Abstract
BRAF protein is a serine/threonine kinase with 766 amino acids. Approximately 15% of human cancers harbor BRAF mutations as well as other BRAF anomalies (amplifications, fusions). Somatic mutations mainly occur in the catalytic kinase domain (CR3), and the predominant mutation is p.V600E which is the substitution of glutamic acid (E) for valine (V) as result of a mutation at codon 600 of the kinase domain. To our knowledge, the vast majority of the cancers have non-germline BRAF mutations. Here we describe a case of a 60-year-old female with a history of hairy cell leukemia (HCL) who presented with aphasia and forgetfulness. A follow-up Brain CT scan showed three distinct brain lesions which were found to be diagnostic of melanoma (confirmed by immunohistochemistry) with no evidence of a concurrent brain involvement by a B-cell neoplasm. Molecular studies confirmed the same BRAF p.V600E mutation in both malignancies (hairy cell leukemia and melanoma). Thereafter the patient was started on BRAF inhibitor treatment and is now symptom-free after one year of follow up. Having two concurrent malignancies with a shared BRAF mutation is extremely rare and makes this an excellent example of a genomic marker-driven treatment in two histologically and immunophenotypically distinct tumors.
Keywords: Leukemia, Hairy Cell; Melanoma; Proto-Oncogene Proteins B-raf
BACKGROUND
BRAF mutation was first described in 1993 and soon after was found to be one of the most frequently mutated protein kinase genes in human tumors.1 Currently, BRAF mutation is commonly tested in pathology practice and has a prognostic and diagnostic value in many tumors including HCL, melanoma, thyroid cancer, colorectal cancers, brain tumors and various other cancers. It has shown to be an effective target for cancer treatment, but a key question is whether targeted drugs approved for one type of histology could be used for other histologic types harboring the same aberration. In 2015, Blachly et al.2 presented the first case of the co-occurrence of malignant melanoma and HCL, both harboring the BRAF p.V600E mutation and its successful treatment with the BRAF inhibitor dabrafenib. Here we also present another rare case of concurrent BRAF p.V600E positive melanoma and HCL which were successfully treated with dabrafenib-trametinib combination targeted therapy on both malignancies.
CASE REPORT
A 62-year-old female presented in 1993 with marked cytopenias and splenomegaly which were subsequently found to be secondary to hairy cell leukemia (HCL) and treated with cladribine. Thereafter she experienced multiple disease relapses while on cladribine and rituximab. Most recently she presented in 2015 with a progressively worsening headache and expressive aphasia. Follow up brain imaging revealed new brain lesions (Figure 1) which were shown to be consistent with metastatic melanoma (Figure 2A to 2D). The follow-up bone marrow showed involvement by the patient's known hairy cell leukemia with no evidence of melanoma.
Figure 1. A – Brain axial computed tomography shows brain masses with significant vasogenic edema; B – Brain magnetic resonance image weighted in T2 shows brain mass with a targetoid appearance and a central non-enhancing lesion, suggestive of necrosis, surrounded by vasogenic edema.

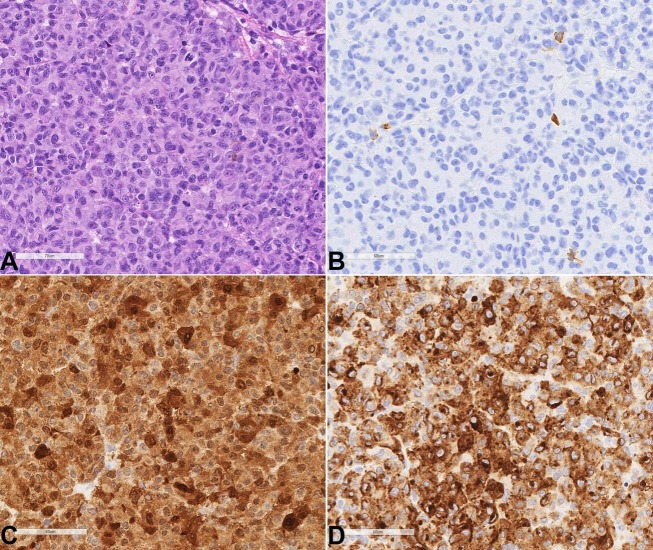
Figure 2. Photomicrography of the brain biopsy with all of the above images being at 400x magnification. A – H&E sections of the excisional biopsy show sheets of neoplastic epithelioid cells with round nuclei and variably prominent red nucleoli; By immunohistochemistry, the tumor cells are negative for CD43 – B; and diffusely positive for S100 – C; and Melan-A – D.
Histology: The H&E slides of the brain mass showed sheets of abnormal tumor cells with round nuclei and prominent red nucleoli (Figure 2A) with an immunophenotype (PAX5 negative, CD20 negative, S100 positive, HMB45 positive, and MelanA positive) which is consistent with melanoma (Figure 2B to 2D).
Flow cytometry of the bone marrow demonstrated a monotypic B-cell population with an immunophenotype (CD19+/CD20++/CD11c+/ dimCD103+ /dimCD25+) consistent with Hairy cell leukemia (Figure 3A and 3B).
Figure 3. A, B – Flow cytometry of the bone marrow shows an abnormal CD19+/CD20++ /CD11c+ /dimCD25+ monotypic B-cell population, consistent with relapsed Hairy Cell Leukemia.
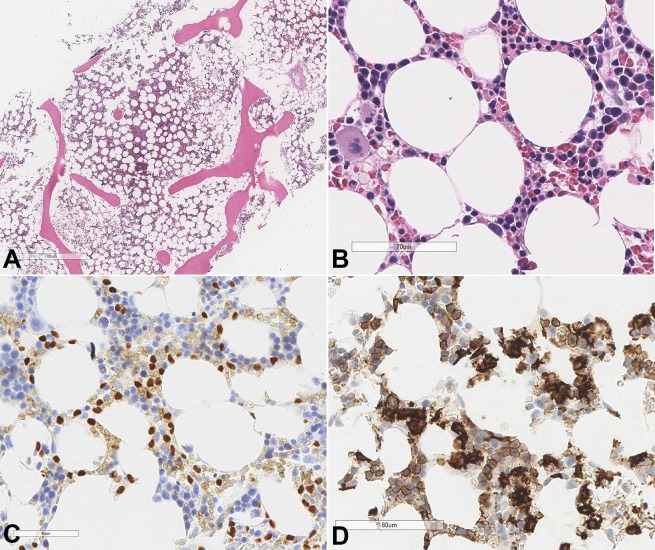
The bone marrow biopsy showed a hypocellular marrow (20-30% of cellularity) with an atypical interstitial B-cell infiltrate (Figure 4A and 4B). By immunohistochemistry, these B-cells were positive for PAX5 and DBA44 (Figure 4C and 4D) as well as Annexin A1. CD34 and CD117 immunostains highlighted rare scattered immature myeloid precursors. S100 and HMB-45 immunostains were negative. Peripheral blood smear showed pancytopenia with rare atypical lymphocytes with villous projections. Overall, given the immunophenotype and morphology, the findings were consistent with relapsed hairy cell leukemia.
Figure 4. Photomicrography of the bone marrow A, B – H&E sections of bone marrow show mildly hypocellular (~30% cellularity) bone marrow with decreased trilineage hematopoiesis and increased abnormal interstitial lymphocytes; C – IHC staining for PAX5 shows positive staining in the abnormal B-cells; D – DBA-44 immunostain highlights the abnormal cells of the HCL.
Molecular Study: BRAF p.V600E mutation (c.1799T>A) was detected by the Real-Time PCR (Quantitative) in both the Hairy Cell Leukemia and the melanoma.
Treatment: Further work-up (physical examination and PET scan) failed to reveal the primary site of the metastatic melanoma brain lesions. Therefore, she underwent CNS radiation therapy in July 2015 and initiated systemic therapy with Mekinist (trametinib) 2 mg tablet (a MAP2K1/2 inhibitor) in combination with Tafinlar (dabrafenib) 75 mg capsule (a BRAF inhibitor). She tolerated treatment well and enjoyed a significant response; all of the patient's brain lesions were resolved radiologically, and her neurologic symptoms diminished in few months. Complete blood count and peripheral blood smear showed no evidence of residual HCL. As of June 2017, she remains clinically stable and reveals no overt evidence of significant clinical disease progression.
To our knowledge case reports do not require IRB review at our institution.
DISCUSSION
BRAF gene is on chromosome 7q34 and has 18 exons. BRAF protein is a serine/threonine kinase with 766 amino acids composed of 3 conserved regions (CR); CR1 and CR2 are regulatory domains, and CR3 contains a catalytic protein kinase domain (Residues 457-717).3 BRAF somatic missense mutations most commonly occur in CR3 region especially at codon 600 which leads to constitutive BRAF protein kinase activity. 80-90% of all V600 mutations are due to NM_004333.4 (BRAF): c.1799T>A (p.Val600Glu), which is the substitution of the wildtype allele, amino acid Valine-600 (Val, V) with a Glutamic acid (Glu, E).4 dbSNP and COSMIC correspondences for this mutation are rs113488022 and COSM18443 respectively. In addition to mutations, other types of BRAF aberrations are found in cancer, including amplification and BRAF fusions.5,6 BRAF germline mutations have also been reported in association with developmental disorders including Noonan syndrome (NS) and Cardio-facio-cutaneous syndrome.7 These germline BRAF mutations are different from those found in cancers.
BRAF is under the RAF (Rapidly Accelerated Fibrosarcoma) protein kinase family. The proto-oncogenes of the RAF family include ARAF, BRAF, and CRAF (currently known as RAF1). BRAF (serine/threonine-protein kinase BRAF) was first isolated in 1993 from a rodent retrovirus, and its malignant potential was discovered when inoculated normal mouse fibroblasts transformed into neoplastic fibrosarcoma cells.1 In 2002, BRAF was first found in human tumors.8 Normally, dividing cells display surface receptors that bind to growth factors present in the surrounding environment. This leads to activation of a family of gene products that stimulate cell growth (RET). Activated RET binds to RAF by a GTP-dependent mechanism. Subsequent phosphorylation of serine and threonine residues by RAF present on the proteins of the mitogen-activated protein kinase pathway (MAPK/ERK) send signals to the nucleus. Mutated RAF leads to constitutive activation of the downstream signaling pathways. This allows the cell to bypass the G1 restriction point of the cell cycle with upregulation of cyclin D1, resulting in unchecked cellular proliferation and survival.9 The MAPK pathway is frequently dysregulated in cancer, often via mutations of its intracellular components or activation of growth factor receptor tyrosine kinases. Among the three forms of RAF kinases, BRAF is the most potent.10
BRAF gene mutations are reportedly associated with papillary thyroid carcinoma (30-70%)9, malignant melanoma (50%), HCL, adenocarcinoma of the lung and colon (5-20%) and ovarian cancer (15-30%).2 The discovery of mutations in BRAF heralded a new era of targeted therapy. HCL and melanoma of particular are noted to have shown a dramatic response to BRAF inhibition.11, 12
Vemurafenib and dabrafenib are two FDA-approved BRAF inhibitors that proved to be effective in the treatment of BRAF p.V600E-mutated melanoma patients.13 However, resistance can occur, and possible mechanisms are due to the decreased negative feedback of the EGFR pathway as a result of BRAF p.V600E inhibition especially in colorectal cancers (CRCs).14 Unlike CRCs, melanoma cells express low levels of EGFR. Therefore, EGFR pathway does not play an important role in BRAF inhibitor resistant melanomas. Another mechanism of resistance could be due to mutations other than p.V600E codon or RAF1 activation. Emerging data suggest that BRAF inhibitors may sometimes be better used in combination therapies rather than sole treatment (e.g., a BRAF inhibitor together with an EGFR or MAP2K1/2 inhibitor) to impacts relevant co-activated pathways.15 Trametinib is currently the only FDA approved MAP2K1/2 inhibitor which also approved in combination use with dabrafenib for melanoma. According to the current studies on the HCL treatment, vemurafenib is on phase II clinical trial, and dabrafenib showed effective responses in the case reports.16
CONCLUSION
The discovery of BRAF mutations in a wide range of cancers shows a great deal of promise in personalized medicine and is a major driver for the rapid drug development of such targeted therapies across a variety of malignancies. However, the approach may ultimately lead to genomic marker-driven treatments independent of their histology and immunophenotype. Our case report was a very rare incidence of two histologically different malignancies with the same BRAF mutation in a patient that clearly showed the effectiveness of such targeted therapy involving different organ systems.
ACKNOWLEDGEMENTS
This study includes data from an abstract that was accepted to for a platform presentation at the International Academy of Pathology/ European Society or Pathology Congress 2016 in Cologne, Germany.
We would like to acknowledge with appreciation Ravindra Prasad, supervisor of Histology at UC Davis Pathology Department for his valuable contribution to this report.
Footnotes
How to cite: Ghorbani-Aghbolaghi A, Lechpammer M, Ali SF, Ku NK, Dwyre DM, Rashidi HH. An extremely rare case of concurrent BRAF V600E mutation driven hairy cell leukemia and melanoma: case report and review of literature. Autops Case Rep [Internet]. 2017;7(3):13-19. http://dx.doi.org/10.4322/acr.2017.032
Financial support: None
REFERENCES
- 1.Sithanandam G, Druck T, Cannizzaro LA, Leuzzi G, Huebner K, Rapp UR. B-raf and a B-raf pseudogene are located on 7q in man. Oncogene. 1992;7(4):795-9. [PubMed] [Google Scholar]
- 2.Blachly JS, Lozanski G, Lucas DM, Grever MR, Kendra K, Andritsos LA. Cotreatment of hairy cell leukemia and melanoma with the BRAF inhibitor dabrafenib. J Natl Compr Canc Netw. 2015;13(1):9-13, quiz 13. http://dx.doi.org/10.6004/jnccn.2015.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sithanandam G, Kolch W, Duh FM, Rapp UR. Complete coding sequence of a human B-raf cDNA and detection of B-raf protein kinase with isozyme specific antibodies. Oncogene. 1990;5(12):1775-80. [PubMed] [Google Scholar]
- 4.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949-54. http://dx.doi.org/10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 5.Tanami H, Imoto I, Hirasawa A, et al. Involvement of overexpressed wild-type BRAF in the growth of malignant melanoma cell lines. Oncogene. 2004;23(54):8796-804. http://dx.doi.org/10.1038/sj.onc.1208152. [DOI] [PubMed] [Google Scholar]
- 6.Ross JS, Wang K, Chmielecki J, et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int J Cancer. 2016;138(4):881-90. http://dx.doi.org/10.1002/ijc.29825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarkozy A, Carta C, Moretti S, et al. Germline BRAF mutations in Noonan, LEOPARD, and cardiofaciocutaneous syndromes: molecular diversity and associated phenotypic spectrum. Hum Mutat. 2009;30(4):695-702. http://dx.doi.org/10.1002/humu.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949-54. http://dx.doi.org/10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 9.Su F, Bradley WD, Wang Q, et al. Resistance to selective BRAF inhibition can be mediated by modest upstream pathway activation. Cancer Res. 2012;72(4):969-78. http://dx.doi.org/10.1158/0008-5472.CAN-11-1875. [DOI] [PubMed] [Google Scholar]
- 10.Pakneshan S, Salajegheh A, Smith R, Lam AK-Y. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45(4):346-56. http://dx.doi.org/10.1097/PAT.0b013e328360b61d. [DOI] [PubMed] [Google Scholar]
- 11.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507-16. http://dx.doi.org/10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietrich S, Glimm H, Andrulis M, von Kalle C, Ho AD, Zenz T. BRAF inhibition in refractory hairy-cell leukemia. N Engl J Med. 2012;366(21):2038-40. http://dx.doi.org/10.1056/NEJMc1202124. [DOI] [PubMed] [Google Scholar]
- 13.Hauschild A, Grob J-J, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, openlabel, phase 3 randomized controlled trial. Lancet. 2012;380(9839):358-65. http://dx.doi.org/10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 14.Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483(7387):100-3. http://dx.doi.org/10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 15.Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J Clin Oncol. 2015;33(34):4023-31. http://dx.doi.org/10.1200/JCO.2015.63.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turski ML, Vidwans SJ, Janku F, et al. Genomically driven tumors and actionability across histologies: BRAF-Mutant cancers as a paradigm. Mol Cancer Ther. 2016;15(4):533-47. http://dx.doi.org/10.1158/1535-7163.MCT-15-0643. [DOI] [PubMed] [Google Scholar]