Abstract
Fanconi anemia (FA) is a rare genetic disorder associated with predisposition to head and neck and gynecological squamous cell cancers. In the general population, these cancers are commonly linked to human papillomavirus (HPV) infection. Antibodies to natural HPV infection and HPV vaccination were evaluated in 63 individuals with FA while considering host immune factors. Approximately 30% of reportedly unvaccinated participants were seropositive (HPV6–38%, HPV11–25%, HPV16–26%, and HPV18–26%). Seropositivity was significantly associated with having had sex regardless of age (p=0.007). Most participants showed seropositivity after HPV vaccination (HPV6–100%, HPV11–100%, HPV16–100% and HPV18–92%). Interestingly, titers for all 4 subtypes were significantly lower in the post-hematopoietic stem cell transplant (HSCT) participants compared to those who received the vaccine, but had not undergone HSCT (HPV6- p=0.030, HPV11-p=0.003, HPV16-p=0.018, HPV18-p=<0.001). It is unclear if these titers sufficiently protect from new infection since protective serologic cut offs have not yet been defined for the HPV vaccine. Individual immune functions were not associated with HPV seropositivity, however, underlying heterogeneous immune deficiency may explain higher rates of seropositivity in our younger unvaccinated participants (age 4–13 years). To better measure the efficacy of HPV vaccination in those with FA and other immune-compromised or cancer-prone disorders, future well-controlled vaccine studies are required.
Keywords: Human papillomavirus, Fanconi anemia, Serology, Vaccination
Introduction
Fanconi anemia (FA) is a genetic disorder that is characterized by genome instability, progressive bone marrow failure and predisposition to gynecological and head and neck squamous cell carcinomas (SCC) [1]. Reports by the International Fanconi Anemia Registry and the German FA registry estimated that the risk of head and neck SCC is over 500-fold higher among individuals with FA compared to the general population. The risk remains high even after hematopoietic stem cell transplantation (HSCT) and the tumors occur at strikingly early ages and carry dismal prognosis [2–4]. Additionally, individuals with FA treated with HSCT who develop graft vs host disease (GVHD) have a higher incidence of head and neck cancers in the ten years following treatment (28% vs 0% in those without GVHD); this finding points to the importance of minimizing the risk of GVHD [5]. Increased risk for GVHD observed in earlier FA studies is now reduced significantly by T-cell depletion of the donor graft [6, 7]. Chemotherapy and radiation are associated with high morbidity and mortality because of underlying DNA repair defects, making treatment of SCC difficult in this population [8–10].
Previous studies have found associations between human papillomavirus (HPV) infection, and cancers of anogenital and oropharyngeal regions in the general population [11–14]. Recent U.S. population-based studies conducted by the Centers for Disease Control and Prevention (CDC) show 62% of oropharyngeal cancers are attributable to HPV types 16 or 18 [15]. Studies of HPV in SCC tumors obtained from individuals with FA have shown contradictory findings [16–18]. The extent to which head and neck SCCs and anogenital cancers are associated with HPV in individuals with FA remains unanswered. However, these contradictory reports have increased interest in studies addressing the role of HPV infection and vaccination in individuals with FA. In a study of individuals with FA living in Brazil, HPV positivity in oral samples was significantly higher than in non-FA controls [19]. Our group has also reported similar findings of higher HPV positivity in oral rinses of individuals with FA compared to their first-degree relatives [20].
Typically, an intact immune system recognizes, eliminates, and protects the body from viral and bacterial infections, as well as from transformed cells (pre-cancer cells) [21, 22]. Nevertheless, molecular and cellular mechanisms responsible for protection from and clearance of HPV infection are not completely understood [23]. Since our group and others have previously shown heterogeneous immune defects in those living with FA, a better understanding of the role of HPV and host immunity would allow for a more thoughtful approach towards prevention of HPV infection and risk reduction for SCC in this vulnerable population particularly as the HPV vaccine is available and recommended [24–27].
Data from clinical trials show that HPV vaccines, when given as a 3-dose series, have very high efficacy for prevention of vaccine type–associated cervical pre-cancers [28–30]. The prophylactic quadrivalent HPV vaccine has also been shown to prevent HPV16- and HPV18-associated vaginal, vulvar, and anal pre-cancers and HPV6- and HPV11-associated anogenital warts [24, 31, 32]. No clinical trial data are currently available to demonstrate efficacy for prevention of oropharyngeal cancers. However, because many of these are attributable to HPV16, the HPV vaccine is likely to offer protection against these cancers as well. The current study sought to measure HPV antibody titers in vaccinated and naturally exposed, unvaccinated individuals with FA while simultaneously evaluating humoral immunity to provide insights into the prevalence of exposure to HPV and response to vaccination.
Methods
Informed Consent
Child and adult participants with FA followed at the Fanconi Anemia Comprehensive Care Center (FACCC) at Cincinnati Children’s Hospital Medical Center (CCHMC) were invited to participate in the study. Individuals with FA participating in the Fanconi Anemia Research Fund (FARF) sponsored Adult or Family Meetings at Camp Sunshine were also offered the opportunity to participate. Informed consent was obtained from participants at least 18 years of age. Parental permission was obtained for children less than 18 years of age. The study was approved by CCHMC’s Institutional Review Board.
Risk Factor Survey
Health related surveys were administered by either paper or using the REDCap (Research Electronic Data Capture) system [33]. The survey collected demographic, socioeconomic, clinical, sexual history, lifestyle and environmental exposure information. Participants ages 15 years and older were asked to complete surveys for themselves unless they were physically or mentally unable to do so, in which case a parent or guardian was asked to complete the survey on their behalf. For children less than 12 years old, a parent/guardian was asked to complete the survey for their participating children. For children between the ages of 12 and 15 years old, a parent/guardian was asked either to complete the survey or assist their child in completing the survey. However, the sexual questions were ascertained by study staff interview whenever possible for this age group provided a parent/guardian gave permission to do so. When available, biological mothers of FA participants were also asked whether they had genital or anal warts, a dysplastic Pap smear, or had a positive HPV test within a 3-year period before their child's birth.
Blood Collection, Processing and HPV Antibody Testing
Venous blood was collected using appropriate universal precautions and aseptic techniques by trained personnel. Approximately 50% of the samples were collected at the FACCC, centrifuged at 2,400 × g and aliquoted, and immediately placed into storage at −80°C (N=33). Samples collected at the FARF-sponsored Family Meeting at Camp Sunshine or the Adult Meeting in Austin Texas were kept at room temperature and centrifuged at 2,400 × g within 8 hours of collection, aliquoted and then stored on dry ice until they were shipped via Same-Day FedEx (N=20). Alternatively, they were shipped by FEDEX priority overnight to CCHMC, centrifuged within 24 hours, aliquoted and stored at −80°C (N=12). An aliquot of each serum (0.5–1.0 mL typically) was sent via Next-Day FedEx to CDC where HPV antibody testing was performed using a multiplex ELISA (M4ELISA) to simultaneously measure antibody responses to HPV 6,11,16 and 18, as well as the pseudovirion-based neutralization assay (PBNA) for HPV16 and 18 as previously described [34, 35]. The RLU (Relative Light Units) at 99th percentile of Johnson-Su probability distribution of children’s sera run at 1:100 (N=49, gift from Dr. Joakim Dillner, Lund University, Sweden) was set as the assay threshold for seropositivity for each HPV type evaluated as previously described [35]. The RLU (signal) threshold calculated using Johnson-Su distribution for these specific assays were 7,998, 3,225, 18,372, and 7,426 for HPV 6,11,16,18 respectively. Inter-assay variation between batches was less than or equal to 25%. Among the 63 total participants, 8 (12.3%) had samples collected at two different time points. Four of these participants were vaccinated and the most recent time point was used in order to allow for the most complete immune profiles.
Immune assays
Participant sera were tested for lymphocyte subsets, B cell panel, immunoglobulin levels (IgG, IgM and IgD) along with tetanus and diphtheria titers. The B cell panel was not performed on the first 9 study participants. Immunoglobulin levels were determined by standard methods in the CCHMC Clinical Laboratory. Tetanus and diphtheria titers were determined by Quantitative Multiplex Bead Assay in CCHMC’s Clinical Diagnostic Laboratory. Remaining assays were performed at CCHMC’s Diagnostic Immunology Laboratories. Results were interpreted with respect to age-appropriate reference ranges established in the laboratories. Evaluation of participant lymphocyte subsets was performed via routine four-color flow cytometric analysis of EDTA preserved whole blood using fluorochrome-labeled monoclonal antibodies to lineage-specific cell surface markers for T cells (CD3, CD4, CD8), B cells (CD19), and natural killer (NK) cells (CD16, CD56). All antibodies were obtained from BD Biosciences (San Jose, CA, USA). Briefly, erythrocytes were lysed by incubation in FACSLyse (BD Biosciences) and then stained with antibody and analyzed on a FACSCalibur flow cytometer (BD Biosciences) using multiset software (BD Biosciences). A similar flow-based approach was applied for assessment of the B-cell panel [23].
Statistical analysis
Statistical analyses were performed using SAS, version 9.3. Fisher’s exact tests were used to 1) compare the demographics and disease characteristics between vaccinated and unvaccinated participants; 2) assess the association between HPV seropositivity and potential risk factors for HPV infection; and 3) compare the measurements of HPV titers in vaccinated participants stratified by immune function. To assess the agreement in seropositivity assayed by different methods, we used Cohen’s Kappa. Since many tests have been performed; a p-value correction is usually needed. However, given the exploratory nature of this study, we opted to use 0.05 as the cutoff p-value for statistical significance.
Results
Population Characteristics
Sixty-five individuals with FA completed the survey and provided blood for serological and immune testing. Two participants received the HPV vaccine before HSCT and had their blood drawn for serological testing approximately 1 year after HSCT. Since we could not ascertain whether the titers were from vaccination or natural infection they were not included in the final analysis. Median age for the remaining 63 participants was 15.0 years (range 3.0 to 42.0 years). Twenty-four participants (38%) reported having received either the Gardasil quadrivalent HPV vaccine (Merck; N=23) or the Cervarix bivalent vaccine (GlaxoSmithKline; N=1) (Table 1).The participant who received the Cervarix vaccine was included in the HPV 16 and 18 analyses only as they were expectantly negative for HPV6 and 11. When comparing the HPV vaccinated and unvaccinated groups, those in the vaccinated group were older (vaccination usually starts between 9 and 11 years of age) with median age of 18.5 years (interquartile range (IQR) 14.5–23.0) compared to a median age of 12.0 years (IQR 8.0–23.0 years) in the unvaccinated group (p=0.019, Wilcoxon Rank sum test). Individuals with FA commonly develop progressive marrow failure, myelodysplastic syndrome and/or acute myeloid leukemia with advancing age, and require treatment with HSCT. In our study population, 58% had undergone HSCT in the vaccinated group compared to 41% in the unvaccinated group (p=0.20, Fisher’s exact test). There were more females than males, more participants with the FA-A complementation group, and more white participants than participants of other races, but no statistically significant differences were observed between the unvaccinated and vaccinated subjects except age. (Table 1).
Table 1.
Population demographic and disease characteristics
| No HPV Vaccine (N=39) |
HPV Vaccine (N=24) |
P | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age (years) | 0.006 | ||||
| 0–8 | 11 | 28% | 0 | 0% | |
| 9–14 | 13 | 33% | 6 | 25% | |
| 15–18 | 4 | 10% | 6 | 25% | |
| >18 | 11 | 28% | 12 | 50% | |
| History of HSCT | 0.20 | ||||
| Yes | 16 | 41% | 14 | 58% | |
| No | 23 | 59% | 10 | 42% | |
| Sex | 0.19 | ||||
| Male | 19 | 49% | 7 | 29% | |
| Female | 20 | 51% | 17 | 71% | |
| FA Complementation Group | 0.25 | ||||
| A | 17 | 44% | 12 | 50% | |
| C | 4 | 10% | 5 | 21% | |
| Other | 5 | 13% | 4 | 17% | |
| Do Not Know | 13 | 33% | 3 | 13% | |
| Race | 0.15 | ||||
| White | 33 | 85% | 24 | 100% | |
| Black | 3 | 8% | 0 | 0% | |
| Other | 3 | 8% | 0 | 0% | |
Note: Frequencies (N) and percentages (%) were provided for all variables and differences were evaluated using Fisher’s exact tests. Bold values indicate that significant differences were observed (p-value ≤ 0.05).
Immune Function
Results for all the immune parameters tested were available in most participants. Twelve participants of 54 tested (22%) had lower percentages of B cells than expected for their age. The numbers of memory B cells were similarly low in 6/52 (12%) participants. Immunoglobulin G (IgG) levels were below normal for age in 14/61 (23%) participants. Diphtheria and tetanus titers were undetectable in 3/62 (5%) and 1/62 (2%) of the participants, respectively. Overall 31 (49%) participants had at least one of the above abnormalities reflecting baseline humoral immune deficiencies in individuals with FA.
Unvaccinated individuals with FA
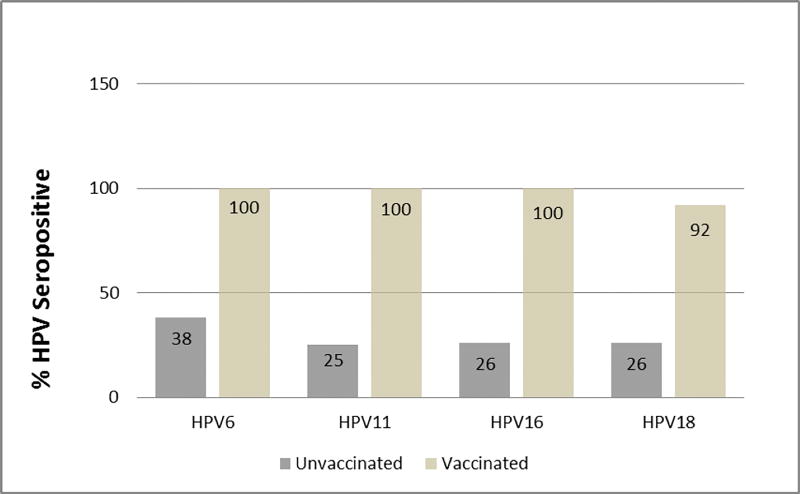
In unvaccinated participants, we detected positive serology by M4ELISA in 38%, 25%, 26% and 26% of individuals, respectively (Figure 1). The geometric mean titers (GMT) of the antibodies were 0.9 AU/ml (95% CL: 0.3–2.8), 0.7 AU/ml (95% CL: 0.1–3.7), 1.7 IU/ml (95% CL: 0.5–6.2) and 1.7 IU/ml (95% CL: 0.6–2.0) for HPV6, 11, 16, and 18, respectively. Neutralizing antibodies detected for HPV16 and HPV18 PBNA was observed in 26% and 15% of individuals. In unvaccinated participants, sexual experience was significantly associated and age was marginally associated with HPV seropositivity (Table 2). To further assess the relationship among sexual experience, age and HPV seropositivity, we tested the effect of age in participants after stratifying by sexual experience. We observed no statistically significant associations between age and HPV seropositivity in either group suggesting that sexual experience may be a more important factor than age for HPV seropositivity in absence of vaccination.
Figure 1. Positive HPV Serology/Titers in Vaccinated and Unvaccinated Participants with FA.
Direct 4-plex HPV VLP IgG enzyme-linked immunosorbent assays (M4ELISA) were performed. Titers were measured in AU/ml for HPV6 and 11, and in IU/ml for HPV16 and 18. Study participants were divided by their reported history of vaccination. All but one vaccinated participant indicated that they had received the quadrivalent Gardasil HPV vaccine. This subject was analyzed as unvaccinated in the analysis for HPV6 and 11 titers.
Table 2.
HPV Risk Factors in Unvaccinated Study Participants with FA
| Variable | Variable Levels |
HPV Seronegative (N=23) |
HPV Seropositive (N=16) |
P | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Age (years) | 0–8 | 9 | 39% | 2 | 13% | 0.06 |
| 9–14 | 9 | 39% | 4 | 25% | ||
| 15–18 | 2 | 9% | 2 | 13% | ||
| >18 | 3 | 13% | 8 | 50% | ||
| Birth Method | Vaginal | 14 | 61% | 9 | 56% | 1.00 |
| C-Section | 9 | 39% | 7 | 44% | ||
| Mother Abnormal Pap | No | 14 | 88% | 10 | 100% | 0.51 |
| Yes | 2 | 13% | 0 | 0% | ||
| Fed Breast Milk as an Infant | No | 9 | 39% | 5 | 33% | 1.00 |
| Yes | 14 | 61% | 10 | 67% | ||
| History of Sexual Experience | No | 21 | 91% | 8 | 50% | 0.007 |
| Yes | 2 | 9% | 8 | 50% | ||
| History of Genital Warts | No | 23 | 100% | 15 | 94% | 0.41 |
| Yes | 0 | 0% | 1 | 6% | ||
| History of Common Warts | No | 15 | 94% | 10 | 77% | 0.30 |
| Yes | 1 | 6% | 3 | 23% | ||
| Reported Primary Smoking | No | 23 | 100% | 15 | 94% | 0.41 |
| Yes | 0 | 0% | 1 | 6% | ||
| SHS Exposure | No | 17 | 77% | 8 | 50% | 0.10 |
| Yes | 5 | 23% | 8 | 50% | ||
| Alcohol Consumption | No | 23 | 100% | 14 | 88% | 0.16 |
| Yes | 0 | 0% | 2 | 13% | ||
| History of Blood Transfusion | No | 10 | 43% | 5 | 31% | 0.52 |
| Yes | 13 | 57% | 11 | 69% | ||
| History of HSCT | No | 14 | 61% | 9 | 56% | 1.00 |
| Yes | 9 | 39% | 7 | 44% | ||
Note: HPV seropositivity was determined by the M4ELISA. HPV seropositive participants were those positive for one or more HPV types. Age at blood draw was evaluated as both a continuous and categorical variable. Frequencies (N) and percentiles (%) were provided for all variables and differences were evaluated using Fisher’s exact tests on non-missing values. Mother abnormal pap was specific to the 3 years prior to childbirth. Mother abnormal pap, secondhand smoke (SHS) exposure, and common warts had missing values which were not included in the percentages. C-section refers to cesarean section. HSCT, hematopoietic stem cell transplant. Bold p-values indicate that significant differences were observed (p-value ≤ 0.05).
Next, we wanted to know if immune dysfunction in the unvaccinated participants played a role in HPV seropositivity or if those who had undergone HSCT were more or less likely to be HPV seropositive. There were no significant differences by HSCT in either immune function or HPV titers (Tables 3 and 4). However, among the 23 unvaccinated individuals younger than 13 years of age and who had no previous history of HPV vaccination or sexual exposure, 5 (21%) were seropositive for one or more HPV types by M4ELISA (Table 5), two of these participants (9%) were seropositive for HPV16 and 4 were seropositive for HPV18 (17%). Examination of lifestyle and disease treatment factors did not reveal any identifiable risk factors for their seropositivity (Table 2).
Table 3.
Immune Function by HSCT in All Unvaccinated Participants
| HSCT=No (N=23) | HSCT=Yes (N=16) | p | |
|---|---|---|---|
|
| |||
| Immune Function | N (%) | N (%) | |
| Total B Cell percent (%) | 0.22 | ||
| Low | 5 (33%) | 2 (13%) | |
| Normal/High | 10 (67%) | 14 (88%) | |
| Total Memory B Cell percent (%) | 0.60 | ||
| Low | 1 (7%) | 3 (19%) | |
| Normal/High | 13 (93%) | 13 (81%) | |
| CD4 Absolute | 0.56 | ||
| Low | 1 (4%) | 2 (13%) | |
| Normal/High | 22 (96%) | 14 (88%) | |
| IgG (mg/dL) | 0.72 | ||
| Low | 5 (24%) | 5 (31%) | |
| Normal/High | 16 (76%) | 11 (69%) | |
| Diphtheria (IU/mL) | 1.00 | ||
| Negative | 1 (5%) | 1 (6%) | |
| Positive | 21 (95%) | 15 (94%) | |
| Tetanus (IU/mL) | - | ||
| Negative | - | - | |
| Positive | 22 (100%) | 16 (100%) | |
Note: Data are shown as frequency (%) and compared using Fisher’s exact test. HSCT, hematopoietic stem cell transplant. IU, international units. Tetanus and diphtheria vaccinations were only included if they were given post-HSCT.
Table 4.
HPV Titers by HSCT in Unvaccinated Participants who were HPV Seropositive
| HSCT=No | HSCT=Yes | P | |
|---|---|---|---|
|
| |||
| HPV Type (response in seropositive) | Median (IQR) | Median (IQR) | |
| HPV6 (AU/ml) | 4.6 (0.7–10.0) N=8 | 7.2 (1.2–29.0) N=7 | 0.69 |
| HPV11 (AU/ml) | 1.5 (1.3–3.5) N=5 | 8.6 (1.4–28.8) N=5 | 0.53 |
| HPV16 (IU/ml) | 21.4 (8.6–30.1) N=5 | 41.0 (20.6–79.9) N=5 | 0.68 |
| HPV18 (IU/ml) | 4.1 (1.4–6.4) N=5 | 12.7 (2.2–18.3) N=5 | 0.53 |
Note: Data are shown as median (IQR) and compared using Wilcoxon rank sum tests. HSCT, hematopoietic stem cell transplant. M4ELISA, titers based on a 4-plex HPV VLP IgG enzyme-linked immunosorbent assay. IU, international units, AU, arbitrary units.
Table 5.
HPV Titers in Young Unvaccinated Children with FA
| Age (years) |
M4ELISA (AU/ml) | M4ELISA (IU/ml) | ||
|---|---|---|---|---|
| HPV6 | HPV11 | HPV16 | HPV18 | |
| 4 | 1.60 | 1.48 | 5.48 | 1.11 |
| 8 | 0.26 | 0 | 0 | 0 |
| 9 | 0.30 | 0.25 | 1.58* | 1.43 |
| 10 | 0.25 | 0.34 | 1.06* | 1.29 |
| 13 | 7.62 | 34.07 | 21.43 | 4.06 |
Note: Only children ≤ 13 years old with at least one seropositive test were included. M4ELISA, titers based on a 4-plex HPV VLP IgG enzyme-linked immunosorbent assay.
Indicates that titers were below the positivity cut-off. IU, international units, AU, arbitrary units.
HPV vaccinated individuals with FA
Twenty-four participants with FA were vaccinated; all but one reported having received the Merck quadrivalent HPV vaccine. In participants vaccinated, for HPV6, 11, 16 and 18, seropositivity was 100%, 100%, 100% and 92% respectively, using M4ELISA (Figure 1). Seropositivity for HPV16 and HPV18 by PBNA was 100% and 92%. Twenty-three vaccinated individuals reported the number of doses received. In the 20 who reported receiving the complete 3 dose series, 89% were seropositive for all HPV vaccine types, while we did not detect seroconversion to HPV18 in 11%. In the 4 individuals who received less than 3 doses, all 4 showed seropositivity to all 4 HPV types (100%). For vaccinated individuals, age, birth method, second hand smoke exposure, alcohol consumption, or history of genital or common warts were not associated with HPV seropositivity. M4ELISA and PBNA results showed complete agreement (HPV16, kappa=1.0, p<0.001, and HPV18 kappa=1.0, p<0.001). The M4ELISA GMTs were 65.6 AU/ml (95%CL: 34.4–125.1), 71.1 AU/ml (95%CL: 36.4–138.8), 249.4 IU/ml (132.9–467.8) and 42.8 IU/ml (17.4–105.4) for HPV6, 11, 16, and 18, respectively. Twelve (38%) vaccinated participants had at least one immune abnormality. Still, all (100%) of these participants were seropositive to all four types.
Of those that had received HSCT, fourteen participants had received the HPV vaccine after HSCT and were included in final analysis for the vaccinated group. Median time from HSCT to vaccine and from vaccine to blood draw for the two groups is provided in Table 6. Time from vaccination to blood draw was similar between those participants who had not received HSCT and those who had undergone HSCT (p=0.57), and timing ranged from within a year of vaccination to 5 years (median=3 years). HPV titers for those participants who had undergone HSCT versus those not transplanted are shown in Table 6. Participants who had not received HSCT had titers in the expected range compared to published titers in individuals without FA [36, 37]. In contrast, interestingly, titers for all 4 types were significantly lower in participants who had undergone HSCT compared to those who had not been transplanted (Table 6). Comparing immune functions and HPV titers in vaccinated individuals stratified by HSCT vs no HSCT, as shown in Table 6, there was no significant difference in immune parameters between the two groups except that total B cell numbers were normal in 100% of participants post-HSCT compared to only 38% of participants who had not received HSCT (p=0.003). Note that these immune function studies were performed at the time of serology sampling and not at the time of vaccination. Interestingly, when we looked closely at those who received HSCT, one participant was on chronic immune suppression for prior kidney transplantation. Three participants received their first HPV vaccine in the same year as their HSCT yet their available immune function parameters were normal at the time of blood draw. Three participants had their blood taken more than 5 years post-vaccination. Only four of the 14 vaccinated participants who had received a HSCT also had at least one immune abnormality. Not all immune parameters were available for each participant.
Table 6.
Titers and Immune Function by HSCT in Participants Vaccinated for HPV
| HSCT=No (N=10) | HSCT=Yes (N=14) | P | |
|---|---|---|---|
| HPV Type | Median (IQR) | Median (IQR) | |
|
| |||
| HPV6 (AU/ml) | 179.5 (120.8–296.8) | 27.0 (17.1–98.2) | 0.030 |
| HPV11 (AU/ml) | 262.6 (155.3–363.5) | 27.8 (20.7–46.1) | 0.003 |
| HPV16 (IU/ml) | 683.2 (446.3–1238.3) | 100.3 (49.0–693.2) | 0.018 |
| HPV18 (IU/ml) | 198.5 (65.4–477.9) | 26.2 (3.7–56.1) | <0.001 |
|
| |||
| Time Variable | Median (IQR) | Median (IQR) | |
|
| |||
| Years from HSCT to vaccination | - | 5.0 (3.0–13.0) | - |
| Years from vaccination to blood draw | 2.0 (1.0–3.0) | 3.0 (1.0–5.0) | 0.57 |
|
| |||
| Vaccination | N (%) | N (%) | |
|
| |||
| # of HPV Vaccine Doses | 0.54 | ||
| <3 shots | 2 (22%) | 1 (7%) | |
| 3 shots | 7 (78%) | 13 (93%) | |
|
| |||
| Immune Function | N (%) | N (%) | |
|
| |||
| Total B Cell percent (%) | 0.003 | ||
| Low | 5 (63%) | 0 (0%) | |
| Normal/High | 3 (38%) | 13 (100%) | |
| Total Memory B Cells (%) | 0.52 | ||
| Low | 0 (0%) | 2 (15%) | |
| Normal/High | 7 (100%) | 11 (85%) | |
| CD4 Absolute | |||
| Low | 2 (20%) | 1 (7%) | 0.55 |
| Normal/High | 8 (80%) | 13 (93%) | |
| IgG (mg/dL) | 1.00 | ||
| Low | 2 (20%) | 2 (17%) | |
| Normal/High | 8 (80%) | 10 (83%) | |
| Diphtheria (IU/mL) | 1.00 | ||
| Negative | 0 (0%) | 1 (8%) | |
| Positive | 9 (100%) | 12 (92%) | |
| Tetanus (IU/mL) | 1.00 | ||
| Negative | 0 (0%) | 1 (8%) | |
| Positive | 9 (100%) | 12 (92%) | |
Note: Data are shown as median (IQR) and compared using Wilcoxon rank sum test, or as frequency (%) and compared using Fisher’s exact test. HSCT, hematopoietic stem cell transplant. IU, international units, AU, arbitrary units. Tetanus and diphtheria vaccinations were only included if they were given post-HSCT.
Discussion
Measurement of HPV serological and neutralizing antibodies in individuals with FA is one of the best approaches to measure the protective effect of HPV vaccines as well as monitoring response to natural infection [38]. The antibody response to HPV is generally type-specific. Important preclinical and early clinical studies indicated the significance of neutralizing antibodies in both protection from virus challenge and natural infection [39, 40]. Clinical studies of the quadrivalent vaccine have shown that the vaccine induces high levels of anti-HPV6, 11, 16 and 18 type specific antibodies. Simpler antibody assays like direct M4ELISA have been shown to correlate well with PBNA methods [41, 42]. In the current study, we elected to measure HPV titers using both M4ELISA and PBNA. Together, the assays showed 100% concordance. We have also previously reported results on 59 of these serum samples tested using a GST-L1 Luminex assay detecting antibodies to HPV L1 (HPV1, 2, 4, 6, 11, 16, 18, 52, and 58) proteins [43]. When comparing the GST-L1 Luminex to these current M4ELISA results, higher agreement for HPV16 (kappa=0.86, p=<0.01) and HPV11 (kappa=0.80, p=<0.01) were observed compared to HPV18 (kappa=0.66, p=<0.01) and HPV6 (kappa=0.69, p=<0.01) (data not shown) [43].
In our study, 5 of 23 (21%) participants from our younger unvaccinated group (≤13 years of age) had positive titers for at least one HPV type. In particular, four of these participants were seropositive for high risk type 18 and two were seropositive for HPV16. One of these subjects was also positive for oral HPV6 and 16 (tested as a part of our previous study). Overall these findings are consistent with our oral HPV studies where 7 of 14 (50%) similar aged young children were HPV positive [20]. Although our numbers are small, these results do bring up the question of why we are detecting higher rates of HPV seropositivity in young, unvaccinated participants with FA. Data in the normal population (without FA) remain limited by the fact that most studies in younger participants have only looked at one high risk type, HPV16. Only 2.4% of 1,316 normal, healthy children ages 6–11 years tested between 1991 and 1994 as part of the National Health and Nutrition Examination Survey III (NHANES III) were seropositive for HPV16 specific IgG antibodies, although a different ELISA platform was used in that study. Seroprevalence was particularly low in children less than 7 years of age (0.4%) in NHANES as well as other populations [44–51]. A recent Australian study testing for HPV types 6, 11, 16 and 18 failed to detect any seropositive cases among 276 children ages 0–9 years old [52]. Among the 214 children ages 10–14 years old, seropositivity was also very low. No cases were detected in males and 1.1% of females were HPV6 and 11 seropositive and 2.1% were seropositive for HPV16 [52]. While non-sexual transmission of HPV in the general population has been proposed [53], further prospective studies are required to confirm the observed higher prevalence of HPV serology in younger FA children compared to non-FA children as well as determine mechanisms of infection. Moreover, it will be particularly important to ascertain if earlier vaccination (before exposure) will mitigate risks associated with infection in this already cancer-prone population. The development of a prophylactic HPV vaccine is a major advance in the prevention of HPV-related cancers.
Out of 24 vaccinated individuals with FA, 23 received the quadrivalent vaccine and 21 (91%) mounted a serological response to all 4 vaccine types. In our study, HPV serology was evaluted on all comers with FA irrespective of timing of their last dose of vaccination (some were 4–8 years post-vaccination). In healthy populations, the one month peak titers decline approximately 10-fold to a plateau level in the next 2 years and are maintained for more than 4 years thereafter [37]. Although the cutoffs for protective titers are not defined, upon evaluting HPV titers in our vaccinated group, the titers appeared to be lower than predicted based on naturally expected decline.
We further examined HPV titers and immune functions stratified by HSCT. To our surprise, HPV titers were significantly lower in transplanted participants compared to those who had not received HSCT. However, due to lack of availability of definite cut offs for protective titers, it is not possible to ascertain if these post-transplant participants are protected or not. While most immune parameters evaluated did not significantly differ between HSCT and non-HSCT groups, more participants who had not undergone HSCT had lower total B cell percents than those who had received HSCT. While one might expect lower HPV titers to be associated with lower B cells, in our study, the lower titers were observed in the HSCT recepients who in fact had normal B cell percents making it difficult to explain this observation.
Post transplant patients usually start reimmunizations once they are no longer on immune suppressive drugs, immunoglobulin supplements (IVIG), and have achieved general immunocompetence. Based on these criteria, patients commonly start re-immunizations approximately 1 year post-transplant to achieve optimal serologic response. Unfortunately, since these are not all our patients, we do not have complete current medication histories (including immunosuppressive medications used for graft versus host disease prophylaxis or treatment, or current IVIG supplementation) available on each of our study participants. However, one subject was on chronic immunosuppression following kidney transplantation, 3 participants had received their first HPV vaccine in the same year as their HSCT, and 3 participants were at least 5 years post-vaccination at the time of blood draw. Others had some evidence of immune dysfunction (e.g. low IgG level, low diptheria and tetanus titers). While we cannot definitely, explain our findings in all post-transplant participants, these heterogenious clinical observations could potentially explain the lower titers observed in some. Often, clinicians assume complete immunocompetence post HSCT. Our results highlight the fact that clinicians need to pay extra attention and assure complete immune reconstitution prior to reimmunizing their HSCT patients with HPV and other vaccines. Further, similar to other clinical vaccines (diptheria and tetanus), clinical monitoring of HPV titers would better assure adequate protection for these high risk patients.
Regardless of the timing of testing post vaccination, it will be important to determine whether the observed vaccine titers offer protection against HPV infection to those with FA following vaccination. Unfortunately, no serologic correlate has been defined for protection afforded by the HPV vaccines administered to the general population. Just as important, HPV titers should be monitored for many years post vaccination to evaluate sustainability and to address the question of need for booster vaccine in this cancer prone population, particularly as some parents of children with FA request to have their younger children vaccinated. Additionally, B cell deficiency and dysfunction were observed in almost half of the participants with FA, a finding confirming previous reports [26, 27]. Immune dysfunction seen in participants with FA can change over time and response to vaccine may differ depending on their age at vaccination. The cross-sectional nature and size of this study prevents examination of temporal associations between HPV seropositivity and changing demographics, sexual experience, age and immune function, emphasizing the need for well-designed prospective studies in FA.
We understand that our study is limited by its retrospective nature, and this in turn brings in additional limitations including the wide age range of our participants, age dependent differences in immune status of our participants, and timing of blood collection after the last dose of vaccine being different for each participant. An additional limitation for the study is that HPV titer and immune function assessment was not at the time of vaccination. It remains unknown whether there were low B cell percent or memory B cells at the time of vaccination, which would have played a role in response to the HPV vaccine. Nevertheless, this is a first step in trying to understand susceptibility to HPV infection and response to HPV vaccination in a rare disease like FA which is known to have baseline increased predisposition to SCC.
In summary, our results show that young unvaccinated children with FA are being exposed to HPV. This finding suggests greater risk of infection prior to the recommended age for initiating HPV immunization. Given the cancer-prone nature of this population, additional studies are required. Indeed, our results support the need to determine if earlier vaccination in younger children (<13 years of age) might be beneficial. Another important observation from our study is the significant difference in HPV titers between transplanted and non-transplanted participants. Given that there are no protective titers defined, it is not possible to confirm if these participants are sufficiently protected. Consequently, clinicians should practice greater awareness of the timing of re-immunization for HPV and consider a possible role for clinical monitoring of HPV titers in order to assure titers are at least in the ranges seen in general population and in pre-HSCT patients with FA. Taken together, future well-controlled longitudinal studies are necessary to define adequacy of serological response in terms of protection from new HPV infection and HPV-related malignancies. Focused studies of individuals with FA are also needed to evaluate the response to HPV infection and vaccination over time accounting for the effect of HSCT, age and/or sexual experience. This will help address the possible need for additional booster HPV vaccine(s) to support or maintain titers.
Highlights.
Young unvaccinated children with FA who have not been sexually exposed are HPV seropositive.
Typical risk factors such as tobacco and alcohol use are not significantly associated with HPV seropositivity in this FA population.
HPV titers are significantly lower in participants with FA who have received HSCT compared to those who had not had HSCT.
Acknowledgments
This study was funded by the Fanconi Anemia Research Fund (FARF) (P.I. PM) and by a NIH R01 HL108102 (P.I. MBK). This work was also supported in part by a Clinical and Translational Science and Training grant support (UL1-RR026314-01 NCRR/NIH). We would like to thank the Care Managers at the FACCC for their help facilitating sample collection. Most importantly, we would like to thank all the individuals with FA and their families who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
The authors declare no potential conflicts of interest.
References
- 1.Tamary H, Alter BP. Current diagnosis of inherited bone marrow failure syndromes. Pediatric hematology and oncology. 2007;24:87–99. doi: 10.1080/08880010601123240. [DOI] [PubMed] [Google Scholar]
- 2.Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003;101:1249–56. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica. 2008;93:511–7. doi: 10.3324/haematol.12234. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg PS, Socie G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood. 2005;105:67–73. doi: 10.1182/blood-2004-04-1652. [DOI] [PubMed] [Google Scholar]
- 5.Guardiola P, Socie G, Li X, Ribaud P, Devergie A, Esperou H, et al. Acute graft-versus-host disease in patients with Fanconi anemia or acquired aplastic anemia undergoing bone marrow transplantation from HLA-identical sibling donors: risk factors and influence on outcome. Blood. 2004;103:73–7. doi: 10.1182/blood-2003-06-2146. [DOI] [PubMed] [Google Scholar]
- 6.Mehta PA, Davies SM, Leemhuis T, Myers K, Kernan NA, Prockop SE, et al. Radiation-free, alternative-donor HCT for Fanconi anemia patients: results from a prospective multi-institutional study. Blood. 2017;129:2308–15. doi: 10.1182/blood-2016-09-743112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhury S, Auerbach AD, Kernan NA, Small TN, Prockop SE, Scaradavou A, et al. Fludarabine-based cytoreductive regimen and T-cell-depleted grafts from alternative donors for the treatment of high-risk patients with Fanconi anaemia. British journal of haematology. 2008;140:644–55. doi: 10.1111/j.1365-2141.2007.06975.x. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman E. Bone marrow transplantation in Fanconi's anemia. Stem Cells. 1993;11(Suppl 2):180–3. doi: 10.1002/stem.5530110829. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman E, Devergie A, Dutreix J. Radiosensitivity in Fanconi anaemia: application to the conditioning regimen for bone marrow transplantation. British journal of haematology. 1983;54:431–40. doi: 10.1111/j.1365-2141.1983.tb02117.x. [DOI] [PubMed] [Google Scholar]
- 10.Gluckman E, Devergie A, Schaison G, Bussel A, Berger R, Sohier J, et al. Bone marrow transplantation in Fanconi anaemia. British journal of haematology. 1980;45:557–64. doi: 10.1111/j.1365-2141.1980.tb07178.x. [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. The New England journal of medicine. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 13.Gillison ML, Chaturvedi AK, Lowy DR. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer. 2008;113:3036–46. doi: 10.1002/cncr.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jemal A, Simard EP, Dorell C, Noone AM, Markowitz LE, Kohler B, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. Journal of the National Cancer Institute. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinau M, Saraiya M, Goodman MT, Peters ES, Watson M, Cleveland JL, et al. Human papillomavirus prevalence in oropharyngeal cancer before vaccine introduction, United States. Emerging infectious diseases. 2014;20:822–8. doi: 10.3201/eid2005.131311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alter BP, Giri N, Savage SA, Quint WG, de Koning MN, Schiffman M. Squamous cell carcinomas in patients with Fanconi anemia and dyskeratosis congenita: a search for human papillomavirus. Int J Cancer. 2013;133:1513–5. doi: 10.1002/ijc.28157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kutler DI, Wreesmann VB, Goberdhan A, Ben-Porat L, Satagopan J, Ngai I, et al. Human papillomavirus DNA and p53 polymorphisms in squamous cell carcinomas from Fanconi anemia patients. Journal of the National Cancer Institute. 2003;95:1718–21. doi: 10.1093/jnci/djg091. [DOI] [PubMed] [Google Scholar]
- 18.van Zeeburg HJ, Snijders PJ, Wu T, Gluckman E, Soulier J, Surralles J, et al. Clinical and molecular characteristics of squamous cell carcinomas from Fanconi anemia patients. Journal of the National Cancer Institute. 2008;100:1649–53. doi: 10.1093/jnci/djn366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Araujo MR, Rubira-Bullen IR, Santos CF, Dionisio TJ, Bonfim CM, De Marco L, et al. High prevalence of oral human papillomavirus infection in Fanconi's anemia patients. Oral Dis. 2011;17:572–6. doi: 10.1111/j.1601-0825.2011.01803.x. [DOI] [PubMed] [Google Scholar]
- 20.Sauter SL, Wells SI, Zhang X, Hoskins EE, Davies SM, Myers KC, et al. Oral human papillomavirus is common in individuals with Fanconi anemia. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24:864–72. doi: 10.1158/1055-9965.EPI-15-0097-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masserot C, Peffault de Latour R, Rocha V, Leblanc T, Rigolet A, Pascal F, et al. Head and neck squamous cell carcinoma in 13 patients with Fanconi anemia after hematopoietic stem cell transplantation. Cancer. 2008;113:3315–22. doi: 10.1002/cncr.23954. [DOI] [PubMed] [Google Scholar]
- 22.Whiteside TL, Miescher S, MacDonald HR, Von Fliedner V. Separation of tumor-infiltrating lymphocytes from tumor cells in human solid tumors. A comparison between velocity sedimentation and discontinuous density gradients. Journal of immunological methods. 1986;90:221–33. doi: 10.1016/0022-1759(86)90079-7. [DOI] [PubMed] [Google Scholar]
- 23.Bleesing JJ. Assays for B cell and germinal center development. Curr Protoc Immunol. 2004 doi: 10.1002/0471142735.im0735s63. Chapter 7:Unit 7 35. [DOI] [PubMed] [Google Scholar]
- 24.Dillner J, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, et al. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. 2010;341:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morbidity and mortality weekly report. 2010;59:626–9. [PubMed] [Google Scholar]
- 26.Myers KC, Bleesing JJ, Davies SM, Zhang X, Martin LJ, Mueller R, et al. Impaired immune function in children with Fanconi anaemia. British journal of haematology. 2011;154:234–40. doi: 10.1111/j.1365-2141.2011.08721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers KC, Sauter S, Zhang X, Bleesing JJ, Davies SM, Wells SI, et al. Impaired immune function in children and adults with Fanconi anemia. Pediatr Blood Cancer. 2017 doi: 10.1002/pbc.26599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman AF, Holland SM. Persistent bacterial infections and primary immune disorders. Curr Opin Microbiol. 2007;10:70–5. doi: 10.1016/j.mib.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, et al. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev Res (Phila) 2009;2:868–78. doi: 10.1158/1940-6207.CAPR-09-0031. [DOI] [PubMed] [Google Scholar]
- 30.Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 31.Joura EA, Leodolter S, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693–702. doi: 10.1016/S0140-6736(07)60777-6. [DOI] [PubMed] [Google Scholar]
- 32.Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Jr, Aranda C, Jessen H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. The New England journal of medicine. 2011;365:1576–85. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 33.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–16. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 35.Panicker G, Rajbhandari I, Gurbaxani BM, Querec TD, Unger ER. Development and evaluation of multiplexed immunoassay for detection of antibodies to HPV vaccine types. Journal of immunological methods. 2015;417:107–14. doi: 10.1016/j.jim.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell K, Dunne EF, Kemper AR, Dolor RJ, Unger ER, Panicker G, et al. Antibody responses among adolescent females receiving the quadrivalent HPV vaccine series corresponding to standard or non-standard dosing intervals. Vaccine. 2015;33:1953–8. doi: 10.1016/j.vaccine.2015.02.058. [DOI] [PubMed] [Google Scholar]
- 37.Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(Suppl 5):F123–38. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dillner J. Toward "serolomics": papillomavirus serology is taking a technologic lead in high-throughput multiplexed antibody analysis. Clinical chemistry. 2005;51:1768–9. doi: 10.1373/clinchem.2005.055483. [DOI] [PubMed] [Google Scholar]
- 39.Ault KA, Giuliano AR, Edwards RP, Tamms G, Kim LL, Smith JF, et al. A phase I study to evaluate a human papillomavirus (HPV) type 18 L1 VLP vaccine. Vaccine. 2004;22:3004–7. doi: 10.1016/j.vaccine.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 40.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. The New England journal of medicine. 2002;347:1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 41.Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Human vaccines. 2008;4:425–34. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 42.Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, et al. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clinical and diagnostic laboratory immunology. 2003;10:108–15. doi: 10.1128/CDLI.10.1.108-115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katzenellenbogen RA, Carter JJ, Stern JE, Butsch Kovacic MS, Mehta PA, Sauter SL, et al. Skin and mucosal human papillomavirus seroprevalence in persons with fanconi anemia. Clinical and vaccine immunology : CVI. 2015;22:413–20. doi: 10.1128/CVI.00665-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.af Geijersstam V, Eklund C, Wang Z, Sapp M, Schiller JT, Dillner J, et al. A survey of seroprevalence of human papillomavirus types 16, 18 and 33 among children. Int J Cancer. 1999;80:489–93. doi: 10.1002/(sici)1097-0215(19990209)80:4<489::aid-ijc1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 45.Cubie HA, Plumstead M, Zhang W, de Jesus O, Duncan LA, Stanley MA. Presence of antibodies to human papillomavirus virus-like particles (VLPs) in 11–13-year-old schoolgirls. Journal of medical virology. 1998;56:210–6. [PubMed] [Google Scholar]
- 46.Dunne EF, Karem KL, Sternberg MR, Stone KM, Unger ER, Reeves WC, et al. Seroprevalence of human papillomavirus type 16 in children. The Journal of infectious diseases. 2005;191:1817–9. doi: 10.1086/430274. [DOI] [PubMed] [Google Scholar]
- 47.Luxton JC, Rose RC, Coletart T, Wilson P, Shepherd PS. Serological and T-helper cell responses to human papillomavirus type 16 L1 in women with cervical dysplasia or cervical carcinoma and in healthy controls. The Journal of general virology. 1997;78(Pt 4):917–23. doi: 10.1099/0022-1317-78-4-917. [DOI] [PubMed] [Google Scholar]
- 48.Manns A, Strickler HD, Wikktor SZ, Pate EJ, Gray R, Waters D. Low incidence of human papillomavirus type 16 antibody seroconversion in young children. The Pediatric infectious disease journal. 1999;18:833–5. doi: 10.1097/00006454-199909000-00020. [DOI] [PubMed] [Google Scholar]
- 49.Marais D, Rose RC, Williamson AL. Age distribution of antibodies to human papillomavirus in children, women with cervical intraepithelial neoplasia and blood donors from South Africa. Journal of medical virology. 1997;51:126–31. doi: 10.1002/(sici)1096-9071(199702)51:2<126::aid-jmv7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 50.Marais DJ, Rose RC, Lane C, Kay P, Nevin J, Denny L, et al. Seroresponses to human papillomavirus types 16, 18, 31, 33, and 45 virus-like particles in South African women with cervical cancer and cervical intraepithelial neoplasia. Journal of medical virology. 2000;60:403–10. doi: 10.1002/(sici)1096-9071(200004)60:4<403::aid-jmv7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 51.Mund K, Han C, Daum R, Helfrich S, Muller M, Fisher SG, et al. Detection of human papillomavirus type 16 DNA and of antibodies to human papillomavirus type 16 proteins in children. Intervirology. 1997;40:232–7. doi: 10.1159/000150552. [DOI] [PubMed] [Google Scholar]
- 52.Newall AT, Brotherton JM, Quinn HE, McIntyre PB, Backhouse J, Gilbert L, et al. Population seroprevalence of human papillomavirus types 6, 11, 16, and 18 in men, women, and children in Australia. Clin Infect Dis. 2008;46:1647–55. doi: 10.1086/587895. [DOI] [PubMed] [Google Scholar]
- 53.Chang F, Syrjanen S, Kellokoski J, Syrjanen K. Human papillomavirus (HPV) infections and their associations with oral disease. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 1991;20:305–17. doi: 10.1111/j.1600-0714.1991.tb00936.x. [DOI] [PubMed] [Google Scholar]