HA was added at 350µg/ml for 30 h. For the RhoA/ROCK inhibition experiments, Y-27632 (R&D systems, Minneapolis, MN) was reconstituted in DMSO and stored at −20 °C as 10mM stock aliquots. Mouse intestinal organoids were incubated with Y-27632 (10µM) or an equal volume of vehicle control for 1 hr prior to HA addition.
4.6. Isolation of human epithelial cells
Human epithelial cells were obtained from tissue samples derived from surgically resected colons of patients with Crohn’s Disease (CD) ulcerative colitis (UC) or non-IBD conditions (control). Tissue was provided by the Department of Surgical Pathology at the Cleveland Clinic under an IRB approved protocol. The method to isolate human epithelial cells was previously described [33]. Briefly, the mucosal layer was dissected, and washed and surface debris removed by blotting the tissue with paper towels multiple times. The mucosal layer was then cut into strips (3~5cm) and placed into HBSS. Strips were incubated in 0.15% DTT solution (Sigma D0632) dissolved in HBSS for 30 min, at RT with gentle stirring. Strips were rinsed once in HBSS and then washed three times in 100ml of HBSS containing 1mM EDTA solution for 1 h each time to remove epithelial cells. Epithelial cells used in experiments were collected from the second EDTA wash by centrifugation at 1500 g for 5 min, and washed once in 10ml of PBS. Cell pellets were snap frozen and kept in −80°C until protein analysis was performed.
4.7. Immunofluorescence staining
Human and mouse colon samples were cut at 1–2cm length and fixed in Histochoice (AMRESCO, Solon, OH) at least 18h prior to paraffin embedding and tissue sectioning. Mouse organoids were fixed in Histochoice overnight and embedded in 1% low melting temperature agarose prior to paraffin embedding and tissue sectioning. Cut tissue sections (5µ) were deparaffinized by sequential immersion in the following solutions: Clear- Rite 3 (2 × 3min), Flex 100 (2min, 1min), Flex 95 (2min, 1min) (Richard-Allan Scientific, Kalamazoo, MI), followed by a tap water rinse of more than 1 min. Sections were then incubated in a blocking solution of HBSS containing 2% Fetal Bovine Serum (HBSS+ 2% FBS) for 30 minutes at room temperature. Primary detection reagents were diluted in HBSS+ 2% FBS, applied to the sections and incubated at 4°C for overnight (~18h) in a humidified chamber. For ZO-1 detection, affinity purified rabbit polyclonal antibody against ZO-1 (ThermoFisher Scientific, Waltham, MA) was used at a 1:50 dilution for tissue sections and 1:100 dilution for organoids sections. For HA detection, HABP biotinylated HA binding protein (Calbiochem, Billerica, MA) was used at 1:100 dilution. For E-cadherin detection, rabbit monoclonal antibody against E-cadherin (1:100) (Cell signaling, Danvers, MA) For CD44 detection, rat monoclonal antibody against murine CD44 (1:100) (Abcam, Cambridge, UK) was used. After primary antibody exposure, sections were washed with HBSS three times for 5 minutes each and then incubated with secondary detection reagents diluted in HBSS+ 2% FBS: for ZO-1, goat anti-rabbit Alexa 568 secondary (ThermoFisher Scientific, Waltham, MA) (1:1000 dilution in HBSS+ 2% FBS); for HA detection Alexa-488 Streptavidin (1:1000); for CD44 detection, Alexa 488 goat anti-rat secondary (1:1000) for 1 hr at room temperature. Slides were again washed in HBSS three times for 5 min each. After washing, nucleus staining and mounting were done using Vectashield with DAPI (Vector laboratories, Burlingame, CA). Single plane CD44 and ZO-1 stained sample images were obtained using a Leica DM5500B upright microscope equipped with a Leica DFC425C camera and LAS software (Leica Microsystems, GmbH, Wetzlar, Germany) and a Retiga SRV Cooled CCD camera and QCapture Plus software (QImaging, Surrey, BC Canada). Confocal images of ZO-1 stained cells were obtained using a Leica TCS-SP5II upright confocal/multi-photon microscope (Leica Microsystems, GmbH, Wetzlar, Germany). The quantification of image fluorescence was conducted using Image-Pro Plus computer software (Media Cybernetics, Rockville, MD).
4.8. Western blotting
Epithelial cell samples were lysed in 1X RIPA buffer (Cell Signaling Technology, Danvers, MA) containing 1X Halt Phosphatase inhibitor cocktail (ThermoFisher Scientific, Waltham, MA) and 1X Protease inhibitor cocktail (P8340, Sigma-Aldrich, St. Louis, MO). A Pellet Pestle cordless motor (Fisherbrand, Pittsburgh, PA) was used to homogenize the cell pellets on ice. Samples were centrifuged for 10 min at 20000 g and the supernatants were collected. Mouse intestinal epithelial organoids were collected from 3 wells per sample. After removing media, 500ml of dissociation buffer was added into each well and organoids from 3 wells were collected into 15ml conical tubes. Samples were then centrifuged at 200 g for 5 min. Pellets were washed with 10ml of PBS and lysed in 1X RIPA buffer containing 1X Halt Phosphatase inhibitor cocktail and 1X Protease inhibitor cocktail. Protein concentrations were measured with Pierce BCA Protein assay (ThermoFisher Scientific, Waltham, MA). Samples containing equal amount of protein were mixed with 5µl of lithium dodecyl sulfate (LDS) sample buffer (4X) and 2µl of Sample reducing agent (10X) (both from ThermoFisher Scientific). Mixed samples were boiled at 95°C for 10 min. Proteins were separated using SDS-PAGE with 4~12% NuPAGE Bis-Tris Protein Gels (ThermoFisher Scientific) run at a constant voltage of 200 V for 40 min. Separated proteins were transferred at 4°C to 0.45µm PVDF membranes (Millipore, Billerica, MA) in a solution of 1X Tris/Glycine buffer (Biorad, Hercules, CA) containing 20% methanol at 30 V for ~18h. PVDF membranes were blocked in PBST (PBS with 0.1% tween) containing 5% milk for one hour and then incubated with following primary antibodies at 4°C overnight on a shaker: a rabbit polyclonal antibody against ZO-1 (Thermo Fisher Scientific, Waltham, MA) at 1:1000; a rabbit monoclonal antibody against E-cadherin (Cell signaling, Danvers, MA) at 1:1000; a rabbit polyclonal antibody against Villin-1 (Cell signaling, Danvers, MA) at 1:2000; a mouse monoclonal antibody against human Layilin (Santa Cruz Biotechnology, C-7, Dallas, TX) at 1:500; and a rabbit polyclonal antibody against CD44 (ab157107, abcam, Cambridge, UK) at 1:1000. Afterwards, membranes were washed with PBST three times, for 10 min each, and then incubated with HRP-conjugated donkey polyclonal anti-rabbit IgG (GE Healthcare, Little Chalfont, UK) at 1:20,000 dilution or anti-mouse IgG at 1:20,000 (Sigma-Aldrich, St. Louis, MO) in PBST containing 5% milk for 1hr at room temperature. Membranes were then washed with PBST three times for 10 min each, and protein bands were visualized using Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare, Little Chalfont, UK).
4.9. Quantitative real time-PCR
RNA was isolated from 1cm samples of mouse colon tissue or from 3 wells of organoids using Qiagen RNeasy Mini Kit following the manufacturer’s instructions. The RNA concentration was measured using a Nanodrop ND-1000 spectrophotometer (Thermo Scientific) and 1µg of RNA was used to make cDNA using Ambion M-MLV transcriptase (Thermo Scientific) with Oligo dT and dNTP and followed the manufacturer’s instructions. Quantitative real-time PCR analysis was performed using the CFX96 Real-Time PCR System (Bio-Rad, Hercules, CA) and cDNA product with the TaqMan Gene Expression Master mix (Applied Biosystems) per the manufacturer’s instructions. TaqMan Probes for mouse genes were as follows: Layn (Mm01176974_m1); 18S (Hs99999901_s1).
4.10. Statistical analysis
The statistical difference between groups was evaluated by the unpaired one-tailed Student's t test. All error bars drawn indicate the standard error of mean (SEM). All graphs and statistical analysis was performed with GraphPad Prism version 5.0a (GraphPad Software, Inc., La Jolla, CA).
Supplementary Material
Highlights.
Hyaluronan 35 kDa (HA35) treatment increases ZO-1 expression in mouse intestinal epithelial organoids in vitro.
HA35-mediated ZO-1 induction is TLR-4 and CD44 independent.
Layilin mediates the induction of ZO-1 expression post HA35 treatment in vitro and in vivo.
ZO-1 expression is reduced but layilin expression is similar in the intestinal epithelium of IBD patients as compared to non-IBD controls.
Acknowledgments
We thank Dr. Kelli VanDussen and Dr. Thaddeus Stappenbeck for providing L-WRN cells and sharing their detailed protocols. We also thank the Cleveland Clinic Lerner Research Institute Imaging Core, which provided histology services.
Funding: This research was supported by the National Institutes of Health (HD061918, R21AA024387, and the Programs of Excellence in Glycosciences Grant HL107147), as well as the Cleveland Clinic Innovators Research Fund (all to C. de la M.). M.L. is supported by Peer Reviewed Medical Research Program Grant PR150084 from the Department of Defense. This work utilized the Leica SP5 confocal/multi-photon microscope that was purchased with partial funding from National Institutes of Health SIG grant [1S10RR026820-01].
Abbreviation
- HA35
Hyaluronan 35 kDa
- ZO-1
Zonula occludens-1
- TLR
Toll-like receptor
- DSS
Dextran sulfate sodium
- IBD
Inflammatory bowel disease
- JAM
Junctional adhesion molecule
- ROS
Reactive oxygen species
- RHAMM
receptor for HA mediated motility
- HARE
HA receptor for endocytosis
- LYVE1
lymphatic vessel endothelial HA receptor 1
- ROCK
Rho-associated protein kinase
- RNAi
RNA interference
- mBD-3
murine beta defensin-3
- Milk HA
Hyaluronan purified from human milk
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neunlist M, Van Landeghem L, Mahé MM, Derkinderen P, des Varannes SB, Rolli-Derkinderen M. The digestive neuronal-glial-epithelial unit: a new actor in gut health and disease. Nature Reviews Gastroenterology and Hepatology. 2013;10:90–100. doi: 10.1038/nrgastro.2012.221. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke J-D, Serino M, et al. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zahm J-M, Milliot M, Bresin A, Coraux C, Birembaut P. The effect of hyaluronan on airway mucus transport and airway epithelial barrier integrity: potential application to the cytoprotection of airway tissue. Matrix Biol. 2011;30:389–395. doi: 10.1016/j.matbio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Ghazi K, Deng-Pichon U, Warnet J-M, Rat P. Hyaluronan fragments improve wound healing on in vitro cutaneous model through P2X7 purinoreceptor basal activation: role of molecular weight. PLoS ONE. 2012;7:e48351. doi: 10.1371/journal.pone.0048351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim Y, Kessler SP, Obery DR, Homer CR, McDonald C, de la Motte CA. Hyaluronan 35kDa treatment protects mice from Citrobacter rodentium infection and induces epithelial tight junction protein ZO-1 in vivo. Matrix Biol. 2016 doi: 10.1016/j.matbio.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur. J. Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Cyphert JM, Trempus CS, Garantziotis S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int J Cell Biol. 2015;2015:563818–8. doi: 10.1155/2015/563818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hascall VC, Wang A, Tammi M, Oikari S, Tammi R, Passi A, et al. The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc. Matrix Biol. 2014;35:14–17. doi: 10.1016/j.matbio.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Y, Wu GD, Sadahiro T, Noh S-I, Wang H, Talavera D, et al. Interaction of CD44 and hyaluronic acid enhances biliary epithelial proliferation in cholestatic livers. AJP: Gastrointestinal and Liver Physiology. 2008;295:G305–12. doi: 10.1152/ajpgi.90229.2008. [DOI] [PubMed] [Google Scholar]
- 10.Wolny PM, Banerji S, Gounou C, Brisson AR, Day AJ, Jackson DG, et al. Analysis of CD44-Hyaluronan Interactions in an Artificial Membrane System INSIGHTS INTO THE DISTINCT BINDING PROPERTIES OF HIGH AND LOW MOLECULAR WEIGHT HYALURONAN. Journal of Biological Chemistry. 2010;285:30170–30180. doi: 10.1074/jbc.M110.137562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nature Medicine. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 12.Bono P, Rubin K, Higgins JM, Hynes RO. Layilina novel integral membrane protein, is a hyaluronan receptor. Mol. Biol. Cell. 2001;12:891–900. doi: 10.1091/mbc.12.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forteza RM, Casalino-Matsuda SM, Falcon NS, Valencia Gattas M, Monzon ME. Hyaluronan and layilin mediate loss of airway epithelial barrier function induced by cigarette smoke by decreasing E-cadherin. Journal of Biological Chemistry. 2012;287:42288–42298. doi: 10.1074/jbc.M112.387795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang B, Yang BL, Savani RC, Turley EA. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. Embo J. 1994;13:286–296. doi: 10.1002/j.1460-2075.1994.tb06261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohda D, Morton CJ, Parkar AA, Hatanaka H, Inagaki FM, Campbell ID, et al. Solution structure of the link module: a hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell. 1996;86:767–775. doi: 10.1016/s0092-8674(00)80151-8. [DOI] [PubMed] [Google Scholar]
- 16.Bono P, Cordero E, Johnson K, Borowsky M, Ramesh V, Jacks T, et al. Layilin a cell surface hyaluronan receptor interacts with merlin and radixin. Exp. Cell Res. 2005;308:177–187. doi: 10.1016/j.yexcr.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Zhuo W, Wang Y, Ao X, An J. Down-regulation of layilin a novel hyaluronan receptor, via RNAinterference inhibits invasion and lymphatic metastasis of human lung A549 cells. Biotechnology and Applied Biochemistry. 2008;50:89–96. doi: 10.1042/BA20070138. [DOI] [PubMed] [Google Scholar]
- 18.Riehl TE, Santhanam S, Foster L, Ciorba M, Stenson WF. CD44 and TLR4 mediate hyaluronic acid regulation of Lgr5+ stem cell proliferation crypt fission and intestinal growth in postnatal and adult mice. AJP: Gastrointestinal and Liver Physiology. 2015;309:G874–G887. doi: 10.1152/ajpgi.00123.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill DR, Rho HK, Kessler SP, Amin R, Homer CR, McDonald C, Cowman MK, de la Motte CA. Human milk hyaluronan enhances innate defense of the intestinal epithelium. Journal of Biological Chemistry. 2013;288:29090–29104. doi: 10.1074/jbc.M113.468629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill DR, Kessler SP, Rho HK, Cowman MK, de la Motte CA. Specific-sized Hyaluronan Fragments Promote Expression of Human -Defensin 2 in Intestinal Epithelium. Journal of Biological Chemistry. 2012;287:30610–30624. doi: 10.1074/jbc.M112.356238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodgers LS, Beam MT, Anderson JM, Fanning AS. Epithelial barrier assembly requires coordinated activity of multiple domains of the tight junction protein ZO-1. J. Cell. Sci. 2013;126:1565–1575. doi: 10.1242/jcs.113399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 23.Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated protein, zonula occludens-1 localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc. Natl. Acad. Sci. U.S.a. 1996;93:10779–10784. doi: 10.1073/pnas.93.20.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nature Protocols. 2013;8:2471–2482. doi: 10.1038/nprot.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 26.Zheng L, Riehl TE, Stenson WF. Regulation of Colonic Epithelial Repair in Mice by Toll-Like Receptors and Hyaluronic Acid. Ygast. 2009;137:2041–2051. doi: 10.1053/j.gastro.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuneki M, Madri JA. CD44 Influences Fibroblast Behaviors Via Modulation of Cell-Cell Cell-Matrix Interactions Affecting Survivin and Hippo Pathways. J. Cell. Physiol. 2016;231:731–743. doi: 10.1002/jcp.25123. [DOI] [PubMed] [Google Scholar]
- 28.Uhlén M, Björling E, Agaton C, Szigyarto CA-K, Amini B, Andersen E, et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell Proteomics. 2005;4:1920–1932. doi: 10.1074/mcp.M500279-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Shao M, Pan H, Wang H, Cheng L, Yang H, et al. Novel role of zonula occludens-1: A tight junction protein closely associated with the odontoblast differentiation of human dental pulp cells. Cell Biol. Int. 2016;40:787–795. doi: 10.1002/cbin.10617. [DOI] [PubMed] [Google Scholar]
- 30.Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermüller N, et al. Inflammatory bowel disease is associated with changes of enterocytic junctions. AJP: Gastrointestinal and Liver Physiology. 2001;281:G216–28. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- 31.Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil Transmigration in Inflammatory Bowel Disease Is Associated with Differential Expression of Epithelial Intercellular Junction Proteins. The American Journal of Pathology. 2001;159:2001–2009. doi: 10.1016/S0002-9440(10)63051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the Tight Junction Protein ZO-1 in Dextran Sulfate Sodium Induced Colitis. Journal of Surgical Research. 2007;140:12–19. doi: 10.1016/j.jss.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 33.Youngman KR, Simon PL, West GA, Cominelli F, Rachmilewitz D, Klein JS, et al. Localization of intestinal interleukin 1 activity and protein and gene expression to lamina propria cells. Gastroenterology. 1993;104:749–758. doi: 10.1016/0016-5085(93)91010-F. [DOI] [PubMed] [Google Scholar]
- 34.Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J. Biol. Chem. 2000;275:26967–26975. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- 35.Saikia P, Bellos D, McMullen MR, Pollard KA, De La Motte C, Nagy LE. miR181b-3p and its target importin α5 regulate TLR4 signaling in Kupffer cells and liver injury in mice in response to ethanol. Hepatology. 2017 doi: 10.1002/hep.29144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shakya S, Wang Y, Mack JA, Maytin EV. Hyperglycemia-Induced Changes in Hyaluronan Contribute to Impaired Skin Wound Healing in Diabetes: Review and Perspective. Int J Cell Biol. 2015;2015:701738–11. doi: 10.1155/2015/701738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borowsky ML, Hynes RO. Layilin a novel talin-binding transmembrane protein homologous with C-type lectins is localized in membrane ruffles. J. Cell Biol. 1998;143:429–442. doi: 10.1083/jcb.143.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danielson BT, Knudson CB, Knudson W. Extracellular processing of the cartilage proteoglycan aggregate and its effect on CD44-mediated internalization of hyaluronan. Journal of Biological Chemistry. 2015;290:9555–9570. doi: 10.1074/jbc.M115.643171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Šamaj J, Baluška F, Voigt B, Schlicht M, Volkmann D, Menzel D. Endocytosis, Actin Cytoskeleton, and Signaling. Plant Physiology. 2004;135:1150–1161. doi: 10.1104/pp.104.040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levayer R, Pelissier-Monier A, Lecuit T. Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nat. Cell Biol. 2011;13:529–540. doi: 10.1038/ncb2224. [DOI] [PubMed] [Google Scholar]
- 42.Sato T, Stange DE, Ferrante M, Vries RGJ, Van Es JH, Van den Brink S, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.