Abstract
Purpose
Etirinotecan pegol (EP) is a long-acting topoisomerase-I inhibitor designed to provide sustained exposure to SN-38 (active metabolite of irinotecan). This phase II study compared EP versus irinotecan as second-line treatment for KRAS-mutant, irinotecan-naïve, metastatic colorectal cancer (mCRC).
Methods
Patients were randomized to EP 145 mg/m2 or irinotecan 350 mg/m2 Q21d until disease progression/unacceptable toxicity. The primary endpoint was progression-free survival (PFS) with response determined by central radiologic review (RECIST version 1.1).
Results
The study was terminated before completing accrual due to evolving standards of care. Eighty-three patients were randomized. Median PFS was longer with EP versus irinotecan (4.0 versus 2.8 months, respectively; HR 0.65; 95% CI 0.40–1.04; P = 0.07). Six-month PFS rates were 32.8 and 15.4%, respectively. Median OS was 9.6 and 8.4 months in EP and irinotecan arms, respectively (HR 0.91; 95% CI 0.56–1.49). ORRs were 10 and 5%, respectively (P = 0.676); median DOR was significantly longer in EP arm (7.9 versus 1.4 months; P = 0.018). The most common grade-3/4 adverse events for EP and irinotecan were diarrhea (21 vs 20%), neutropenia (10 vs 22%), abdominal pain (14 vs 5%), nausea (14 vs 2%), and vomiting (12 vs 7%), respectively.
Conclusion
EP is active and safe for second-line treatment of KRAS-mutant, irinotecan-naïve mCRC.
Keywords: Etirinotecan pegol, Metastatic colorectal cancer, Irinotecan, KRAS mutant, Chemotherapy
Introduction
Colorectal cancer is the third most commonly diagnosed cancer in the United States, and it is the second and third most fatal cancer in men and women, respectively [1]. Approximately, 20% of patients present with metastatic disease, and 40–50% of patients with earlier stage disease recur after surgical resection or develop distant metastases [2, 3]. The current management of metastatic colorectal cancer (mCRC) involves the administration and sequencing of several different active drugs, alone or in combination, with EGFR-targeted agents restricted to KRAS wild-type disease [4]. Median survival for patients with mCRC now exceeds 2 years; however, treatment remains challenging given that it occurs along a continuum in which patients are ideally exposed to all active, yet toxic, agents over time [5].
Irinotecan is a topoisomerase-I inhibitor initially approved as a salvage therapy for mCRC and is used world-wide alone and in combination for the treatment of mCRC [6–14]. Irinotecan is a prodrug that is metabolized in vivo to SN-38 (7-ethyl-10-hydroxycamptothecin), which has 100- to 1000-fold more potent cytotoxicity in vitro relative to the parent compound [15]. The clinical utility of irinotecan, however, may be limited by suboptimal pharmacokinetics [16]. When given at the recommended dose of 350 mg/m2 by intravenous (IV) infusion over 90 min, irinotecan produces a relatively high peak plasma concentration, which may be responsible for the cholinergic reactions and severe myelosuppression seen in clinical practice with this regimen [17–20]. Moreover, the short terminal half-life of SN-38 from irinotecan can result in undetectable plasma levels within 7 days [17, 21, 22]. Regardless of irinotecan dosing schedule (whether weekly or every 2–3 weeks), the pharmacokinetic profile of irinotecan results in short and intermittent tumor exposure to its S-phase–active metabolite during each treatment cycle, limiting the exposure of tumor cells to topoisomerase inhibition as they enter mitosis [23].
Etirinotecan pegol (EP) is a prodrug that was specifically engineered to reduce peak irinotecan and SN-38 plasma concentrations, and extend the SN-38 effective half-life providing prolonged systemic and increased tumor exposure to the active metabolite. Preclinical studies of EP demonstrate accumulation and retention of SN-38 in tumor tissue secondary to localization and retention of EP in tumor tissue, enhanced antitumor activity, and reduced hematopoietic toxicity relative to irinotecan [24–26]. In the initial phase I clinical trial conducted in patients with refractory solid tumors, EP administration resulted in sustained and controlled exposure to SN-38 and the mean terminal half-life of SN-38 was 50 days [27]. The confirmed partial response (PR) rate was 11%, including a response in one patient with colon cancer whose disease had progressed on a prior irinotecan-based regimen. The early-onset, cholinergic diarrhea commonly seen with irinotecan was not observed in this study. Phase II and III studies conducted in women with heavily pretreated ovarian cancer and advanced breast cancer provide further evidence of the clinical activity and favorable tolerability profile of EP [28–30]. This randomized phase II study was designed to compare the safety and efficacy of EP to conventional irinotecan as second-line therapy for patients with KRAS-mutant, irinotecan-naïve mCRC.
Materials and methods
Patient population
Patients were eligible for study inclusion if they had the following: histologically confirmed, KRAS-mutant, metastatic colorectal adenocarcinoma with at least 1 uni-dimensionally measurable lesion (per RECIST version 1.1); mutation centrally determined from tumor tissue, either the primary or metastatic lesion; received at least one but no more than one prior fluoropyrimidine-containing regimen in the metastatic setting and had not received prior irinotecan in any setting; Eastern Cooperative Oncology Group performance status of 0 or 1; recovery from the toxic effects of prior radiotherapy; adequate organ and bone marrow function at the screening visit; and were at least 18 years of age.
Patients were excluded from the study if they had received chemotherapy or radiotherapy within the 4 weeks (6 weeks for nitrosoureas or mitomycin C) prior to day 1 of cycle 1, and had not, as deemed by the investigator, recovered to National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3.0 Grade 0 or 1 toxicity (any grade of alopecia was allowed) associated with previous treatment, irrespective of the interval from the last treatment; had major surgery within 4 weeks or minor surgery within 2 weeks of day 1 of cycle 1; received biologic agents (including antibodies such as bevacizumab) or investigational drugs within 28 days of study drug initiation; received prior treatment with a camptothecin derivative (eg, topotecan, SN-38 investigational agent); had known or suspected brain metastases; were pregnant or lactating; had liver cirrhosis, interstitial pneumonitis, inflammatory bowel disease, unresolved bowel issues, or chronic or acute gastrointestinal disorders with diarrhea as a major symptom, or any other significant comorbid conditions the investigator believed would impair study participation or cooperation; or had a history of hypersensitivity or intolerance to other pegylated drugs or the excipients of irinotecan. Additionally, patients could not have received select cytochrome P450 3A4 inhibitors or inducers within 2 weeks of study drug initiation or during the study. All patients underwent genotyping for UGT1A1 polymorphism status at a central laboratory. All patients provided signed informed consent before undergoing any protocol-mandated procedure. The study was conducted in accordance with and approved by local institutional review boards and/or ethics committees.
Study design and treatment schedule
This was a multicenter, open-label, randomized phase II study. Patients were randomized 1-to-1 to receive EP or irinotecan. Randomization was stratified by age (18–64 versus ≥ 65 years), extent of metastatic disease (two or more organs versus one organ), and type of first-line therapy in the metastatic setting (single-agent fluoropyrimidine [5-fluorouracil (5-FU) or capecitabine] versus combination therapy [eg, with bevacizumab or oxaliplatin]). Etirinotecan pegol 145 mg/m2 was administered IV over 90 min every 21 days without premedication requirements. Irinotecan 350 mg/m2 was administered IV over 90 min every 21 days (atropine as premedication could be used at investigator’s discretion); patients age 65 and older and those with prior abdominal or pelvic irradiation received a lower starting dose of irinotecan (300 mg/m2).
Treatment was continued until disease progression, unacceptable toxicity, death, withdrawal of consent by the patient, investigator decision, patient loss to follow-up, protocol violation, or termination of the study by the sponsor. Specific dose modifications were recommended based on hematologic parameters and/or the development of diarrhea or other drug-related non-hematologic toxicities (Supplementary Material Appendix 1). A new treatment cycle was not initiated until recovery to an absolute neutrophil count ≥ 1500/μL, platelet count ≥ 100,000/μL, hemoglobin value ≥ 9.0 g/dL, and resolution of treatment-related diarrhea to grade 0 for at least 48 h without supportive antidiarrheal measures (this was increased to 7 days approximately half-way through the study to ensure the safety of redosing EP with its extended half-life). Prophylactic antidiarrheal medication was not allowed for either treatment group; however, patients were instructed to have loperamide readily available and to begin treatment at the first episode of poorly formed or loose stools or the earliest onset of bowel movements more frequent than normally expected for the patient.
Assessments
Tumor measurements were documented during screening by radiologic imaging [computed tomography (CT) scans or magnetic resonance imaging (MRI)] and then approximately every 6 weeks from day 1 of cycle 1 using the same assessment method for each patient (CT or MRI) until documented disease progression, start of new anticancer therapy, death, or end of study participation. A standardized imaging protocol was prepared; all images were forwarded to an independent, blinded central radiologist for review (VirtualScopics, NY). Following the end-of-treatment visit, patients were contacted every 3 months to assess survival status and disease progression, receipt of subsequent therapy for cancer, and resolution of all treatment-attributable toxicity. Quarterly follow-up continued until the patient died, withdrew from the study, or was lost to follow-up. Prior to termination of the study by the sponsor, one final survival sweep was conducted in which patients who were not known to have died or withdrawn consent for follow-up were contacted.
Statistical analysis
The primary endpoint was PFS, defined as the time from the date of randomization to the date of progressive disease (PD) as determined by central radiologic review based on RECIST version 1.1 or death from any cause. The primary PFS analysis required 123 events to achieve 80% power to detect a hazard ratio (HR) of 0.6 (median PFS from 3 months in the irinotecan arm to 5 months with EP) using a two-sided log-rank test at the α = 0.05 significance level. The target sample size was 174 patients (87 patients per treatment arm) to account for accrual duration and loss to follow-up. Secondary efficacy endpoints included OS, defined as the time from the date of randomization until death from any cause; ORR, defined as the number of patients with a confirmed complete response (CR) or PR as determined by central radiologic review; and response duration, measured from the time of first documented PR or CR (whichever was recorded first) until the date of objectively documented progressive disease or death.
Efficacy analyses were performed on the intent-to-treat (ITT) population, which included all randomized patients who underwent baseline evaluation and randomized treatment assignment. The Clopper–Pearson exact two-sided 95% confidence interval (CI) was calculated for each ORR point estimate. Time-to-event outcomes (PFS, OS, and duration of response) were estimated using the Kaplan–Meier method. The P value from a two-sided log-rank test was used for the analysis of PFS and OS, while hazard ratios were generated by a Cox regression model. For PFS, patients who were alive or started new cancer therapy and were without disease progression were censored at the time of last tumor assessment. For OS, patients who were alive were censored at the time of last contact.
Safety endpoints included the incidence and duration of toxicities according to NCI-CTCAE version 3.0. The safety population consisted of all randomized patients who received at least one dose of the study drug to which they were randomized. No inferential statistical testing was planned for safety analyses. Exploratory analyses included assessment of UGT1A1 polymorphism status and its association with diarrhea and neutropenia.
Results
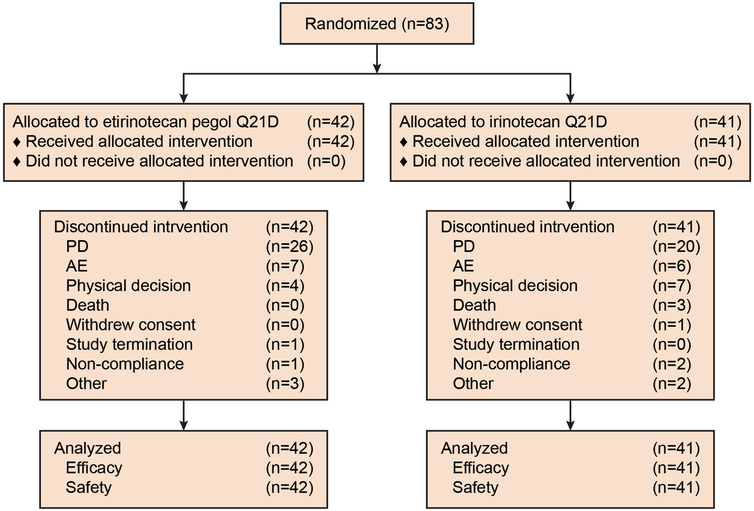
Between February 2009 and March 2014, 83 patients were enrolled at 23 investigative sites in six countries (Fig. 1). The baseline patient demographics and clinical characteristics were generally balanced between treatment arms (Table 1). Notably, the target sample size was not reached. At the time the trial was initiated, single-agent irinotecan was considered an acceptable second-line option for a subset of patients with KRAS mutant mCRC; however, combination therapy became the standard of care before accrual was complete (including combination therapy with 5-FU/leucovorin, zivaflibercept and/or bevacizumab). Accrual was discontinued in March 2014, the database was locked on 2 December 2014, and efficacy and safety analyses were then performed for the 83 patients who comprise the ITT population.
Fig. 1.
CONSORT diagram
Table 1.
Baseline patient demographic and clinical characteristics
| Characteristic | Etirinotecan pegol (n = 42) | Irinotecan (n = 41) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Median age, years (range) | 60 (40–80) | 56 (30–83) | ||
| ≥65 | 12 | 29 | 11 | 27 |
| Male sex | 24 | 57 | 25 | 61 |
| Race | ||||
| White | 29 | 69 | 32 | 78 |
| Black/African American | 3 | 7 | 1 | 2 |
| Asian | 10 | 24 | 7 | 17 |
| Native Hawaiian/Pacific Islander | 0 | 0 | 1 | 2 |
| ECOG PS | ||||
| 0 | 21 | 50 | 25 | 61 |
| 1 | 21 | 50 | 16 | 39 |
| UGT1A1 status | ||||
| Poor | 3 | 7 | 5 | 12 |
| Intermediate | 21 | 50 | 13 | 32 |
| Normal | 12 | 29 | 20 | 49 |
| Inconclusive | 1 | 2 | 0 | 0 |
| Missing | 5 | 12 | 3 | 7 |
| Median time since initial diagnosis, years (range) | 1.3 (0–9) | 1.0 (0–8) | ||
| Median time since metastatic disease, years | 1.0 | 0.9 | ||
| No. of metastatic sites | ||||
| 1 | 16 | 38 | 9 | 22 |
| 2 or more | 26 | 62 | 32 | 78 |
| No. of metastatic organs | ||||
| 0 | 2 | 4.8 | 0 | 0 |
| 1 | 20 | 48 | 13 | 32 |
| 2 or more | 20 | 48 | 28 | 68 |
| No. of prior therapies | ||||
| 1 | 36 | 86 | 34 | 83 |
| 2 | 5 | 12 | 7 | 17 |
| 3 or more | 1 | 2 | 0 | 0 |
| No. of prior regimens in metastatic setting | ||||
| 1 | 42 | 100 | 41 | 100 |
ECOG PS Eastern cooperative oncology group performance status, UGT1A1 uridine diphosphate-glucuronosyl transferase 1A1
All patients in both treatment arms received at least one dose of study drug. The median duration of exposure in the EP arm was 89.5 days (range 21–380 days) versus 49.0 days (range 21–369 days) in the irinotecan arm. The median number of cycles was doubled in the EP arm (four cycles; range 1–17 cycles) relative to the irinotecan arm (two cycles; range 1–15). All patients had discontinued treatment by the date of the data lock. The primary reasons for discontinuation included disease progression (EP 62%; irinotecan 49%), adverse events (AEs) (EP 17%; irinotecan 15%), investigator decision (EP 9.5%; irinotecan 17%), and death (EP 0%; irinotecan 7.3%).
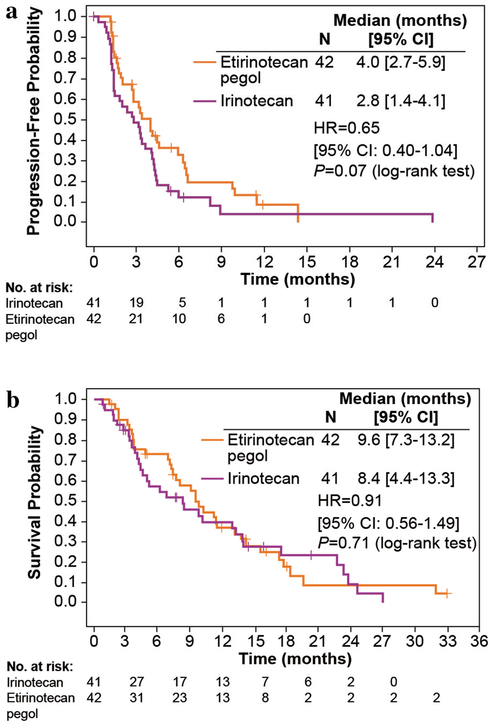
In the primary efficacy analysis, PFS as determined by central radiologic review was prolonged in the patients receiving EP compared to those receiving irinotecan; however, the difference did not reach clinical significance (median PFS, 4.0 versus 2.8 months, respectively; HR 0.65; 95% CI 0.40–1.04; P = 0.07; Fig. 2a). At 3 months, 58.9% of patients in the EP arm were alive and progression free compared with 48.7% in the irinotecan arm (Table 2). Proportions at 6 months were 32.8 and 15.4%, respectively. Nine patients (21.4%) in the EP arm and 4 (9.8%) in the irinotecan arm were censored from the PFS analysis.
Fig. 2.
a Progression-free survival by independent review and b Overall survival by treatment group in patients with KRAS-mutant, irinotecan-naive metastatic colorectal cancer
Table 2.
Primary and secondary efficacy outcomes in the ITT population (central radiologic review)
| Endpoint | Etirinotecan pegol (n = 42) | Irinotecan (n = 41) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| PFS | ||||
| Median, months | 4.0 | 2.8 | ||
| 95% CI | 2.7–5.9 | 1.4–4.1 | ||
| 3-month PFS, % | 58.9 | 48.7 | ||
| 95% CI | 41.8–72.5 | 32.5–63.2 | ||
| 6-month PFS, % | 32.8 | 15.4 | ||
| 95% CI | 18.2–8.2 | 6.2–28.3 | ||
| OS | ||||
| Median, months | 9.6 | 8.4 | ||
| 95% CI | 7.3–13.2 | 4.4–13.3 | ||
| 6-month PFS, % | 73.2 | 57.6 | ||
| 95% CI | 56.9–84.2 | 40.3–71.5 | ||
| 12-month PFS, % | 36.8 | 39.8 | ||
| 95% CI | 22.1–51.6 | 23.9–55.3 | ||
| RECIST best tumor response* | ||||
| ORR | 4 | 9.8 | 2 | 5.0 |
| 95% CI | 2.7–23.1 | 0.6–16.9 | ||
| CR | 0 | 0 | 0 | 0 |
| PR | 4 | 9.8 | 2 | 5.0 |
| SD | 23 | 56.1 | 16 | 40.0 |
| PD | 10 | 24.4 | 15 | 37.5 |
| Inevaluable** | 4 | 9.8 | 7 | 17.5 |
| Duration of response | ||||
| Median, months | 7.9 | 1.4 | ||
| 95% CI | 1.5–11.6 | 1.4–1.4 | ||
| P value (log-rank) | 0.018 | |||
ITT intention-to-treat, PFS progression-free survival, CI confidence interval, OS overall survival, ORR objective response rate, CR complete response, PR partial response, SD stable disease, PD progressive disease
Two patients (one per treatment arm) who did not have measurable lesions by central review were excluded from the analysis
These are patients who did not have post-baseline tumor assessments 42 days or later after study drug administration and also did not have disease progression during the study
Median OS did not differ between treatment arms (9.6 versus 8.4 months; HR 0.91; 95% CI 0.56–1.49; P value = 0.71; Fig. 2b). The proportion of patients alive at 6 months was numerically higher in the EP arm relative to irinotecan (73.2 versus 57.6%, respectively; Table 2), and the proportions at 12 months were similar (36.8 versus 39.8%).
Response data are summarized in Table 2. Two patients (one in each treatment arm) were excluded from the ORR calculation due to lack of measurable lesions for central radiologic review. Of the remaining 81 patients, four in the EP arm and two in the irinotecan arm responded to treatment (10 and 5%, respectively). The median duration of response was significantly longer in the EP arm relative to irinotecan (7.9 versus 1.4 months, respectively; P = 0.018). The majority of patients in the EP arm experienced stable disease as their best overall response (56.1%). Sixteen patients (40%) treated with irinotecan experienced stable disease as their best overall response.
Rates of AEs were generally similar between treatment arms, with the exception of alopecia, which appeared to be reduced with EP treatment (17 versus 59% with irinotecan) (Table 3). The most common treatment-emergent AEs of grades ≥ 3, shown in Table 4, included diarrhea (EP 21%; irinotecan 20%), neutropenia (including neutropenia, decreased neutrophils, and febrile neutropenia) (EP 10%; irinotecan 22%), and abdominal pain (EP 14%; irinotecan 5%). Serious AEs were slightly higher in the irinotecan arm (59 vs 45% for EP; Supplementary Material Table 1). The most common serious AEs were diarrhea (EP 10%; irinotecan 17%), dehydration (EP 7%; irinotecan 7%), and intestinal obstruction (EP 2%; irinotecan 10%).
Table 3.
The most common (≥ 10%) treatment-emergent adverse events by treatment arm
| Adverse event | Etirinotecan pegol (n = 42) | Irinotecan (n = 41) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Patients with ≥ 1 TEAE | 42 | 100 | 41 | 100 |
| Diarrhea | 26 | 62 | 31 | 76 |
| Nausea | 23 | 55 | 24 | 59 |
| Vomiting | 18 | 43 | 21 | 51 |
| Fatigue | 20 | 48 | 18 | 44 |
| Abdominal pain | 18 | 43 | 14 | 34 |
| Alopecia | 7 | 17 | 24 | 59 |
| Anorexia | 13 | 31 | 10 | 24 |
| Constipation | 12 | 29 | 10 | 24 |
| Weight decreased | 13 | 31 | 6 | 15 |
| Dehydration | 6 | 14 | 8 | 20 |
| Dizziness | 7 | 17 | 7 | 17 |
| Neutropeniaa | 11 | 26 | 12 | 29 |
| Hypokalemia | 9 | 21 | 4 | 10 |
| Anemia | 6 | 14 | 6 | 15 |
| Pyrexia | 4 | 10 | 7 | 17 |
| Asthenia | 7 | 17 | 3 | 7 |
| Upper abdominal pain | 5 | 12 | 4 | 10 |
| Decreased hemoglobin | 2 | 5 | 6 | 15 |
| Hyponatremia | 5 | 12 | 3 | 7 |
| Lethargy | 3 | 7 | 5 | 12 |
| Leukopenia | 5 | 12 | 2 | 5 |
| Abdominal distention | 1 | 2 | 5 | 12 |
| Headache | 5 | 12 | 1 | 2 |
| Blurred vision | 5 | 12 | 1 | 2 |
| Anxiety | 5 | 12 | 0 | 0 |
TEAE treatment-emergent adverse events
Includes neutropenia, decreased neutrophils, and febrile neutropenia
Table 4.
The most common (≥ 5%) treatment-emergent adverse events of grades ≥ 3 by treatment arm
| Adverse event | Etirinotecan pegol (n = 42) | Irinotecan (n = 41) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Patients with ≥ 1 TEAE | 26 | 62 | 26 | 63 |
| Diarrhea | 9 | 21 | 8 | 20 |
| Neutropeniaa | 5 | 12 | 9 | 22 |
| Abdominal pain | 6 | 14 | 2 | 5 |
| Dehydration | 4 | 10 | 4 | 10 |
| Vomiting | 5 | 12 | 3 | 7 |
| Nausea | 6 | 14 | 1 | 2 |
| Hypokalemia | 3 | 7 | 3 | 7 |
| Fatigue | 4 | 10 | 1 | 2 |
| Intestinal obstruction | 1 | 2 | 4 | 10 |
| Leukopenia | 3 | 7 | 2 | 5 |
TEAE treatment-emergent adverse events
Includes neutropenia, decreased neutrophils, and febrile neutropenia
Diarrhea was the most common treatment-emergent AE in both arms, observed in 62% of patients in the EP arm and in 76% of those in the irinotecan arm (Table 3). Although a similar number of patients received some type of antidiarrheal treatment (64 and 76%, respectively), fewer patients treated with EP received loperamide (43 versus 66% for irinotecan), and slightly more received diphenoxylate atropine (36 and 22%, respectively). Grade 3 diarrhea was observed in 9 (21%) EP recipients and 7 (17%) irinotecan recipients. There were no cases of grade 4 diarrhea in the EP arm, and 1 case in the irinotecan arm. The median time to onset of grade 3/4 diarrhea was 68 days (range 5–181 days) in the EP arm and 16 days (range 2–73) in the irinotecan arm. The median duration of grade 3/4 diarrhea was similar in both arms (6 versus 5 days, respectively).
Eleven patients in the EP arm experienced at least one episode of neutropenia [grade 1/2, seven patients; grade 3/4, four patients] compared to 12 patients in the irinotecan arm [grade 1/2, three patients; grade 3/4, nine patients]. The median durations of grade 3/4 neutropenia were similar (7 days with EP versus 5 days with irinotecan), while the time to onset of grade 3/4 neutropenia was longer in the EP arm (109 versus 21 days for irinotecan).
Eighteen (43%) patients treated with EP required at least one dose delay due to toxicity, and 13 (31%) required at least one dose reduction (Supplementary Material Table 2). Twelve (29%) and 15 (37%) patients treated with irinotecan required at least one dose delay and/or reduction, respectively. Discontinuation of treatment due to AEs was similar in both arms: seven in the EP arm (two cases of grade 3 diarrhea, one case each of: grade 3 asthenia, grade 3 abdominal pain, grade 2 neutropenia, grade 2 fatigue, and grade 2 liver abscess) and six in the irinotecan arm (one case each of: grade 4 colitis, grade 3 vomiting, grade 3 hyperbilirubinemia, grade 3 hypogastric abdominal pain, grade 2 diarrhea, and grade 2 fatigue). There were two treatment-related fatal AEs: one death due to acute prerenal azotemia in the EP arm and one death due to sepsis secondary to neutropenia in the irinotecan arm.
Patients were classified as poor or intermediate/normal risk based on their UGT1A1 status. Given the sample size and limited observations of grade 3 diarrhea and neutropenia, no firm conclusions can be drawn regarding an association between these toxicities and UGT1A1 allele for patients treated with EP.
Discussion
In this randomized phase II trial, single-agent EP showed clinical activity in patients with previously-treated KRAS-mutant mCRC. The study was originally designed to demonstrate the superiority of EP to single-agent irinotecan in the second-line setting; however, enrollment to the trial ended prematurely due to changes in clinical practice that made recruitment to single-agent camptothecin-based therapy in this population difficult. Thus, the trial only enrolled 83 of the planned 174 patients over 5 years, and the reduced enrollment limited the statistical power of the trial. Point estimates for all efficacy outcomes favored EP, but in the primary analysis, the increase in PFS (from 2.8 to 4.0 months) narrowly missed achieving statistical significance despite the reduced enrollment to the study (P = 0.07). At 6 months, 32.8% of patients randomized to EP were progression free compared to 15.4% of patients randomized to irinotecan. Four patients in the EP arm and 2 in the irinotecan arm achieved PRs. Notably, the median duration of response was 6.5 months longer in the EP arm, and this difference was statistically significant (P = 0.018); it should be noted that this analysis was not pre-specified in any hierarchical testing sequence. Overall survival was similar (median OS, 9.6 months for EP and 8.4 months for irinotecan), although the trial was neither designed nor sufficiently powered to show a difference in this outcome.
EP was generally well tolerated when dosed at 145 mg/m2 every 21 days. The most commonly reported grade 3/4 AEs in this treatment arm were diarrhea, abdominal pain, nausea, and vomiting. Diarrhea was also the most commonly reported grade 3/4 AE in the irinotecan arm; however, the onset of irinotecan-induced diarrhea was early in the course of treatment (median time to onset, 16 days). With EP, grade 3/4 diarrhea had a delayed onset (median time to onset, 68 days), consistent with the pharmacokinetic profile of this agent, which eliminates the high peak plasma drug concentrations associated with certain toxicities, such as early-onset cholinergic diarrhea with the potential for late onset toxicities. Of note, the diarrheal supportive care guidelines evolved during the course of the phase II program: all protocols now mandate a 7-day window (as opposed to 48-h) in which patients must have normal bowel function prior to redosing. In addition, with grade 2 diarrhea, the dose of EP is reduced one dose level to 120 mg/m2 and with a second occurrence the dose level is further reduced to 90 mg/m2. This has led to a reduction in the incidence of grade 3 and higher diarrhea [31].
Similarly, there appeared to be a lower incidence and longer time to the onset of grade 3/4 neutropenia in the EP arm (10% and time to onset of 109 days) relative to the irinotecan arm (22% and time to onset of 21 days). This finding is consistent with preclinical data that demonstrated reduced myelosuppression for EP relative to irinotecan [24, 26]. A relatively low rate of serious neutropenia has also been reported with EP in other patient populations [28, 29].
The clinical development of EP continues to progress in breast cancer as evidenced by an ongoing phase III trial of EP versus treatment of physician’s choice in patients with metastatic breast cancer who have stable brain metastases and have been previously treated with an anthracycline, a taxane, and capecitabine. In the mCRC setting, due to changes in the standard of care in the second-line setting, the clinical development of EP should include its evaluation in combination regimens. Because irinotecan is commonly administered with infusional 5-FU and leucovorin, a phase I trial was conducted in parallel with this randomized phase II trial.32 The phase I trial established a recommended dose of EP of 75 mg/m2 given every 2 weeks with leucovorin (400 mg/m2 IV over 2 h), followed by 5-FU (IV bolus 400 mg/m2 IV over 1–2 min then 2400 mg/m2 IV by continuous infusion over 46 h). Results of a randomized phase II trial of nanoliposomal irinotecan (PEP02 MM-398) in combination with leucovorin plus 5-FU as second-line therapy in patients with mCRC support the combination strategy as evidenced by a median PFS of 5.0 months and a median OS of 14.6 months [33]. It is recommended that future studies of EP for the second-line treatment of mCRC should use combination therapy instead of single-agent therapy.
Conclusions
In conclusion, single-agent EP produced encouraging PFS, OS, and independently confirmed responses, with a favorable toxicity profile, in patients with previously treated KRAS-mutant mCRC. The current findings support the integration of EP into contemporary regimens in place of irinotecan for the treatment of patients with mCRC.
Supplementary Material
Acknowledgements
Gregg Olsen, MD, East Valley Hematology and Oncology Medical Center, Sherman Oaks, CA, USA for enrolling patients; Phillips Gilmore Oncology Communications, Inc. for medical writing assistance, funded by Nektar Therapeutics.
Footnotes
Conflict of interest Research support provided by Nektar Therapeutics. Philip Philip reports grant support from Bayer, Merck, Novartis, Celgene, Incyte, Lilly. Mary Tagliaferri and Ute Hoch report employment with Nektar Therapeutics. Alison Hannah reports consultancy fees from Nektar Therapeutics. The remaining authors have nothing to report.
Electronic supplementary material The online version of this article (doi:10.1007/s00280-017-3438-y) contains supplementary material, which is available to authorized users.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30 [DOI] [PubMed] [Google Scholar]
- 2.Surveillance, epidemiology, and end results program: SEER stat facts sheet: colon and rectum cancer. http://seer.cancer.gov/stat-facts/html/colorect.html. Cited 8 Feb 2017
- 3.Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll HJ et al. (2015) A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer 14:1–10 [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network: clinical practice guidelines in oncology (NCCN guidelines®): colon cancer version 1.2017, 11/16 update. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Cited 8 Feb 2017
- 5.Lucas AS, O’Neil BH, Goldberg RM (2011) A decade of advances in cytotoxic chemotherapy for metastatic colorectal cancer. Clin Colorectal Cancer 10:238–244 [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Pyrhönen S, James R, Hickish TF, Heikkila R et al. (1998) Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 352:1413–1418 [DOI] [PubMed] [Google Scholar]
- 7.Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R et al. (1998) Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 352:1407–1412 [DOI] [PubMed] [Google Scholar]
- 8.André T, Louvet C, Maindrault-Goebel F, Couteau C, Mabro M, Lotz JP et al. (1999) CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. GERCOR. Eur J Cancer 35:1343–1347 [DOI] [PubMed] [Google Scholar]
- 9.Fuchs CS, Moore MR, Harker G, Villa L, Rinaldi D, Hecht JR (2003) Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol 21:807–814 [DOI] [PubMed] [Google Scholar]
- 10.Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M et al. (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 25:4779–4786 [DOI] [PubMed] [Google Scholar]
- 11.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C et al. (2007) on behalf of Gruppo Oncologico Nord Ovest. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 25:1670–1676 [DOI] [PubMed] [Google Scholar]
- 12.Haller DG, Rothenberg ML, Wong AO, Koralewski PM, Miller WH Jr, Bodoky G et al. (2008) Oxaliplatin plus irinotecan compared with irinotecan alone as second-line treatment after single-agent fluoropyrimidine therapy for metastatic colorectal carcinoma. J Clin Oncol 26:4544–4550 [DOI] [PubMed] [Google Scholar]
- 13.Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T et al. (2012) Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 30:3499–3506 [DOI] [PubMed] [Google Scholar]
- 14.Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L et al. (2014) Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 371:1609–1618 [DOI] [PubMed] [Google Scholar]
- 15.Kawato Y, Animimi M, Hirota Y, Kuga H, Sato K (1991) Intra-cellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res 51:4187–4191 [PubMed] [Google Scholar]
- 16.Perez EA, Hillman DW, Mailliard JA, Ingle JN, Ryan JM, Fitch TR et al. (2004) Randomized phase II study of two irinotecan schedules for patients with metastatic breast cancer refractory to an anthracycline, a taxane, or both. J Clin Oncol 22:2849–2855 [DOI] [PubMed] [Google Scholar]
- 17.Kehrer DFS, Yamamoto W, Verweij J, de Jonge MJA, de Bruijn P, Sparreboom A (2000) Factors involved in prolongation of the terminal disposition phase of SN-38: clinical and experimental studies. Clin Cancer Res 6:3451–3458 [PubMed] [Google Scholar]
- 18.Masi G, Falcone A, Di Paolo A, Allegrini G, Danesi R, Barbara C et al. (2004) A phase I and pharmacokinetic study of irinotecan given as a 7-day continuous infusion in metastatic colorectal cancer patients pretreated with 5-fluorouracil or raltitrexed. Clin Cancer Res 10:1657–1663 [DOI] [PubMed] [Google Scholar]
- 19.Takimoto CH, Morrison G, Harold N, Quinn M, Monahan BP, Band RA et al. (2000) Phase I and pharmacologic study of irinotecan administered as a 96-hour infusion weekly to adult cancer patients. J Clin Oncol 18:659–667 [DOI] [PubMed] [Google Scholar]
- 20.Herben VM, Schellens JH, Swart M, Gruia G, Vernillet L, Beijnen JH et al. (1999) Phase I and pharmacokinetic study of irinotecan administered as a low-dose, continuous intravenous infusion over 14 days in patients with malignant solid tumors. J Clin Oncol 17:1897–1905 [DOI] [PubMed] [Google Scholar]
- 21.Chabot CG (1997) Clinical pharmacokinetics of irinotecan. Clin Pharmacokinet 33:245–259 [DOI] [PubMed] [Google Scholar]
- 22.Pitot HC, Goldberg RM, Reid JM, Sloan JA, Skaff PA, Erlichman C et al. (2000) Phase I dose-finding and pharmacokinetic trial of irinotecan hydrochloride (CPT-11) using a once-every-three-week dosing schedule for patients with advanced solid tumor malignancy. Clin Cancer Res 6:2236–2244 [PubMed] [Google Scholar]
- 23.Goldwasser F, Bae I, Valenti M, Torres K, Pommier Y (1995) Topoisomerase I related parameters and camptothecin activity in the colon carcinoma cell lines from the National Cancer Institute anticancer screen. Cancer Res 55:2116–2121 [PubMed] [Google Scholar]
- 24.Hoch U, Staschen C-M, Johnson RK, Eldon MA (2014) Non-clinical pharmacokinetics and activity of etirinotecan pegol (NKTR-102), a long-acting topoisomerase 1 inhibitor, in multiple cancer models. Cancer Chemother Pharmacol 74:1125–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adkins CE, Nounou MI, Hye T, Mohammad AS, Terrell-Hall T, Mohan NK et al. (2015) NKTR-102 Efficacy versus irinotecan in a mouse model of brain metastases of breast cancer. BMC Cancer 15:685. doi: 10.1186/s12885-015-1672-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persson H, Barker T, Eldon M et al. (2008) NKTR-102, a novel pegylated-irinotecan, has an enhanced pharmacokinetic profile with reduced gastrointestinal and hematopoietic toxicity compared to irinotecan with repeat dosing in dogs In: Presented at the 2008 American Association for Cancer Research Annual Meeting, San Diego, 12–16 April (abstr 5741) [Google Scholar]
- 27.Jameson GS, Hamm JT, Weiss GJ, Alemany C, Anthony S, Basche M et al. (2013) A multicenter, phase 1, open-label, dose-escalation study to assess the safety, tolerability, and pharmacokinetics of etirinotecan pegol in patients with refractory solid tumors. Clin Cancer Res 19:268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vergote IB, Garcia A, Micha J, Pippitt C, Bendell J, Spitz D et al. (2013) Randomized multicenter phase II trial comparing two schedules of etirinotecan pegol (NKTR-102) in women with recurrent platinum-resistant/refractory epithelial ovarian cancer. J Clin Oncol 10:4060–4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Awada A, Garcia AA, Chan S, Jerusalem GH, Coleman RE, Huizing MT, NKTR-102 Study Group et al. (2013) Two schedules of etirinotecan pegol (NKTR-102) in patients with previously treated metastatic breast cancer: a randomised phase 2 study. Lancet Oncol 14:1216–1225 [DOI] [PubMed] [Google Scholar]
- 30.Perez EA, Awada A, O’Shaughnessy J, Rugo HS, Twelves C, Im SA et al. (2015) Etirinotecan pegol (NKTR-102) versus treatment of physician’s choice in women with advanced breast cancer previously treated with an anthracycline, a taxane, and capecitabine (BEACON): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 16:1556–1568 [DOI] [PubMed] [Google Scholar]
- 31.Cortés J, Rugo HS, Twelves C, Awada A, Perez EA, Im SA et al. (2016) Safety and tolerability of etirinotecan pegol in advanced breast cancer: analysis of the randomized, phase 3 BEACON trial. Springerplus 5:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnamurthi S, Manpreet C, Rodal MB et al. (2014) A phase 1 study of etirinotecan pegol in combination with 5-fluorouracil and leucovorin in patients with advanced cancer. J Clin Oncol 32(suppl 3) (abstr 550) [Google Scholar]
- 33.Chibaudel B, Maindrault-Gœbel F, Bachet JB, Louvet C, Khalil A, Dupuis O et al. (2016) PEPCOL: a GERCOR randomized phase II study of nanoliposomal irinotecan PEP02 (MM-398) or irinotecan with leucovorin/5-fluorouracil as second-line therapy in metastatic colorectal cancer. Cancer Med 5:676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.