Abstract
A 10-year-old French bulldog presented with an abdominal tumor. Triple-phase helical computed tomography was performed, revealing a hepatic tumor, an enlarged hepatic lymph node, and no masses in other organs. The hepatic tumor demonstrated marked enhancement, similar to that of the aorta in the arterial phase. The tumor had rich vascularization and a hepatic arterio-venous shunt formed between the hepatic artery and middle hepatic vein. The hepatic tumor was surgically removed and histological diagnosis revealed a hepatic carcinoid tumor. During surgery, rapid massive arterial hemorrhage occurred from the site of the incision. The animal died without improvement post-surgery. In the case of an arterio-venous shunt in a hepatic tumor, it is important to be careful to avoid perioperative bleeding.
Keywords: abdomen, arterio-venous shunt, canine, computed tomography, liver tumor
The advent of multidetector helical computed tomography (CT) allows fast scanning and improved image resolution and quality [4]. Due to advances in CT, detailed investigation of various diseases, including vascular abnormalities and tumors, has become possible. One of the advances in CT, triple-phase helical CT, can be used to scan the whole abdomen rapidly, in 3 phases [2, 4, 9, 14]. In triple-phase helical CT, the images are obtained during the arterial phase, followed by the portal venous phase, and delay phase images, after a single bolus injection [2, 4, 14]. Moreover, triple-phase helical CT allows evaluation of both the hemodynamics of abdominal masses and the reconstructed three-dimensional (3D) image by scanning the whole body in both phases [4, 14].
In humans, hepatic tumors may form an arterio-venous shunt. Arterio−venous shunts in hepatic tumors are classified as arterio-portal vein shunts and arterio−hepatic venous shunts. In humans, the former occurs frequently, while the latter type is rare [6]. The formation of an arterio−hepatic venous shunt in tumor is attributed to complex anatomy and various pathological conditions, including tumor growth and vascularization [5]. An arterio−venous shunt in a tumor may cause severe hypertension, splenomegaly, ascites, esophagogastric varices and hemorrhage.
In veterinary medicine, arterio−hepatic venous shunts in hepatic tumors have not yet been reported. This report describes for the first time an arterio−hepatic venous shunt in a hepatic tumor that was investigated by triple-phase helical CT.
A 10-year-old male French bulldog, weighing 11.2 kg, was referred to the Synergy Animal General Hospital after accidental ingestion of a towel and vomiting. The complete blood cell count (CBC) revealed anemia (hematocrit value: 26.5%, reference range: 40−55%). In the serum biochemistry profile, alkaline phosphatase (>1,000 U/l, reference range: 17−78 U/l), gamma glutamyltransferase (18 U/l, reference range: 5−14 U/l), alanine aminotransferase (348 U/l, reference range: 47−254 U/l), and aspartate aminotransferase (966 U/l, reference range: 17−44 U/l) levels had increased. Ultrasound examination revealed a hepatic tumor of mixed echogenicity. Thoracic and abdominal radiographs were unremarkable. Barium sulfate was used for investigation of obstruction, but none was found, and most barium sulfate was present in the colon and rectum at 22 hr after administration (30 min before CT examination).
Triple-phase helical CT was performed with a 16-slice multidetector CT scanner (SOMATOM Emotion, Siemens, Munich, Germany). The scanning parameters were as follows: rotation time 0.6 sec, slice thickness 1 mm, reconstruction interval 1 mm, table speed 15 mm/rotation, helical pitch 15.0, X-ray tube voltage 130 kV, and X-ray tube current 150 mA. Iopamidol (Oypalomin 300, Fuji Pharma Co., Tokyo, Japan) was used as a contrast medium and was administered at a dose of 2.5 ml/kg (750 mg iodine/kg) via the cephalic vein, by means of a power injector (Stellant, Nihon MEDRAD, Osaka, Japan). The injection time was fixed at 15 sec. Pre-contrast (before the injection of contrast medium), arterial phase (20 sec after the start of the injection), portal venous phase (40 sec after the start of the injection) and delay phase (120 sec after the start of the injection) scans were obtained as described previously [4, 14].
The CT findings showed that the tumor was located in the hepatic quadrate lobe and the right medial lobe (Fig. 1). The maximum size of the mass was calculated to be 6.3 × 5.4 × 6.4 cm in the CT image of the arterial phase. In the arterial phase, the contrast enhancement pattern was heterogeneous, and the maximum contrast value of the tumor was approximately 320 HU, a value similar to the contrast value of the aorta (Fig. 1B). The mass was shown to connect to the middle hepatic vein. The middle hepatic vein and caudal vena cava more cranial than the liver were enhanced. In 3D reconstruction imaging of the arterial phase, the mass was directly connected to the hepatic artery (Fig. 2). The mass was hypervascular and formed an arterio−hepatic venous shunt, indicating that the hepatic artery and middle hepatic vein were connected within the mass. In the portal venous phase and delay phase, the hepatic mass also appeared to be heterogeneous and the maximum contrast value of the hepatic mass was higher than that of the surrounding liver parenchyma (Fig. 1C and 1D). The maximum contrast values of the hepatic mass in the portal venous phase and delay phase decreased rapidly in comparison with those in the arterial phase (portal venous phase: approximately 170 HU, delay phase: approximately 140 HU).
Fig. 1.
A contrast computed tomography image demonstrating the heterogeneous pattern evident in all phases. A hepatic tumor (black arrow) was located in the quadrate lobe and right medial lobe. (A) Precontrast computed tomography image. (B) Arterial phase. The tumor showed marked enhancement. The middle hepatic vein, which was connected to the tumor, and the caudal vena cava were enhanced, but the left hepatic vein was not enhanced. (C) Portal phase. (D) Delay phase. The tumor showed washout in the portal venous and delay phases. The left hepatic vein was enhanced from the portal vein. MHV: Middle hepatic vein, LHV: Left hepatic vein.
Fig. 2.
Multiplanar reconstruction image (A) and 3D image (B) in arterial phase. The tumor was directly connected to the hepatic artery. There was a hypervascular formation in the tumor; the hepatic artery, medial hepatic artery, and middle hepatic vein were connected in tumor. Black arrow and purple region: Hepatic tumor. White arrow: Barium sulfate in colon. AO: Aorta, CVC: Caudal vena cava, HA: Hepatic artery, H LYM: Hepatic lymph node.
The hepatic lymph nodes were enlarged, according to sizes calculated from the CT image (sizes of hepatic lymph node, right: 1.4 × 1.8 × 2.0 cm, left: 1.5 × 1.7 × 2.1 cm; Fig. 3). The contrast patterns of the right hepatic lymph node were heterogeneous (the marginal region was richly enhanced and the center region was poorly enhanced) in all phases. The contrast patterns of the left hepatic lymph node were homogeneous. The portal vein was excluded by two enlarged hepatic lymph nodes.
Fig. 3.
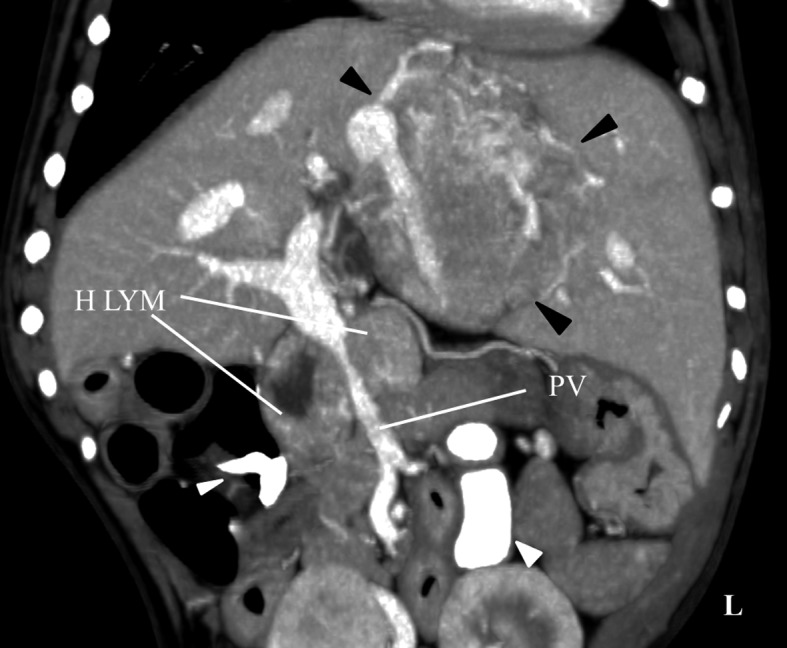
Contrast computed tomography image demonstrating enlarged hepatic lymph nodes. The right hepatic lymph node showed a heterogeneous pattern (the marginal region was markedly enhanced and the center region was poorly enhanced), while the left hepatic lymph node showed homogeneous enhancement. The portal vein showed exclusion by two enlarged hepatic lymph nodes. Black arrow: Hepatic tumor. White arrow: Barium sulfate in colon. H LYM: Hepatic lymph node, PV: Portal vein.
No other organs, including the lungs, the gastrointestinal system and the pancreas, showed the presence of masses. No abdominal lymph nodes other than the hepatic lymph node showed specific findings, such as the abnormal contrast pattern or enlargement. Barium sulfate was present in the colon and rectum. According to these CT findings, the mass was deemed likely to be a hepatic tumor with an arterio−hepatic venous shunt in the tumor and hepatic lymph node metastasis.
During surgery, the hepatic mass was removed by means of lobectomy of the whole quadrate hepatic lobe and partial lobectomy of the right medial lobe. The hepatic mass adhered to the surrounding tissue (gall bladder and medial left lobe). When the hepatic mass was dissected from the surrounding tissue, arterial hemorrhage was caused at the site of incision. Hypotension, bradycardia, depression of arterial oxygen saturation followed, due to rapid and massive arterial hemorrhage. Subsequently, blood transfusion was performed, but cardiac arrest occurred after the hepatic tumor was removed. Despite cardiopulmonary resuscitation, the animal died without improvement. The two enlarged hepatic lymph nodes were not removed.
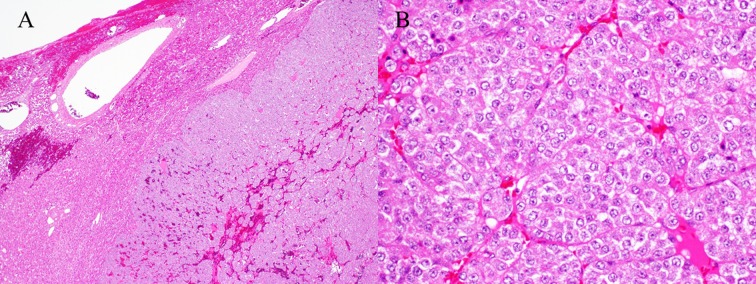
Histologically (Fig. 4), the hepatic mass was composed of nests and packets of polyhedral cells, separated by fine fibrous connective tissue. The neoplastic cells had moderate amounts of eosinophilic, granular cytoplasm and round vesiculated nuclei. There was mild to moderate anisocytosis and anisokaryosis. Mitoses averaged 2 per 10 high-power fields. Within the mass, there were multifocal, small areas of hemorrhage and necrosis. These findings were consistent with a carcinoid.
Fig. 4.
Histological findings of the mass. The mass was composed of nests and packets of polyhedral cells (A). The neoplastic cells had moderate amounts of eosinophilic, granular cytoplasm and round vesicular nuclei (B). A ×40, B ×200: Hematoxylin and eosin stain.
In the 3D reconstructed image of the arterial phase, the arterio−hepatic venous shunt was connected directly to the hepatic artery and the middle hepatic vein in the hepatic tumor. An arterio−hepatic venous shunt had formed within the tumor, creating hypervascularity in the tumor. Therefore, massive arterial hemorrhage occurred at the site of incision in the tumor. Hemorrhage is a major complication in hepatic tumor resection [10]. This case suggests that an arterio−hepatic venous shunt in a tumor could be a risk factor for massive arterial hemorrhage during hepatic tumor resection.
Recently, it was reported that various methods of hepatic vascular occlusion during hepatic resection can be useful to reduce hepatic blood flow and prevent hemorrhage during hepatic resection in the dog [1, 10, 11, 13]. Those methods have been reported in dogs with hepatic arteriovenous fistulas, intrahepatic portosystemic shunts and hepatic tumors involving the caudal vena cava [1, 11, 13]. Those methods could be required for resection of hepatic tumors with an arterio−hepatic venous shunt.
In humans, transarterial chemoembolization, an arterio−hepatic venous shunt in hepatocellular carcinoma is a risk factor for systemic embolization [3, 8]. This finding is one of the important criteria for selecting methods in interventional radiology, including radiofrequency ablation and drug-eluting bead transarterial chemoembolization [8]. With the increased use of CT in veterinary medicine, reports of arterio−venous shunts in tumors may increase in future. The CT finding of an arterio-hepatic venous shunt may become the criterion for selecting new methods for treating hepatic masses in dogs.
Primary hepatic carcinoid tumors are rare in dogs. Reports of hepatic carcinoid tumors in dogs have also shown that the liver is the most common site for carcinoid metastases, particularly when the carcinoid arises from the gastrointestinal tract or pancreas [7]. In our study, the dog did not have a mass in any organ other than the tumor in the liver. Hence, we diagnosed the hepatic tumor as a primary hepatic carcinoid tumor, and suspected that the enlarged hepatic lymph nodes represented metastasis.
In our study, the hepatic tumor exhibited a heterogeneous pattern in all phases. The contrast in the tumor demonstrated marked enhancement, similar to that of the aorta, in the arterial phase, which decreased rapidly in the portal venous phase. However, the maximum contrast values of the hepatic tumor were higher than that of the surrounding liver parenchyma in the portal venous and delay phase. In humans, the characteristic contrast patterns of primary hepatic carcinoid tumors include marked enhancement in the arterial phase [12]. Such carcinoid tumors also show washout in the portal phase and higher enhancement in the delay phase than the surrounding liver parenchyma. The contrast pattern of the primary carcinoid tumor in our study was therefore similar to that in humans. Nevertheless, in a previous study, hepatocellular carcinoma showed a heterogeneous pattern [4]. In humans, arterio-hepatic venous shunts are most frequently observed in patients with hepatocellular carcinoma [5, 6, 8]. Because this report was based on a single dog, a future large-scale study is necessary to determine whether the characteristic findings of primary hepatic carcinoid tumors in dogs include arterio-hepatic venous shunts.
The present case indicates that hepatic tumors may show marked enhancement, similar to that of the aorta in the arterial phase. Such hepatic tumors may have rich vascularization and may involve a hepatic artery−hepatic vein shunt. Furthermore, 3D reconstructed images of the arterial phase are helpful for detecting arterio−venous shunts in tumors. Moreover, when a hepatic mass is detected with an arterio−venous shunt within the tumor, it is indicative of the risk of massive arterial hemorrhage. In such cases, it is important to select surgical methods that can prevent hemorrhage, and to be careful to avoid perioperative bleeding.
REFERENCES
- 1.Breznock E. M., Berger B., Pendray D., Wagner S., Manley P., Whiting P., Hornof W., West D.1983. Surgical manipulation of intrahepatic portocaval shunts in dogs. J. Am. Vet. Med. Assoc. 182: 798–805. [PubMed] [Google Scholar]
- 2.Fukushima K., Fujiwara R., Yamamoto K., Kanemoto H., Ohno K., Tsuboi M., Uchida K., Matsuki N., Nishimura R., Tsujimoto H.2016. Characterization of triple-phase computed tomography in dogs with pancreatic insulinoma. J. Vet. Med. Sci. 77: 1549–1553. doi: 10.1292/jvms.15-0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H. C., Suk K. T., Kim D. J., Yoon J. H., Kim Y. S., Baik G. H., Kim J. B., Kim C. H., Sung H., Choi J. Y., Han K. H., Park S. H.2014. Transarterial chemoembolization in Barcelona Clinic Liver Cancer Stage 0/A hepatocellular carcinoma. World J. Gastroenterol. 20: 745–754. doi: 10.3748/wjg.v20.i3.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kutara K., Seki M., Ishikawa C., Sakai M., Kagawa Y., Iida G., Ishigaki K., Teshima K., Edamura K., Nakayama T., Asano K.2014. Triple-phase helical computed tomography in dogs with hepatic masses. Vet. Radiol. Ultrasound 55: 7–15. doi: 10.1111/vru.12099 [DOI] [PubMed] [Google Scholar]
- 5.Luo M. Y., Shan H., Jiang Z. B., Li L. F., Huang H. Q.2003. Study on hepatocellular carcinoma-associated hepatic arteriovenous shunt using multidetector CT. World J. Gastroenterol. 9: 2455–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngan H., Peh W. C.1997. Arteriovenous shunting in hepatocellular carcinoma: its prevalence and clinical significance. Clin. Radiol. 52: 36–40. doi: 10.1016/S0009-9260(97)80303-0 [DOI] [PubMed] [Google Scholar]
- 7.Patnaik A. K., Lieberman P. H., Hurvitz A. I., Johnson G. F.1981. Canine hepatic carcinoids. Vet. Pathol. 18: 445–453. doi: 10.1177/030098588101800404 [DOI] [PubMed] [Google Scholar]
- 8.Pua U.2015. High-Flow Arterio-Hepatic Venous Shunt in Hepatocellular Carcinoma: Use of multi-electrode radiofrequency for shunt obliteration. Cardiovasc. Intervent. Radiol. 38: 1330–1334. doi: 10.1007/s00270-014-0990-2 [DOI] [PubMed] [Google Scholar]
- 9.Scialpi M., Volterrani L., Mazzei M. A., Cappabianca S., Barberini F., Piscioli I., Brunese L., Lupattelli L.2009. Small (< or = 2 cm) atypical hepatic haemangiomas in the non-cirrhotic patient: pattern-based classification scheme for enhancement at triple-phase helical CT. Radiol. Med. (Torino) 114: 935–947. doi: 10.1007/s11547-009-0427-1 [DOI] [PubMed] [Google Scholar]
- 10.Seki M., Asano K., Kanno N., Teshima K., Edamura K., Tanaka S.2009. Hemodynamic and biochemical changes during total hepatic vascular exclusion in dogs. J. Vet. Med. Sci. 71: 1285–1289. doi: 10.1292/jvms.001285 [DOI] [PubMed] [Google Scholar]
- 11.Seki M., Asano K., Ishigaki K., Iida G., Teshima K., Watari T., Tanaka S.2011. En block resection of a large hepatocellular carcinoma involving the caudal vena cava in a dog. J. Vet. Med. Sci. 73: 693–696. doi: 10.1292/jvms.10-0199 [DOI] [PubMed] [Google Scholar]
- 12.Wang L. X., Liu K., Lin G. W., Jiang T.2015. Primary hepatic neuroendocrine tumors: comparing CT and MRI features with pathology. Cancer Imaging 15: 13. doi: 10.1186/s40644-015-0046-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiting P. G., Breznock E. M., Moore P., Kerr L., Berger B., Gregory C., Hornof W.1986. Partial hepatectomy with temporary hepatic vascular occlusion in dogs with hepatic arteriovenous fistulas. Vet. Surg. 15: 171–180. doi: 10.1111/j.1532-950X.1986.tb00198.x [DOI] [Google Scholar]
- 14.Yoshida O., Kutara K., Seki M., Ishigaki K., Teshima K., Ishikawa C., Iida G., Edamura K., Kagawa Y., Asano K.2016. Preoperative differential diagnosis of canine adrenal tumors using triple-phase helical computed tomography. Vet. Surg. 45: 427–435. doi: 10.1111/vsu.12462 [DOI] [PubMed] [Google Scholar]