Abstract
The present study aimed to explore the therapeutic effects of the Tiaogeng Yijing decoction on patients with poor ovarian response (POR) undergoing in vitro fertilization-embryo transfer (IVF-ET), in addition to the underlying molecular mechanisms of these effects. A total of 40 patients were randomly and equally assigned to the treatment or control group. Patients in the treatment group received the Tiaogeng Yijing decoction continuously for three menstrual cycles in addition to microstimulation, while patients in the control group underwent microstimulation only. The following molecules were measured following treatment: Serum levels of sex hormones, including follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2) and anti-mullerian hormone (AMH); follicular fluid levels of cytokines, including growth differentiation factor (GDF)-9, transforming growth factor (TGF)-β1, leukemia inhibitory factor (LIF), granulocyte-colony stimulating factor (G-CSF) and vascular endothelial growth factor (VEGF); and endometrial levels of cytokines, including integrin αVβ3, TGF-β1, LIF, G-CSF and VEGF. In addition, the antral follicle count (AFC), mean ovarian diameter (MOD) and pregnancy outcomes were measured. The results revealed that the Tiaogeng Yijing decoction significantly decreased serum levels of FSH and E2, and significantly increased serum AMH levels, the AFC, follicular fluid levels of GDF-9, TGF-β1 and VEGF, and endometrial levels of integrin αVβ3, TGF-β1 and VEGF, in addition to pregnancy outcomes (all P<0.05 vs. the control group). However, no significant differences were found in the MOD or levels of LH, LIF and G-CSF. In conclusion, the present study demonstrated that the Tiaogeng Yijing decoction promotes pregnancy outcomes in patients with POR undergoing IVF-ET, and that this effect may be associated with the upregulation of TGF-β1 and VEGF in the follicular fluid and endometrium.
Keywords: Tiaogeng Yijing decoction, poor ovarian response, pregnancy outcomes, in vitro fertilization-embryo transfer, transforming growth factor β1, vascular endothelial growth factor
Introduction
Poor ovarian response (POR) is characterized by a decreased production of follicles/oocytes after controlled ovarian hyperstimulation (COH) in vitro fertilization (IVF) treatment (1,2). POR is one of the most difficult and challenging problems in the field of IVF, with a prevalence of 5.6–35.1% (3). Compared with normal responders, patients with POR exhibit decreased fertilization rates and lower embryo quality (4). In addition, POR is associated with high IVF cycle cancellation rates and diminished pregnancy rates, reducing the overall success rate of IVF and decreasing cost-effectiveness (5). Methods to improve endometrial receptivity and retrieve high quality oocytes are required to increase pregnancy rates following IVF-embryo transfer (ET) in patients with POR.
Traditional Chinese medicine (TCM), including acupuncture and herbal medicine, has received attention for the treatment of reproductive system diseases in Asian countries (6–12). The Yijing decoction, documented in Fu Qingzhu Nüke (13), has been reported to possess significant therapeutic effects. For example, Wang et al (14) reported that the Yijing decoction could improve sperm motility and vitality, in addition to sperm count. The Yijing decoction has also been demonstrated to improve asthenozoospermia (15). Additionally, the administration of a modified Yijing decoction has been revealed to improve ovarian reserves and regulate sex hormone levels (16). Furthermore, the Bushen Yijing decoction has been reported to decrease myosin heavy chain α and β gene expression with aging (17). The Tiaogeng Yijing decoction is a modified form of the Yijing decoction, which contains 15 Chinese herbal medicines (14). However, little information is available regarding the effects of the Tiaogeng Yijing decoction on POR.
The present study aimed to investigate the therapeutic effects of the Tiaogeng Yijing decoction on patients with POR undergoing IVF-ET, in addition to the underlying molecular mechanisms of these effects. The results of the present study provide novel insights into potential adjuvant therapies to improve POR in patients undergoing IVF-ET.
Materials and methods
Patients
A total of 40 female patients with POR preparing to receive IVF-ET treatment at the Reproductive Medicine Center of Yantai Yuhuangding Hospital (Yantai, China) and The Affiliated Qingdao Hiser Hospital of Qingdao University (Qingdao, China) between July 2015 and December 2015 were included in the present study. Patients were randomly assigned to the treatment group or the control group. POR was diagnosed according to the European Society of Human Reproduction and Embryology consensus (18) and the American Society for Reproductive Medicine guidelines (19). The inclusion criteria of the present study were that patients with POR underwent microstimulation to promote ovulation, but for whom ET had failed at least once. The exclusion criteria were as follows: i) The partner of the patient had oligoasthenozoospermia; ii) patients with immunological infertility; iii) patients with malignant or benign tumors of the reproductive system requiring surgery; iv) patients who had received sexual hormone therapy in the past 3 months; v) patients with heart, liver, kidney or hematopoietic disease, mental illness or other serious diseases; vi) patients with previous poor compliance; and vii) patients with incomplete clinical data. The present study was approved by the Ethics Committee of Yantai Yuhuangding Hospital and all patients provided written informed consent.
Formula of the Tiaogeng Yijing decoction
The formula of the Tiaogeng Yijing decoction was as follows: Radix Rehmanniae Preparata, 15 g; Radix Morindae Officinalis, 12 g; stir-baked Rhizoma Dioscoreae, 18 g; stir-baked Cortex Eucommiae with salt solution, 12 g; alcohol processed Radix Angelica Sinensis, 15 g; stir-baked Radix Paeoniae Alba, 15 g; Jiu Salvia Miltiorrhiza Bge., 15 g; Caulis Spatholobi, 15 g; Bupleurum Chinense, 9 g; Cyathula, 15 g; prepared Rhizoma Cyperi with vinegar, 12 g; Cuscuta chinensis Lam., 18 g; alcohol processed Rhizoma Polygonat, 12 g; Radix Codonopsis, 12 g; and Rhizoma Atractylodis Macrocephalae stir-fried with wheat bran, 12 g. The Tiaogeng Yijing decoction was provided by The Affiliated Qingdao Hiser Hospital of Qingdao University.
Treatment
Patients in the treatment group were orally administered the Tiaogeng Yijing decoction twice a day for 2 weeks with boiling water (decoction of 200 ml), starting on the seventh day of their menstrual cycle. After three cycles of this treatment, the patients underwent microstimulation, as described below, to promote ovulation. By contrast, patients in the control group underwent microstimulation without any prior treatment.
Microstimulation of ovulation
Clomiphene (50 mg; Beijing Double-Crane Pharmaceutical Co., Ltd., Beijing, China) was administered orally once daily to the patients from the second day of their menstrual cycle for 5 days. This was followed by a low-dose gonadotropin (Gn) (Livzon Pharmaceutical Group, Inc., Zhuhai, China), such as human menopausal Gn or Gn releasing hormone agonist. The initial dosage of Gn was quantitatively determined according to the patient's age, height, weight and ovarian reserve function (20). The concentrations of Gn ranged from 100–150 IU per day starting on cycle day 8. During the COH, ovarian follicle development and endometrial thickness were monitored by transvaginal ultrasound (TVU). In addition, the levels of serum estradiol (E2), luteinizing hormone (LH) and progesterone were measured as described below. The dosage of Gn was adjusted according to these results. If the diameter of the dominant follicle in the ovaries reached >18 mm, Gn was stopped and human chorionic gonadotropin (HCG) (5,000–10,000 IU per day) (Livzon Pharmaceutical Group, Inc.) was administered starting on cycle day 10. After 36 h of administration of HCG, patients underwent ultrasound-guided transvaginal oocyte retrieval (TVOR).
IVF-ET
After TVOR, the oocyte corona cumulus complex obtained was placed in a culture dish containing 2 ml medium 199 supplemented with 20% fetal bovine serum (both Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 10 IU/ml pregnant mare serum Gn (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 10 IU/ml HCG in an incubator at 37°C with 5% CO2 for 48 h. Thereafter, oocytes were denuded with 0.1% hyaluronidase solution for 1 min at room temperature and their maturity was determined under a phase-contrast microscope. The matured oocytes were inseminated by intracytoplasmic sperm injection. Fertilization was evaluated by assessing oocytes 12–14 h after injection for the presence of distinct pronuclei and two polar bodies. At 24 h after insemination, the ratio of embryo cleavage was assessed under a phase-contrast microscope. All embryos were frozen at −196°C using an automatic freezer (TK 3000, Uberaba, Brazil) and then ET was performed on the next menstrual cycle.
Pregnancy outcomes
The pregnancy outcomes were compared between the two groups including the number of oocytes retrieved following ovarian stimulation, the percentage of high-quality oocytes, the percentage of high-quality embryos and the pregnancy rate. These oocytes and embryos were examined by phase-contrast microscopy. High quality oocytes were chosen according to the cumulus investment and morphology for IVF. High-quality embryos were selected based on the following characteristics: Embryos had 4 blastomeres and 8–10 blastomeres on days 2 and 3 of development, respectively; the embryos had <15% fragmentation, and symmetric and mononucleated blastomeres.
Measurements of hormone and follicular fluid cytokines
Serum (5 ml) and follicular fluid (5 ml) were obtained from the patients prior to and following treatment with the Tiaogeng Yijing decoction. Follicular fluid was acquired by trans-vaginal ultrasound-guided puncture and aspiration of >18 mm diameter follicles. The serum was frozen in aliquots and stored at −20°C until required. Serum anti-mullerian hormone (AMH) was assessed using the AMH/mullerian-inhibiting substance ELISA kit (CSB-E12756h; Cusabio Biotech Co., Ltd., Wuhan, China) according to the manufacturer's protocol. Additionally, expression levels of the follicular fluid cytokines growth differentiation factor 9 (GDF)-9 (CSB-E12925h), transforming growth factor (TGF)-β1 (CSB-E04725h), leukemia inhibitory factor (LIF) (CSB-E04651h), vascular endothelial growth factor (VEGF) (CSB-E04760h), and granulocyte-colony stimulating factor (G-CSF) (CSB-E04563h) were measured using ELISA kits (Cusabio Biotech Co., Ltd.) according to the manufacturer's protocol. Serum levels of follicle-stimulating hormone (FSH), LH and E2 were determined by electrochemiluminescence immunoassay using the Elecsys 2010 Immunoassay Analyzer (Roche Diagnostics, Indianapolis, IN, USA) as previously described (21). Progesterone was determined by a chemiluminescent immunoassay (Immulite 2000 Progesterone assay; Siemens Healthcare GmbH, Erlangen, Germany) using a specific monoclonal antibody against progesterone, according to the manufacturer's protocol. The antral follicle count (AFC) and mean ovarian diameter (MOD) were measured by TVU.
Western blotting
Endometrial samples were obtained from the patients in the two groups after microstimulation of ovulation with a suction curette from the corpus of the uteri. Total protein was extracted from the endometrium of patients in the two groups using radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Haimen, China). The amount of protein was quantified using a BCA Protein assay kit (Beyotime Institute of Biotechnology). The protein samples (20 µg per lane) were separated on a 10–12% gel using SDS-PAGE and then transferred to nitrocellulose membranes (Merck KGaA). The membranes were then blocked with 5% nonfat milk in Tris-buffered saline with Tween 20 for 1 h at room temperature. Subsequently, the membranes were treated overnight at 4°C with primary antibodies directed against the following proteins: Integrin αVβ3 (cat. no. ab78289; 1:1,000 dilution), TGF-β1 (cat. no. ab92486; 1:1,000 dilution), LIF (cat. no. ab34427; 1:1,000 dilution), G-CSF (cat. no. ab9691; 1:1,000 dilution) and VEGF antibody (cat. no. ab69479; 1:1,000 dilution) (all Abcam, Cambridge, UK). GAPDH (cat. no. ab8245; 1:1,000 dilution; Abcam) was used as a loading control and incubated overnight at 4°C. The membranes were then washed and incubated with horseradish peroxidase-conjugated anti-mouse secondary antibody (ab131368; 1:2,000 dilution; Abcam) or anti-rabbit antibody (ab191866; 1:2,000 dilution; Abcam) for 2 h at room temperature. Protein bands were visualized with Enhanced Chemiluminescence Western Blotting Substrate (Merck KGaA) and quantified using ImageJ software (version 1.46; National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are expressed as the mean ± standard deviation, or as percentages. Statistical analyses were conducted using SPSS software (version 19.0; IBM Corp., Armonk, NY, USA). Comparisons between groups were analyzed using a paired t-test or the χ2 test. P<0.05 was determined to indicate a statistically significant difference.
Results
Characteristics of the patients
The general characteristics of the patients are presented in Table I. The mean age of the patients was 35.10±7.39 and 34.80±7.53 years in the treatment and control groups, respectively. There was no significant difference in the age of patients between the treatment and control groups (P=0.083). In addition, no significant differences were observed between the treatment and control groups, respectively, in infertility duration (4.85±1.22 vs. 4.90±1.59 years; P=0.878) and body mass index (22.97±1.68 vs. 23.47±1.58 kg/m2; P=0.353). The factors indicate that the results of the two groups were comparable.
Table I.
Characteristic of patients with poor ovarian response included in the present study.
| Group | ||||
|---|---|---|---|---|
| Parameter | Control (n=20) | Treatment (n=20) | t-value | P-value |
| Ages (years) | 34.80±7.53 | 35.10±7.39 | 1.831 | 0.083 |
| Infertility duration (years) | 4.90±1.59 | 4.85±1.22 | −0.156 | 0.878 |
| BMI (kg/m2) | 23.47±1.58 | 22.97±1.68 | −0.952 | 0.353 |
Parameters were compared using a paired t-test. BMI, body mass index.
Tiaogeng Yijing regulates serum sex hormones levels and increases the AFC of patients with POR
To explore the therapeutic effects of the Tiaogeng Yijing decoction on the pregnancy outcomes of patients with POR undergoing IVF-ET, the levels of sex hormones, and the AFC and MOD, in the treatment group (before and after treatment) and in the control group (after treatment) were compared. As shown in Table II, the results demonstrated that the levels of FSH and E2, and the ratio of FSH/LH, were significantly decreased after treatment with the Tiaogeng Yijing decoction compared with the levels before treatment (all P<0.05), while the AMH level and the AFC were significantly increased (both P<0.05 vs. before treatment). However, no significant differences were identified in LH and the MOD before and after treatment with the Tiaogeng Yijing decoction. Furthermore, compared with the control group, the levels of FSH, E2 and FSH/LH were significantly reduced after treatment with Tiaogeng Yijing decoction (all P<0.05). By contrast, the AMH level and the AFC were significantly increased after treatment compared with the control group (both P<0.05). There were no significant differences in LH and the MOD after treatment compared with the control group. These results indicate that the Tiaogeng Yijing decoction regulates sex hormones levels and increases the AFC.
Table II.
Comparison of serum sex hormones levels, and the AFC and MOD, between the control and treatment groups.
| Group | |||
|---|---|---|---|
| Treatment (n=20) | |||
| Parameter | Control (n=20) | Before treatment | After treatment |
| FSH (mIU/ml) | 12.81±1.43 | 12.63±1.36b | 8.89±1.56d |
| LH (mIU/ml) | 5.05±0.60 | 5.01±0.71 | 4.98±0.21 |
| FSH/LH | 2.56±0.40 | 2.58±0.41b | 1.77±0.33d |
| E2 (pg/ml) | 81.25±0.35 | 80.2±0.35a | 73.2±1.35c |
| AMH (ng/ml) | 0.96±0.28 | 0.95±0.22b | 1.03±0.21d |
| AFC (number) | 4.61±0.35 | 4.64±0.42a | 5.28±0.29c |
| MOD (cm3) | 2.21±0.87 | 2.15±0.89 | 2.29±1.34 |
Parameters were compared using a paired t-test.
P<0.05
P<0.01 vs. after treatment
P<0.05
P<0.01 vs. the control group. FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; AMH, anti-mullerian hormone; AFC, antral follicle count; MOD, mean ovarian diameter.
Tiaogeng Yijing increases the follicular fluid levels of GDF-9, TGF-β1 and VEGF in patients with POR
The levels of GDF-9, TGF-β1, LIF, G-CSF and VEGF in the follicular fluid of patients in the treatment and control groups were compared. The results of this analysis are shown in Table III. The results revealed that the levels of GDF-9 (P<0.001), TGF-β1 (P=0.004) and VEGF (P=0.005) were significantly increased in the treatment group compared with the control group. However, there were no significant differences in expression levels of LIF (P=0.329) and G-CSF (P=0.172) between the treatment and control groups. These results suggest that the Tiaogeng Yijing decoction increases follicular fluid levels of GDF-9, TGF-β1 and VEGF.
Table III.
Comparison of follicular fluid cytokine levels between the control and treatment groups.
| Group | |||
|---|---|---|---|
| Cytokine (pg/ml) | Treatment (n=20) | Control (n=20) | P-value |
| GDF-9 | 397.00±212.85 | 208.40±124.26 | <0.001 |
| TGF-β | 0.24±0.2541 | 0.108±0.0987 | 0.004 |
| LIF | 18.86±9.74 | 13.75±9.93 | 0.329 |
| G-CSF | 0.4179±1.38 | 0.3919±0.41 | 0.172 |
| VEGF | 1171±624.19 | 533.2±417.90 | 0.005 |
Cytokine levels were compared using a paired t-test. GDF, growth differentiation factor; TGF, transforming growth factor; LIF, leukemia inhibitory factor; G-CSF, granulocyte-colony stimulating factor; VEGF, vascular endothelial growth factor.
Tiaogeng Yijing increases endometrial levels of integrin αVβ3, TGF-β1 and VEGF in patients with POR
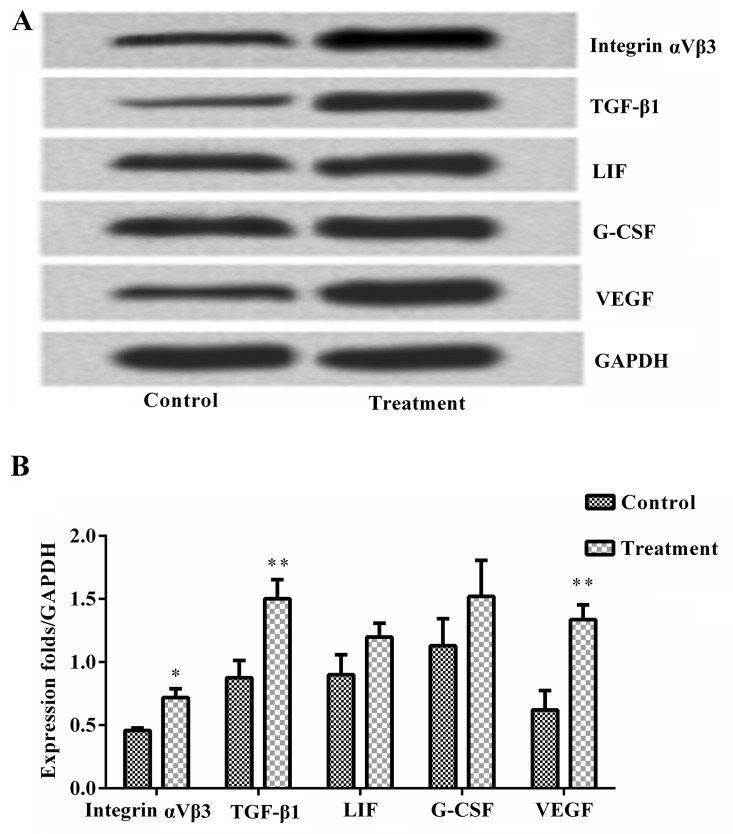
Next, the levels of endometrial cytokines between the two groups were compared. As shown in Fig. 1, the results of western blotting analysis revealed that the protein expression levels of integrin αVβ3, TGF-β1 and VEGF were significantly increased in the treatment group compared with the control group (all P<0.05). However, there were no significant differences in the protein levels of LIF and G-CSF between the two groups. These results suggest that the Tiaogeng Yijing decoction increases endometrial levels of integrin αVβ3, TGF-β1 and VEGF.
Figure 1.
Tiaogeng Yijing increases endometrial levels of integrin αVβ3, TGF-β1 and VEGF in patients with poor ovarian response. Endometrial protein expression levels of integrin αVβ3, TGF-β1, LIF, G-CSF and VEGF were measured via western blot analysis in the control and treatment groups. (A) Western blot and (B) western blot quantification revealed that the Tiaogeng Yijing decoction significantly increased the levels of integrin αVβ3, TGF-β1 and VEGF, but not LIF and G-CSF, compared with the control group. Data are expressed as the mean ± standard deviation and were compared by paired t-test. *P<0.05, **P<0.01 vs. the control group. TGF, transforming growth factor; LIF, leukemia inhibitory factor; G-CSF, granulocyte-colony stimulating factor; VEGF, vascular endothelial growth factor.
Tiaogeng Yijing improves pregnancy outcomes in patients with POR
At 3 months after ET, pregnancy outcomes between the treatment and control groups were compared. The pregnancy outcomes measured included the number of oocytes retrieved, the percentage of high-quality oocytes, the percentage of high-quality embryos and the pregnancy rate. As shown in Table IV, the results demonstrated that the number of retrieved oocytes (P=0.002), high-quality oocytes (P=0.004) and clinical pregnancy rate (P=0.024) were significantly increased in the treatment group compared with the control group. However, there was no significant difference in the percentage of high quality embryos between the two groups (P=0.801). These results indicate that the Tiaogeng Yijing decoction improves pregnancy outcomes.
Table IV.
Comparison of pregnancy outcomes between the control and treatment groups.
| Group | ||||
|---|---|---|---|---|
| Pregnancy outcome | Treatment (n=20) | Control (n=20) | t-value or χ2 | P-value |
| Number of retrieved oocytes | 2.89±1.04 | 2.05±0.97 | 3.618 | 0.002 |
| High-quality oocytes (%) | 68.07±19.05 | 36.40±31.33 | 3.274 | 0.004 |
| High-quality embryos (%) | 57.01±23.78 | 50.50±21.39 | 1.791 | 0.801 |
| Clinical pregnancy rate (%) | 25 | 0 | 5.714 | 0.024 |
Number of retrieved oocytes, quality of oocytes and quality of embryos were compared using a paired t-test. Clinical pregnancy rate was compared using a χ2 test.
Discussion
The present study explored the therapeutic effects of the Tiaogeng Yijing decoction on patients with POR undergoing IVF-ET, in addition to the underlying mechanisms of these effects. The results revealed that oral administration of the Tiaogeng Yijing decoction significantly lowered serum levels of FSH and E2, and significantly increased serum AMH levels, the AFC, follicular fluid levels of GDF-9, TGF-β1 and VEGF, and endometrial levels of integrin αVβ3, TGF-β1 and VEGF. In addition, the Tiaogeng Yijing decoction significantly increased the number of oocytes retrieved, the percentage of high quality oocytes and the pregnancy rate of patients with POR in the present study.
According to the TCM theory, POR belongs to the categories of ‘infertility’, ‘hypomenorrhea’, ‘amenorrhea’ and ‘blood depletion’ (22). In TCM, POR is associated with kidney qi deficiency and deficiency of kidney essence (23). Fu Qingzhu Nüke, a famous book published during the Qing dynasty, states that the basic physiological processes of females are associated with the kidneys (13). In addition, stagnation of liver qi is thought to be responsible for POR (13). Therefore, improving the physical condition of the liver and kidneys may improve POR. The present study investigated the effect of the Tiaogeng Yijing decoction on POR. The Tiaogeng Yijing decoction is derived from the Yijing decoction and includes 15 types of Chinese herbal medicines. In TCM, this mixture is thought to tonify the kidney, invigorate the circulation of blood, smooth the liver and invigorate the spleen. Therefore, it was hypothesized that the Tiaogeng Yijing decoction may be beneficial for patients with POR.
A total of 40 patients with POR preparing to undergo IVF-ET were included in the present study. The patients were randomly assigned to the treatment group (Tiaogeng Yijing decoction treatment in addition to microstimulation) or the control group (microstimulation only). At 3 months after ET, the serum levels of sex hormones, follicular fluid growth factors and endometrial cytokines, in addition to pregnancy outcomes, were measured. FSH and LH are required for normal reproductive function in mammals. It is well known that FSH stimulates ovarian follicle development and that the FSH/LH ratio is a useful marker of ovarian reserves (24). Serum AMH levels serve an important role in predicting ovarian response; this is reflected in the size of the primordial follicle pool and the quality of the oocytes produced (25). AMH has been reported to be a more sensitive marker of ovarian function compared with AFC on day 3 of the menstrual cycle (26). In the present study, FSH, FSH/LH and E2 levels were significantly decreased following Tiaogeng Yijing decoction treatment, and AMH and the AFC were significantly increased, indicating an increase in ovarian reserve function. However, the Tiaogeng Yijing decoction had no effect on LH levels or the MOD.
Follicular fluid provides the microenvironment for oocyte development, which is formed by ultrafiltration of the plasma through the blood-follicle barrier, and the secretion of granule cells and membrane cells. Therefore, numerous molecules in the follicular fluid are thought to reflect the status of the metabolism and development of oocytes, and steroid synthesis (27). It has been reported that the expression and secretion of several cytokines is altered in the follicular fluid during COH (28). Endometrial receptivity is a limiting step in the success of IVF (29). Improved endometrial receptivity is responsible for increased pregnancy rates and reduced early pregnancy failure (30,31). Cytokines and growth factors are associated with endometrial receptivity and implantation (32,33). Thus, the present study measured the levels of GDF-9, integrin αVβ3, TGF-β1, LIF, G-CSF and VEGF in the follicular fluid and endometria of patients with POR. It was observed that the levels of TGF-β1 and VEGF were significantly increased in the follicular fluid and endometrium following the administration of the Tiaogeng Yijing decoction.
TGF-β1 is a multifunctional cytokine, which can regulate cell growth and differentiation, promote the formation of the extracellular matrix, and enhance angiogenesis and immune function. TGF-β1 released by granulosa and theca cells reduces steroid α-hydroxylase activity and mitochondrial cholesterol content, inhibiting the production of androgens, in addition to inhibiting the TGF-α-induced proliferation of theca cells (34). In addition, TGF-β1 can enhance the sensitivity of granulosa cells to FSH stimulation, increasing sex hormone production (35). TGF-β1, together with sex hormones, regulates the growth of follicles and maturation of oocytes, thus serving an important role in fertilization and early embryonic development (35,36). VEGF is a potent vascular endothelial cell-specific mitogen that serves an essential role in angiogenesis. In humans VEGF is highly expressed during preimplantation embryonic development, allowing the implanting embryo to immediately induce angiogenesis at the implantation site (37). VEGF has also been reported to be associated with the regulation of intrafollicular oxygen levels, and patients with low levels of VEGF have been demonstrated to have lower fertilization and pregnancy rates after ET (38). TGF-β1 may be an upstream cytokine of VEGF, thus regulating the function of VEGF in angiogenesis.
In conclusion, the results of the present study suggest that the Tiaogeng Yijing decoction improves the pregnancy outcomes of patients with POR undergoing IVF-ET. These effects may be associated with the upregulation of TGF-β1 and VEGF in the follicular fluid and endometrium. However, further studies are required to confirm these results.
Acknowledgements
The present study was supported by the 2015–2016 Annual Traditional Chinese Medicine Science and Technology Development Program of Shandong Province (grant no. 2015-355).
References
- 1.Turhan NO. Poor response-the devil is in the definition. Fertil Steril. 2006;85:e1. doi: 10.1016/j.fertnstert.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Keay SD, Liversedge NH, Mathur RS, Jenkins JM. Assisted conception following poor ovarian response to gonadotrophin stimulation. Br J Obstet Gynaecol. 1997;104:521–527. doi: 10.1111/j.1471-0528.1997.tb11525.x. [DOI] [PubMed] [Google Scholar]
- 3.Oudendijk JF, Yarde F, Eijkemans MJ, Broekmans FJ, Broer SL. The poor responder in IVF: Is the prognosis always poor? A systematic review. Hum Reprod Update. 2012;18:1–11. doi: 10.1093/humupd/dmr037. [DOI] [PubMed] [Google Scholar]
- 4.Mahutte NG, Arici A. Poor responders: Does the protocol make a difference? Curr Opin Obstet Gynecol. 2002;14:275–281. doi: 10.1097/00001703-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins JM, Davies DW, Devonport H, Anthony FW, Gadd SC, Watson RH, Masson GM. Comparison of ‘poor’ responders with ‘good’ responders using a standard buserelin/human menopausal gonadotrophin regime for in-vitro fertilization. Hum Reprod. 1991;6:918–921. doi: 10.1093/oxfordjournals.humrep.a137459. [DOI] [PubMed] [Google Scholar]
- 6.Huang ST, Chen AP. Traditional Chinese medicine and infertility. Curr Opin Obstet Gynecol. 2008;20:211–215. doi: 10.1097/GCO.0b013e3282f88e22. [DOI] [PubMed] [Google Scholar]
- 7.Rubin Hullender LE, Opsahl MS, Wiemer KE, Mist SD, Caughey AB. Impact of whole systems traditional Chinese medicine on in-vitro fertilization outcomes. Reprod Biomed Online. 2015;30:602–612. doi: 10.1016/j.rbmo.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin LH, Opsahl M, Wiemer K, Humphrey A, Allen P, Mist S, Ackerman D. The effects of adjuvant whole-systems traditional chinese medicine on in vitro fertilization live births: A retrospective cohort study. J Alt Compl Med. 2014;20:12–13. doi: 10.1089/acm.2014.5029.abstract. [DOI] [Google Scholar]
- 9.Cao H, Han M, Ng EH, Wu X, Flower A, Lewith G, Liu JP. Can Chinese herbal medicine improve outcomes of in vitro fertilization? A systematic review and meta-analysis of randomized controlled trials. PloS One. 2013;8:e81650. doi: 10.1371/journal.pone.0081650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo J, Li D, Liu C, Ji X, Li R, Du X. Effects of Chinese herbs combined with in vitro fertilization and embryo transplantation on infertility: A clinical randomized controlled trial. J Tradit Chin Med. 2014;34:267–273. doi: 10.1016/S0254-6272(14)60089-3. [DOI] [PubMed] [Google Scholar]
- 11.Zheng CH, Huang GY, Zhang MM, Wang W. Effects of acupuncture on pregnancy rates in women undergoing in vitro fertilization: A systematic review andmeta-analysis. Fertil Steril. 2012;97:599–611. doi: 10.1016/j.fertnstert.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, Feng X, Mi H, Yao Y, Zhao Y, Li J, Jiao J, Gong A, Sun W, Deng X. Effects of transcutaneous electrical acupoint stimulation on ovarian reserve of patients with diminished ovarian reserve in in vitro fertilization and embryo transfer cycles. J Obstet Gynaecol Res. 2015;41:1905–1911. doi: 10.1111/jog.12810. [DOI] [PubMed] [Google Scholar]
- 13.Fu QZ. Fu Qing-zhu's Gynecology. Blue Poppy Enterprises Inc; 1992. [Google Scholar]
- 14.Wang Q, Ning KQ, Huang XF. Efficacy Observation of Self-made Yijing Decoction for Treating Oligoasthenotspermia. Liaoning J Trad Chin Med. 2012;10:034. (In Chinese) [Google Scholar]
- 15.Wang X, Zheng J. Curative effect observation on Yijing decoction in treatment of Qi stagnation and blood stasis type of Oligoasthenotspermia. J Liaoning Univ Trad Chin Med. 2013;15 (In Chinese) [Google Scholar]
- 16.XU HJ, Sun YC, Yan JM. Clinical observation and study on modified yijing decoction in treating premature ovarian failure. Chine Arch Trad Chine Med. 2012;30 (In Chinese) [Google Scholar]
- 17.Tong PD, Wang XL, Dong HX, Lin SM. Effect of retarding aging chinese drug bushen yijing decoction on rat left ventricular myosin heavy chain (MHC) mRNA change with aging. Chin J Basic Med Trad Chin Med. 2001;7:34–36. (In Chinese) [Google Scholar]
- 18.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE working group on Poor Ovarian Response Definition: ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: The Bologna criteria. Hum Reprod. 2011;26:1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 19.A practice committee report. ASRD; Birmingham, AL: 1999. American Society for Reproductive Medicine (ASRD): Guidelines on Number of Embryos Transferred. [Google Scholar]
- 20.Zhou XP, Hu XL, Zhu YM, Qu F, Sun SJ, Qian YL. Comparison of semen quality and outcome of assisted reproductive techniques in Chinese men with and without hepatitis B. Asian J Androl. 2011;13:465–469. doi: 10.1038/aja.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian GX, Sun Y, Pang CJ, Tan AH, Gao Y, Zhang HY, Yang XB, Li ZX, Mo ZN. Oestradiol is a protective factor for non-alcoholic fatty liver disease in healthy men. Obes Rev. 2012;13:381–387. doi: 10.1111/j.1467-789X.2011.00978.x. [DOI] [PubMed] [Google Scholar]
- 22.Xia T, Ma RH, Mu W, Hu MD, Ma SH, Mao SW, Fu Y. Traditional chinese medicine for diminished ovarian reserve: A systematic review and meta-analysis. Chine Herb Med (CHM) 2014;6:93–102. doi: 10.1016/S1674-6384(14)60014-9. [DOI] [Google Scholar]
- 23.Zelicha K, Luria U. Poor ovarian reserve and high FSH levels. Lantern. 2013;7:6–10. [Google Scholar]
- 24.Mukherjee T, Copperman AB, Lapinski R, Sandler B, Bustillo M, Grunfeld L. An elevated day three follicle-stimulating hormone:Luteinizing hormone ratio (FSH:LH) in the presence of a normal day 3 FSH predicts a poor response to controlled ovarian hyperstimulation. Fertil Steril. 1996;65:588–593. doi: 10.1016/S0015-0282(16)58159-X. [DOI] [PubMed] [Google Scholar]
- 25.Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-lechner E, Tews G. Basal level of anti-Müllerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022–2026. doi: 10.1093/humrep/del127. [DOI] [PubMed] [Google Scholar]
- 26.Fanchin R, Schonäuer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18:323–327. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 27.Nikolettos N, Asimakopoulos B, Nicolettos N, Efthimiadou A, Mourvati E, Demirel C. Evaluation of leptin, interleukin-1beta, tumor necrosis factor-alpha and vascular endothelial growth factor in serum and follicular fluids of women undergoing controlled ovarian hyperstimulation as prognostic markers of ICSI outcome. In Vivo. 2004;18:667–673. [PubMed] [Google Scholar]
- 28.Chen XY, Chen J, Wang ZY, Yu XH, Wei BX, Wu XH. Effects of modified Shoutaiwai recipe on integrin β3 and leukemia-inhibitory factor in endometrium of controlled ovarian hyperstimulation mice during the implantation window. Genet Mol Res. 2015;14:2970–2977. doi: 10.4238/2015.April.10.6. [DOI] [PubMed] [Google Scholar]
- 29.Valbuena D, Jasper M, Remohí J, Pellicer A, Simón C. Ovarian stimulation and endometrial receptivity. Hum Reprod. 1999;14(Suppl 2):S107–S111. doi: 10.1093/humrep/14.suppl_2.107. [DOI] [PubMed] [Google Scholar]
- 30.Aplin JD. Embryo implantation: The molecular mechanism remains elusive. Reprod Biomed Online. 2006;13:833–839. doi: 10.1016/S1472-6483(10)61032-2. [DOI] [PubMed] [Google Scholar]
- 31.Rahiminejad ME, Moaddab A, Ebrahimi M, Rabiee S, Zamani A, Ezzati M, Shamshirsaz Abdollah A. The relationship between some endometrial secretion cytokines and in vitro fertilization. Iran J Reprod Med. 2015;13:557–562. [PMC free article] [PubMed] [Google Scholar]
- 32.Singh M, Chaudhry P, Asselin E. Bridging endometrial receptivity and implantation: Network of hormones, cytokines and growth factors. J Endocrinol. 2011;210:5–14. doi: 10.1530/JOE-10-0461. [DOI] [PubMed] [Google Scholar]
- 33.von Grothusen C, Lalitkumar S, Boggavarapu NR, Gemzell-Danielsson K, Lalitkumar PG. Recent advances in understanding endometrial receptivity: Molecular basis and clinical applications. Am J Reprod Immunol. 2014;72:148–157. doi: 10.1111/aji.12226. [DOI] [PubMed] [Google Scholar]
- 34.Carr BR, McGee EA, Sawetawan C, Clyne CD, Rainey WE. The effect of transforming growth factor-beta on steroidogenesis and expression of key steroidogenic enzymes with a human ovarian thecal-like tumor cell model. Am J Obstet Gynecol. 1996;174:1109–1117. doi: 10.1016/S0002-9378(96)70652-X. [DOI] [PubMed] [Google Scholar]
- 35.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 36.Juengel JL, Mcnatty KP. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum Reprod Update. 2005;11:143–160. doi: 10.1093/humupd/dmh061. [DOI] [PubMed] [Google Scholar]
- 37.Krüssel JS, Behr B, Milki AA, Hirchenhain J, Wen Y, Bielfeld P, Polan Lake M. Vascular endothelial growth factor (VEGF) mRNA splice variants are differentially expressed in human blastocysts. Mol Hum Reprod. 2001;7:57–63. doi: 10.1093/molehr/7.1.57. [DOI] [PubMed] [Google Scholar]
- 38.Van Blerkom J, Antczak M, Schrader R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: Association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod. 1997;12:1047–1055. doi: 10.1093/humrep/12.5.1047. [DOI] [PubMed] [Google Scholar]