Abstract
Background
This study was designed as an external evaluation of potentially relevant models for acute myocardial infarction (AMI) with extracorporeal cardiopulmonary resuscitation (E-CPR).
Material/Methods
Twenty AMI adults that met criteria were retrospectively analyzed from January 2009 to January 2015. Six possible models – ENCOURAGE, SAVE, ECPR, GRACE, SHOCK, and a simplified risk chart – were identified by literature review and model scores calculated based on original data. Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment, commonly used in intensive care units, served as controls. A receiver operating characteristic curve was used to compare the models’ discriminative power for predicting survival to discharge.
Results
The ECPR model showed the best discriminative performance, with an area under the curve (AUC) of 0.893 (95% confidence interval [CI], 0.733–1.530, p=0.006); the cutoff was 12.5 points, with 66.7% sensitivity and 100% specificity. The “clinical” SHOCK model (including infarct site) showed weaker but still good discriminative power, with an AUC of 0.804 (95% CI, 0.580–1.027, p=0.035); the cutoff was 45.5 points, with 83.3% sensitivity and 71.4% specificity. The remaining models did not show significant discriminative power for predicting survival to discharge. Risk stratifications indicated that a statistically significant difference was observed in the distribution of patients into the ECPR group with different prognoses when stratified by its cutoff (p=0.003), while a trend of significant difference was shown when applied to the SHOCK model (p=0.05).
Conclusions
The ECPR and SHOCK models possess important abilities to predict intrahospital outcomes of AMI patients treated with E-CPR.
MeSH Keywords: Cardiopulmonary Resuscitation; Death, Sudden, Cardiac; Extracorporeal Circulation; Extracorporeal Membrane Oxygenation; Myocardial Infarction
Background
Acute myocardial infarction (AMI) complicated with cardiogenic shock (CS) refers to a pathophysiological state in which major organs are hypoperfused by rapid cardiac contractility decreases due to acute coronary thromboembolism, accounting for ~6–10% of events in the whole AMI population [1]. Cardiac arrest (CA), one of the worst prognoses in this population, is the main cause of sudden adult death in developed countries [2] and mainly occurs in unprotected left main coronary artery or double-vessel occlusions. As cardiac output yield from conventional cardiopulmonary resuscitation (C-CPR) is only 25–30% of the normal value [3], it is difficult to achieve restoration of spontaneous circulation (ROSC) before the culprit vessel is re-opened. Prolonged C-CPR not only affects the success rate of resuscitation, but also relates to the incidence of post-resuscitation, permanent, central nervous system injury [4].
Extracorporeal cardiopulmonary resuscitation (E-CPR) is defined as a rescue process in which extracorporeal membrane oxygenation (ECMO) is utilized to obtain ROSC in cardiac-arrested patients unresponsive to C-CPR [5]. As the burden of systemic perfusion is relieved by mechanical circulatory support during E-CPR, the success rate of resuscitation is higher compared to C-CPR. Furthermore, it provides stable and continuous perfusion for critical organs, maintains homeostasis in vivo, and creates favorable conditions for subsequent treatment.
The utilization of E-CPR in the rescue of cardiac-arrested AMI in China is still in its preliminary phase, and the overall survival rate has been relatively low [6,7]. Construction of survival-predictive models in this area might help clinicians in improving their rational utilization of E-CPR in these seriously imperiled patients. Six possible models have been asssembled, called ENCOURAGE (prEdictioN of Cardiogenic shock OUtcome foR AMI patients salvaGed by VA-ECMO), SAVE (Survival After Veno-arterial ECMO), ECPR (ECMO-assisted cardiopulmonary resuscitation), GRACE (Global Registry of Acute Coronary Events), and SHOCK (Should we emergently revascularize occluded coronaries for cardiogenic sHOCK), as well as a simple risk chart (hereinafter called CHART) based on “acute coronary syndrom”, “CS/CA,” and “survival-predictive model” as keywords [8–13].
The purpose of this study was to perform an external evaluation of these models’ discriminative power regarding AMI patients with E-CPR from our institute. These results were then compared with 2 classic risk-predicting scores – ”Acute Physiology and Chronic Health Evaluation II (APACHE II)” and ”Sequential Organ Failure Assessment (SOFA)” – in an intensive care unit (ICU) to identify predictive models valuable for intrahospital outcomes at an early stage.
Material and Methods
Patient selection
All consecutive AMI adults (>18 years old) treated with E-CPR after a futile C-CPR in our institute were retrospectively analyzed from January 2010 to January 2015. Our institute is a tertiary university hospital with primary percutaneous coronary intervention (PPCI) capacity for ~350 AMI cases annually and a 24-7 facility for PPCI and ECMO treatments. The study protocol was approved by our hospital research ethics committee, and all participants provided signed consent forms by their relatives prior to participation.
The AMI diagnosis criteria referred to the third universal definition of myocardial infarction [14] and were confirmed by subsequent coronary angiography. CA in all patients, including ventricular fibrillation, pulseless electrical activity, and asystole, were confirmed by electrocardiogram or hemodynamic monitoring. C-CPR includes continuous chest compressions, intravenous injection of vasoactive drugs (e.g., atropine, epinephrine), invasive mechanical ventilation, and repeated defibrillation.
Inclusion and exclusion criteria
According to the Extracorporeal Life Support Organization [15], V-A ECMO establishment should be considered when the CA time before C-CPR was <5 min and spontaneous circulation could not be restored by C-CPR for 5–30 min. The exclusion criteria were as follows: AMI with non-coronary atherosclerotic lesions; ROSC but CS after C-CPR; CA length was >5 min or the C-CPR length was >90 min; and well-known ECMO contraindications.
Establishment and management of ECMO
Venous-arterial ECMO was established under the premise of uninterrupted C-CPR and the initial blood flow set at 4–5 L/min. The details of our device and techniques for ECMO have been described in our previous studies [6,16,17]. The criterion for successful E-CPR was defined as restoration of spontaneous heart beat (ROSB) after ECMO initiation [9]. After extracorporeal lift support (ECLS) deployment, patients were sent for coronary revascularization to immediately resolve culprit lesions. After crucial interventions, primary therapeutic targets of ECLS, such as sufficient systemic perfusion, were maintained by titration of ECLS volume and inotrope dosage. Intra-aortic balloon counterpulsation or continuous renal replacement therapy was utilized when necessary. Pressure-controlled ventilation was maintained during ECLS and the parameters of ventilator and ECMO adjusted on a timely basis, based on blood gas analysis from the right radial artery. Concentrated blood products were supplemented when necessary. ECLS efforts were reduced gradually until cessation when hemodynamic stability and improvement of the left ventricular ejection fraction (LVEF) were achieved. Figure 1 summarizes our E-CPR protocol and primary therapeutic targets during rescue.
Figure 1.
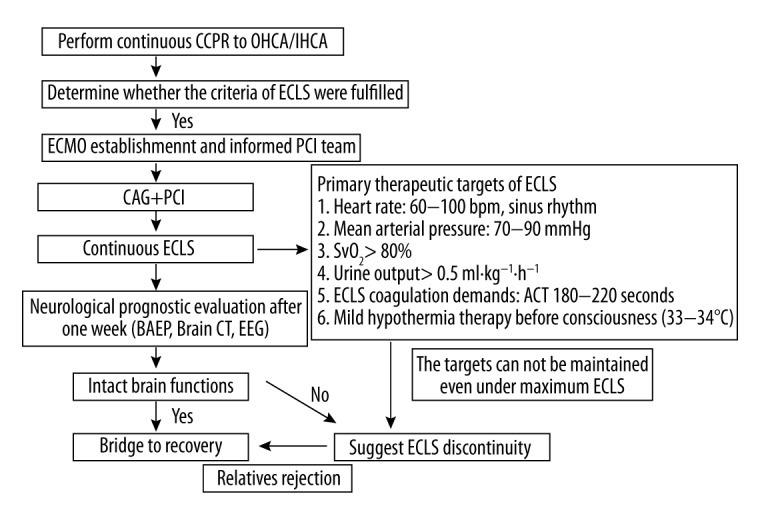
Flow chart for extracorporeal cardiopulmonary resuscitation in cardiac-arrested AMI patients. OHCA – out-of-hospital cardiac arrest; IHCA – in-hospital cardiac arrest; ECLS – extracorporeal lift support; ECMO – extracorporeal membrane oxygenation; PCI – percutaneous coronary intervention; SvO2 – Mixed Venous Oxygen Saturation; BAEP – brainstem auditory evoked potential; EEG – electroencephalograph.
Data collection
Demographic characteristics, physiologic, laboratory, therapeutic details, and outcomes during hospitalization were recorded. ENCOURAGE, SAVE, ECPR, GRACE, SHOCK, and CHART scores were calculated using published descriptions [8–13]. It was noteworthy that the SHOCK model consisted of MODEL I, in which early clinical parameters were entered (one of the indicators was “AMI location or LVEF”, with one selected) and MODEL II in which invasive hemodynamic parameters were embedded. MODEL I was evaluated in the present study, considering its rapidity and simplicity in noninvasive assessment at early stages of a critical event.
Statistical analysis
SPSS Statistics 23.0 software (IBM, Chicago, IL, USA) was utilized for data analysis. The normal distributed quantitative data are expressed as a mean ± standard deviation (SD); the comparison between 2 groups was determined via t-test or t’ test. Abnormally distributed quantitative data are expressed with median and inter-quartile ranges and the Mann-Whitney U-tests were applied for comparison. The qualitative data were expressed by frequency and composition. The differences in constituent ratio between 2 groups were compared via Fisher’s exact test. All P values were two-sided and considered statistically significant if P<0.05. The discriminative performances for intrahospital outcomes of these models were assessed by receiver operating characteristic (ROC) curve analysis.
Results
Patient characteristics
A total of 20 refractory cardiac-arrested AMI patients were selected for their E-CPR rescue and enrolled in this analysis. Nineteen cases were intrahospital CA. All patients achieved ROSB after E-CPR implementation and received subsequent successful PPCI treatment. The rate of successful ECMO weaning and survival to discharge were 40% and 30%, respectively. Two patients died after ECMO withdrawal, one from septic shock and the other from multiple-organ failure. Hypoxic encephalopathy (75%) and coagulation dysfunction (62.5%) were the top 2 complications in ECMO assistance. Table 1 lists more details of general clinical characteristics.
Table 1.
General characteristics.
| Parameter | Value |
|---|---|
| Age | 58.8±13.9 |
| BMI (kg/m2) | 26.8±2.8 |
| Male, n (%) | 17 (85%) |
| C-CPR (min) | 46.7±22.2 |
| Hypertension, n (%) | 9 (45%) |
| Diabetes, n (%) | 6 (30%) |
| OMI, n (%) | 7 (35%) |
| Active smoke, n (%) | 15 (75%) |
| E-CPR site, n (%) | |
| Emergency room | 8 (40%) |
| Coronary care unit | 9 (45%) |
| Catheterization lab | 3 (15%) |
| Initial rhythm, n (%) | |
| Ventricular fibrillation | 16 (80) |
| Pulseless electrical activity | 3 (15%) |
| Asystole | 1 (5%) |
| Door to balloon (min) | 116.2±32.4 |
| Duration of ECLS (h) | 102.3±66.6 |
| ICU stay (d) | 6.0 (2.3, 15.8) |
BMI – body mass index; C-CPR – conventional cardiopulmonary resuscitation; ECLS – extracorporeal life support; OMI – old myocardial infarction; E-CPR – extracorporeal cardiopulmonary resuscitation.
Univariate analysis of risk factors for intrahospital mortality
Table 2 lists univariate comparisons of demographics and critical ECLS features of patients with different prognoses. Compared to non-survivors, survivors tended to have shorter C-CPR times, higher rates of successful ECMO removal, longer ICU stays, higher mean arterial pressures (MBP), and lower arterial blood lactic acid concentrations after 48 h of ECLS (p<0.01 or 0.05). Also, there was a significant difference in the distribution of culprit vessels between the 2 groups (p<0.05) (Table 2). Univariate Spearman correlation analysis showed that successful ECMO removal (r=0.802, p<0.01), length of ICU stay (r=0.609, p<0.01), MBP value after 48 h of ECLS (r=0.558, p<0.05), and right coronary artery culprit vessel (r=0.491, p<0.05) were all factors positively correlated with survival to discharge.
Table 2.
Univariate analysis of risk factors for intrahospital mortality.
| Parameter | Non-survivors (n=14) | Survivors (n=6) | Statistics | P |
|---|---|---|---|---|
| Age | 57.5±15.0 | 61.7±11.8 | −0.603 | 0.554 |
| BMI (kg/m2) | 26.7±2.3 | 27.1±3.9 | 0.247 | 0.808 |
| Male, n (%) | 12 (85.7) | 5 (83.3) | – | 1.000 |
| APACHE II score | 27.4±7.1 | 21.3±6.4 | 1.815 | 0.086 |
| Hypertension, n (%) | 4 (28.6) | 2 (33.3) | – | 1.000 |
| Diabetes, n (%)) | 6 (42.9) | 3 (50) | – | 1.000 |
| OMI, n (%)) | 5 (35.7) | 2 (33.3) | – | 1.000 |
| C-CPR (min) | 51.0±24.5 | 29.2±4.9 | 1.730 | 0.006 |
| GCS score <6, n (%) | 5 (35.7) | 1 (16.7) | – | 0.613 |
| Syntax score | 28.9±8.2 | 34.1±10.6 | −1.194 | 0.248 |
| Culprit vessel, n (%) | – | 0.044 | ||
| LAD | 8 (57.1) | 1 (16.7) | ||
| Lcx | 0 (0) | 1 (16.7) | ||
| RCA | 1 (7.1) | 3 (50) | ||
| LM | 4 (28.6) | 0 (0) | ||
| LAD+RCA | 1 (7.1) | 1 (16.7) | ||
| Duration of ECLS (h) | 86.7±56.1 | 138.7±79.8 | −1.675 | 0.111 |
| ICU stay (d) | 3.0 (2.0, 11.0) | 16.0 (9.5, 37.8) | −2.353 | <0.01 |
| Weaned from ECLS, n (%)) | 2 (14.3) | 6 (100) | – | 0.001 |
| Measurement just after ROSB | ||||
| Heart beat (beat/min) | 101.3±23.2 | 100.3±25.6 | 0.082 | 0.936 |
| MBP (mmHg) | 56.0±12.8 | 63.8±8.5 | −1.345 | 0.195 |
| PH of ABG | 7.08±0.10 | 7.19±0.06 | −2.509 | 0.022 |
| Lactate of ABG (mmol/L) | 13.2±4.6 | 8.4±3.4 | 2.264 | 0.036 |
BMI – body mass index; OMI – old myocardial infarction; C-CPR – conventional cardiopulmonary resuscitation; GCS – Glasgow coma scale; LAD – left anterior descending branch; Lcx – left circumflex; RCA – right coronary artery; LM – left main artery; ECLS – extracorporeal life support; CCU – coronary care unit; ECMO – extracorporeal membrane oxygenation; ROSB – return of spontaneous beating; MBP – Mean arterial pressure; ABG – arterial blood gas. ‘−’ – no data.
Discriminative performance of models in intrahospital outcome
ROC curve analysis of the scoring systems for predicting survival to discharge is shown in Table 3 and Figure 2. Overall, the ECPR model showed the best discriminative performance, with an area under the curve (AUC) of 0.893 (95% confidence interval, CI, 0.733–1.530; p=0.006). The cutoff was 12.5 points, and the sensitivity and specificity were 66.7% and 100%, respectively. The SHOCK scoring system showed weaker but still good discriminative power, with an AUC of 0.804 (95% CI, 0.580–1.027, p=0.035) when the parameter of AMI location was entered or 0.774 when LVEF was entered as a substitute (95% CI, 0.514–1.033, p=0.058). The cutoffs for the SHOCK score under the above 2 conditions were 45.5 points and 60% of the expected survival rate, which showed 83.3 and 71.4% sensitivity and 85.7 and 66.7% specificity, respectively. The remaining models did not show significant discriminative power for predicting survival to discharge. Risk stratifications were made among the participants with different prognoses according to cutoffs of the ECPR and SHOCK models. A statistically significant difference was observed in the distribution of patients into ECPR group with different prognoses when stratified by its cutoff (p=0.003), while a trend of significant difference was shown when applied to the SHOCK model (p=0.05) (Table 4).
Table 3.
ROC analysis of the validated models for predicting survival to discharge.
| Predicted model | AUC | p | 95% CI | Cutoff | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| ENCOURAGE | 0.571 | 0.621 | 0.315–0.828 | – | – | – |
| SAVE | 0.631 | 0.364 | 0.346–0.916 | – | – | – |
| CHART | 0.548 | 0.741 | 0.255–0.840 | – | – | – |
| GRACE | 0.512 | 0.934 | 0.193–0.831 | – | – | – |
| SHOCK (AMI site) | 0.804 | 0.035 | 0.580–1.027 | 45.5 | 83.3 | 71.4 |
| SHOCK (LVEF) | 0.774 | 0.058 | 0.514–1.033 | – | – | – |
| ECPR | 0.893 | 0.006 | 0.733–1.53 | 12.5 | 66.7 | 100 |
| APACHE II | 0.720 | 0.127 | 0.477–0.964 | – | – | – |
| SOFA | 0.512 | 0.943 | 0.253–0.771 | – | – | – |
AMI – acute myocardial infarction; LVEF – left ventricular ejection fraction. ‘−’ – no data.
Figure 2.
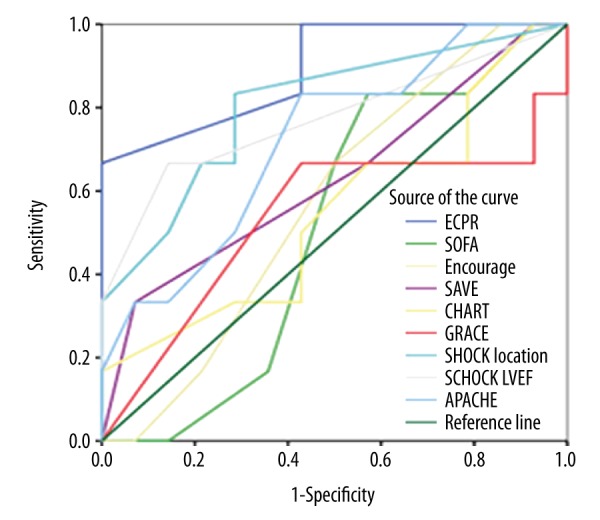
Comparison of the receiver-operating characteristic curves for all risk-prediction tools (n=20).
Table 4.
Distribution of patients into ECPR and SHOCK groups with different intrahospital prognoses.
| Risk group | Total (n=20) | Survivors (n=6) | Non-survivors (n=14) | P |
|---|---|---|---|---|
| ECPR model | 0.003 | |||
| Low-risk group (>12.5) | 4 (20) | 4 (100) | 0 (0) | |
| High risk group (<12.5) | 16 (80) | 2 (12.5) | 14 (87.5) | |
| SHOCK model | 0.05 | |||
| Low-risk group (<45.5) | 9 (45) | 5 (55.6) | 4 (44.4) | |
| High risk group (>45.5) | 11 (55) | 1 (9.1) | 10 (90.9) |
AMI – acute myocardial infarction; LVEF – left ventricular ejection fraction.’−’ – no data.
Discussion
Although the treatment level of critical care medicine and the PPCI rate have increased significantly in recent decades, there has been no significant improvement in the 30-day mortality rate in cardiac-arrested AMI patients [9]. As an effective alternative to C-CPR when it is futile, E-CPR provides continuous perfusion to critical organs, increases the rate of successful defibrillation, and creates conditions for interventional therapy [6,18]. However, several recently-published studies have found that mechanical circulatory support devices achieve potential survival benefits only in the early stages of shock, when organ functions are still in their reversible phases, rather than on a routine basis [19–21]. Recently, some scholars have proposed several survival-predictive models for CS/CA, some of which are especially designed for CS with ECMO rescue [8,10,12]. Their internal validations showed that these models help select the appropriate candidates, predict their intrahospital prognoses, and facilitate risk-adjusted comparison of individual center outcomes [22]. Actually, candidate selection, initiation timing, and sequential ECLS management in Chinese E-CPR practice have been less effective when compared to foreign counterpart experiences. One possible explanation for this difference is the lack of effective survival-predictive models that are suitable for Chinese E-CPR clinical practice. Taking into account differences in scale, therapeutic strategy, and data collection among these original studies, some areas of heterogeneity exist in their application scopes and discriminative power. Thus, it was not clear whether some of these models were valuable for domestic practice. To the best of our knowledge, we report here treatment experiences of AMI with E-CPR from a Chinese single-center cohort and the first comprehensive external evaluation to identify potentially useful, survival-predictive models for this population. Evaluation of the results demonstrated that the ECPR and SHOCK models both possessed significant predictive values for intrahospital outcomes. In fact, these 2 models proved more valuable than 2 classical ICU severity scoring systems for decision-making in which E-CPR has been considered.
ROC curve analysis of all scoring systems for predicting survival to discharge is shown in Figure 2 and Table 3. Overall, the ECPR model was found to have the best discriminative performance. It is noteworthy that patients from the original study were uniformly in-hospital CA with E-CPR treatment, which was the population in this study most similar to populations examined in other validated models. Some parameters included in this model, such as a shockable arrest rhythm and shorter low perfusion time before E-CPR, were key and specific factors to a prognosis. This might have been an important reason for the model’s outstanding performance. Internal evaluation of the ECPR model showed that the low-risk group, scoring more than 10 points, exhibited a higher survival rate to discharge, while our cutoff was 12.5 points. This might be explained by the etiologic heterogeneity between the 2 studies. Compared with original research, CA etiology in the present study was exclusively AMI, which presented the worst prognosis subgroup among all possible etiologies [23]. The higher cutoff reflected calibration to this etiologic heterogeneity. Although the mean ages, etiology constitutions, and treatment approaches were totally equivalent to the original study, this study showed higher rates of ROSB, early revascularization, and shorter ECLS times compared to the former. This benefit might be attributed to the fact that a rapid response ECMO team and coronary intervention team were established that allowed us to achieve around-the-clock ECMO rescue and PPCI treatment. In our opinion, all patients who underwent successful E-CPR procedures should be treated with PPCI as long as there are no contraindications to anticoagulation. Our results indicated that this treatment paradigm enhanced the efficiency of health economics while also obtaining a survival rate equivalent to that of experienced foreign counterparts.
The SHOCK model also exhibited good prognostic differentiation according to our present evaluation, which included 2 sets of parameters that brought in early clinical parameters or hemodynamic indicators. The former was selected to examine the possibility that there might be advantages for noninvasive evaluation in the early stages of shock. Our evaluation demonstrated that the “clinical” model with infarct location showed weaker but still good discriminative power, with an AUC of 0.804 (p=0.035), and better performance than with LVEF (AUC 0.774, p=0.058). The corresponding cutoff for the “clinical” model was 45.5 points with sensitivity and specificity of 83.8 and 71.4%, respectively. Compared with other scoring systems, this model’s main advantage was that its original research covered the entire risk stratification of AMI patients with CS, including those with cardiopulmonary resuscitation or hypoxic encephalopathy, who were exclusively excluded from randomized, controlled trials. This might have been a reason why the SHOCK model showed a higher predictive value in this study. Additionally, our data showed that the survival group showed a higher proportion of right coronary artery and a lower proportion of left anterior descending culprit vessels as well as better hemodynamics and tissue perfusion after 48 h of ECLS, which was coincident with findings from SHOCK research.
Unlike other scoring systems, the SHOCK model assigned appropriate weights to risk stratification according to whether early coronary revascularization (ECR) was performed. Although current guidelines did not provide a definitive recommendation that PPCI should be performed on severe AMI patients after E-CPR treatment whenever possible, some observational data indicated that ECR shows a significant positive effect on intrahospital and one-year survival rate improvements [6,24,25]. The SHOCK study also demonstrated that a higher risk group tended to benefit more from ECR. Our patients represented the highest risk subgroup in the CS population. Inspiringly, ECR were successfully performed without exception in these extremely severe patients after effective E-CPR, which might have provided a more positive effect on the prognosis than in more common CS patients. However, it should be kept in mind that, as it might be difficult to make accurate judgments regarding indicators of hypoxic brain damage in this model at early stages after CA attack, the assessment results might be biased and influence prospective predictions in the real world.
Negative findings were demonstrated in the evaluations of the GRACE, ENCOURAGE, SAVE, and CHART models. (1) This might have been because the GRACE model was derived from a large, international, multicenter registry for acute coronary syndrome. Despite that GRACE had advantages from the largest sample size and broadest disease spectrum coverage, the nature of the registration made this study exclude a large number of high-risk patients, such that CA subjects only accounted for 1.5% of the population and those who died within 24 h after admission were almost entirely excluded from the result analysis. This selection bias reduced the overall risk level of enrolled patients and also affected the choice of medications and interventional therapy. In addition, the subjects in the GRACE study were more elderly and had higher proportions of congestive heart failure, old myocardial infarction, and medication history compared with the present study. Finally, the GRACE study was carried out in 1999–2001 and, compared with thrombolytic therapy, PPCI was not widely performed at that time. The heterogeneity of patients and therapeutic strategies might have affected its discriminative prognosticating performance. (2) The CHART study was specified to AMI patients with CS and the proportion of CA was up to 27% despite its registry nature. Cheng et al. have found that the CHART model had a higher discriminative ability for the 30-day survival rate in ST-segment elevation myocardial infarction patients with CS when compared with GRACE scores (AUC 0.75 vs. 0.66, respectively, p=0.009) (9). Surprisingly, discriminative power was not further improved after the addition of information regarding coronary angiography and clinical data, which might be explained by the fact that the selection bias from those who underwent PPCI partially affected the relationship between clinical characteristics and their mortality. (3) The applicable groups of patients for the SAVE and ENCOURAGE models were both CS patients receiving ECMO support. The derived cohort of the SAVE model was a prospective cohort of 3846 patients from an international multicenter, but the AMI etiology only accounted for 29% of this group and the proportion of pre-ECMO CA was only ~32%. The multivariate, logistic, regression analysis from the original research demonstrated that pre-ECMO CA and prolonged mechanical ventilation were independently predictors of mortality. However, the derivation and validation cohorts both excluded E-CPR patients and treatments with mechanical ventilation and ECLS that were completed successively within a short period in our study. Meanwhile, we believe that its scoring rules need to be further improved. Specifically, zero point was endowed to the item of “shock diagnosis” for AMI diagnosis, but 2 points could be added when the criterion of “refractory ventricular tachycardia/ventricular fibrillation” was met. As most of our patients belonged to the AMI combined with refractory ventricular fibrillation group, 2 points were awarded in this item for most of our patients. This scoring rule made AMI etiology, which indicated a poorer prognosis in shock, barely reflect its survival-predicting value and might have weakened the ultimate discriminative power of this model.
The ENCOURAGE study included only 138 patients from 2 French ICUs. Despite a higher proportion of pre-ECMO CA (57%) compared with that of the SAVE model, the proportion of E-CPR was ~14%. Notably, the pre-ECMO low perfusion time, an important factor for successful resuscitation and long-term outcome [26–28], was significantly shorter than in our study (16 min vs. 47 min, respectively). Therefore, the small sample size and heterogeneity in baseline characteristics and therapeutic strategy might explain this model’s poor discriminative performance despite a larger AUC than the SAVE model.
This study possessed some limitations. The retrospective nature and small number of patients limited the statistical power of analysis. Also, as the case number for Chinese ECMO applications has increased in recent decades, the absolute volume remains relatively small, especially for indications of cardiopulmonary resuscitation. For this reason, we believe that establishment of a regional cooperative network for E-CPR referral treatment is urgently needed.
Conclusions
E-CPR is an effective rescue method for cardiac-arrested AMI patients unresponsive to C-CPR. The ECPR and “clinical” SHOCK models with infarct sites were shown by our evaluation to have significant discriminative performance for predicting survival to discharge. As there were some limitations because of the small E-CPR case numbers and a relatively low overall survival rate in China, future research should include a larger trial with either more patients from a single-center or integration of multicenter data. In addition, the development of a scoring system based on national characteristics might be warranted.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Source of support: This work was partly supported by a grant from the Key Project of Tianjin Health and Family Planning Commission (14KG112) and the Key Program of Tianjin Science and Technology Development Plan (16YFZCSY01060)
References
- 1.Thiele H, Ohman EM, Desch S, et al. Management of cardiogenic shock. Eur Heart J. 2015;36:1223–30. doi: 10.1093/eurheartj/ehv051. [DOI] [PubMed] [Google Scholar]
- 2.Noc M, Radsel P. Urgent invasive coronary strategy in patients with sudden cardiac arrest. Curr Opin Crit Care. 2008;14:287–91. doi: 10.1097/MCC.0b013e3282f85bb0. [DOI] [PubMed] [Google Scholar]
- 3.Morimura N, Sakamoto T, Nagao K, et al. Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: A review of the Japanese literature. Resuscitation. 2011;82:10–14. doi: 10.1016/j.resuscitation.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Matos RI, Watson RS, Nadkarni VM, et al. Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation. 2013;127:442–51. doi: 10.1161/CIRCULATIONAHA.112.125625. [DOI] [PubMed] [Google Scholar]
- 5.Chen YS, Chao A, Yu HY, et al. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol. 2003;41(2):197–203. doi: 10.1016/s0735-1097(02)02716-x. [DOI] [PubMed] [Google Scholar]
- 6.Huang L, Liu Y, Li T, et al. Effect and related factors of extracorporeal cardiopulmonary resuscitation combined with emergent percutaneous coronary intervention on cardiac arrest patients due to acute myocardiaI infarction. Chin J Cardiol. 2016;44(7):570–76. doi: 10.3760/cma.j.issn.0253-3758.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, He FL, Li BF, et al. The clinical report of Chinese extracorporeal life support. Chin J ECC. 2011;9:1–5. [Google Scholar]
- 8.Muller G, Flecher E, Lebreton G, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med. 2016;42(3):370–78. doi: 10.1007/s00134-016-4223-9. [DOI] [PubMed] [Google Scholar]
- 9.Cheng JM, Helming AM, van Vark LC, et al. A simple risk chart for initial risk assessment of 30-day mortality in patients with cardiogenic shock from ST-elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2016;5(2):101–7. doi: 10.1177/2048872615568966. [DOI] [PubMed] [Google Scholar]
- 10.Park SB, Yang JH, Park TK, et al. Developing a risk prediction model for survival to discharge in cardiac arrest patients who undergo extracorporeal membrane oxygenation. Int J Cardiol. 2014;177(3):1031–35. doi: 10.1016/j.ijcard.2014.09.124. [DOI] [PubMed] [Google Scholar]
- 11.Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–53. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: The survival after veno-arterial-ECMO(SAVE)-score. Eur Heart J. 2015;36(33):2246–56. doi: 10.1093/eurheartj/ehv194. [DOI] [PubMed] [Google Scholar]
- 13.Sleeper LA, Reynolds HR, White HD, et al. A severity scoring system for risk assessment of patients with cardiogenic shock: A report from the SHOCK Trial and Registry. Am Heart J. 2010;160(3):443–50. doi: 10.1016/j.ahj.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Extracorporeal Life Support Organization (ELSO) Guidelines for ECPR Cases. Available from: https://www.elso.org/Resources/Guidelines.aspx.
- 16.Huang L, Liu YW, Li T, et al. Resuscitation efficacy of extracorporeal membrane oxygenation in non-postcardiotomy adult patients with cardiac arrest. Chin J Cardiol. 2016;44(11):945–50. doi: 10.3760/cma.j.issn.0253-3758.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Huang L, Li T, Xu L, et al. Extracorporeal membrane oxygenation outcomes in acute respiratory distress treatment: Case study in a Chinese referral center. Med Sci Monit. 2017;23:741–50. doi: 10.12659/MSM.900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagawa E, Dote K, Kato M, et al. Should we emergently revascularize occluded coronaries for cardiac arrest?: Rapid-response extracorporeal membrane oxygenation and intra-arrest percutaneous coronary intervention. Circulation. 2012;126:1605–13. doi: 10.1161/CIRCULATIONAHA.111.067538. [DOI] [PubMed] [Google Scholar]
- 19.Cheng JM, den Uil CA, Hoeks SE, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: A meta-analysis of controlled trials. Eur Heart J. 2009;30:2102–8. doi: 10.1093/eurheartj/ehp292. [DOI] [PubMed] [Google Scholar]
- 20.Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–96. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 21.Werdan K, Gielen S, Ebelt H, et al. Mechanical circulatory support in cardiogenic shock. Eur Heart J. 2014;35:156–67. doi: 10.1093/eurheartj/eht248. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt M, Combes A, Pilcher D. What’s new with survival prediction models in acute respiratory failure patientsrequiring extracorporeal membrane oxygenation. Intensive Care Med. 2014;40:1155–58. doi: 10.1007/s00134-014-3342-4. [DOI] [PubMed] [Google Scholar]
- 23.Wu MY, Lee MY, Lin CC, et al. Resuscitation of non-postcardiotomy cardiogenic shock or cardiac arrest with extracorporeal life support: the role of bridging to intervention. Resuscitation. 2012;83(8):976–81. doi: 10.1016/j.resuscitation.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Nikolaou NI, Christou AH. Cardiac aetiology of cardiac arrest: Percutaneous coronary interventions during and after cardiopulmonary resuscitation. Best Pract Res Clin Anaesthesiol. 2013;27:347–58. doi: 10.1016/j.bpa.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region out of Hospital Cardiac Arrest) registry. Circ Cardiovasc Interv. 2010;3:200–7. doi: 10.1161/CIRCINTERVENTIONS.109.913665. [DOI] [PubMed] [Google Scholar]
- 26.Stub D, Bernard S, Pellegrino V, et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial) Resuscitation. 2015;86:88–94. doi: 10.1016/j.resuscitation.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Haneya A, Philipp A, Diez C, et al. A 5-year experience with cardiopulmonary resuscitation using extracorporeal life support in non-postcardiotomy patients with cardiac arrest. Resuscitation. 2012;83:1331–37. doi: 10.1016/j.resuscitation.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Cardarelli M, Young A, Griffith B. Use of extracorporeal membrane oxygenation for adults in cardiac arrest (E-CPR): A meta-analysis of observational studies. ASAIO J. 2009;55:581–86. doi: 10.1097/MAT.0b013e3181bad907. [DOI] [PubMed] [Google Scholar]


