Abstract
Major psychiatric disorders are associated with dysregulated glutamate homeostasis and deficits in the omega-3 fatty acid docosahexaenoic acid (DHA). This study determined the effects of dietary-induced alterations in brain DHA accrual on cortical glutamate homeostasis in the adult rat brain. Adolescent rats were fed a control diet (n=20), a n-3 fatty acid-deficient diet (DEF, n=20), or a fish oil-fortified diet containing preformed DHA (FO, n=20). In adulthood 1H MRS scans were performed with voxels in the prefrontal cortex (PFC) and thalamus. Compared with controls, erythrocyte, PFC, and thalamus DHA levels were significantly lower in DEF rats and significantly higher in FO rats. In the PFC, but not the thalamus, glutamate was significantly elevated in DEF rats compared with controls and FO rats. Glutamine did not differ between groups and the glutamine/glutamate ratio was lower in DEF rats. No differences were observed for markers of excitotoxicity (NAA, GFAP), or astrocyte glutamate transporter (GLAST, GLT-1) or glutamine synthetase expression. Across diet groups, PFC DHA levels were inversely correlated with PFC glutamate levels and positively correlated with GLAST expression. Together these findings demonstrate that rat cortical DHA accrual during adolescence impacts glutamate homeostasis in the adult PFC.
Keywords: Omega-3 fatty acids, Docosahexaenoic acid (DHA), Glutamate, Glutamine synthetase, Glutamate transporter, Rat
1. Introduction
Converging evidence indicates that mood and psychotic disorders are associated with abnormalities in glutamate neurotransmission. Postmortem brain studies suggest that patients with psychiatric disorders exhibit altered glutamate transporter and N-methyl-d-aspartate (NMDA) receptor subunit expression (Beneyto et al., 2008; Feyissa et al., 2009; Matute et al., 2005; Rao et al., 2012; Uezato et al., 2009) and glutamate concentrations (Hashimoto et al., 2007). The non-competitive glutamate NMDA receptor antagonist ketamine has psychotogenic effects (Lahti et al., 1995; Malhotra et al., 1997), as well as rapid antidepressant effects (McGirr et al., 2015), and increases cortical glutamine in healthy human subjects (Rowland et al., 2005) and glutamate in the rat PFC (Kim et al., 2011). Meta-analyses of 1H magnetic resonance spectroscopy (1H MRS) studies indicate that schizophrenia (Merritt et al., 2016) and bipolar disorder (Gigante et al., 2012) are associated with elevated, and major depressive disorder (MDD)(Yildiz-Yesiloglu and Ankerst, 2006) with lower, glutamate and/or glutamine levels in cortical and subcortical brain regions. While these findings suggest that a dysregulation in glutamate homeostasis is associated with the pathophysiology of mood and psychotic disorders, little is known about pathogenic mechanisms and associated risk factors.
A separate body of translational evidence has identified a deficiency in omega-3 polyunsaturated fatty acids (n-3 PUFA), including docosahexaenoic acid (DHA), as a potential neurodevelopmental risk factor for psychiatric disorders (McNamara et al., 2015; Teseia et al., 2017). Meta-analyses indicate that bipolar I disorder (McNamara and Welge, 2016), MDD (Lin et al., 2010), and schizophrenia (van der Kemp et al., 2012) are associated with erythrocyte (red blood cell) membrane DHA deficits. DHA is the most abundant n-3 PUFA in the mammalian brain, and erythrocyte and frontal cortex DHA levels are positively correlated in adult humans (Carver et al., 2001). DHA increases rapidly in the human frontal cortex (Carver et al., 2001) and rat brain (Green et al., 1999) during active periods of synaptic maturation, and emerging evidence suggests that DHA is required for the structural and functional maturation of glutamatergic synapses (Cao et al., 2009; Moreira et al., 2010; Yoshida et al., 1997). Furthermore, astrocytes play a key role in maintaining glutamate homeostasis (Hertz and Zielke, 2004), and DHA is required for optimal astrocyte maturation (Champeil-Potokar et al., 2004, 2006; Joardar et al., 2006) and modulates astrocyte glutamate transporter activity and expression (Champeil-Potokar et al., 2015; Berry et al., 2005; Grintal et al., 2009; Harbeby et al., 2012). However, the effects of altering DHA levels on glutamate homeostasis in brain have not been systematically evaluated in vivo using1H MRS.
The present study investigated the effects of alterations in rat brain DHA accrual during adolescent development on in vivo glutamate and glutamine levels in the young adult PFC and thalamus using 1H MRS at 7 Tesla. Three distinct groups of rats were generated with high, medium (control), and low blood and regional brain DHA levels. Following scanning, postmortem erythrocyte, PFC, and thalamus DHA levels, and astrocyte glutamate transporters GLAST (EAAT1) and GLT-1 (EAAT2), glutamine synthetase (GS), and glial fibrillary acidic protein (GFAP) expression were determined. Based on the translational evidence reviewed above, our specific prediction was that blood and brain DHA levels would be inversely correlated with glutamate and glutamine levels.
2. Methods
2.1. Animals and diets
Post-weaning (P20) male Long-Evans hooded rats from different nulliparous dams were purchased (Harlan Farms, Indianapolis, IN), and randomized to one of three diets (n=20/diet group) from P21 until young adulthood (P90). Control (CON) rats were maintained on an α-linolenic acid (ALA, 18:3n-3)-fortified diet (TD.04285, Harlan-TEKLAD, Madison, WI). Deficient (DEF) rats were maintained on an ALA-free diet (TD.04286), and n-3 PUFA enriched rats were maintained on diet containing 1.1% fish oil in place of ALA (FO, TD.110837, Harlan-TEKLAD, Madison, WI). Diets were closely matched for all non-fat nutrients and fatty acid composition with the exception of ALA, which was absent from the DEF and FO diets, and DHA and eicosapentaenoic acid (EPA, 20:5n-3) which were present in the FO diet but not the CON and DEF diets (Supplemental Table 1). Rats were housed 2 per cage with food and water available ad libitum, and were maintained under standard vivarium conditions on a 12:12 h light:dark cycle. All experimental procedures were approved by the University of Cincinnati and Children’s Hospital Institutional Animal Care and Use Committees, and adhere to the guidelines set by the National Institutes of Health.
2.2. 1H MRS
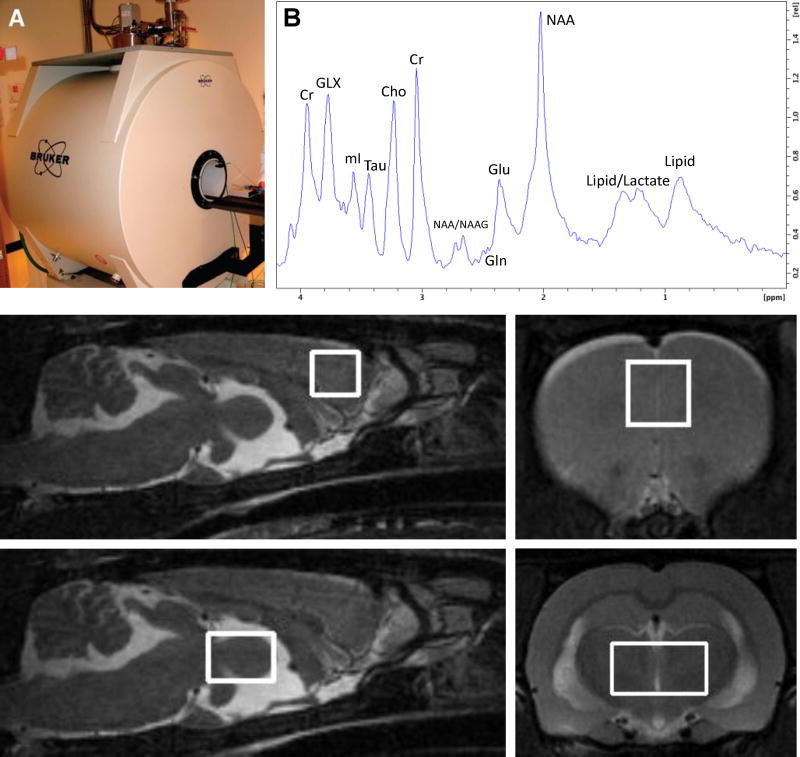
Adult (P90) male rats were anesthetized with 2.5–3.5% isoflurane in air, positioned supine with their teeth in a bite bar, and scanned in a 7T Bruker Biospec system (Bruker BioSpin, Ettlingen, Germany)(Fig. 1A). Respiration was monitored and body temperature was maintained at 36–38°C using an animal monitoring system (SAI Inc., Stony Brook, NY). The head was centered inside a 38 mm Litz coil (Doty Scientific, Inc., Columbia, SC), and a set of localizers from each orthogonal plane were collected. Following acquisition of these localizers, fast spin echo (RARE) images (effective TE 45 ms, TR 3300 ms, RARE factor 16, matrix 256 × 256, FOV 35 mm, 20 slices in the axial direction and 10 slices in the sagittal direction) were collected for voxel placement. Voxels were placed in the bilateral mPFC (3 mm × 3 mm × 3 mm, 256 averages, Fig. 1C,D) and thalamus (6 mm × 4 mm × 4 mm, 128 averages, Fig. 1E,F). The voxel was shimmed using FASTMAP to an average line width of 10.5 Hz. Water suppressed data were acquired using VAriable Pulse Power and Optimized Relaxation delays (VAPOR) water suppression with a 120 Hz bandwidth followed by Point RESolved Spectroscopy (PRESS) with Outer Volume Suppression (OVS) localization with a TE of 20 ms, TR of 2500 ms, 2048 points, and a spectral width of 3301 Hz. A 1H MRS spectrum acquired from the rat mPFC is illustrated in Fig. 1B. Unsuppressed data were acquired by turning the VAPOR RF pulses off and acquiring 4 averages. The spectra were imported into LCModel for quantitation, using the unsuppressed acquisition for phasing, eddy-current correction, and an internal water reference. A provided simulated basis set was used to fit the spectra to obtain peak areas which were scaled to the water area (assumed concentration 45 mM) to provide concentration estimates. These estimates were not corrected for metabolite relaxation times and are reported in institutional units (IU). Estimates were retained if the Cramer-Rao lower bound (CRLB) reported by LCModel was less than 25%. Primary measures of interest were glutamate, glutamine, glutamate+glutamine (Glx), and the glutamine/glutamate ratio. A secondary measure of interest was N-acetyl aspartate (NAA) which served as an index of excitotoxicity (Roffman et al., 2000).
Figure 1.
The 7T Bruker Biospec Imaging System (A), a 1H MRS spectrum from a control mPFC (B), voxel placement in mPFC in the sagittal (C) and coronal (D) orientations, and voxel placement in thalamus in the sagittal (E) and coronal (F) orientations. Cr, creatine; Glx, glutamate+glutamine; mI, myo-inositol; Tau, protein; Cho, choline; NAA, N-acetyl aspartate; NAAG, N-acetyl aspartylglutamate; Gln, glutamine; Glu, glutamate.
2.3. Tissue collection
Immediately following scanning, isoflurane-anesthetized rats were sacrificed by decapitation. Whole venous trunk blood was collected into EDTA-coated tubes, and centrifuged at 4°C for 20 min (1,500 × g). Plasma and buffy coat were then removed and erythrocytes washed 3 times with 0.9% NaCl and stored at −80°C. The brain was dissected on ice to isolate the left PFC and thalamus for fatty acid analyses, and the right PFC isolated for gene expression assays.
2.4. Gas chromatography
The gas chromatography procedure has been described in detail previously (McNamara et al., 2009). Briefly, erythrocyte, PFC, and thalamus total fatty acid composition were determined with a Shimadzu GC-2014 equipped with an auto-injector (Shimadzu Scientific Instruments Inc., Columbia MD). The column was a DB-23 (123–2332): 30 m (length), I.D. 0.32 mm wide bore, film thickness of 0.25 µM (J&W Scientific, Folsom CA). Fatty acid identification was determined using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Analysis of fatty acid methyl esters is based on areas calculated with EZstart 7.4 software. Fatty acid composition is expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids). All samples were processed by a technician blinded to treatment. The primary measure of interest was DHA.
2.5. qRT-PCR
Total RNA was isolated using the RNeasy Mini Kit and potential DNA contamination removed using RNase-free DNase (Qiagen, Valencia, CA). cDNA was prepared using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Targets were amplified in quadruplicate wells of a 384 well TaqMan low density microfluidic card (Thermo Fisher, Waltham, MA) on an ABI 7900HT500 Real Time PCR System (Applied Biosystems, Foster City, CA). Primary genes of interest were the astrocyte glutamate-aspartate transporter (GLAST, Slc1a3, NM_001289941), glial-specific glutamate transporter-1 (GLT-1, Slc1a2, NM_001035233), glutamine synthetase (GS, Glul, NM_017073), and glial fibrillary acidic protein (GFAP, NM_017009). Data were analyzed as normalized cycle threshold values (ΔCT) using PPIA (NM_017101) as the housekeeping gene.
2.6. Statistical analyses
Diet group differences neurochemical, fatty acid, and qRT-PCR data were evaluated in each brain region separately with a one-way ANOVA. Post hoc comparisons were made with unpaired t-tests (2-tailed, α=0.05). Pearson (linear) correlation analyses were performed to determine relationships between brain fatty acid, gene expression, and neurochemical concentration data. Statistical analyses were performed with GB-STAT software (Dynamic Microsystems, Inc., Silver Springs MD).
3. Results
3.1. Fatty acid composition
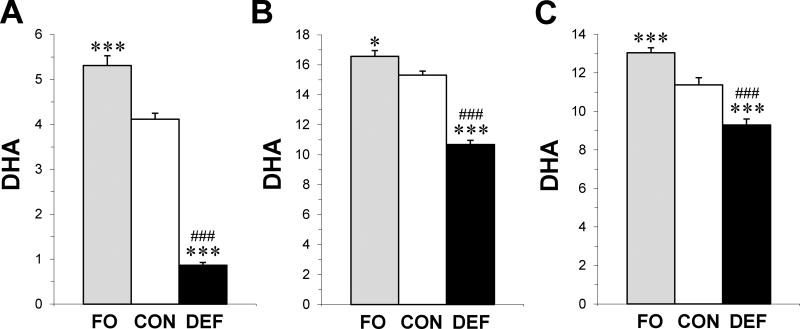
Significant group effects were observed for erythrocyte (F2,57 = 110.2, p≤0.0001), PFC (F2,57 = 85.4, p≤0.0001), and thalamus (F2,57 = 35.9, p≤0.0001) DHA levels. Compared with controls, erythrocyte DHA was significantly lower in adult rats fed the DEF diet (4-fold, p≤0.0001) and significantly higher in FO rats (+19%, p=0.003)(Fig. 2A). PFC DHA levels were significantly lower in adult rats fed the DEF diet (−30%, p≤0.0001), and significantly higher in FO rats (+8%, p=0.01), compared with controls (Fig. 2B). Thalamus DHA levels were significantly lower in adult rats fed the DEF diet (−17%, p≤0.0001), and significantly higher in FO rats (+14%, p≤0.0001), compared with controls (Fig. 2C). Across diet groups erythrocyte DHA levels were positively correlated with DHA levels in the PFC (r = +0.81, p≤0.0001) and thalamus (r = +0.61, p≤0.0001).
Figure 2.
Erythrocyte (A), PFC (B), and thalamus (C) DHA composition in adult rats maintained on the control diet (CON), n-3-free diet (DEF), and fish oil-fortified diet (FO)(n=20/group) during peri-adolescent development. Values are group mean composition (mg fatty acid/100 mg fatty acids) ± S.E.M. *P≤0.05, ***P≤0.0001 vs. CON, ###P≤0.0001 vs. FO.
3.2. 1H MRS
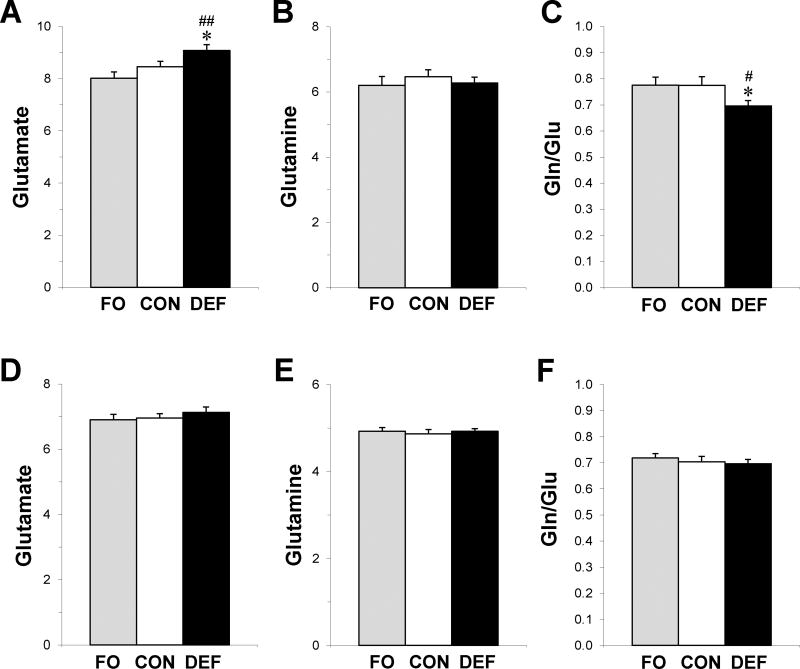
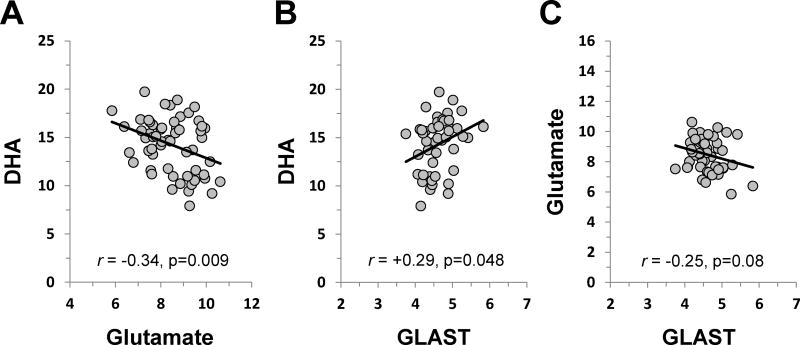
In the PFC the group effect was significant for glutamate (F2,57 = 5.5, p=0.006), which was higher in DEF rats compared with controls (+7%, p=0.05) and FO rats (+12%, p=0.003)(Fig. 3A). The group effect was not significant for glutamine (F2,57 = 0.37, p=0.69)(Fig. 3B). The glutamine/glutamate ratio was significant (F2,57 = 3.2, p=0.04) and was lower in DEF rats compared with controls (−10%, p=0.05) and FO rats (−10%, p=0.04)(Fig. 3C). There was non-significant trend for Glx (F2,57 = 2.5, p=0.09), and no significant group differences were observed for NAA (F2,57 = 0.5, p=0.79). In the thalamus, there were no significant group differences for glutamate (F2,57 = 0.6, p=0.57)(Fig. 3D), glutamine (F2,57 = 0.2, p=0.83)(Fig. 3E), the glutamine/glutamate ratio (F2,57 = 0.4, p=0.68)(Fig. 3F), Glx (F2,57 = 0.5, p=0.63), or NAA (F2,57 = 0.2, p=0.85). Across diet groups, PFC DHA levels were inversely correlated with PFC glutamate levels (r = −0.34, p=0.009)(Fig. 5A), but not PFC glutamine levels (r = −0.07, p=0.58) or the glutamine/glutamate ratio (r = 0.16, p=0.22). Erythrocyte DHA levels were also inversely correlated with PFC glutamate levels (r = −0.28, p=0.039).
Figure 3.
Concentrations of glutamate (Glu), glutamine (Gln), and the Gln/Glu ratio in the PFC (A,B,C) and thalamus (D,E,F) of adult rats maintained on the control diet (CON), n-3-free diet (DEF), and fish oil-fortified diet (FO) during peri-adolescent development. Values are group mean institutional units (IU) ± S.E.M. (n=20/group). *P≤0.05 vs. CON, #P≤0.01, ##P≤0.001 vs. FO.
Figure 5.
Relationships between PFC DHA and glutamate levels (A), PFC DHA and GLAST mRNA expression levels (B), and PFC glutamate and GLAST mRNA expression levels. Pearson correlation coefficients and associated p-values (two-tailed) are presented.
3.3. qRT-PCR
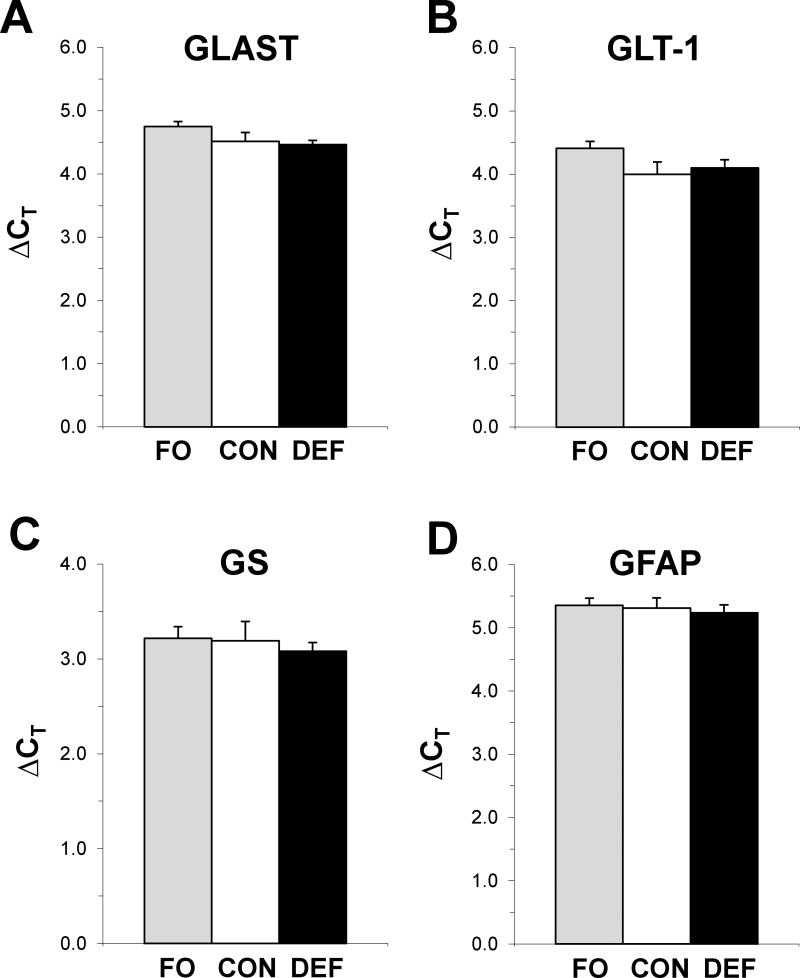
In the PFC there were no significant group differences for the housekeeping gene PPIA (F2,50 = 0.5, p=0.60), GLAST (F2,50 = 2.2, p=0.13), GLT-1 (F2,50 = 2.1, p=0.14), GS (F2,50 = 0.2, p=0.81), or GFAP (F2,50 = 0.2, p=0.83)(Fig. 4). Across diet groups, PFC DHA levels were positively correlated with PFC GLAST expression (r = +0.29, p=0.048)(Fig. 5B), and there was a non-significant trend for an inverse correlation between PFC GLAST expression and PFC glutamate levels (r = −0.25, p=0.08)(Fig. 5C).
Figure 4.
Expression of astrocyte glutamate-aspartate transporter (GLAST)(A), glial-specific glutamate transporter-1 (GLT-1)(B), glutamine synthetase (GS)(C), and glial fibrillary acidic protein (GFAP)(D) in the PFC of adult rats maintained on the control diet (CON), n-3-free diet (DEF), and fish oil-fortified diet (FO) during peri-adolescent development. Values are group mean ΔCT ± S.E.M. (n=16–18/group).
4. Discussion
This study investigated whether alterations in brain DHA accrual during adolescence would impact glutamate homeostasis in the adult rat brain using 1H MRS. Specifically, we tested the prediction that blood and brain DHA levels would be inversely correlated with glutamate and glutamine levels. Compared with controls, DHA levels were significantly lower in the PFC and thalamus of adult rats fed the DEF diet and significantly higher in rats fed the FO diet. Consistent with our prediction, glutamate levels were significantly higher in DHAdeficient rats compared with controls and FO rats, and PFC glutamate levels were inversely correlated with DHA levels across diet groups. This pattern was not observed in the thalamus. Contrary to our prediction, glutamine did not differ between groups. We also found that the glutamine/glutamate ratio was lower in DEF rats, though this was primarily driven by elevated glutamate levels. Although astrocyte glutamate-aspartate transporters (GLAST,GLT-1) did not differ significantly between groups, PFC GLAST expression was positively correlated with PFC DHA levels across diet groups. Differences in PFC glutamate levels were not associated with markers of excitotoxicity (NAA, GFAP) across groups. These findings demonstrate that rat cortical DHA accrual during adolescence is an important determinant of glutamate homeostasis in the young adult PFC, and suggest that this effect is not associated with excitotoxicity and may be mediated in part by alterations in astrocyte-mediated glutamate uptake.
The peri-adolescent dietary protocol used in the present study resulted in three distinct groups of rats with high, medium, and low blood and regional brain DHA levels. The magnitude of the PFC DHA deficit observed in DEF rats compared with controls was similar to the DHA deficits we observed in the postmortem PFC of patients with psychiatric disorders (McNamara et al., 2007, 2007, 2008). It is notable that the DHA deficit observed in DEF rats compared with controls was greater in the PFC (−30%) than the thalamus (−17%), a finding that is consistent with a previous study observing region-specific differences in DHA accrual and loss (Xiao et al., 2005). Erythrocyte DHA levels were highly correlated with regional brain DHA levels across all diet groups, a finding also observed in a human postmortem study (Carver et al., 2001). Rats receiving fish oil fortification exhibited greater erythrocyte and regional brain DHA levels compared with rats maintained on diet containing only the n-3 fatty acid precursor ALA, which has also been observed in human subjects (Barceló-Coblijn et al., 2008). These findings demonstrate that habitual dietary n-3 PUFA levels during the periadolescent period is an important determinant of rat blood and regional brain DHA levels in young adulthood.
A primary finding of this study is that PFC DHA accrual during adolescence is inversely correlated with glutamate levels in the young adult rat PFC. In contrast to the PFC, glutamate levels were not significantly altered in the thalamus, though a similar, albeit less robust, pattern was observed. While it is not known why more robust changes were not observed in the thalamus, this region also exhibited less robust changes in DHA levels compared with the PFC. It is also possible that the dynamic maturational changes that occur within the mPFC during peri-adolescent development, including a substantial pruning of afferents to the amygdala (Cressman et al., 2010), may render it more vulnerable to dysregulation. Relative to CON rats, we did not observe significant glutamate changes in the PFC of FO rats. It is possible that the increase in PFC DHA levels observed in FO rats relative to CON rats (+8%) was not sufficiently robust to impact on glutamate homeostasis. Nevertheless, in the PFC there was a pattern of decreasing glutamate levels in the FO group, and among all rats there was an inverse correlation between PFC DHA and glutamate levels. Lastly, we did not observe alterations in NAA, which decreases in the rat mPFC following excitotoxic lesions (Roffman et al., 2000), or GFAP, a marker of reactive gliosis that is up-regulated following excitotoxic lesions (Chang et al., 2008). These data suggest that elevated glutamate levels in the PFC of DEF rats were not associated with excitotoxicity.
In contrast to glutamate, PFC glutamine levels did not differ across groups. This finding suggests that elevated glutamate levels were not accompanied by a corresponding increase in glutamine biosynthesis within astrocytes. We also found that the astrocyte glutamate-aspartate transporter GLAST expression was positively correlated with DHA in the PFC, and there was a trend for an inverse correlation between GLAST expression and glutamate levels in the PFC. Taken in conjunction with prior evidence that reduced GLAST expression is associated with elevated extracellular glutamate levels (Rothstein et al., 1996), these associations suggest that astrocyte-mediated glutamate uptake may mediate the effects of DHA on PFC glutamate levels. However, additional studies will be required to more definitively evaluate this mechanism.
This study has a number of limitations. First, rats were anesthetized with isoflurane during 1H MRS acquisition which may have artificially altered the signal. However, all groups were anesthetized with isoflurane, and an in vivo microdialysis study found that extracellular glutamate levels are not altered in the mouse hippocampus in response to isoflurane anesthesia (Horn and Klein, 2010). Second, in vivo 1H MRS cannot discriminate whether the glutamate signal is localized to neuronal, astrocyte, and/or extracellular glutamate. Therefore, follow-up studies employing 13C MRS which can discriminate neuronal versus astrocytic contributions to glutamate/glutamine cycling are warranted. Third, our preliminary assessment of glutamate transporter expression did not investigate protein levels or activity indices. Fourth, this study only investigated glutamate homeostasis under basal conditions, and additional studies are warranted to investigate dynamic responses e.g., in response to ketamine (Kim et al., 2011). Moreover, we did not incorporate behavioral endpoints to investigate functional impact. However, it is notable that a previous study using a similar peri-adolescent feeding paradigm observed elevated behavioral indices of depression and aggression in DEF rats (DeMar et al., 2001). Strengths of this study include high resolution (7 Tesla) determination of regional brain glutamate and glutamine levels in vivo, and postmortem evaluation of regional fatty acid levels and glutamate-related gene expression.
In conclusion, the present findings demonstrate that rat brain DHA accrual during adolescence is an important determinant of glutamate levels in the young adult PFC. This effect was more robust in the PFC compared with the thalamus, was not accompanied by changes in markers of excitotoxicity, and may be mediated in part by alterations in astrocyte-mediated glutamate transporter expression. These observations add to a growing body of evidence from rodent developmental studies implicating brain DHA in the structural and functional integrity of glutamatergic synapses as well as astrocyte-mediated glutamate uptake. From a translational perspective, the present 1H MRS findings provide an empirical foundation in support of the hypothesis that elevated PFC glutamate levels and DHA deficits observed in patients with psychiatric disorders may be linked. It will therefore be of interest to investigate whether increasing erythrocyte, and presumably PFC, DHA levels through dietary fortification can correct or prevent elevated PFC glutamate levels in individuals with or at risk for developing these disorders.
Supplementary Material
Highlights.
Major psychiatric disorders are associated with dysregulated glutamate homeostasis and deficits in the omega-3 fatty acid docosahexaenoic acid (DHA).
This study determined the effects of dietary-induced alterations in brain DHA accrual on glutamate homeostasis in the adult rat brain.
In the prefrontal cortex (PFC), but not the thalamus, glutamate was elevated, and the glutamine/glutamate ratio was lower, in DHA-deficient rats.
Across diet groups, PFC DHA levels were inversely correlated with PFC glutamate levels and positively correlated with GLAST expression.
Rat cortical DHA accrual during adolescence is a determinant of glutamate homeostasis in the adult prefrontal cortex.
Acknowledgments
This work was supported in part by National Institute of Health grants MH107378, DK097599, and MH097818 to R.K.M. R.K.M. has received research support from NARSAD, Martek Biosciences Corporation, Ortho-McNeil Janssen, AstraZeneca, Eli Lilly, Kyowa Hakko Bio Co., LTD, Royal DSM Nutritional Products, LLC, and the Inflammation Research Foundation (IRF), was a member of the IRF scientific advisory board, and served as a paid consultant for VAYA Pharma Inc., and Vifor Pharma Inc. The NIH did not have any role in the design, implementation, analysis or interpretation of the research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The other authors do not have any financial disclosures to report.
References
- Barceló-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK. Flaxseed oil and fish-oil capsule consumption alters human red blood cell n-3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n-3 fatty acid. Am. J. Clin. Nutr. 2008;88:801–809. doi: 10.1093/ajcn/88.3.801. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology. 2008;33:2175–2186. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- Berry CB, Hayes D, Murphy A, Wiessner M, Rauen T, McBean GJ. Differential modulation of the glutamate transporters GLT1, GLAST and EAAC1 by docosahexaenoic acid. Brain Res. 2005;1037:123–133. doi: 10.1016/j.brainres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, et al. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J. Neurochem. 2009;111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res. Bull. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- Champeil-Potokar G, Chaumontet C, Guesnet P, Lavialle M, Denis I. Docosahexaenoic acid (22:6n-3) enrichment of membrane phospholipids increases gap junction coupling capacity in cultured astrocytes. Eur. J. Neurosci. 2006;24:3084–3090. doi: 10.1111/j.1460-9568.2006.05185.x. [DOI] [PubMed] [Google Scholar]
- Champeil-Potokar G, Denis I, Goustard-Langelier B, Alessandri JM, Guesnet P, Lavialle M. Astrocytes in culture require docosahexaenoic acid to restore the n-3/n-6 polyunsaturated fatty acid balance in their membrane phospholipids. J. Neurosci. Res. 2004;75:96–106. doi: 10.1002/jnr.10817. [DOI] [PubMed] [Google Scholar]
- Champeil-Potokar G, Hennebelle M, Latour A, Vancassel S, Denis I. Docosahexaenoic acid (DHA) prevents corticosterone-induced changes in astrocyte morphology and function. J. Neurochem. 2015 doi: 10.1111/jnc.13510. [DOI] [PubMed] [Google Scholar]
- Chang YC, Kim HW, Rapoport SI, Rao JS. Chronic NMDA administration increases neuroinflammatory markers in rat frontal cortex: cross-talk between excitotoxicity and neuroinflammation. Neurochem. Res. 2008;33:2318–2323. doi: 10.1007/s11064-008-9731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, et al. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J. Comp. Neurol. 2010;518:2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMar JC, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J. Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigante AD, Bond DJ, Lafer B, Lam RW, Young LT, Yatham LN. Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar Disord. 2012;14:478–487. doi: 10.1111/j.1399-5618.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- Green P, Glozman S, Kamensky B, Yavin E. Developmental changes in rat brain membrane lipids and fatty acids. The preferential prenatal accumulation of docosahexaenoic acid. J. Lipid Res. 1999;40:960–966. [PubMed] [Google Scholar]
- Grintal B, Champeil-Potokar G, Lavialle M, Vancassel S, Breton S, Denis I. Inhibition of astroglial glutamate transport by polyunsaturated fatty acids: evidence for a signalling role of docosahexaenoic acid. Neurochem. Int. 2009;54:535–543. doi: 10.1016/j.neuint.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Harbeby E, Jouin M, Alessandri JM, Lallemand MS, Linard A, Lavialle M, et al. n-3 PUFA status affects expression of genes involved in neuroenergetics differently in the fronto-parietal cortex compared to the CA1 area of the hippocampus: effect of rest and neuronal activation in the rat. Prostaglandins Leukot. Essent. Fatty Acids. 2012;86:211–220. doi: 10.1016/j.plefa.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol. Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27:735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Horn T, Klein J. Lactate levels in the brain are elevated upon exposure to volatile anesthetics: a microdialysis study. Neurochem Int. 2010;57:940–947. doi: 10.1016/j.neuint.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Joardar A, Sen AK, Das S. Docosahexaenoic acid facilitates cell maturation and beta-adrenergic transmission in astrocytes. J. Lipid Res. 2006;47:571–581. doi: 10.1194/jlr.M500415-JLR200. [DOI] [PubMed] [Google Scholar]
- Kim SY, Lee H, Kim HJ, Bang E, Lee SH, Lee DW, et al. In vivo and ex vivo evidence for ketamine-induced hyperglutamatergic activity in the cerebral cortex of the rat: Potential relevance to schizophrenia. NMR. Biomed. 2011;24:1235–1242. doi: 10.1002/nbm.1681. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- Matute C, Melone M, Vallejo-Illarramendi A, Conti F. Increased expression of the astrocytic glutamate transporter GLT-1 in the prefrontal cortex of schizophrenics. Glia. 2005;49:451–455. doi: 10.1002/glia.20119. [DOI] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol. Med. 2015;45:693–704. doi: 10.1017/S0033291714001603. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Able JA, Jandacek RJ, Rider T, Tso P. Inbred C57BL/6J and DBA/2J mouse strains exhibit constitutive differences in regional brain fatty acid composition. Lipids. 2009;44:1–8. doi: 10.1007/s11745-008-3244-8. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Hahn CG, Jandacek RJ, Rider T, Tso P, Stanford KE, et al. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol. Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Jandacek RJ, Rider T, Tso P, Richtand NM, Stanford KE. Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: Gender differences and partial normalization with antipsychotic medications. Schizophr. Res. 2007;91:37–50. doi: 10.1016/j.schres.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek RJ, Rider T, Tso P, Stanford KE, Hahn CG, et al. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160:285–299. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Vannest JJ, Valentine CJ. Role of perinatal long-chain omega-3 fatty acids in cortical circuit maturation: Mechanisms and implications for psychopathology. World J. Psychiatr. 2015;5:15–34. doi: 10.5498/wjp.v5.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Welge JA. Meta-analysis of erythrocyte polyunsaturated fatty acid biostatus in bipolar disorder. Bipolar Disord. 2016;18:300–306. doi: 10.1111/bdi.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of glutamate alterations in schizophrenia: A meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry. 2016;73:665–674. doi: 10.1001/jamapsychiatry.2016.0442. [DOI] [PubMed] [Google Scholar]
- Moreira JD, Knorr L, Ganzella M, Thomazi AP, de Souza CG, de Souza DG, et al. Omega-3 fatty acids deprivation affects ontogeny of glutamatergic synapses in rats: relevance for behavior alterations. Neurochem. Int. 2010;56:753–759. doi: 10.1016/j.neuint.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Rao JS, Kellom M, Reese EA, Rapoport SI, Kim HW. Dysregulated glutamate and dopamine transporters in postmortem frontal cortex from bipolar and schizophrenic patients. J. Affect. Disord. 2012;136:63–71. doi: 10.1016/j.jad.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Roffman JL, Lipska BK, Bertolino A, Van Gelderen P, Olson AW, Khaing ZZ, et al. Local and downstream effects of excitotoxic lesions in the rat medial prefrontal cortex on In vivo 1H-MRS signals. Neuropsychopharmacology. 2000;22:430–439. doi: 10.1016/S0893-133X(99)00143-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am. J. Psychiatry. 2005;162:394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- Tesei A, Crippa A, Ceccarelli SB, Mauri M, Molteni M, Agostoni C, et al. The potential relevance of docosahexaenoic acid and eicosapentaenoic acid to the etiopathogenesis of childhood neuropsychiatric disorders. Eur. Child. Adolesc. Psychiatry. 2017 doi: 10.1007/s00787-016-0932-4. [DOI] [PubMed] [Google Scholar]
- Uezato A, Meador-Woodruff JH, McCullumsmith RE. Vesicular glutamate transporter mRNA expression in the medial temporal lobe in major depressive disorder, bipolar disorder, and schizophrenia. Bipolar Disord. 2009;11:711–725. doi: 10.1111/j.1399-5618.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- van der Kemp WJ, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. A meta-analysis of the polyunsaturated fatty acid composition of erythrocyte membranes in schizophrenia. Schizophr. Res. 2012;141:153–161. doi: 10.1016/j.schres.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Huang Y, Chen ZY. Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Br. J. Nutr. 2005;94:544–550. doi: 10.1079/bjn20051539. [DOI] [PubMed] [Google Scholar]
- Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida S, Yasuda A, Kawazato H, Sakai K, Shimada T, Takeshita M, et al. Synaptic vesicle ultrastructural changes in the rat hippocampus induced by a combination of alpha-linolenate deficiency and a learning task. J. Neurochem. 1997;68:1261–1268. doi: 10.1046/j.1471-4159.1997.68031261.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.