Abstract
A significant proportion of patients admitted to intensive care units require tracheostomies for a variety of indications. Continual cuff inflation to facilitate mechanical ventilatory support may mean patients find themselves awake, cooperative and attempting to communicate but unable to do so effectively. Resulting frustration and anxiety can negatively impact upon care. Through participation in the Global Tracheostomy Collaborative, our unit rapidly implemented novel techniques facilitating communication in such patients. In carefully selected and controlled situations, the subglottic suction port of routinely available tracheostomy tubes can be used to deliver a retrograde flow of gas above the cuff to exit via the larynx, facilitating speech. The resulting above cuff vocalisation is described in detail for five general ICU patients at our institution, highlighting the benefits of multidisciplinary care and the increasingly important role of the speech and language therapists in the critically ill.
Keywords: Tracheostomy, communication, vocalisation, rehabilitation of speech and language disorders
Introduction
Tracheostomies are used as artificial airway devices in around 10% of all mechanically ventilated intensive care unit (ICU) admissions in the UK, with exact numbers dependent on the local case mix and to some extent, local practice.1–6 One of the advantages of tracheostomy is that patients can have reductions or cessation of sedative medication, but may find themselves in a situation where they are alert, yet still dependent on positive pressure ventilatory support. This almost universally requires an inflated tube cuff in order to deliver the pressure generated by the ventilator to the lungs, although increasingly it is recognised that patients can be ventilated and indeed weaned successfully with increasing periods of cuff deflation.7
For the majority of these patients who remain significantly ventilator-dependent or have a substantial aspiration risk that prevents cuff deflation, the continued presence of the inflated cuff by necessity ‘seals off’ the upper airway, preventing effective oral communication. Critical care nursing, medical and allied health staff are familiar with attempts to communicate non-verbally with patients in this situation by lip-reading, facial expressions, gestures, attempted writing, typing or the use of communication boards, ranging from simple charts through to complex interactive devices. At present, these interactive systems are often imprecise, cumbersome, costly and prone to breakage.8,9
The nature of critical illness means that patients are often fatigued, with subtle peripheral myopathies and with limb movements further hampered by peripheral vascular or monitoring devices, resulting in frustrating attempts at communication for staff, relatives and most importantly, the patient.10,11 The effect on psychological well-being, delirium and depression of emerging from sedation and finding yourself unable to speak, swallow or communicate are unknown but likely to be additive to the well-documented adverse effects of critical illness.7,12,13 Frustration, anger and low mood can lead to withdrawal from interaction with family and carers and reduced participation in treatment, rehabilitation and the recovery process.14 The role of experienced critical care speech and language therapists (SLT) as part of the multidisciplinary ICU team is key in attempting to overcome communication barriers.15
Endotracheal and tracheostomy tubes that have the ability to drain subglottic secretions via dedicated additional ports are increasingly recognised as one of the effective components of strategies to reduce ventilator-associated pneumonias.16 Through participation in an international tracheostomy quality improvement project (The Global Tracheostomy Collaborative (GTC), www.globaltrach.org) our hospital collaborated with colleagues from the Tracheostomy Review And Management Service (TRAMS) team, based at Austin Healthcare, Melbourne, Australia. Some of TRAMS’s work is with high spinal injury patients, and they were using the same Smiths Medical (Ashford, Kent, UK) Blue Line Ultra Subglottic Suction (SGS) tracheostomy tubes that are in routine use on our ICU in Manchester, UK. TRAMS were using the SGS port in an innovative way to allow speech for patients who were fully ventilated. The GTC QI project supports rapid adoption of international best practices and led to us quickly sharing protocols and experience in this technique. By directing a retrograde flow of gas via the SGS tube to exit above the cuff, patients can theoretically vocalise, with the technique described as above cuff vocalisation (ACV), as demonstrated in Figures 1 and 2. We describe a case series of the innovative use of the SGS port of tracheostomy tubes to facilitate communication in five general ICU patients. Our patients were awake, cooperative and attempting to communicate, but were unable to do so effectively due to the requirement for continually inflated tracheostomy tube cuff for ongoing mechanical ventilatory support.
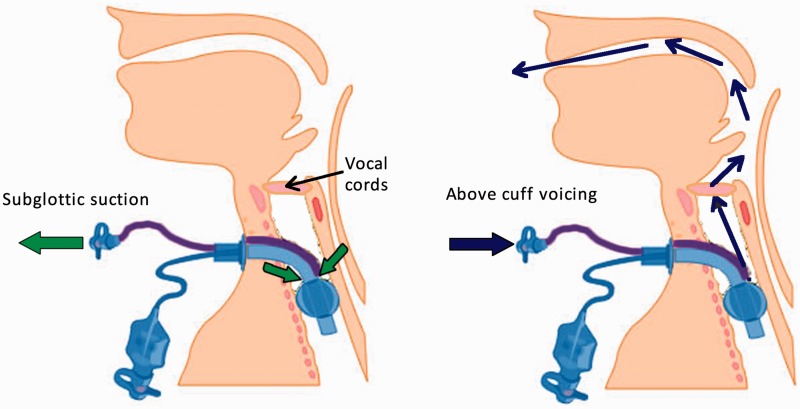
Figure 1.
Tracheostomy tube in situ with subglottic suction ports and tubing indicated. Left-hand figure demonstrates the usual removal of secretions by aspiration. Right-hand figure demonstrates the flow of gas to the upper airways via the larynx when additional gas flow is directed into the subglottic port.
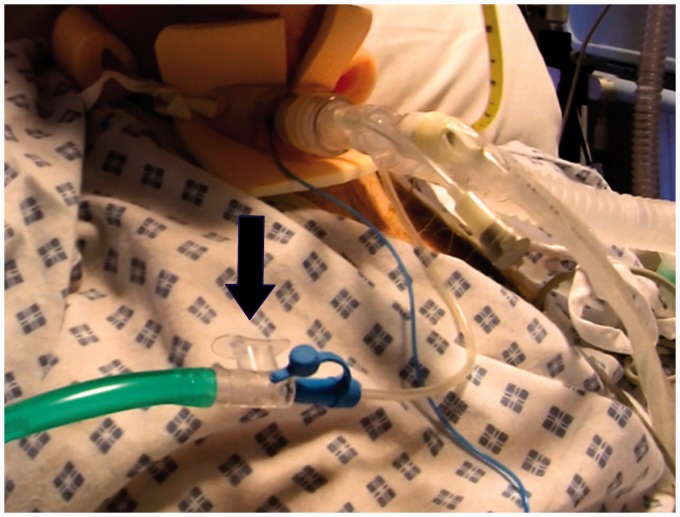
Figure 2.
One of the patients described with cuffed Blue Line Ultra Subglottic suction Tracheostomy tube in situ with the cuff inflated. The green oxygen tubing is connected to the subglottic suction port via the clear open valve (arrow), occluding which will facilitate ACV (with permission).
Case reports
Case 1
A 76-year-old man with a history of asthma and Parkinson’s disease was admitted to hospital with shortness of breath, confusion and suspected infective exacerbation of asthma. He quickly developed Type 2 respiratory failure due to Influenza A and was transferred to ICU for invasive ventilation. He deteriorated further and required partial extracorporeal CO2 removal using a Hemolung® RAS (ALung Technologies, Pittsburgh, US). After seven days of intubation he was tracheostomised and was already showing signs of critical illness myopathy. Three days later he was referred to SLT, alert but confused and unable to follow commands. He was noted to have pulmonary oedema, remaining on ventilator pressure support of 23 cm H2O with the cuff inflated. He demonstrated occasional cuff leak and a whisper voice whilst attempting to mouth words. Two days later he was much more cooperative but high ventilator pressure support precluded cuff deflation or speaking valve trials. Although he had audible upper airway secretions, none were retrieved on SGS. With consent, ACV was trialled and he was able to phonate at 5 l/min flow rate with an audibly wet, breathy voice quality rated 2 on the Therapy Outcome Measure for Voice Impairment scale (TOMS, see Table 1).17 This represented an improvement from TOMS 0 without ACV. His speech was intelligible and although his voice was not ‘normal’ it was sufficient to enable him to speak audibly. Nursing staff and SLT continued with regular 5 min spells of ACV in order to facilitate communication with staff, family and visitors for a further three days until he began to tolerate cuff deflation and his wean progressed rapidly to decannulation two days later. In addition to the benefits of communication and laryngeal sensitivity, effects on swallowing were observed during fibreoptic endoscopic evaluation of swallowing (FEES).18 Without ACV, he silently aspirated secretions and oral fluids but with ACV, aspiration became overt with an active cough response. The laryngeal airflow not only revived vocal fold vibration producing voice but also appeared to improve glottic closure reflexes.19
Table 1.
Therapy outcome measure for voice impairment.
| Score | Description |
|---|---|
| 0 | Severe persistent aphonia Unable to phonate. Does not phonate |
| 1 | Consistent dysphonia Occasional phonation. May be dysphonic with aphonic episodes |
| 2 | Moderate dysphonia Can phonate but frequent episodes of marked vocal impairment occurring |
| 3 | Moderate/mild dysphonia Less frequent episodes of dysphonia (e.g. occurs some time each day/or slight persistent ‘huskiness’) |
| 4 | Mild dysphonia Occasional episodes of dysphonia occurring |
| 5 | No dysphonia Appropriate modal voice consistently used |
Source: Adapted from Enderby.17
Case 2
A 41-year-old man with severe Chronic Obstructive Pulmonary Disease (COPD) following smoking and cannabis use was admitted to hospital for a double lung transplant. He remained sedated and invasively ventilated via an oral endotracheal tube for 12 days post-transplant, requiring veno-arterial and veno-venous extra-corporeal membrane oxygenation (VA and VV ECMO) for eight days of this period. He was tracheostomised at day 12 and sedation was reduced and stopped by day 14. Ventilator weaning was very prolonged due to critical illness myopathy, pleural effusions and anxiety and he required continual cuff inflation to facilitate positive pressure support. By five weeks post-transplant, he was getting frustrated with mouthing words and he was referred to SLT for communication and swallowing assessment. SLT performed an ACV trial and he achieved audible voice, easily scoring a TOMS 4. On FEES he was observed to have a sluggish left vocal cord but this hardly impacted on voice quality. He continued to use ACV for four further days communicating very effectively until cuff deflation was established and he was decannulated a few days later. At times he was observed to burp which may have been a consequence of ACV airflow causing aerophagia.20
Case 3
A 76-year-old lady with a background of COPD, hypertension, presbycusis (age-related hearing loss) and non-insulin dependent diabetes underwent a left upper lobectomy for lung carcinoma. She had a failed extubation post-operatively and was re-intubated then tracheostomised two days later. Within five days she was awake but intermittently drowsy and was attempting to communicate by mouthing words with variable intelligibility. She was too confused and agitated to lip-read or use writing or picture/word charts and had unreliable yes/no head nod and shake responses. Following SLT assessment, an ACV trial was agreed, given her high ventilation requirements and need for cuff inflation. On 3 and 5 l/min flow rates she achieved audible whisper but no voice and airflow sounded turbulent and restricted. Despite this, her speech became more intelligible over the following two days, communication interactions became easier and no desaturation or respiratory distress occurred. Her hearing impairment did impact but she benefitted from communication partners writing down conversation.
Because of her limited voice, we considered a potential problem with upper airway patency and ACV was stopped. However, FEES was not carried out as cuff deflation followed quickly and swallow function was assessed by SLT as safe. With cuff deflation, she tolerated the speaking valve poorly and experienced ongoing dysphonia (gruff, strained voice). This prompted ENT assessment and the vocal folds were visualised and found in a paramedian position and weak, confirming the signs observed. She was decannulated 10 days later and her dysphonia gradually improved, suggesting causation as likely intubation trauma and recurrent laryngeal nerve trauma during thoracic surgery.21 Upper airway obstruction is a likely contraindication for using ACV although further research is needed on the optimal timing and use of endoscopy for establishing this prior to trials.
Case 4
A 41-year-old man with community-acquired pneumonia was admitted to ICU following deterioration requiring intubation and ventilation but developed acute respiratory distress syndrome necessitating VV ECMO for 34 days. He was previously fit and well, but profoundly deaf since birth, resulting in him communicating as both a deaf speaker and sign language user. He developed severe critical illness myopathy related to high dose steroids and was unable to use his upper limbs or even lift his head off the pillow. He was tracheostomised following 11 days of intubation and was totally ventilator dependent. Once awake he was attempting to communicate by mouthing words and was referred to SLT for communication and swallowing advice. He had bulbar signs of myopathy reducing his intelligibility and was becoming increasingly frustrated and angry with his communication difficulties, impacting his compliance with nursing and medical care. Since cuff deflation was impossible due to a ventilator pressure support of 25 cm H2O, a trial of ACV on 6 l/min was carried out and produced a completely normal voice at the first use within a few minutes (TOMS 5). ACV was subsequently performed every day for 5 min spells to facilitate communication with staff and relatives. A small amount of secretions were removed by SGS each time and a communication partner was required to occlude the airflow port but otherwise no difficulties were encountered. He continued to use ACV for the next month as tolerance of cuff deflation remained poor despite sprint weaning and he sustained a strong, loud voice. Interestingly, he was initially totally aphagic due to myopathy of swallowing musculature and prolonged cuff intubation and inflation but his swallow function improved quicker than anticipated. It is postulated but not clinically proven as yet, that the repeated airflow on ACV facilitated re-sensitisation of the larynx, improving airway protection and swallow strength.19
Case 5
A 30-year-old homeless man sustained 45% burns to face, neck, both arms and hands following a fire in his tent. He was previously well with a background of alcohol and substance abuse. He was admitted to the burns unit 24 h after the event after being found by a passer-by. In addition to dermal burns, he presented with stridor, hoarseness, periorbital oedema and underwent a difficult intubation. Bronchoscopy revealed inhalation burns to the posterior tongue, pharynx, glottis, vocal cords and trachea with soot and slough in the pharynx and larynx. He was sedated and ventilated and repeat bronchoscopy showed minimal laryngeal oedema but carbonaceous sputum. He had a failed extubation at day 8 but was successfully extubated onto high-flow nasal cannula oxygen at day 10, and assessed by SLT regularly over the following week. He was drooling, with a weak voice, unsafe swallow and agitation. At day 20, he was re-intubated and tracheostomised due to respiratory failure, high sputum load and secretion aspiration. He was ventilated with the cuff inflated for a further week and found communication extremely frustrating, increasing his agitation and non-compliance with treatments. Bronchoscopy and ENT fibreoptic nasendoscopy showed significant improvement in laryngopharyngeal inhalation injury and it was decided to trial ACV in order to facilitate communication. He was unable to achieve voice (TOMS 0) and secretions were propelled up into the oropharynx stimulating a cough and swallow. Minimal benefit was perceived from ACV and cooperation was limited, which extended to all therapeutic communication strategies suggested by SLT. He remained aphonic and severely dysphagic following decannulation five days later. Repeat FEES demonstrated poor glottic closure, epiglottic and vocal fold oedema and silent aspiration of secretions and oral intake as a result of intubation and inhalation trauma. He was discharged with a percutaneous endoscopic gastrostomy and remained aphonic due to a permanently altered larynx. This case illustrates the need for cooperation and intact laryngeal function for ACV to be successful, although in such complex cases options for communication are very limited.
Videos of some of these patients using ACV (with appropriate permissions) and animations describing the flow of gas are available via the Health Foundation website at www.health.org.uk/blog/giving-voice-critically-ill-patients-literally.
Discussion
We have described how verbal communication is possible in typical ICU patients in a carefully controlled and directly supervised environment using Blue Line Ultra SGS tracheostomy tubes in common use. We are not the first group to describe the successful use of additional airflow to facilitate ACV in specialist populations, but have recognised the potential application of this technique as part of the armoury of communication aids available to the general ICU population managed with tracheostomies. The following is a brief overview of related techniques to facilitate oral communication in the tracheostomy-ventilated patient.
In order to achieve adequate voice, a subglottic (tracheal) pressure of a least 2 cm H2O is required as a minimum, with tracheal pressures of 5--10 cm H2O described during normal speech with flows of 50–300 ml/s (3–18 l/min).22,23 Whilst it is not possible to replicate these physiological conditions in the patient with a cuffed tracheostomy, strategies exist to allow the patient enough glottic airflow to vocalise. These can be considered as scenarios and systems where the cuff remains inflated or the cuff is at least partially deflated. In practice, different options may be appropriate for patients at different stages of their critical illness.
Cuff deflation
For a patient to tolerate cuff deflation, they must have an appropriate secretion load and adequate clearance, be at an acceptable risk of aspiration into the airway and have adequate respiratory mechanics to be able to tolerate the reduction in positive pressure support. Speech can be achieved by simple cuff deflation, assuming that there is enough space between the outer tracheostomy tube and the trachea for adequate flows of exhaled gas to pass, that the larynx is functional and that the upper airways are patent. This method of vocalisation is often referred to as ‘leak speech’. Using a fenestrated tracheostomy tube system may increase the amount of gas passing through the larynx. Fenestrated tubes or cuff deflation effectively divert gas flow away from the patients lungs, which in most cases will reduce the effectiveness of ventilation (Figure 3). Specific ventilator strategies and ventilators that will tolerate and compensate for such volume loss can be used in this situation.24
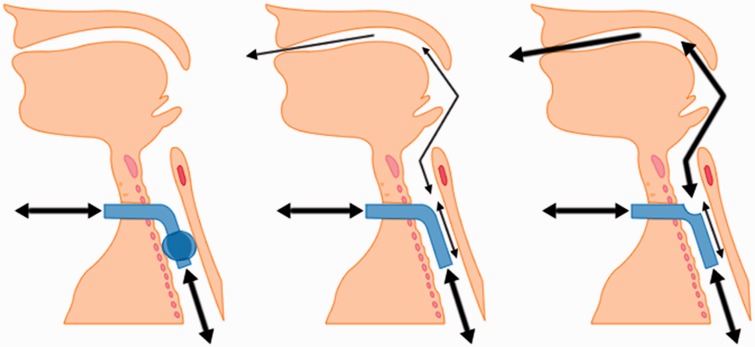
Figure 3.
Gas flows through correctly positioned tracheostomy tubes: Left-hand figure shows a cuff-inflated tube with gas flow excluded from the upper airway. Centre figure shows gas flow in ‘leak speech’, with the cuff deflated (or not present). Gas flows via the tracheostomy tube, but also a small amount via the upper airways. Right-hand figure shows increased airflow via the upper airways by adding a fenestration to the tracheostomy tube.
The amount of airflow that exits via the upper airways and larynx may be increased by the use of a one-way speaking valve.25 These valves are placed between the ventilator and tracheostomy tube and are ‘open’ in inspiration, allowing normal ventilation of the lungs. In expiration, however, the valve closes and gas flow cannot exit via the lumen of the tracheostomy tube. Gas must therefore pass around the tube in the trachea to the upper airways, traversing the larynx and potentially allowing speech. The obvious requirements in this situation are a deflated (or no) cuff and a patent upper airway, and significant harm has been described when using these valves.26 These valves can be used with or without a ventilator, although any ventilator must be able to compensate for the significantly increased leakage and escape of gas from the circuit via the upper airways.25,27 If the patient is able to co-ordinate respiration and movement, simple digital occlusion of the tracheostomy tube will drive expired airflow through the larynx. Newer tubes have been described that incorporate the one-way valve mechanism within a dedicated ‘speaking’ fenestrated inner cannula.28
Cuff remains at least partially inflated
If the cuff cannot be continually deflated for clinical reasons, there remain a number of options to facilitate speech. Dedicated ‘speaking tubes’ have dynamic cuffs whereby during inspiration, the cuff expands and provides an effective seal of the airway. The cuff deflates in expiration and allows exhaled gas to at least partially bypass the tracheostomy tube. Gas is exhaled via both the upper airways and the tracheostomy tube itself.29
With a fenestrated tracheostomy tube (with an appropriately fenestrated inner cannula in situ) gas flow is directed partially via the upper airways from the lumen of the tracheostomy tube. The cuff can remain inflated. In this configuration, positive pressure ventilation will be at least partially directed via the upper airways in inspiration and exhaled gas will similarly exit. Vocalisation is theoretically possible therefore in inspiration and expiration. The tracheostomy tube does not protect from aspiration as there remains an open communication from the upper airways into the lungs. A number of commercially available cuffed fenestrated tubes exist, and modifications to existing tubes have also been described by fashioning bespoke fenestrations to facilitate speech.30 Tubes with dynamic fenestrations are also commercially available which allow the fenestrations to open in expiration only.31
If the cuff remains permanently inflated and the tube is un-fenestrated, vocalisation is possible using an additional gas supply that exits above the cuff. There have been a number of these types of tubes described, with ‘Speaking tracheostomy tubes with the cuff inflated’ reported as early as 1975.32 Other variations on this technique were described using single or multiple gas ports and generally reported successful quiet speech in small case series.33–37 Because laryngeal gas flow and mechanical ventilation of the lungs continue independently by two separate gas supplies, this technique effectively decouples speech and breathing. There is no loss of ventilation during speech with this device, and the inflated cuff reduces the risk of aspiration.23 This classification of vocalisation strategy has become known as ACV.
The potential for ACV in the acutely ill critical care population
With the increasing use of SGS tubes in an attempt to reduce VAP, many tracheostomised ICU patients will have a device in situ that could allow attempts at vocalisation in certain circumstances. Husain described the use of Blue Line Ultra SGS tubes for ACV in spinal injury patients in 2011.38 Although this particular tube was designed for dedicated aspiration of secretions from the subglottic space, there may be advantages over some other tubes specifically designed for ACV.20 The ‘speech lumen’ (SGS port) diameter is larger, the inner cannula is not corrugated and the SGS lumen can be flushed with saline for patency. Perhaps the biggest advantage is that the tracheostomy tube initially inserted to facilitate ongoing airway maintenance can be used for attempts at ACV without having to change the tube for another device. This reduces the need for a specific tube change to facilitate speech, which is a disadvantage of other dedicated speaking tubes.23
There are a number of potential limitations to the use of ACV via a SGS or additional port for vocalisation. Voice quality may be limited and in our patients voice quality was limited to little more than a whisper in some, as reported in other series. Voice quality may improve with higher translaryngeal gas flows but this can be associated with a potentially greater risk of airway injury.37,39 If the resistance to airflow retrograde through the stoma is less than that through the upper airway, much of the added flow may leak from the stomal site and not be available for speech.36,40 This may be less of a problem with percutaneously formed stomas.36 The TRAMS team pragmatically does not recommend the use of ACV before 72 h following new tracheostomy and limit gas flows to 5 l/min. It should be stressed that complications should be minimised by appropriate bedside supervision of trials of ACV by an experienced multidisciplinary ICU team, including an appropriately trained SLT.
The delivery of gas flow via the SGS tube may also cause potential problems. Dry gas or high concentrations of oxygen are likely to have a drying effect on the laryngeal mucosa and hyperadduction of the vocal folds in response to translaryngeal airflow may occur. Humidifying gas delivered via a small calibre SGS tube is possible but requires modifications to existing systems. The delivery of gas into the subglottic region relies on a patent upper airway to decompress the space. Gas may be swallowed or potentially be delivered into the soft tissues and cause subcutaneous emphysema if the tube becomes displaced. Most systems have an open connector between the SGS port and the external gas supply that requires occlusion in order to facilitate speech. This requires a degree of strength and co-ordination in the ICU patient, or assistance from staff or carers, although automated systems have been described.35 If a closed system were used that delivers continual flow to the subglottic space, a (low) pressure relief valve would be a sensible addition to the circuit. In keeping with previous reports, our experience suggests that several days of ACV use may be necessary before the patient is able to develop voice with ACV and in some patients, voice may not be possible.23,37,39 From our limited observational data, there were no clear predictors of early, successful voicing.
Finally, upper airway secretions may also interfere with the quality of voice, and secretions above the cuff can lead to a blocked gas flow line.23 This problem should be minimised by the continued use of the SGS port for secretion clearance. We have observed anecdotally that the management of oral and laryngeal secretions during ACV seemed to improve in the two patients who underwent concomitant FEES during a trial of ACV. This phenomenon is worthy of further study as the flow of gas through the larynx may facilitate the clearance of subglottic secretions. This could be an additional benefit of ACV over and above that benefit from earlier laryngeal recovery after intubation that would be expected from encouraging translaryngeal gas flow.
In conclusion, we have described how effective verbal communication is possible in a carefully controlled and directly supervised environment using Blue Line Ultra SGS tracheostomy tubes that are in common use amongst critical care patients. This report also highlights the benefits of collaboration through networks such as the GTC in driving rapid adoption of techniques that translate immediate benefits to patients. Guidelines for more widespread safe use of these and other tracheostomy tubes should be developed jointly between SLT practitioners and the multidisciplinary ICU team. In addition to communication benefits, the effect of ACV on secretion management, recovery of laryngeal function, decannulation times and ventilator-associated pneumonias are currently unknown. Further detailed studies are required to determine parameters for safe use, clarify patient selection and techniques to ensure our patients have the best chance of successful verbal communication during their ICU management.
Acknowledgements
The authors gratefully acknowledge the assistance of the Global Tracheostomy Collaborative (www.globaltrach.org) in sharing of experience and protocols regarding the use of ACV, especially that of Mrs Tanis Cameron and the TRAMS team, Austin Health, Melbourne (www.tracheostomyteam.org).
Declaration of Conflicting Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This work was carried out as part of UHSMs Health Foundation Funded ‘Shine’ Tracheostomy Quality Improvement Project.
BAM is the current European Lead of the Global Tracheostomy Collaborative.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors have received unrestricted funding from Smiths Medical to evaluate BLUS tubes for the purposes of ACV in a future study.
Consent
All patients discussed in this case series have given their written consent to publication, have been offered a draft of the final manuscript and the Trust Cadicott Guardian has given her written consent to publication.
References
- 1.Wilkinson KA, Martin IC, Freeth, et al. NCEPOD: on the right Trach? www.ncepod.org.uk/2014tc.htm (accessed 20 October 2014).
- 2.Fischler L, Erhart S, Kleger GR, et al. Prevalence of tracheostomy in ICU patients. A nation-wide survey in Switzerland. Intensive Care Med 2000; 26: 1428–1433. [DOI] [PubMed] [Google Scholar]
- 3.Blot F, Melot C. Commission d’Epidémiologie et de Recherche Clinique. Indications, timing, and techniques of tracheostomy in 152 French ICUs. Chest 2005; 127: 1347–1352. [DOI] [PubMed] [Google Scholar]
- 4.Nathens AB, Rivara FP, Mack CD, et al. Variations in rates of tracheostomy in the critically ill trauma patient. Crit Care Med 2006; 34: 2919–2924. [DOI] [PubMed] [Google Scholar]
- 5.Veenith T, Ganeshamoorthy S, Standley T, et al. Intensive care unit tracheostomy: a snapshot of UK practice. Int Arch Med 2008; 1: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGrath BA, Ramsaran R, Columb MO. Estimating the number of tracheostomies performed in critical care in England. Br J Anaesth 2012; 109: 662P. [Google Scholar]
- 7.Engels PT, Bagshaw SM, Meier M, et al. Tracheostomy: from insertion to decannulation. Can J Surg 2009; 52: 427–433. [PMC free article] [PubMed] [Google Scholar]
- 8.Adam SI, Srinet P, Aronberg RM, et al. Verbal communication with the Blom low profile and Passy-Muir one-way tracheotomy tube speaking valves. J Commun Disord 2015; 56: 40–46. [DOI] [PubMed] [Google Scholar]
- 9.Hashmi NK, Ransom E, Nardone H, et al. Quality of life and self-image in patients undergoing tracheostomy. Laryngoscope 2010; 120: S196. [DOI] [PubMed] [Google Scholar]
- 10.Flinterud SI, Andershed B. Transitions in the communication experiences of tracheostomised patients in intensive care: a qualitative descriptive study. J Clin Nurs 2015; 24: 2295–2304. [DOI] [PubMed] [Google Scholar]
- 11.Happ MB, Seaman JB, Nilsen ML, et al. The number of mechanically ventilated ICU patients meeting communication criteria. Heart Lung 2015; 44: 45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalaila R, Zbidat W, Anwar K, et al. Communication difficulties and psychoemotional distress in patients receiving mechanical ventilation. Am J Crit Care 2011; 20: 470–479. [DOI] [PubMed] [Google Scholar]
- 13.Bach JR, Gonçalves MR, Rodriguez PL, et al. Cuff deflation: rehabilitation in critical care. Am J Phys Med Rehabil 2014; 93: 719–723. [DOI] [PubMed] [Google Scholar]
- 14.Magnus VS, Turkington L. Communication interaction in ICU-patient and staff experiences and perceptions. Intensive Crit Care Nurs 2006; 22: 167–180. [DOI] [PubMed] [Google Scholar]
- 15.McGrath BA, Wallace S. The UK National Tracheostomy Safety Project and the role of speech and language therapists. Curr Opin Otolaryngol Head Neck Surg 2014; 22: 181–187. [DOI] [PubMed] [Google Scholar]
- 16.Frost SA, Azeem A, Alexandrou E, et al. Subglottic secretion drainage for preventing ventilator associated pneumonia: a meta-analysis. Aust Crit Care 2013; 26: 180–188. [DOI] [PubMed] [Google Scholar]
- 17.John A, Enderby P. Reliability of speech and language therapists using therapy outcome measures. Int J Lang Commun Disord 2000; 35: 287–302. [DOI] [PubMed] [Google Scholar]
- 18.Kelly AM, McLaughlin C, Wallace S, et al. Fibreoptic endoscopic evaluation of swallowing (FEES): the role of speech and language therapy. Royal College of Speech and Language Therapists Position Paper RCSLT 2015, www.rcslt.org (accessed 4 July 2015).
- 19.Leder SB and Suiter DM. Deglutition in patients with tracheostomy, nasogastric tubes and orogastric tubes. In: Shaker R, Belfasky PC, Postma GN, Easterby C (eds) Principles of deglutition: a multidisciplinary text for swallowing and its disorders. New York: Springer-Verlag, 2013, pp.461–483.
- 20.Pandian V, Smith CP, Cole TK, et al. Optimizing communication in mechanically ventilated patients. J Med Speech Lang Pathol 2014; 21: 309–318. [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamin B. Laryngeal trauma from intubation: endoscopic evaluation and classification. In: Pasha (ed.) Otolaryngology head & neck surgery. 3rd ed. St Louis: Plural Publishing, 1998, pp.2013–2035.
- 22.Holmberg EB, Hillman RE, Perkell JS. Glottal airflow and transglottal air pressure measurements for male and female speakers in soft, normal, and loud voice. J Acoust Soc Am 1988; 84: 511–529. [DOI] [PubMed] [Google Scholar]
- 23.Hess DR. Facilitating speech in the patient with a tracheostomy. Respir Care 2005; 50: 519–525. [PubMed] [Google Scholar]
- 24.Conway DH, Mackie C. The effects of tracheostomy cuff deflation during continuous positive airway pressure. Anaesthesia 2004; 59: 652–657. [DOI] [PubMed] [Google Scholar]
- 25.Sutt A-L, Cornwell P, Mullany D, et al. The use of tracheostomy speaking valves in mechanically ventilated patients results in improved communication and does not prolong ventilation time in cardiothoracic intensive care unit patients. J Crit Care 2015; 30: 491–494. [DOI] [PubMed] [Google Scholar]
- 26.Thomas AN, McGrath BA. Patient safety incidents associated with airway devices in critical care: a review of reports to the UK National Patient Safety Agency. Anaesthesia 2009; 64: 358–365. [DOI] [PubMed] [Google Scholar]
- 27.Egbers PH, Bultsma R, Middelkamp H, et al. Enabling speech in ICU patients during mechanical ventilation. Intensive Care Med 2014; 40: 1057–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adam SI, Srinet P, Aronberg RM, Rosenberg G, Leder SB. Verbal communication with the Blom low profile and Passy-Muir one-way tracheotomy tube speaking valves. Journal of Communication Disorders 2015; 56: 40–46. [DOI] [PubMed] [Google Scholar]
- 29.Nomori H. Tracheostomy tube enabling speech during mechanical ventilation. Chest 2004; 125: 1046–1051. [DOI] [PubMed] [Google Scholar]
- 30.la Cruz de M, Islam S, Cloyes R. Novel modification of tracheostomy tube to allow speech and manage tracheal stenosis. BMJ Case Rep 2013. http://casereports.bmj.com/content/2013/bcr-2013-200622.full.pdf (accessed 4 July 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunduk M, Appel K, Tunc M, et al. Preliminary report of laryngeal phonation during mechanical ventilation via a new cuffed tracheostomy tube. Respir Care 2010; 55: 1661–1670. [PubMed] [Google Scholar]
- 32.Safar P, Grenvik A. Speaking cuffed tracheostomy tube. Crit Care Med 1975; 3: 23–26. [DOI] [PubMed] [Google Scholar]
- 33.Saul A, Bergström B. A new permanent tracheostomy tube-speech valve system. Laryngoscope 1979; 89: 980–983. [PubMed] [Google Scholar]
- 34.Kluin KJ, Maynard F, Bogdasarian RS. The patient requiring mechanical ventilatory support: use of the cuffed tracheostomy “talk” tube to establish phonation. Otolaryngol Head Neck Surg 1984; 92: 625–627. [DOI] [PubMed] [Google Scholar]
- 35.Levine SP, Koester DJ, Kett RL. Independently activated talking tracheostomy systems for quadriplegic patients. Arch Phys Med Rehabil 1987; 68: 571–573. [PubMed] [Google Scholar]
- 36.Sparker AW, Robbins KT, Nevlud GN, et al. A prospective evaluation of speaking tracheostomy tubes for ventilator dependent patients. Laryngoscope 1987; 97: 89–92. [DOI] [PubMed] [Google Scholar]
- 37.Leder SB, Traquina DN. Voice intensity of patients using a Communi-Trach I cuffed speaking tracheostomy tube. Laryngoscope 1989; 99: 744–747. [PubMed] [Google Scholar]
- 38.Husain T, Gatward JJ, Harris RD. Use of subglottic suction port to enable verbal communication in ventilator-dependent patients. Am J Respir Crit Care Med 2011; 184: 384. [DOI] [PubMed] [Google Scholar]
- 39.Leder SB. Verbal communication for the ventilator-dependent patient: voice intensity with the Portex “Talk” tracheostomy tube. Laryngoscope 1990; 100: 1116–1121. [DOI] [PubMed] [Google Scholar]
- 40.Leder SB, Astrachan DI. Stomal complications and airflow line problems of the Communi-Trach I cuffed talking tracheotomy tube. Laryngoscope 1989; 99: 194–196. [DOI] [PubMed] [Google Scholar]