Abstract
Aims
Optical frequency domain imaging (OFDI) is a recently developed, light-based, high-resolution intravascular imaging technique. Intravascular ultrasound (IVUS) is a widely used, conventional imaging technique for guiding percutaneous coronary intervention (PCI). We aimed to demonstrate the non-inferiority of OFDI-guided PCI compared with IVUS-guided PCI in terms of clinical outcomes.
Methods and results
We did a prospective, multicentre, randomized (ratio 1:1), active-controlled, non-inferiority study to compare head-to-head OFDI vs. IVUS in patients undergoing PCI with a second generation drug-eluting stent. The primary endpoint was target vessel failure defined as a composite of cardiac death, target-vessel related myocardial infarction, and ischaemia-driven target vessel revascularization until 12 months after the PCI. The major secondary endpoint was angiographic binary restenosis at 8 months. We randomly allocated 829 patients to receive OFDI-guided PCI (n = 414) or IVUS-guided PCI (n = 415). Target vessel failure occurred in 21 (5.2%) of 401 patients undergoing OFDI-guided PCI, and 19 (4.9%) of 390 patients undergoing IVUS-guided PCI, demonstrating non-inferiority of OFDI-guided PCI to IVUS-guided PCI (hazard ratio 1.07, upper limit of one-sided 95% confidence interval 1.80; Pnon-inferiority = 0.042). With 89.8% angiographic follow-up, the rate of binary restenosis was comparable between OFDI-guided PCI and IVUS-guided PCI (in-stent: 1.6% vs. 1.6%, P = 1.00; and in-segment: 6.2% vs. 6.0%, P = 1.00).
Conclusion
The 12-month clinical outcome in patients undergoing OFDI-guided PCI was non-inferior to that of patients undergoing IVUS-guided PCI. Both OFDI-guided and IVUS-guided PCI yielded excellent angiographic and clinical results, with very low rates of 8-month angiographic binary restenosis and 12-month target vessel failure.
Clinical registration
ClinicalTrials.gov, number NCT01873027.
Keywords: Drug-eluting stent, Intravascular ultrasound, Optical coherence tomography, Percutaneous coronary intervention
Introduction
Percutaneous coronary intervention (PCI) is a catheter-based therapy performed to open narrowed or blocked coronary arteries to restore blood flow to the ischaemic myocardium. Imaging of the coronary arteries plays an important role in guiding optimal PCI strategies and stent deployment. Intravascular ultrasound (IVUS) is the most commonly used intracoronary imaging technique during the PCI procedure. Intravascular ultrasound is useful to determine adequate size of the balloon and stent; to optimize stent expansion, extension, and apposition; and to identify and treat possible complications after stent implantation.1 Recent studies have shown that IVUS-guided PCI was superior to conventional angiography-guided PCI, especially in particular diseases such as long lesion and chromic total occlusion.2,3 IVUS-guided PCI have a potential to reduce cardiac death, major adverse cardiac events, stent thrombosis, and target lesion revascularization as compared with angiography-guided PCI.4
Optical coherence tomography (OCT) is a novel intravascular imaging technique that uses near infrared light rather than ultrasound to generate images. The strength of OCT lies in its high resolution of 10–20 µm, while the resolution afforded by IVUS is approximately 100–200 μm. Optical coherence tomography is capable of providing more detailed morphological information to monitor the PCI procedure than IVUS.5 Optical frequency domain imaging (OFDI: LUNAWAVE®, Terumo Corporation, Tokyo, Japan) is the most recently developed OCT technology, which enables high-frame-rate (158 frame/s) real-time imaging of long coronary segments (up to 150 mm) within a few seconds, in combination with rapid spiral pullback (40 mm/s) and contrast injection into a coronary artery through a guiding catheter. Optical frequency domain imaging is becoming increasingly widespread as an adjunctive imaging technique for the PCI procedure. Like IVUS, OFDI guidance is expected to improve procedural and clinical results.6 However, 2014 European guideline (European Society of Cardiology) for myocardial revascularization provides a lower-grade recommendation for the use of OCT (Class IIb, Level of Evidence: C) in selected patients to optimize stent implantation as compared with the use of IVUS (Class IIa, Level of Evidence: B).7 Therefore, we undertook the OPtical frequency domain imaging vs. INtravascular ultrasound in percutaneous coronary InterventiON (OPINION) trial powered to evaluate the non-inferiority of OFDI-guided PCI compared with IVUS-guided PCI in terms of clinical outcomes.
Methods
Study design and patients
The design of the OPINION trial has been described elsewhere.8 OPINION is a prospective, multicentre, randomized, open-label, parallel group, active-controlled, non-inferiority trial comparing clinical outcomes after OFDI-guided PCI with those after IVUS-guided PCI with a second generation drug-eluting stent. The study was done in 42 medical centres in Japan (see Supplementary material online, Appendix). An independent data and safety monitoring committee monitored the safety of the trial. Wakayama Medical University (Wakayama, Japan), one of the clinical sites, undertook co-ordination of this trial. Translational Research Informatics Center (Kobe, Japan) undertook data management, statistical analysis and site management. Adult patients (20–85 years of age) scheduled for PCI with a second generation drug-eluting stent to a de novo native coronary artery lesion were eligible for inclusion in the OPINION trial. Patients with myocardial infarction in the previous 3 months, cardiogenic shock, congestive heart failure, chronic kidney disease [estimated glomerular filtration rate ≤30 mL/min/1.73 m2 or serum creatinine level ≥133 μmol/L (1.5 mg/dL)], haemodialysis or peritoneal dialysis, planned surgery within 1 year after PCI, scheduled PCI with bare metal stent, triple-vessel disease, left main coronary artery disease, aorto-ostial lesion arising within 3 mm of the origin of a coronary artery, chronic total occlusion, small vessel disease (reference vessel diameter <2.5 mm), coronary bypass graft, in-stent restenosis, and participation in the other clinical studies were not eligible. All participants provided written informed consent. Local research ethics boards approved the protocol.
Randomization and masking
All recruited patients were randomly allocated in a 1:1 ratio to receive either OFDI-guided or IVUS-guided PCI with a second generation drug-eluting stent. Randomization was performed by a web-based allocation system (eClinical Base®) and was stratified by age, presence of diabetes mellitus, and participating medical centre. Participants and investigators were not masked to the allocation.
Procedures
The detailed information regarding the study procedures according to either OFDI-guided or IVUS-guided PCI has been described elsewhere.8 The biolimus-eluting stent (NOBORI®, Terumo Corporation, Tokyo, Japan) was used in this trial. In cases of mismatch between lengths of the biolimus-eluting stent and lesion, use of the other second generation DES was allowed. In the OFDI-guided PCI group, proximal and distal reference sites were set at cross-sections adjacent to the target lesion that had the most normal appearance and was free of lipidic plaque.9 Stent diameter was determined by measuring lumen diameter at the proximal and distal reference sites, and stent length was determined by measuring the distance from the distal to proximal reference site. In the IVUS-guided PCI group, proximal and distal reference sites were set at cross-sections adjacent to the target lesion that had the largest lumen and a plaque burden of <50%. Stent diameter was determined by measuring vessel diameter (approximated by the external elastic membrane diameter) at the proximal and distal reference sites, and stent length was determined by measuring the distance from the distal to proximal reference site. The stent was implanted with adequate inflation pressure. Immediately after stent implantation, post-dilatation was allowed on the basis of the angiographic findings. Following stent implantation and/or post-dilatation, iterative intravascular imaging was performed to evaluate the initial results in either group. When incomplete stent expansion, incomplete stent apposition, asymmetric stent expansion, plaque or thrombus protrusion with potential to provoke flow disturbance, or stent edge dissection with potential to provoke flow disturbance were identified, additional procedures were performed, if deemed safe and feasible. The additional procedures were followed by further intravascular imaging. The detailed algorithms of OFDI-guided PCI and IVUS-guided PCI are shown in the Supplementary material online, Appendix.
We assessed the PCI procedural results and OFDI/IVUS procedure-related complications (defined as a composite of acute coronary occlusion, air embolization, slow flow, distal embolization, side branch occlusion, coronary dissection, thrombus formation, vasospasm, and ventricular arrhythmia). Detailed definitions of the individual procedure-related complications are shown in the Supplementary material online, Appendix. The recommended antiplatelet regimen is aspirin (≥81 mg daily) indefinitely and thienopyridine (75 mg clopidogrel daily) for 12 months. We did clinical follow-up at discharge, 8 months, and 12 months after the PCI, and angiographic follow-up at 8 months.
An angiographic core laboratory (Cardiocore, Tokyo, Japan), masked to the treatment allocations, analysed all the angiograms of the patients in the study and provided the data for patients with events comprising the study endpoints to the clinical event assessment committee for accurate adjudication. The detailed information of the angiographic analyses has been described elsewhere.8
Outcomes
The primary endpoint of the study was target vessel failure (defined as a composite of cardiac death, target-vessel related myocardial infarction, and ischaemia-driven target vessel revascularization). Secondary endpoints of the study were cardiac death, myocardial infarction, target-vessel related myocardial infarction, ischaemia-driven target vessel revascularization, ischaemia-driven target lesion revascularization, major adverse cardiac event (defined as a composite of cardiac death, myocardial infarction, or ischaemia-driven target lesion revascularization), stent thrombosis, restenosis, stroke, and renal dysfunction (contrast-induced nephropathy). Detailed definitions of the endpoints are shown in the Supplementary material online, Appendix. The clinical event committee, masked to the treatment allocations, adjudicated all the endpoints.
Statistical analysis
With the assumption of the target vessel failure rate of 9% at 12 months after IVUS-guided PCI with biolimus-eluting stent, and a non-inferiority margin of 7% (corresponding to a hazard ratio of 1.85), we calculated that 387 patients in each study group would be required to have 80% power to detect the non-inferiority of OFDI-guided PCI to IVUS-guided PCI, at a one-sided alpha level of 0.05. Assuming a dropout rate of 3%, a total of 800 patients were required.
The study was designed to test the hypothesis that OFDI-guided PCI would be non-inferior to IVUS-guided PCI with respect to the primary endpoint. The criteria for non-inferiority required that the upper limits of the one-sided 95% confidence interval were below the pre-specified non-inferiority margin of 1.85 for the hazard ratio. The primary analysis was evaluated in the per-protocol set, which included all patients who received OFDI-guided PCI or IVUS-guided PCI and did not have any major protocol deviations, and a non-inferiority test was performed with a Cox proportional hazard model. In addition, target vessel failure-free survival and 12-month failure rate were calculated using the Kaplan–Meier method, and the difference between OFDI-guided and IVUS-guided PCI was compared using the log-rank test. We also performed sensitivity analyses of the primary endpoint in the full-analysis set, which included all randomized patients who were allocated to the study groups.
The secondary analysis was evaluated in the full-analysis set. To assess the difference in event rates, the hazard ratio and corresponding 95% confidence interval were estimated with the use of the Cox proportional hazard model. We did analyses for baseline characteristics, PCI procedural results, and quantitative coronary angiography analysis results in the full-analysis set. We presented categorical data as numbers and percentages, and compared them using Fisher’s exact test or χ2 test. We presented continuous data as mean (±SD) and compared them using the Wilcoxon rank-sum test. Except for the non-inferiority test of the primary endpoint, all reported P values are two-sided; those under 0.05 are considered to be statistically significant. All analyses were conducted with SAS software, version 9.3 (SAS Institute).
Role of the funding source
The sponsor of the study had no role in the design, collection, analysis, or interpretation of the data, in the writing of the report, or in the decision to submit the article for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Trial profile
Between 10 June 2013, and 1 July 2014, we randomly allocated 829 patients to receive OFDI-guided PCI (n = 414) or IVUS-guided PCI (n = 415). 817 (98.6%) patients comprised the full-analysis set (OFDI-guide PCI [n = 412] and IVUS-guide PCI [n = 405]) and 791 (95.4%) patients comprised the per-protocol set (OFDI-guide PCI [n = 401] and IVUS-guide PCI [n = 390]) (Figure 1).
Figure 1.
Trial profile.
Baseline features and procedures
Patient clinical characteristics were well balanced between the groups of OFDI-guided and IVUS-guided PCI (Table 1). Angiographic lesion characteristics were similar between the two groups except for significantly lower frequency of moderate or severe calcification in the OFDI-guided PCI group.
Table 1.
Baseline characteristics
| OFDI-guided PCI (n = 412) | IVUS-guided PCI (n = 405) | P-value | |
|---|---|---|---|
| Age (years) | 69 ± 9 | 68 ± 9 | 0.68 |
| Male | 315 (76.5) | 322 (79.5) | 0.31 |
| Coronary risk factor | |||
| Hypertension | 315 (76.5) | 299 (73.8) | 0.42 |
| Dyslipidaemia | 316 (76.7) | 321 (79.3) | 0.40 |
| Diabetes mellitus | 169 (41.0) | 165 (40.7) | 0.94 |
| Family history of coronary artery disease | 40 (9.7) | 58 (14.3) | 0.05 |
| Current smoker | 67 (16.3) | 73 (18.0) | 0.45 |
| Prior myocardial infarction | 70 (17.0) | 61 (15.1) | 0.51 |
| Prior PCI | 140 (34.0) | 140 (34.6) | 0.88 |
| Prior coronary artery bypass graft | 7 (1.7) | 9 (2.2) | 0.62 |
| Clinical presentation | 0.60 | ||
| Stable angina | 363 (88.1) | 352 (86.9) | |
| Unstable angina | 48 (11.7) | 53 (13.1) | |
| Coronary arteries | 0.27 | ||
| Right coronary artery | 102 (24.8) | 117 (28.9) | |
| Left anterior descending artery | 223 (54.1) | 197 (48.6) | |
| Left circumflex artery | 84 (20.4) | 87 (21.5) | |
| Lesion characteristics | |||
| Thrombus | 4 (1.0) | 5 (1.2) | 0.75 |
| Bifurcation | 154 (37.4) | 157 (38.8) | 0.72 |
| Moderate or heavy calcification | 29 (7.0) | 51 (12.6) | 0.009 |
| Long lesion (lesion length >28 mm) | 56 (13.6) | 54 (13.3) | 0.92 |
| ACC/AHA lesion type B2 or C | 329 (79.9) | 319 (78.8) | 0.70 |
Data are n (%) or mean ± SD.
OFDI, optical frequency domain imaging; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention; ACC/AHA, American College of Cardiology/American Heart Association.
Stent diameter was significantly smaller in the OFDI-guided PCI group compared with the IVUS-guided PCI group (Table 2). Total stent length tended to be longer and maximum balloon diameter tended to be smaller in the OFDI-guided PCI group compared with the IVUS-guided PCI group. Other PCI procedures were similar between the two groups (Table 2 and Supplementary material online, Appendix). Mean (±SD) number of OFDI/IVUS procedures was 3.0 ± 1.1 per patient in either group. Solely on the basis of the OFDI/IVUS evaluation, aggressive PCI procedures for lesion preparation before stenting and PCI optimization after stenting were conducted in 156 (37.9%) of 412 patients in the OFDI-guided PCI group and 146 (36.0%) of 405 patients in the IVUS-guided PCI group. The rate of PCI procedure success was very high and comparable between the two groups. The rate of OFDI/IVUS procedure-related complication was very low and comparable between the two groups (Table 2 and Supplementary material online, Appendix). The total amount of contrast medium was significantly greater in the OFDI-guided PCI group compared with the IVUS-guided PCI group. Medical therapies at discharge were similar between the two groups (see Supplementary material online, Appendix).
Table 2.
Percutaneous coronary intervention procedure results
| OFDI-guided PCI (n = 412) | IVUS-guided PCI (n = 405) | P-value | |
|---|---|---|---|
| Stent diameter (mm) | 2.92 ± 0.39 | 2.99 ± 0.39 | 0.005 |
| Total stent length (mm) | 25.9 ± 13.2 | 24.8 ± 13.2 | 0.06 |
| Multiple stenting | 68 (16.5) | 59 (14.6) | 0.50 |
| Pre-dilatation | 316 (76.7) | 316 (78.0) | 0.67 |
| Post-dilatation | 316 (76.7) | 304 (75.1) | 0.62 |
| Balloon dilatation of side-branch | 39 (9.5%) | 41 (10.1%) | 0.81 |
| Maximum balloon diameter (mm) | 3.1 ± 0.8 | 3.3 ± 1.2 | 0.06 |
| Maximum inflation pressure, atmosphere | 16.0 ± 4.2 | 16.0 ± 4.2 | 0.70 |
| No. of OFDI/IVUS procedure | 3.0 ± 1.1 | 3.0 ± 1.1 | 0.14 |
| Aggressive PCI procedure based on OFDI/IVUS | 156 (37.9) | 146 (36.0) | 0.61 |
| Lesion preparation before stenting | |||
| Pre-dilatation with larger balloon or higher inflation pressurea | 34 (8.3) | 31 (7.7) | 0.80 |
| Rotablator usea | 6 (1.5) | 8 (2.0) | 0.60 |
| Cutting balloon usea | 4 (1.0) | 7 (1.7) | 0.38 |
| Thrombus aspiration | 0 (0) | 1 (0.2) | 0.50 |
| PCI optimization after stenting | |||
| Post-dilatation with larger balloon or higher inflation pressureb | 128 (31.1) | 114 (28.1) | 0.40 |
| Additional stentingc | 16 (3.9) | 11 (2.7) | 0.43 |
| Thrombus aspiration | 1 (0.2) | 2 (0.5) | 0.62 |
| PCI procedure success | 406 (98.5) | 399 (98.5) | 0.68 |
| OFDI/IVUS procedure related complications | 3 (0.7) | 1 (0.3) | 0.62 |
| Total amount of contrast (mL) | 164 ± 66 | 138 ± 56 | <0.001 |
Data are n (%) or mean ± SD.
OFDI, optical frequency domain imaging; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention.
Because of severe calcified lesion.
Because of incomplete stent expansion or incomplete stent apposition.
Because of stent edge dissection or residual stenosis.
Angiographic diameter stenosis at pre-PCI was similar between the OFDI-guided PCI group and the IVUS-guided PCI group (Table 3). In-stent acute gain achieved by PCI was significantly smaller in the OFDI-guided PCI group, while in-segment acute gain was comparable between the two groups. In-stent and in-segment diameter stenosis at post-PCI were similar between the two groups.
Table 3.
Quantitative coronary angiography
| OFDI-guided PCI | IVUS-guided PCI | P-value | |
|---|---|---|---|
| Pre-PCI | |||
| Number of lesion | 409 | 400 | |
| Lesion length (mm) | 17.73 ± 10.14 | 17.56 ± 10.99 | 0.47 |
| Reference vessel diameter (mm) | 2.62 ± 0.53 | 2.59 ± 0.57 | 0.26 |
| Minimum lumen diameter (mm) | 0.94 ± 0.37 | 0.89 ± 0.38 | 0.10 |
| Diameter stenosis (%) | 64 ± 12 | 65 ± 13 | 0.16 |
| Post-PCI | |||
| Number of lesions | 409 | 400 | |
| In-stent reference vessel diameter (mm) | 2.91 ± 0.46 | 2.94 ± 0.51 | 0.74 |
| In-stent minimum lumen diameter (mm) | 2.57 ± 0.43 | 2.61 ± 0.46 | 0.23 |
| In-stent diameter stenosis (%) | 11 ± 6 | 10 ± 6 | 0.14 |
| In-stent acute gain (mm) | 1.63 ± 0.47 | 1.72 ± 0.50 | 0.019 |
| In-segment reference vessel diameter (mm) | 2.86 ± 0.56 | 2.89 ± 0.56 | 0.63 |
| In-segment minimum lumen diameter (mm) | 2.25 ± 0.53 | 2.27 ± 0.52 | 0.78 |
| In-segment diameter stenosis (%) | 22 ± 10 | 22 ± 9 | 0.89 |
| In-segment acute gain (mm) | 1.32 ± 0.54 | 1.38 ± 0.54 | 0.15 |
| 8-month follow-up | |||
| Number of lesion | 369 | 365 | |
| In-stent reference vessel diameter (mm) | 2.83 ± 0.47 | 2.88 ± 0.51 | 0.22 |
| In-stent minimum lumen diameter (mm) | 2.39 ± 0.51 | 2.45 ± 0.51 | 0.10 |
| In-stent diameter stenosis (%) | 16 ± 11 | 15 ± 9 | 0.80 |
| In-stent late loss (mm) | 0.19 ± 0.29 | 0.17 ± 0.25 | 0.58 |
| In-stent binary restenosis | 6 (1.6) | 6 (1.6) | 1.00 |
| In-segment reference vessel diameter (mm) | 2.82 ± 0.53 | 2.85 ± 0.56 | 0.59 |
| In-segment minimum lumen diameter (mm) | 2.16 ± 0.56 | 2.17 ± 0.55 | 0.75 |
| In-segment diameter stenosis (%) | 24 ± 13 | 24 ± 12 | 0.54 |
| In-segment late loss (mm) | 0.10 ± 0.46 | 0.10 ± 0.44 | 0.72 |
| In-segment binary restenosis | 23 (6.2) | 22 (6.0) | 1.00 |
Data are number or mean ± SD.
OFDI, optical frequency domain imaging; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention.
Clinical outcomes
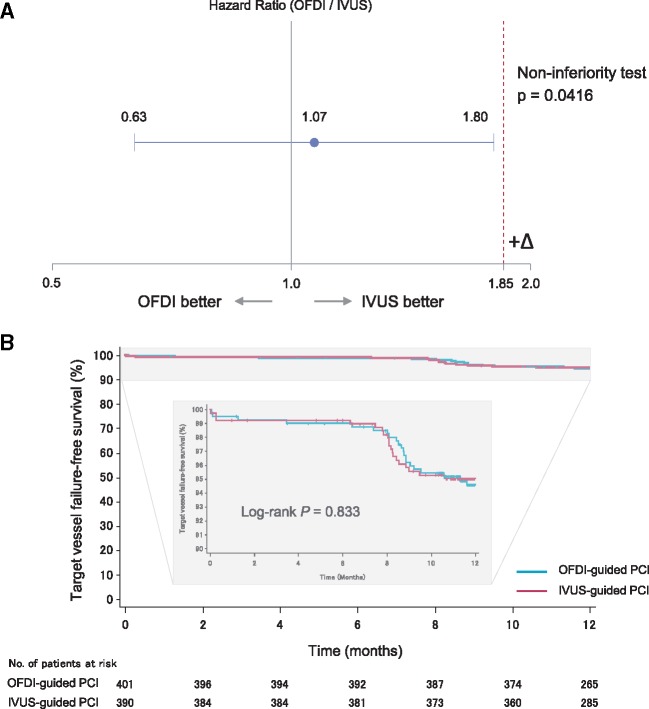
Within 12 months, the target vessel failure (primary endpoint) occurred in 21 (5.2%) of 401 patients in the OFDI-guided PCI group and in 19 (4.9%) of 390 in the IVUS-guided PCI group, with a hazard ratio in the OFDI-guided PCI group of 1.07 (upper limit of one-sided 95% confidence interval 1.80; P = 0.042 for non-inferiority) (Summarizing figure). A sensitivity analyses for the full-analysis set did not alter the results for non-inferiority (Pnon-inferiority=0.045) (see Supplementary material online, Appendix).
Summarizing figure.
Non-inferiority test for the target vessel failure (primary endpoint) based on hazard ratio (A) and target vessel failure-free survival curves through 12-month follow-up (B).
The rate of cardiac death, myocardial infarction, target-vessel related myocardial infarction, ischaemia-driven target vessel revascularization, ischaemia-driven target lesion revascularization, major adverse cardiac event, stent thrombosis, and stroke (secondary endpoints) was similar between the OFDI-guided PCI group and the IVUS-guided PCI group (Table 4). In addition, contrast-induced nephropathy did not occur either in the OFDI-guided PCI group or the IVUS-guided PCI group.
Table 4.
Clinical outcomes at 12 months (secondary endpoints)
| OFDI-guided PCI (n = 412) | IVUS-guided PCI (n = 405) | HR (95% CI) | P-value | |
|---|---|---|---|---|
| Cardiac death | 0 (0) | 1 (0.2) | 0.98 (0.00–18.68)a | 0.99 |
| Myocardial infarction | 2 (0.5) | 3 (0.7) | 0.65 (0.05–5.74) | 0.98 |
| Target-vessel related myocardial infarction | 2 (0.5) | 3 (0.7) | 0.65 (0.05–5.74) | 0.98 |
| Ischaemia-driven target vessel revascularization | 20 (4.9) | 17 (4.2) | 1.16 (0.57–2.41) | 0.78 |
| Ischaemia-driven target lesion revascularization | 11 (2.7) | 12 (3.0) | 0.90 (0.35–2.25) | 0.97 |
| Major adverse cardiac event | 12 (2.9) | 14 (3.5) | 0.84 (0.35–1.98) | 0.81 |
| Stent thrombosis | 1 (0.2) | 2 (0.5) | 0.49 (0.01–9.46) | 0.99 |
| Stroke | 4 (1.0) | 1 (0.2) | 3.96 (0.39–195.53) | 0.38 |
| Contrast-induced nephropathy | 0 (0) | 0 (0) | — | — |
Data are n (%).
OFDI, optical frequency domain imaging; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention; CI, confidence interval; HR, hazard ratio.
Based on the median unbiased estimator.
Angiographic outcomes
In-stent and in-segment diameter stenosis at 8-month follow-up were similar between the two groups (Table 3). The rates of in-stent and in-segment binary restenosis at 8-month follow-up (secondary endpoints) were comparable between the two groups.
Discussion
This is the first prospective, randomized, large-scale, multicentre study comparing head-to-head OCT vs. IVUS during PCI with a second generation drug eluting stent in terms of clinical outcomes. The principal findings of the present study were the following: (1) the 12-month clinical outcome in patients undergoing OFDI-guided PCI was non-inferior to that in patients undergoing IVUS-guided PCI and (2) the rate of target vessel failure, a composite of cardiac death, target vessel-related myocardial infarction, and ischaemia-driven target vessel revascularization, at 12 months, was very low in patients undergoing OFDI-guided PCI (5.2%) as well as in those undergoing IVUS-guided PCI (4.9%).
Intravascular ultrasound has been used clinically for two decades and its utility for guiding PCI has been well established.1 In recent years, OCT has emerged as a reliable diagnostic technique capable of assisting PCI procedures.10 Either technique has its distinct advantages and disadvantages. IVUS allows more complete vessel and plaque visualization in return for the relatively coarse resolution. On the other hand, OCT provides very much higher resolution images, although the visible range is limited to the vessel surface. Until now, it has been a topic of debate as to which technique is better for the guidance of PCI.
Several studies have reported the superior ability of OCT to provide precise measurement of coronary dimension and accurate detection of suboptimal results of stent deployment including incomplete stent apposition, tissue protrusion, intra-stent thrombus and stent edge dissection as compared with IVUS.10 However, there is little evidence showing the impact of OCT guidance in PCI on clinical outcomes. To the best of our knowledge, only one retrospective, small-scale, double-centre study has demonstrated similar rates of cardiac death, myocardial infarction, or target lesion revascularization and stent thrombosis at 12 months between the patients undergoing OCT-guided PCI and IVUS-guided PCI.11 Therefore, we designed the present OPINION trial to demonstrate the non-inferiority of OFDI-guided PCI compared with IVUS-guided PCI in terms of clinical outcomes.
A further debate arising from previous studies was about the difference of stent expansion between OCT-guided PCI and IVUS-guided PCI. A previous study showed that stent expansion was smaller in the OCT guidance compared with the IVUS guidance.12 In OCT, use of the visible lumen as a reference rather than the vessel itself might lead to smaller stent size selection and lower inflation pressure for stent optimization. Therefore, a recent study, ILUMIEN III used an alternative OCT algorithm for optimal stent selection, based on the measurement of the external elastic lamina diameter, and achieved similar post-PCI minimum stent area between the OCT and IVUS guidance.13 Regrettably, both studies were not designed to detect the difference in clinical outcomes between OCT-guided and IVUS-guided stent implantation. In the present study, we used lumen as a reference according to our daily clinical practice and then the selected stent diameter and angiographic acute gain achieved by PCI were slightly, but significantly, smaller in the OCT-guided PCI group compared with the IVUS-guided PCI group. However, no difference in angiographic late lumen loss, percent diameter stenosis and binary restenosis rate was observed at 8 month, and more importantly, there was no difference in the rate of target vessel failure within 12 months. In the second generation drug-eluting stent era, those slight differences in stent diameter and angiographic acute gain between OCT-guided PCI and IVUS-guided PCI were considered to have no influence on the clinical outcomes.
Information from OCT and IVUS exerts a considerable impact on the PCI procedure. In the present study, both OCT and IVUS guidance modified the stent optimization procedure in approximately one-third of the patients. Most of the additional procedure was post-dilatation with a larger balloon or higher inflation pressure, which was intended to improve incomplete stent expansion or incomplete stent apposition. Those aggressive PCI procedures guided by OCT and IVUS might contribute to reducing the risk of post-stenting restenosis and thrombosis.
Safety is an important concern in OCT as an invasive imaging technique. Unlike IVUS, OCT for image acquisition requires intracoronary injection of contrast media, which may lead to adverse events. In the present study, however, the rate of procedure-related complications in patients undergoing OCT-guided PCI was extremely low, and was similar to the rate found in those undergoing IVUS-guided PCI. In addition, although the total amount of contrast medium was significantly greater in patients undergoing OCT-guided PCI compared with IVUS-guided PCI, no patients exhibited contrast-induced nephropathy. Thus, our data supports the safety of OCT guidance in PCI as well as IVUS guidance.
The present study had several limitations. First, we did not investigate clinical outcome in patients undergoing PCI guided by angiography alone. To date, only one retrospective, large-scale, multicentre registry study has demonstrated that OCT-guided PCI compared with angiography-guided PCI resulted in a significantly lower rate of cardiac death or myocardial infarction at 1 year.14 An additional prospective randomized study is warranted to determine the superiority of OCT-guided PCI over angiography-guided PCI in terms of clinical outcome. Second, we cannot deny that both OCT and IVUS are bystander in the present non-inferiority endpoint analysis without the presence of an angiographic control group. It remains unknown whether the additional procedures on the basis of the OCT or IVUS evaluation reduce the incidence of the target vessel failure in relatively simple lesions. Third, we did not analyse the images of OFDI and IVUS. Therefore, we do not know the success rate of optimal stent implantation according to our OCT and IVUS criteria and the frequency of inadequate stent findings immediately after PCI. Fourth, our OCT and IVUS criteria may not be applicable to complex lesions. Further studies are needed to establish universal OCT and IVUS criteria for optimal stent implantation that leads to favorable clinical outcomes. Fifth, we do not necessarily support the routine use of OCT or IVUS during PCI. The expected effectiveness of OCT or IVUS guidance is limited by some factors, including the cost of the imaging catheter, the additional time to perform repetitive imaging procedures, and the need for training in order to accurately acquire and interpret the images. Sixth, the event-free survival curves clearly suggest the effect of repeat angiography on outcomes. Finally, regarding sample size calculation, the estimated event rate of the primary endpoint was overestimated. One of the reasons for the low event rate might be that the trial design excluded extremely high-risk patients for intravascular imaging guidance of PCI.
Conclusion
The 12-month clinical outcome in patients undergoing OFDI-guided PCI was non-inferior to that of patients undergoing IVUS-guided PCI. Both OFDI-guided and IVUS-guided PCI yielded excellent angiographic and clinical results, with very low rates of 8-month angiographic binary restenosis and 12-month target vessel failure.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This study was funded by Terumo Corporation.
Conflict of interest: T.K. has received lecture fees from Terumo Corporation. T.S. has received lecture fees from Terumo Corporation. J.S. has received lecture fees from Terumo Corporation. T.A. has received lecture fees and research funds from Terumo Corporation. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Supplementary Material
References
- 1. Mintz GS, Weissman NJ.. Intravascular ultrasound in the drug-eluting stent era. J Am Coll Cardiol 2006;48:421–429. [DOI] [PubMed] [Google Scholar]
- 2. Hong SJ, Kim BK, Shin DH, Nam CM, Kim JS, Ko YG, Choi D, Kang TS, Kang WC, Her AY, Kim YH, Hur SH, Hong BK, Kwon H, Jang Y, Hong MK.. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. Jama 2015;314:2155–2163. [DOI] [PubMed] [Google Scholar]
- 3. Galassi AR, Sumitsuji S, Boukhris M, Brilakis ES, Di Mario C, Garbo R, Spratt JC, Christiansen EH, Gagnor A, Avran A, Sianos G, Werner GS.. Utility of intravascular ultrasound in percutaneous revascularization of chronic total occlusions: an overview. JACC Cardiovasc Interv 2016;9:1979–1991. [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Farooq V, Garcia-Garcia HM, Bourantas CV, Tian N, Dong S, Li M, Yang S, Serruys PW, Chen SL.. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention 2012;8:855–865. [DOI] [PubMed] [Google Scholar]
- 5. Prati F, Guagliumi G, Mintz GS, Costa M, Regar E, Akasaka T, Barlis P, Tearney GJ, Jang IK, Arbustini E, Bezerra HG, Ozaki Y, Bruining N, Dudek D, Radu M, Erglis A, Motreff P, Alfonso F, Toutouzas K, Gonzalo N, Tamburino C, Adriaenssens T, Pinto F, Serruys PW, Di Mario C.. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J 2012;33:2513–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wijns W, Shite J, Jones MR, Lee SW, Price MJ, Fabbiocchi F, Barbato E, Akasaka T, Bezerra H, Holmes D.. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur Heart J 2015;36:3346–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A.. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 8. Kubo T, Shinke T, Okamura T, Hibi K, Nakazawa G, Morino Y, Shite J, Fusazaki T, Otake H, Kozuma K, Akasaka T.. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): study protocol for a randomized controlled trial. J Cardiol 2016;68:455–460. [DOI] [PubMed] [Google Scholar]
- 9. Ino Y, Kubo T, Matsuo Y, Yamaguchi T, Shiono Y, Shimamura K, Katayama Y, Nakamura T, Aoki H, Taruya A, Nishiguchi T, Satogami K, Yamano T, Kameyama T, Orii M, Ota S, Kuroi A, Kitabata H, Tanaka A, Hozumi T, Akasaka T.. Optical coherence tomography predictors for edge restenosis after everolimus-eluting stent implantation. Circ Cardiovasc Interv 2016;9:pii:e004231.. [DOI] [PubMed] [Google Scholar]
- 10. Kubo T, Akasaka T, Shite J, Suzuki T, Uemura S, Yu B, Kozuma K, Kitabata H, Shinke T, Habara M, Saito Y, Hou J, Suzuki N, Zhang S.. OCT compared with IVUS in a coronary lesion assessment: the OPUS-CLASS study. JACC Cardiovasc Imaging 2013;6:1095–1104. [DOI] [PubMed] [Google Scholar]
- 11. Kim IC, Yoon HJ, Shin ES, Kim MS, Park J, Cho YK, Park HS, Kim H, Nam CW, Han SW, Kim YN, Kim KB, Hur SH.. Usefulness of frequency domain optical coherence tomography compared with intravascular ultrasound as a guidance for percutaneous coronary intervention. J Interv Cardiol 2016;29:216–224. [DOI] [PubMed] [Google Scholar]
- 12. Habara M, Nasu K, Terashima M, Kaneda H, Yokota D, Ko E, Ito T, Kurita T, Tanaka N, Kimura M, Ito T, Kinoshita Y, Tsuchikane E, Asakura K, Asakura Y, Katoh O, Suzuki T.. Impact of frequency-domain optical coherence tomography guidance for optimal coronary stent implantation in comparison with intravascular ultrasound guidance. Circ Cardiovasc Interv 2012;5:193–201. [DOI] [PubMed] [Google Scholar]
- 13. Ali ZA, Maehara A, Généreux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, Guagliumi G, Meraj PM, Alfonso F, Samady H, Akasaka T, Carlson EB, Leesar MA, Matsumura M, Ozan MO, Mintz GS, Ben-Yehuda O, Stone GW.. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet 2016;388:2618–2628. [DOI] [PubMed] [Google Scholar]
- 14. Prati F, Di Vito L, Biondi-Zoccai G, Occhipinti M, La Manna A, Tamburino C, Burzotta F, Trani C, Porto I, Ramazzotti V, Imola F, Manzoli A, Materia L, Cremonesi A, Albertucci M.. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention 2012;8:823–829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.