Abstract
A 30-year-old immunocompetent female presented with right flank pain since 3 years. MRI revealed a large well-defined T1 and T2 hypointense mildly enhancing lesion in the right anterior pararenal space displacing the right kidney and encasing the right ureter with T2 hyperintense wall thickening of the left renal pelvis and ureter. A provisional diagnosis of solitary fibrous tumour was kept. Bilateral double J stenting was done for hydronephrosis. Surgical debulking of the lesion was done with biopsy from the left periureteral wall thickening and was found to be myelolipoma on histopathological examination. This case is a novel variety of myelolipoma which is lipid poor, extra-adrenal and in bilateral perirenal and periureteric location.
Keywords: radiology, hematuria, urological surgery
Background
Myelolipoma is a benign non-functioning tumour which comprises mature adipose tissue with varying amounts of haematopoietic cells. It is an unusual tumour with an overall incidence of <1% at autopsy.1 The most common site for this tumour is the adrenal gland. Rarely, it occurs in extra-adrenal location, most commonly in the presacral space.2–6 It is difficult to distinguish between myelolipoma and other tumours containing adipose tissue and haematopoietic precursors on imaging as well as histopathology. Frozen section study is also difficult in such cases owing to the lipomatous component.7
The present case is a new variety of extra-adrenal myelolipoma which was found to be lipid poor on histopathological examination. Unlike other cases of bilateral extra-adrenal myelolipomas which are usually in perirenal location, this tumour was encasing both ureters as well.8
Case presentation
A 30-year-old female presented to us with complaint of right flank pain since 3 years and history of two episodes of mild self-resolving haematuria during this period. There was no abdominal distension or lump and no history of passage of stones in urine. She was evaluated elsewhere with ultrasound of the abdomen which revealed bilateral hydronephrosis and dilated upper ureters with smooth tapering. For this, she also underwent bilateral double J stenting elsewhere before presenting to us. At presentation, the abdominal examination was unremarkable.
Investigations
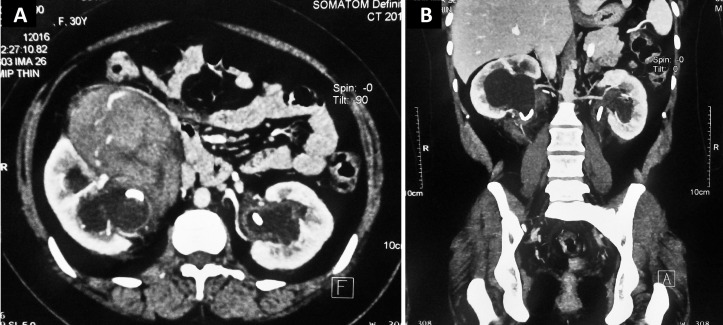
Patient’s serum creatinine was 1.1 mg/dL (normal: 0.6–1.2 mg/dL) and haemoglobin was 11.5 g/dL (normal: 12.0–16.0 g/dL). Contrast-enhanced CT abdomen revealed enhancing soft tissue density mass in the retroperitoneum in bilateral perirenal and periureteric location along with bilateral hydroureteronephrosis (figure 1). The largest lesion was present in the right perinephric space (12×8×6 cm). There was no midline communication. Contrast-enhanced MRI was done for better evaluation of the lesion. It revealed a large well-defined T1 and T2 hypointense mild inhomogeneously enhancing lesion in the right anterior pararenal space, compressing and displacing the right ureter posterolaterally (figures 2 and 3). The lesion encased the right mid-ureter just above the pelvic brim and displaced the ascending colon anteriorly. No internal heterogeneity or hyperintense areas were seen within the lesion. Thin rim of T2 hyperintense fluid was seen surrounding the lesion. There was T2 hyperintense enhancing thickening along the left proximal and mid-ureter. Diffusion-weighted imaging showed areas of mild restricted diffusion within the lesion on both sides. Apparent diffusion coefficient was 1.2×10−3 mm2/s. Bilateral hydronephrosis was still seen along with double J stents in situ. Urinary bladder was normal. There was no retroperitoneal or pelvic lymphadenopathy.
Figure 1.
Contrast-enhanced CT abdomen in (A) axial and (B) coronal plane showing enhancing soft tissue density mass in the retroperitoneum in bilateral perirenal and periureteric location along with bilateral hydroureteronephrosis. Bilateral double J stents are seen in situ.
Figure 2.

(A) and (B) T2-weighted images and T2 fat-saturated axial image of abdomen at the level of lower pole of kidney demonstrate a well-defined hypointense lesion in the right anterior pararenal space. The lesion abuts the inferior vena cava and displaces the descending colon anteriorly. There is also diffuse T2 hyperintense wall thickening of left ureter (arrow). (C) and (D) T1-weighted Fast Spoiled Gradient-Echo (FSPGR) precontrast and postcontrast images at the same level demonstrate the lesion to be T1 hypointense with mild inhomogeneous enhancement. There is also mild enhancement of the left ureteric wall thickening (arrow).
Figure 3.

(A) and (B) T1-weighted Liver Acquisition with Volume Acquisition (LAVA) postcontrast coronal and sagittal images demonstrate the extent of the lesion from the lower pole of right kidney to the level of mid ureter. (C) Diffusion-weighted imaging axial image demonstrates areas of mild restricted diffusion within the lesion on the right side and in the mural thickening of left upper ureter. (D) Heavily T2-weighted maximum intensity projection image in coronal plane demonstrates marked compression of the right proximal ureter with displacement of the mid-ureter laterally. Bilateral hydronephrosis and irregularity of the left ureter are also noted.
Differential diagnosis
Benign mesenchymal tumour such as visceral solitary fibrous tumour: suspected due to the imaging findings and insidious clinical presentation.
Lymphoma: suspected because of bilateral retroperitoneal soft tissue involvement.
Extramedullary haematopoiesis: suspected due to the typical age of patient and location of mass.
Treatment
Ultrasound-guided trucut biopsy of the lesion was done which revealed inflammatory infiltrate only. Next, laparoscopic biopsy was done which revealed lymphocyte and plasma cell rich infiltrates. Immunohistochemistry for IgG4 levels was negative.
The patient underwent exploratory laparotomy which revealed a large mass densely adhered to the ureter. Debulking of the mass was done and biopsy was taken from the left periureteral lesion.
Histopathological evaluation of the excised mass revealed sheets of haematopoietic cells comprising occasional megakaryocytes, few myeloid cells, clusters of normoblasts, lymphocytes and plasma cells intermixed with mature adipose tissue on H&E staining (figure 4). Areas of haemorrhage were also seen. No malignant cells were seen. These findings were confirmatory for myelolipoma.
Figure 4.
Histopathological examination after staining with H&E at (A) 40× magnification and (B) 100× magnification showing sheets of haematopoietic cells, lymphocytes and plasma cells intermixed with mature adipose tissue.
Outcome and follow-up
Postoperative period was uneventful. The patient was discharged on fourth postoperative day. Double J stents were removed 4 weeks later. Serum creatinine at 6 months was 0.9 mg/dL. Follow-up imaging at 6 months with contrast-enhanced CT showed residual soft tissue mass with mild to moderate hydronephrosis (figure 5). The patient remains asymptomatic till the last follow-up at 9 months after surgery. Further follow-up is planned at every 6 months after last imaging with serum creatinine and ultrasonography or earlier if symptomatic.
Figure 5.
Postoperative contrast-enhanced CT abdomen in (A) axial and (B) coronal plane showing residual lesion in the perirenal and periureteric location on both sides.
Discussion
Extra adrenal location of myelolipoma is a rare occurrence. Retroperitoneal extra-adrenal myelolipoma is usually found in relation with the kidneys. The renal sinus and perirenal location are more common than renal parenchymal location.4 8–10
The typical age of presentation is sixth to eighth decade, with a female predominance.2 8 11 12 The exact aetiology is still not known. It has been proposed that during embryonic development, some of the haematopoietic cells may get misdirected and get ectopically placed in the peritoneal cavity/retroperitoneum. Postnatally, under periods of stress such as severe anaemia, reactivation of these cells may be responsible for extra-adrenal myelolipoma. Alternatively, it may be possible that adrenal mesenchymal cells may undergo metaplasia to give rise to myelolipoma. If this happens in the usual location of the adrenals, it would give rise to adrenal myelolipoma while in an ectopic location, it would form extra-adrenal myelolipoma.13 A neoplastic theory says that myelolipoma arises as a part of clonal proliferation of cells sharing the same chromosomal translocations as acute myelogenous leukaemia and myelodysplastic syndrome.14 However, unlike these tumours, myelolipoma is not associated with malignant behaviour.15
Since it is rarely associated with any symptoms unless large, extra-adrenal myelolipoma is usually diagnosed as an incidental finding on imaging performed for other indications.16 Symptoms are usually seen owing to the size or haemorrhage. The imaging characteristics of myelolipoma are dependent on the composition of fat within. When present in the adrenal gland, the presence of an encapsulated mass with macroscopic fat along with soft tissue regions on CT is suggestive of myelolipoma.15 Pseudocapsule is a feature of both adrenal and extra-adrenal myelolipomas.17 Rarely, there may be calcification within the lesion.18 After contrast administration, the soft tissue region shows enhancement while the fat is typically non-enhancing. The imaging features of extra-adrenal myelolipoma are similar to its adrenal counterpart. However, retroperitoneal myelolipomas may be difficult to differentiate with the most common retroperitoneal sarcoma—liposarcoma. Both tumours are well defined and contain abundant fat. On MRI, fat shows a high intensity on T1-weighted images, while the myeloid component shows a high intensity on T2-weighted images. Contrast enhancement is similar to that seen on CT. In adrenal imaging, differentiation of lipid-poor adenomas from malignancy is difficult and requires specialised imaging in the form of CT washout studies or chemical shift MRI. Histologically, the tumour contains varying amounts of mature fat and haematopoietic tissue. The amount of fat and cellularity of haematopoietic tissue both will vary with areas of calcification and haemorrhage within.11
Lipid-poor myelolipomas are extremely rare. They have not been reported in extra-adrenal location so far. Nor there is any report of lesion in bilateral periureteral location. The amount of macroscopic fat within the lesion is very variable. Probably that is the reason conventional T1-weighted and T2-weighted images in the present case did not reveal any areas of fat signal intensity within the lesion. The presence of microscopic fat with haematopoietic tissue on histopathological examination, however, clinched the diagnosis. Elsayes et al proposed a MRI-based classification of adrenal myelolipoma based on the amount of fat and myeloid tissue. In group 1, fat predominates, leading to homogenous hyperintense lesions on T1-weighted images. In group 2, there is a mixture of fatty and myeloid components, causing heterogeneous contrast enhancement. In group 3, myeloid cells predominate, leading to nodules with hypointensity on T1, moderate intensity on T2 and enhancement after contrast administration.19
The treatment of myelolipoma is no different from the other benign masses of the adrenal gland and the indications of surgery are also the same. Melck et al compared immediate laparoscopic adrenalectomy with observation for adrenal masses <6 cm with lipid-poor imaging characteristics.20 He concluded that the cost of surgery was similar to the cost of observation initially although after 9 years, the cumulative cost of surveillance was higher than immediate surgery. Given the benign nature of myelolipoma, it is conceivable that these patients would require a long follow-up, and since routine imaging may not reliably distinguish a benign lipid-poor mass from a malignant one, an initial surgical approach seems justified.
An unusual association in the present case was the presence of haematuria. There was external compression of both the ureters by the myelolipoma. Although haematuria is one of the rarest presentations of obstruction of the collecting system, it has been seen in ureteropelvic junction obstruction due to extrinsic compression.21
Learning points.
Retroperitoneal myelolipoma is a rare cause of bilateral perirenal or periureteral mass.
Lipid poor extra-adrenal myelolipoma has not been reported previously.
Due to the absence of macroscopic fat, identification of lipid poor myelolipoma on ultrasonography and routine CT or MRI studies is difficult and diagnosis may only be made on histopathology.
Lipid poor retroperitoneal myelolipoma must be differentiated from solitary fibrous tumour, lymphoma and extramedullary hematopoiesis.
Footnotes
Handling editor: Seema Biswas
Twitter: @Priyank_urology
Contributors: SKS and HL conceived and edited the manuscript. SKS and PY were the operating surgeons. SY and PY prepared the manuscript. SY and HL provided the images. All the authors read and approved the final draft.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Olsson CA, Krane RJ, Klugo RC, et al. Adrenal myelolipoma. Surgery 1973;73:665–70. [PubMed] [Google Scholar]
- 2.Hajiran A, Morley C, Jansen R, et al. Perirenal extra-adrenal myelolipoma. World J Clin Cases 2014;2:279–83. 10.12998/wjcc.v2.i7.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao P, Kenney PJ, Wagner BJ, et al. Imaging and pathologic features of myelolipoma. Radiographics 1997;17:1373–85. 10.1148/radiographics.17.6.9397452 [DOI] [PubMed] [Google Scholar]
- 4.Kumar M, Duerinckx AJ. Bilateral extraadrenal perirenal myelolipomas: an imaging challenge. AJR Am J Roentgenol 2004;183:833–6. 10.2214/ajr.183.3.1830833 [DOI] [PubMed] [Google Scholar]
- 5.Shanbhogue AK, Fasih N, Macdonald DB, et al. Uncommon primary pelvic retroperitoneal masses in adults: a pattern-based imaging approach. Radiographics 2012;32:795–817. 10.1148/rg.323115020 [DOI] [PubMed] [Google Scholar]
- 6.Butori N, Guy F, Collin F, et al. Retroperitoneal extra-adrenal myelolipoma: appearance in CT and MRI. Diagn Interv Imaging 2012;93:204–7. 10.1016/j.diii.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 7.Brandler TC, Reder I, Kahn L. Perirenal myelolipoma diagnosed on imprint: case report and review of the literature. Diagn Cytopathol 2015;43:230–3. 10.1002/dc.23178 [DOI] [PubMed] [Google Scholar]
- 8.Temizoz O, Genchellac H, Demir MK, et al. Bilateral extra-adrenal perirenal myelolipomas: CT features. Br J Radiol 2010;83:e198–e199. 10.1259/bjr/28801968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talwalkar SS, Shaheen SP. Extra-adrenal myelolipoma in the renal hilum: a case report and review of the literature. Arch Pathol Lab Med 2006;130:1049–52.doi:10.1043/1543-2165(2006)130[1049:EMITRH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 10.Cox A, Offman SL, Merrimen JL, et al. Bilateral renal sinus myelolipomas. Can Urol Assoc J 2010;4:164–8. 10.5489/cuaj.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xuefeng T, Rui C, Jianping X, et al. Myelolipoma of the kidney: a seldom site for a rare extra-adrenal tumor. Journal of Medical Colleges of PLA 2010;25:317–20. 10.1016/S1000-1948(11)60019-1 [DOI] [Google Scholar]
- 12.Conley A, Klein E, Edhayan E, et al. Extra-adrenal myelolipoma presenting as efferent limb obstruction. Case Rep Surg 2012;2012:1–4. 10.1155/2012/718383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin MB, Tickoo SK, Schultz D. Myelolipoma of the renal sinus. An unusual site for a rare extra- adrenal lesion. Arch Pathol Lab Med 1999;123:631–4. [DOI] [PubMed] [Google Scholar]
- 14.Chang KC, Chen PI, Huang ZH, et al. Adrenal myelolipoma with translocation (3;21)(q25;p11). Cancer Genet Cytogenet 2002;134:77–80. 10.1016/S0165-4608(01)00592-1 [DOI] [PubMed] [Google Scholar]
- 15.Kammen BF, Elder DE, Fraker DL, et al. Extraadrenal myelolipoma: MR imaging findings. AJR Am J Roentgenol 1998;171:721–3. 10.2214/ajr.171.3.9725304 [DOI] [PubMed] [Google Scholar]
- 16.Zieker D, Königsrainer I, Miller S, et al. Simultaneous adrenal and extra-adrenal myelolipoma - an uncommon incident: case report and review of the literature. World J Surg Oncol 2008;6:72 10.1186/1477-7819-6-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenney PJ, Wagner BJ, Rao P, et al. Myelolipoma: CT and pathologic features. Radiology 1998;208:87–95. 10.1148/radiology.208.1.9646797 [DOI] [PubMed] [Google Scholar]
- 18.Ghaouti M, Znati K, Jahid A, et al. Renal myelolipoma: a rare extra-adrenal tumor in a rare site: a case report and review of the literature. J Med Case Rep 2013;7:92 10.1186/1752-1947-7-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsayes KM, Mukundan G, Narra VR, et al. Adrenal masses: mr imaging features with pathologic correlation. Radiographics 2004;24(Suppl 1):S73–S86. 10.1148/rg.24si045514 [DOI] [PubMed] [Google Scholar]
- 20.Melck AL, Rosengart MR, Armstrong MJ, et al. Immediate laparoscopic adrenalectomy versus observation: cost evaluation for incidental adrenal lesions with atypical imaging characteristics. Am J Surg 2012;204:462–7. 10.1016/j.amjsurg.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 21.Grasso M, Caruso RP, Phillips CK. UPJ obstruction in the adult population: are crossing vessels significant? Rev Urol 2001;3:42–51. [PMC free article] [PubMed] [Google Scholar]