Abstract
Children with Autism Spectrum Disorder (ASD) have basic motor impairments in balance, gait, and coordination as well as autism-specific impairments in praxis/motor planning and interpersonal synchrony. Majority of the current literature focuses on specific motor behaviors or domains. Additionally, the relationship between cognition, ASD severity, and motor performance in ASD is unclear. We used a comprehensive set of measures to compare gross and fine motor, praxis/imitation, motor coordination, and interpersonal synchrony skills across three groups of children between 5 and 12 years of age: children with ASD with high IQ (HASD), children with ASD with low IQ (LASD), and typically developing (TD) children. We used the Bruininks-Oseretsky Test of Motor Proficiency and the Bilateral Motor Coordination subtest of the Sensory Integration and Praxis Tests to assess motor performance and praxis skills respectively. Children were also examined while performing simple and complex rhythmic upper and lower limb actions on their own (solo context) and with a social partner (social context). Both ASD groups had lower gross and fine motor scores, greater praxis errors in total and within various error types, lower movement rates, greater movement variability, and weaker interpersonal synchrony compared to the TD group. In addition, the LASD group had lower gross motor scores and greater mirroring errors compared to the HASD group. Overall, a variety of motor impairments are present across the entire spectrum of children with ASD, regardless of their IQ scores. Both, fine and gross motor performance significantly correlated with IQ but not with autism severity; however, praxis errors (mainly, total, overflow, and rhythmicity) strongly correlated with autism severity and not IQ. Our study findings highlight the need for clinicians and therapists to include motor evaluations and interventions in the standard-of-care of children with ASD and for the broader autism community to recognize dyspraxia as an integral part of the definition of ASD.
Keywords: Autism, children, motor, coordination, praxis, interpersonal synchrony
1. Introduction
Autism Spectrum Disorder (ASD) is a multisystem neurodevelopmental disorder characterized by primary deficits in social communication skills and highly repetitive and restricted behaviors and interests (American Psychiatric Association, 2013). In addition to these diagnostic impairments, around 50–85% children with ASD demonstrate consistent deficits in several aspects of perceptuo-motor performance (Bhat, Landa, & Galloway, 2011; Chukoskie, Townsend, & Westerfield, 2013; Green et al., 2009; Hilton et al., 2012). A systematic examination of motor deficits in ASD is warranted for several reasons. First, motor symptoms are some of the earliest identifiable impairments noticed in infants and toddlers who may go on to develop a diagnosis of ASD (Landa & Garrett-Mayer, 2006; Flanagan, Bhat, & Landa, 2012; Bhat, Galloway, & Landa, 2012, Bedford et al., 2016). Second, there is substantial evidence suggesting that motor skill development is intimately linked to social communication and cognitive development in children with ASD (Dziuk et al., 2007; Dowell et al., 2009; Leary & Hill, 1996; Bhat et al., 2016). Lastly, a detailed examination of the motor system can provide insights into the functioning of the well-understood neural substrates underlying motor performance as well as its neighboring brain regions responsible for social performance, and ultimately the neuropathology of ASD (Gizzonio et al., 2015; Dowell et al., 2009). Therefore, in the current study, we used a comprehensive set of measures to assess different axes of motor ability in school-age children with ASD with a wide range of intellectual abilities and autism severity.
Motor Impairments in ASD
Research on motor impairments has suggested that children with ASD have substantial deficits in basic motor control skills as well as specific impairments in praxis (Fournier et al., 2010; Hallett et al., 1993; Noterdaeme et al., 2002; Jansiewicz et al., 2006; Dewey et al., 2007; Mostofsky et al., 2006; Srinivasan et al., 2013). In terms of basic motor skills, children with ASD demonstrate poor postural control (Minshew et al., 2004; Freitag et al., 2007; Teitelbaum et al., 1998), gait abnormalities (Vilensky et al., 1981; Hallett et al., 1993; Rinehart et al., 2006), as well as impairments in bilateral coordination (Fournier et al., 2010; Kaur et al., 2013; Isenhower et al., 2012; Marsh et al., 2013). Children also demonstrate poor fine motor control including manual dexterity, handwriting, object control, and visuo-motor integration skills (Provost et al., 2009; Berkeley et al., 2001; Green et al., 2002; Miyahara et al., 1997; Sacrey et al., 2014; Khuski et al., 2011; Fuentes et al., 2009; Mayes & Calhoun, 2003, McPhilips et al., 2014). Children with ASD received lower scores on multiple standardized tests of basic motor performance compared to typically developing (TD) children as well as children with other developmental diagnoses (Whyatt & Craig, 2012; Manjiviona & Prior, 1995; Miyahara et al., 1997; Ghaziuddin & Butler, 1998; Green et al., 2009; Hilton et al., 2007; Provost et al., 2007; Staples & Reid, 2010; Barbeau et al., 2015; Ament et al., 2015; Jansiewicz et al., 2006; Liu & Breslin, 2013). In addition to these basic motor deficits, children with ASD also demonstrate dyspraxia, i.e. impaired performance of skilled motor gestures during imitation, on verbal command, and during tool use, that cannot be wholly explained by basic perceptuo-motor deficits (Dewey, Cantell, & Crawford, 2007; Dowell, Mahone, and Mostofsky, 2009; Dziuk et al., 2007; Ham et al., 2011; Mostofsky et al., 2006; Carmo et al., 2013; Vanvuchelen et al., 2007; Srinivasan et al., 2013). While impairments in basic gross and fine motor skills are also found in children with other developmental disorders including children with Attention Deficit Hyperactivity Disorder and Developmental Coordination Disorder, impairments in praxis seem to be specific to autism (Dewey et al., 2007; MacNeill et al., 2012). Children with ASD demonstrated both spatial (incorrect body positioning, body-part-for-tool errors) and temporal (poor movement timing, increased time to initiate movement) errors during imitation/praxis tasks (Mostofsky et al., 2006; Dewey et al., 2007; Vanvuchelen et al., 2007; Gizzonio et al., 2015; Salowitz et al., 2013). Overall, there is considerable evidence for the presence of robust impairments in non-specific basic motor skills as well as more specific motor functions such as praxis abilities in ASD.
Neural mechanisms underlying motor impairments in ASD
The broad range of motor impairments in gait, posture, coordination, and imitation/praxis in ASD could be attributed to abnormal brain connectivity reported in this population (Courchesne, Campbell, & Solso, 2010; Just et al., 2012). There is mounting evidence to support the presence of excessive short-range connectivity within cortical regions (such as the frontal and parietal cortices) as well as poor long-range connectivity between cortico-cortical structures (such as the frontal and occipital cortices) or cortico-subcortical structures (including the sensori-motor, premotor, and supplementary motor cortices with the cerebellum or basal ganglia) (Turner et al., 2006; Mostofsky et al., 2009). For instance, atypical long-range functional connectivity between the motor areas and the visual areas of the brain is thought to underlie imitation skills in individuals with ASD (Nebel et al., 2016). Similarly, greater movement variability and poor movement timing may be related to abnormal cortico-cerebellar and cortico-striatal connections (Mostofsky et al., 2009; Rinehart et al., 2006). In fact, the pattern of movement slowing in ASD has been likened to bradykinesia seen in patients with Parkinson’s disease (Mari et al., 2003; Vilensky et al., 1981; Hallett et al. 1998). The more specific praxis impairments in ASD have been attributed to deficits in the bilaterally distributed brain network thought to underlie praxis comprising areas V1 and V5 of the occipital cortex, the superior temporal sulcus, inferior parietal region, premotor region, and the primary motor cortex (Mahajan et al., 2016; Nebel et al., 2016; Mostofsky & Ewen, 2011).
Linkages between motor and social/cognitive skills in ASD
In addition to understanding the nature of perceptuo-motor impairments, considerable research has been directed towards examining the impact of motor impairments on social, communication and behavioral skills in individuals with ASD (Freitag et al., 2007; Hilton et al., 2007; Fuentes & Bastian, 2009; Fitzpatrick et al., 2017; Nebel et al., 2016). Some have proposed a fundamental role of motor deficits in contributing to the classical symptoms of ASD (Bhat et al., 2011; Whyatt et al., 2013; Leary & Hill, 1996; Fitzpatrick et al., 2017; Marsh et al., 2013; Koehne et al., 2016). For instance, Whyatt and colleagues propose a sensory-motor theory of ASD suggesting that individuals with ASD demonstrate a basic perception-action coupling deficit; which not only affects spatio-temporal aspects of movement control but also forms the basis of more complex social communication and cognitive behaviors (Whyatt et al., 2013). Similarly, Fitzpatrick and colleagues argue that social motor coordination in the form of both imitation and interpersonal synchrony is a critical prerequisite for successful social interactions (Fitzpatrick et al., 2013, 2016, and 2017). The authors found that in a pendulum coordination paradigm, high functioning adolescents with ASD synchronized to a lesser degree with their parents both spontaneously and intentionally, compared to TD controls (Fitzpatrick et al., 2016). Moreover, spontaneous social-motor synchronization was associated with responsive joint attention, cooperation, and theory of mind skills, whereas intentional coordination was associated with initiating joint attention and theory of mind skills in ASD (Fitzpatrick et al., 2017).
Neural mechanisms explaining motor – social/cognitive linkages in ASD
Neuroscientific studies supporting for the links between social and motor skills in ASD suggest impairments in the mirror neuron systems (MNS) (Oberman et al., 2005; Dapretto et al., 2006; Williams et al., 2006; Theoret et al., 2005). The core mirror neuron network comprises of neurons in the inferior frontal gyrus and inferior parietal lobule that are active both when we watch a social partner perform an action and also when we ourselves perform the same action (Iacobani et al., 1999; Rizzolati & Craighero, 2004). This common coding between perception and action enables the observer to encode the goal of the action as well as its motor characteristics, thereby playing a primary role in motor imitation of a social partner (Rizzolati, Fogasi, & Gallese, 2001). More recently, a broader mirror neuron network comprising multiple areas in the frontal, parietal, and temporal cortices has been proposed to underlie several other social-cognitive and emotional skills including theory of mind (Frith & Frith, 1999; Hamilton & Grafton, 2006; Iacoboni et al., 2005), empathy (Oberman & Ramachandran, 2007; Dapretto et al., 2005), interpersonal synchrony (Vicaria and Dickens, 2016), language (Gallese, 2008), and intentional understanding (Iacoboni et al., 2005). Dysfunction in the MNS has therefore been linked directly to dyspraxia as well as broader deficits in social communication and emotional skills in ASD (Dowell et al., 2009; Hamilton, 2013).
Relationship between motor measures, intelligence, and autism severity
An area of considerable debate in the ASD literature is the relationship between motor skills, intellectual abilities, as well as autism severity in children with ASD. From a neuroscience perspective, significant correlations between cognitive and motor abilities may point towards impaired brain systems that contribute to both motor and cognitive functioning in individuals with ASD (Green et al., 2009). Alternatively, the presence of motor deficits even after controlling for IQ levels may shed light on motor impairments that are specific to the ASD diagnosis and are considered true deficits independent of task understanding (Whyatt & Craig, 2013). Around 70–75% of children with ASD demonstrate co-occurring moderate to severe intellectual disability (Charman et al., 2011). Several studies assessing motor performance in ASD used subjects with intelligence quotient (IQ) scores above 70 (Green et al., 2002; Hilton et al., 2007; Miyahara et al., 1997; Jansiewicz et al., 2006; Whyatt & Craig, 2012). However, this amounts to selective sampling of a highly heterogeneous population and does not provide a holistic overview of the range of impairments observed across the autism spectrum. Out of the studies that systematically assessed the impact of IQ on motor skills in ASD, some studies found a significant positive correlation between IQ and motor function (Ghaziuddin & Butler, 1998; Manjiviona & Prior, 1995; Green et al., 2009; Mari et al., 2003; Papadopoulos et al., 2012), whereas other studies found motor impairments in individuals with ASD regardless of their IQ scores (Mostofsky et al., 2006; Dziuk et al., 2009; Vanvuchelen et al., 2007; Barbeau et al., 2015). Fournier and colleagues (2010) in their meta-analysis of motor coordination deficits in this population also acknowledged this lack of consensus pertaining to the association between motor and cognitive skills in ASD. Moreover, an added limitation is that a majority of the above-mentioned studies assessed the association between motor and cognitive skills in ASD using a single test of motor performance.
In terms of the relation between motor skills and measures of autism severity, multiple studies have reported a correlation between praxis performance and autism severity (Dziuk et al., 2007; Ham et al., 2011; Gizzonio et al., 2015). Dziuk and colleagues (2009) found that overall praxis performance was significantly correlated with total scores on the Autism Diagnostic Observation Schedule, a standardized measure for autism severity. Furthermore, praxis errors were also correlated with individual components of the ADOS including the reciprocal social interaction, communication, and stereotyped/repetitive behaviors subdomains (Dziuk et al., 2009). However, there is currently limited knowledge on how other aspects of motor functioning (including imitation, praxis, gross and fine motor function, and interpersonal synchrony) in children with ASD relate to autism severity.
Aims of current study
In the current study, we used a variety of measures for the comprehensive profiling of the motor system in children with ASD. As discussed in the previous sections, few studies to date have holistically assessed motor function in ASD using both standardized assessments and experimental tasks (Whyatt & Craig, 2013; Barbeau et al., 2015). We used a battery of standardized tests and behavioral paradigms to assess gross and fine motor performance, praxis, bilateral motor coordination including solo and social motor coordination, as well as interpersonal/social synchrony in school-age children with ASD. To understand the relationship between motor skills and cognitive abilities, we included three groups of children between 5 and 12 years of age – children with ASD with low IQ scores (IQ ≤ 70) (LASD group), children with ASD with high IQ scores (IQ > 70) (HASD group), and TD children. In addition, we also correlated motor performance with IQ and ADOS scores in the ASD group. We hypothesized that both the ASD groups would receive lower scores than the TD group on all the tested motor measures. In line with previous evidence, we hypothesized that in children with ASD, overall gross and fine motor performance may correlate with IQ scores whereas praxis scores may correlate with ADOS scores.
2. Methods
2.1. Participants
The sample reported in the current study was part of a larger randomized controlled trial (RCT) conducted in children with ASD to examine the effects of novel movement-based interventions in ASD (xxx). Twenty-four children with ASD between 5 and 12 years of age were included in the current study, along with a convenience sample of 12 age-matched TD children (Table 1). The participant demographics including age, gender, social economic status (SES), and ethnicity are provided in Table 1. We calculated SES scores using the Hollingshead scale (Hollingshead, 1975). As seen in Table 1, the groups were age-matched but differed in terms of gender and SES scores. Both ASD groups had a greater proportion of males compared to females, which is not surprising given the higher prevalence of ASD in males, 4.5 times than females (Christensen et al., 2016). Furthermore, both ASD groups had lower average SES scores compared to TD families perhaps due to the financial burden imposed on families by the diagnosis (Montes & Halterman, 2008).
Table 1.
Participant demographics.
| Group | Age (years) (Mean ± SE) | N, F:M | ABIQ | SES | Ethnicity | ADOS-2 Comparison Scores |
|---|---|---|---|---|---|---|
|
| ||||||
| TD | 7.75 ± 0.55 | 12, 3:9 | NA | 59.38 ± 2.08 | 7 C, 5A | NA |
|
| ||||||
| HASD | 7.44 ± 0.57 | 12, 0:12 | 115.86 ± 17.03 | 51.17 ± 2.98 | 10 C, 2 O | 7.91±1.76 |
|
| ||||||
| LASD | 8.74 ± 0.59 | 12, 2:10 | 60.07 ± 9.50 | 47.46 ± 2.97 | 6 C, 5 AA, 1 A | 9.27±1.10 |
|
| ||||||
| p-value (t-test/chi square) | ||||||
| TD vs. HASD | ns | 0.05 | NA | 0.04 | NA | |
| TD vs. LASD | ns | ns | NA | <0.01 | NA | |
| HASD vs. LASD | ns | ns | <0.01 | ns | 0.04 | |
N, number of participants; F:M, number of females and males; ABIQ: Abbreviated IQ; SES, Socio Economic Status; NA, Not Assessed; C, Caucasian; AA, African American; A, Asian; O, Others; ns, not significant with p>0.05.
All parents were interviewed using a screening questionnaire to collect information about the child’s birth, development, and medical history. All TD children in this sample were studying in public or private schools in grade levels appropriate for their age (i.e., they were not delayed in their classroom placements), had no special needs, and were not receiving any special services at the time. In addition, the TD group had no significant birth history, family history of ASD, or a history of developmental delays. Further, none of the participating children had any other genetic/neurological/orthopedic disorders, or any vision and hearing impairments that could have restricted study participation. Children were recruited through local day care centers, autism service providers such as clinics and schools, web postings, and by word of mouth. Parents signed the informed consent approved by the xxxx before the children participated in the study and children above 7 years of age also provided assent to participation.
2.2. Materials and Procedures
We videotaped all children during two testing visits each lasting for around 45 minutes to 1 hour. Trained and experienced doctoral students with a background in physical therapy conducted both sessions with training and supervision from the last author. During the first visit, children performed the gross and fine motor items of the BOT-2. During the second visit, children completed the Bilateral Motor Coordination subtest of the SIPT (SIPT-BMC) and an experimental paradigm assessing bilateral coordination and interpersonal synchrony. For children with ASD only, a research-reliable clinical psychology graduate student conducted a third visit to confirm the diagnosis of the child and to obtain their IQ scores. We used various strategies throughout the sessions to ensure task comprehension especially in children with ASD, including visual demonstration of the activity by the tester, use of simple instructions such as “can you do this?”, use of pictures to illustrate the activity, as well as provision of a practice trial with manual feedback, if required.
2.2.1. Bruininks-Oseretsky Test of Motor Proficiency- 2nd Edition (BOT-2, Figure 1a)
Figure 1.
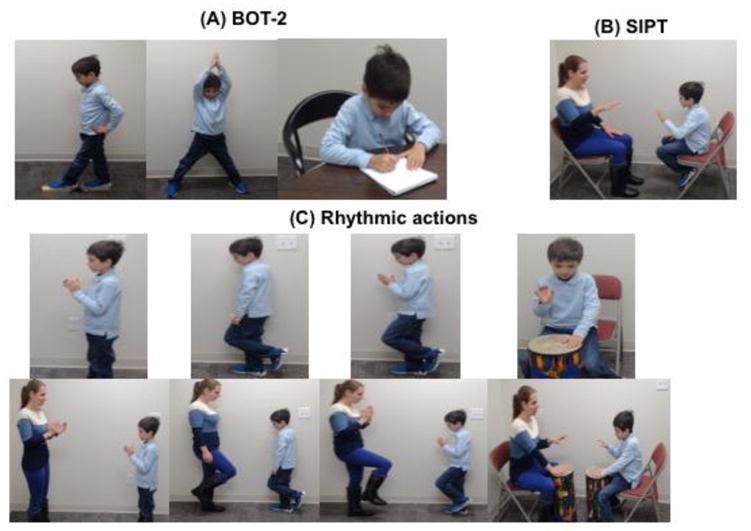
A typically developing child performing (A) gross motor and fine motor actions from the standardized motor test, BOT-2, (B) action sequences from the Bilateral Motor Coordination subtest of the SIPT, and (C) simple and complex clap, march, march clap, and drum actions in solo and social contexts.
BOT-2 is a reliable and valid measure of fine and gross motor performance in individuals between 4 and 21 years of age (Bruininks & Bruininks, 2005). We used five of the BOT-2 subtests to assess gross and fine motor performance in the children. The balance and bilateral coordination subtests were administered to get the composite score for gross motor control (i.e. body coordination score). The fine motor precision and fine motor integration subtests were administered to get the composite score for fine motor control (i.e. fine manual score). In addition, we also included the manual dexterity subtest. Finally, we used the short form of the BOT-2 (BOT-SF) to get a comprehensive estimate of overall motor performance in our sample. We did not include the strength, running & agility, and upper-limb coordination subtests due to time constraints and as they did not directly contribute to the calculation of the gross and fine motor composites within the scoring system of the BOT-2. We are reporting on the standard scores for the BOT-SF, body coordination composite, and fine manual control composite, as well as scaled scores for the manual dexterity subtest.
2.2.2. Bilateral Motor Coordination subtest of the Sensory Integration and Praxis Test (SIPT-BMC, Figure 1b)
SIPT is a comprehensive assessment tool for evaluating praxis and sensory integration in children between 4 and 8.11 years of age (Ayres, 1988). We used the bilateral motor coordination subtest of the SIPT (SIPT-BMC); which includes 22 action sequences involving either the upper limbs or the lower limbs. Out of these 22 action sequences, 14 actions involve the upper limbs whereas 8 actions involve the lower limbs. Some exemplar actions include alternate tapping of hands on the thighs, hand tapping followed by clapping, alternate leg stomping, alternate foot tapping, etc. The tester and the child sat across each other and the child was instructed to copy and mirror the tester’s actions i.e., if the adult used the right hand, the child was asked to use the left hand. A practice trial was completed for the first action so that the children understood the “mirroring” principle. We manually coded children’s videos in real time for various spatio-temporal errors, including rhythmicity, mirroring, and overflow based on the error classification proposed by Dewey (Dewey, 1993; Dewey et al., 2007) and Dziuk et al. (2009) (see Table 2). Physical therapy doctoral students trained in the administration of the praxis subtests coded recorded videos of children from the testing visit for movement errors mentioned above. Each child received a score of 0 (no error) or 1 (error) based on the absence/presence of an error for each action sequence. We then summed the child’s scores across all 22 action sequences for each error type to calculate the rhythmicity, mirroring, and overflow error score. In addition, we summed scores on all error types across all actions to obtain a total error score. Lastly, time to completion was recorded for each action sequence using a stopwatch. We calculated an average time to completion measure across all individual action sequences.
Table 2.
Praxis error classification for the SIPT-BMC actions.
| Error type | Definition | |
|---|---|---|
| Spatial | Mirroring | Inability to mirror the tester’s actions (ideally, child should use right hand/leg if the tester uses left hand/leg) |
| Overflow | Additional movements towards the end of the action sequence beyond what the tester performed | |
| Total | Summation of rhythmicity, mirroring, and overflow errors | |
| Temporal | Rhythmicity | Inability to imitate rhythm of the action sequence |
| Time to completion | Time in seconds to complete the action sequence from start to finish |
2.2.3. Motor Coordination Experimental Paradigm (Figure 1c)
During the second visit, children performed various simple and complex rhythmic actions requiring coordination of the two sides of the body in 2 contexts – (1) solo context where children moved on their own while synchronizing to a metronome beat, and (2) social context where children were asked to synchronize their actions with those of an adult tester while following the metronome beat. In both contexts, children performed test actions including clapping, marching, marching and clapping, and drumming. We deliberately chose a combination of dual and multilimb, symmetrical and asymmetrical actions to capture the range of movements children perform every day. In addition, these actions have been used in similar work conducted in TD children and children with ASD (Getchell, 2006, 2007; Getchell & Whitall, 2003; Kaur et al., 2013; Isenhower et al., 2012). We ensured that the test actions included both upper and lower limb actions of varying complexity in terms of bilateral coordination. For instance, although all test actions were bilateral in nature, drumming, clapping, and marching were dual limb movements whereas the march-clap action was a multi-limb movement. Similarly, clapping requires symmetrical movements of both upper limbs, whereas the other 3 test actions involved asymmetrical movements of upper and/or lower limbs. The drumming test involved two actions: simple drumming that involved an alternate drumming pattern where a hit with one hand was followed by a hit with the other hand, and complex drumming involving an asymmetrical quarter-eighth or 2:1 pattern where 2 hits were performed by 1 hand followed by 1 hit by the other hand. For the multi-limb march-clap action, we separately analyzed the clap and march components and termed them complex clap and complex march (as opposed to the simple clapping and simple marching mentioned above). Overall, for the purpose of analysis we had 6 different actions in each context – (1) simple clapping, (2) complex clapping, (3) simple marching, (4) complex marching, (5) simple drumming, and (6) complex drumming. Movement speeds were controlled using a metronome beat of 120 beats/minute.
The order of actions was fixed across all children: clapping, followed by marching, then march-clap, then alternate drumming, and lastly the quarter-eighth drumming pattern. These actions were first conducted in the solo context followed by the social context. For each action, the tester first demonstrated the action, and then allowed the child a brief practice bout of 10 seconds, followed by the test trial for that action. For each activity, we collected a single trial of 50 seconds. A rest-break was provided after completion of all solo trials before the social portion of the test.
We recorded video data from children as they performed the coordination test paradigm. We completed second-to-second coding of the video data using open source Datavyu coding software (www.datavyu.org). Specifically, we coded the time frame for each event i.e. clap (contact between hands), march (right and left stomp), and drum hit (right and left hand hit) within a trial. The coded files were run through a custom-developed MATLAB program to calculate inter-event duration in seconds i.e., the time between two successive claps, stomps or drum hits. Next, we calculated the Coefficient of Variation (CV) of the inter-event duration to obtain a variability measure for each action, with lower CV indicating greater consistency in movement and hence, better performance. We calculated Movement Rates as the number of events per second for each action. As mentioned above, our metronome beat was set to 120 beats/min. Therefore, if the child was perfectly synchronizing to the metronome beat then he/she would move at the rate of two movements per second or 100 movements in each 50-second trial, except for the drumming actions. In case of the drumming actions, we evaluated the right-hand hits only; hence, perfect synchronization to the metronome beat would result in one hit per second or 50 hits per trial. Lastly, we coded interpersonal synchrony for the social context of the rhythmic actions, i.e. the percentage of time the child perfectly synchronized his/her actions with the tester (in synchrony) or not (out of synchrony). This was coded by tagging the start and end time frames of “in-synchrony” periods with remaining seconds in the trial considered “out-of-synchrony”.
2.2.4. Autism Diagnosis Observation Schedule – 2nd Edition (ADOS-2)
The ADOS-2 is a gold standard assessment to confirm autism diagnosis of children (Lord et al., 2012). It involves semi-structured play-based activities for assessing social interactions and repetitive/restricted behaviors of children. The scoring of the ADOS-2 provides a comparison score of 1–10 to gauge the severity of the disorder, with higher scores indicating greater severity. Autism diagnosis was confirmed for all 24 children with ASD. Details of ADOS comparison scores in the 2 ASD groups provided in Table 1.
2.2.5 Stanford Binet Intelligence Scales - 5th Edition (SBIQ)
We obtained the Intelligence Quotient (IQ) scores using the SBIQ (Becker, 2003) in the ASD group. The SBIQ is a standardized test to measure non-verbal and verbal cognitive skills of individuals between 2 and 90 years of age on five different domains - fluid reasoning, knowledge base, quantitative reasoning, visual-spatial skills, and working memory. For the purpose of this study, we used the abbreviated IQ score that is a quick IQ measure that can be calculated using the two routing subtests of the SBIQ (non-verbal fluid reasoning and verbal knowledge). Based on the abbreviated IQ scores of children with ASD, we divided them into two groups: a) children with ASD with average to high IQ scores (HASD), i.e. IQ>70 and b) children with ASD with low IQ scores (LASD), i.e. IQ≤70 (Table 1).
2.3. Statistical analysis
To establish intra-rater and inter-rater reliability among the coders, we calculated Intra-class correlations coefficients (ICC) using 25% of the dataset for each of the testing measures. The ICC values were >93% for the intra-rater and >80% for the inter-rater comparisons (Table 3). Since our data did not satisfy the assumptions of parametric statistics, we used non-parametric Kruskal-Wallis tests to compare the dependent variables across the three groups. In case of a significant Kruskal-Wallis test, we further used Mann-Whitney U tests to identify the specific groups that differed significantly from each other on our dependent variables. We also conducted pairwise Pearson’s correlations to examine the relationship between IQ, autism severity, and all the motor measures. Significance level was set at p≤0.05.
Table 3.
Results of the Intraclass Correlation Coefficients (ICC) used for calculating reliability of coding for dependent variables
| Subtest | Inter-rater ICC | Intra-rater ICC | |
|---|---|---|---|
| BOT-2 | Gross motor | 0.99 | 0.99 |
| Fine motor | 0.98 | 0.98 | |
|
| |||
| SIPT-BMC | Rhythmicity | 0.96 | 0.97 |
| Mirroring | 0.99 | 0.97 | |
| Overflow | 0.80 | 0.95 | |
| Time | 0.80 | 0.99 | |
|
| |||
| Variability, Rate | Clap | 0.99 | 0.99 |
| March | 0.92 | 0.99 | |
| Drum | 0.98 | 0.99 | |
|
| |||
| IPS | Clap | 0.97 | 0.99 |
| March | 0.96 | 0.93 | |
| Drum | 0.84 | 0.99 | |
3. Results
3.1. BOT-2
The Kruskal-Wallis test revealed a significant group effect for the BOT-SF, body coordination composite, fine manual composite, and manual dexterity subtest scores (Table 4). The post-hoc Mann-Whitney U-tests indicated that both the ASD groups scored lower than the TD group on all the BOT-2 outcome measures (p values below 0.006, see Figure 2). In terms of differences between the two ASD groups, the LASD group received lower scores than the HASD group on the BOT-SF and body coordination subtests. Both groups demonstrated equally poor performance on the fine manual composite and manual dexterity subtest (p values ≤ 0.004, Figure 2).
Table 4.
Results of the Kruskal-Wallis Tests.
| Variable | χ2 | df | p-values | |
|---|---|---|---|---|
| BOT-2 | BOTSF | 21.76 | 2 | <0.001 |
| Body coordination | 23.67 | 2 | <0.001 | |
| Fine manual | 19.93 | 2 | <0.001 | |
| Manual Dexterity | 21.10 | 2 | <0.001 | |
|
| ||||
| SIPT-BMC | Rhythmicity | 8.13 | 2 | 0.017 |
| Mirroring | 12.25 | 2 | 0.002 | |
| Overflow | 10.33 | 2 | 0.006 | |
| Total Error | 12.05 | 2 | 0.002 | |
| Time to Completion | 18.17 | 2 | <0.001 | |
|
| ||||
| Movement Variability | Simple clap- Solo | 5.73 | 2 | ns |
| Complex clap- Solo | 9.75 | 2 | 0.008 | |
| Simple march- Solo | 12.52 | 2 | 0.002 | |
| Complex march- Solo | 14.12 | 2 | 0.001 | |
| Simple drum- Solo | 7.65 | 2 | 0.02 | |
| Complex drum- Solo | 0.64 | 2 | ns | |
| Simple clap- Social | 16.72 | 2 | <0.001 | |
| Complex clap- Social | 11.52 | 2 | 0.003 | |
| Simple march- Social | 13.36 | 2 | 0.001 | |
| Complex march- Social | 14.36 | 2 | 0.001 | |
| Simple drum- Social | 16.80 | 2 | <0.001 | |
| Complex drum- Social | 1.99 | 2 | ns | |
|
| ||||
| Movement Rate | Simple clap- Solo | 6.55 | 2 | 0.04 |
| Complex clap- Solo | 7.22 | 2 | 0.03 | |
| Simple march- Solo | 12.99 | 2 | 0.002 | |
| Complex march- Solo | 5.85 | 2 | 0.05 | |
| Simple drum- Solo | 0.72 | 2 | ns | |
| Complex drum- Solo | 3.71 | 2 | ns | |
| Simple clap- Social | 10.23 | 2 | 0.006 | |
| Complex clap- Social | 14.58 | 2 | 0.001 | |
| Simple march- Social | 10.97 | 2 | 0.004 | |
| Complex march- Social | 11.23 | 2 | 0.004 | |
| Simple drum- Social | 2.81 | 2 | ns | |
| Complex drum- Social | 0.08 | 2 | ns | |
|
| ||||
| Interpersonal synchrony | Simple clap | 11.16 | 2 | 0.004 |
| Complex clap | 13.77 | 2 | 0.001 | |
| Simple march | 13.43 | 2 | 0.001 | |
| Complex march | 13.35 | 2 | 0.001 | |
| Simple drum | 9.13 | 2 | 0.01 | |
| Complex drum | 9.26 | 2 | 0.01 | |
χ2, Chi-square; df, degree of freedom; ns, not significant with p>0.05
Figure 2.
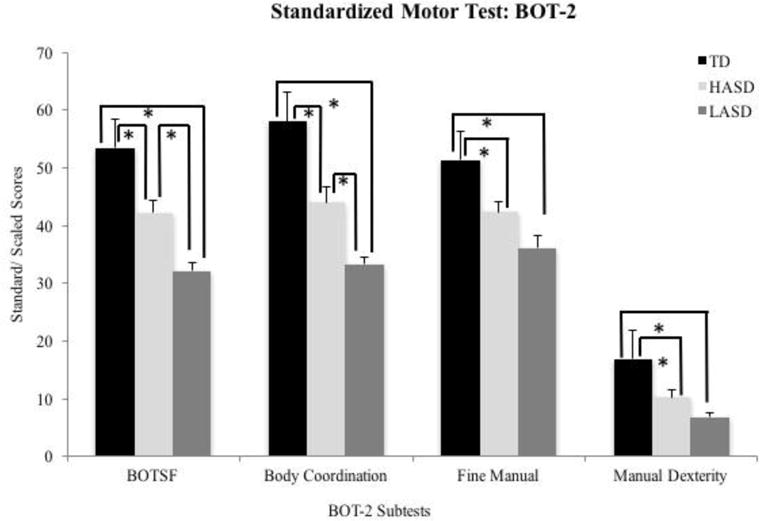
Standard/scaled scores for BOT-2 subtests in the TD, HASD, and LASD groups.
Note: BOT-2 standard scores have a mean of 50 and standard deviation of 10. BOT-2 scaled scores have a mean of 15 and standard deviation of 5.
3.2. SIPT-BMC
The Kruskal Wallis test indicated a significant group effect for the rhythmicity, mirroring, overflow, and total error scores as well as for the time to completion variable (Table 4). Post-hoc Mann-Whitney-U tests suggested that the LASD group had significantly greater rhythmicity, mirroring, overflow, and total errors compared to the TD group (p = 0.001 to 0.006), whereas the HASD group only showed greater overflow errors compared to the TD group (p = 0.01, Figure 3). Additionally, the two ASD groups differed in terms of mirroring errors (p = 0.01) and total errors (p = 0.03) with greater errors in the LASD compared to the HASD group (see Figure 3). Lastly, both the LASD and the HASD groups took longer to complete the action sequences compared to the TD group (p-values < 0.001, LASD = 5.42±0.33 seconds; HASD = 4.54±0.24 seconds; TD = 3.39±0.17 seconds), with the LASD group demonstrating a trend for slower movements compared even to the HASD group (p=0.05).
Figure 3.
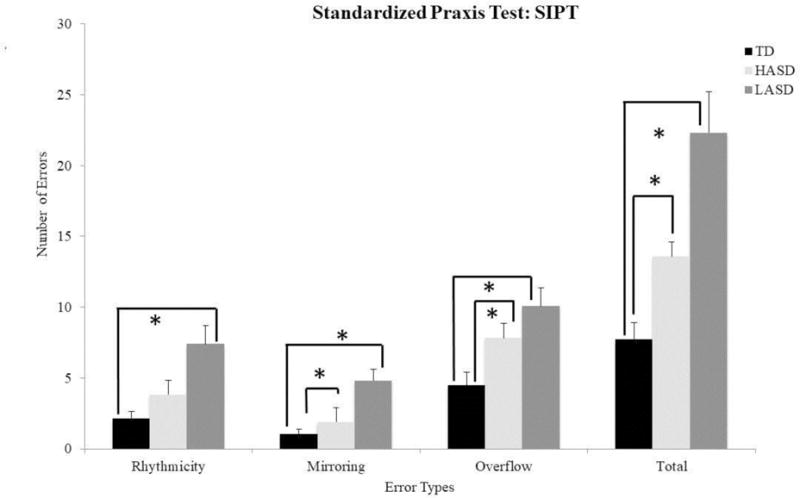
Praxis errors for SIPT-BMC in the TD, HASD, and LASD groups.
3.3. Movement variability (CV)
The Kruskal-Wallis tests showed a significant group effect for 9 out of 12 actions (social context of simple clap, solo and social contexts of complex clap, simple march, complex march, and simple drum), and no differences for the remaining 3 actions (solo context of simple clap, solo and social contexts of complex drum, see Table 4). The subsequent Mann Whitney U-tests indicated that both the LASD and the HASD groups had greater movement variability compared to the TD group for all the aforementioned actions, except for the simple drum action in the solo context, for which only the HASD group had greater movement variability compared to the TD group (p-values = 0.001 – 0.05, Figure 4). There were no significant differences in movement variability between the two ASD groups for any of the actions.
Figure 4.
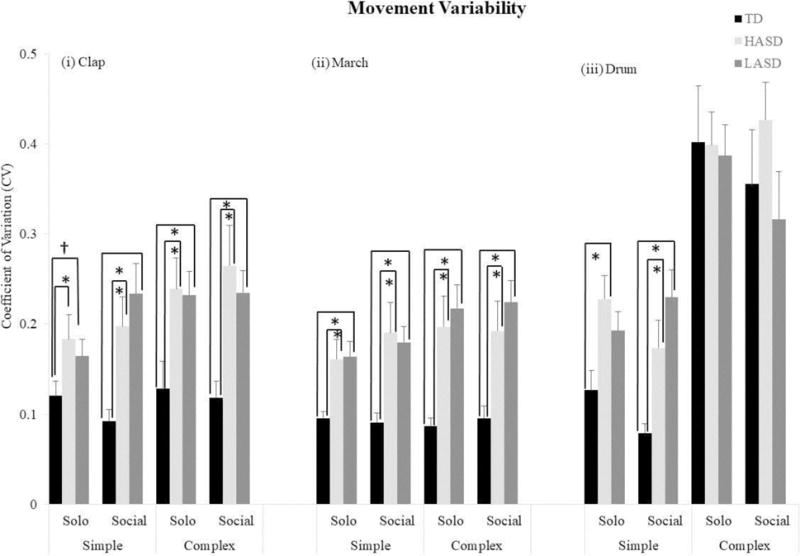
Movement variability or Coefficient of Variation for the rhythmic actions in the TD, HASD, and LASD groups.
3.4. Movement rate
The Kruskal-Wallis tests revealed a significant group effect for 8 actions (solo and social contexts of simple clap, complex clap, simple march, and complex march), and no differences for 4 actions (solo and social contexts of simple drum and complex drum; Table 4). The post-hoc Mann-Whitney U tests indicated that the LASD group had slower movement rates compared to the TD group for all 8 actions listed above. The HASD group had slower movement rates for only 3 actions – solo context of complex clap and social contexts of simple and complex march actions (p values = 0.001 to 0.05, see Figure 5). Additionally, the LASD group had a slower movement rate compared to the HASD group for only 1 action - solo context of the simple clap action (p=0.02).
Figure 5.
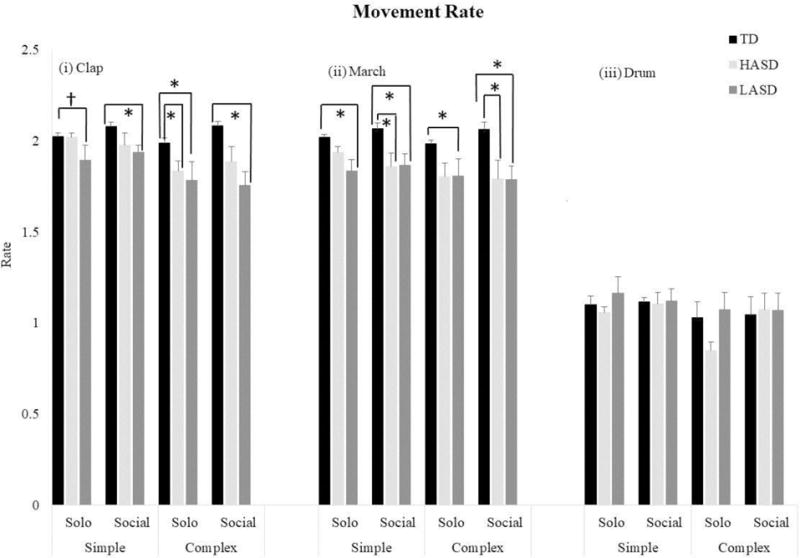
Movement rates for the rhythmic actions in the TD, HASD, and LASD groups.
3.5. Interpersonal synchrony
The Kruskal-Wallis tests revealed a significant group effect for all actions (Table 4). The post-hoc Mann Whitney-U tests indicated that both the LASD and the HASD groups spent less time in-synchrony with their adult partner compared to the TD group for all actions (LASD: p values = 0.001 to 0.01; HASD: p values = 0.001 to 0.04) (see Figure 6). There were no significant differences between the two ASD groups on this dependent variable.
Figure 6.
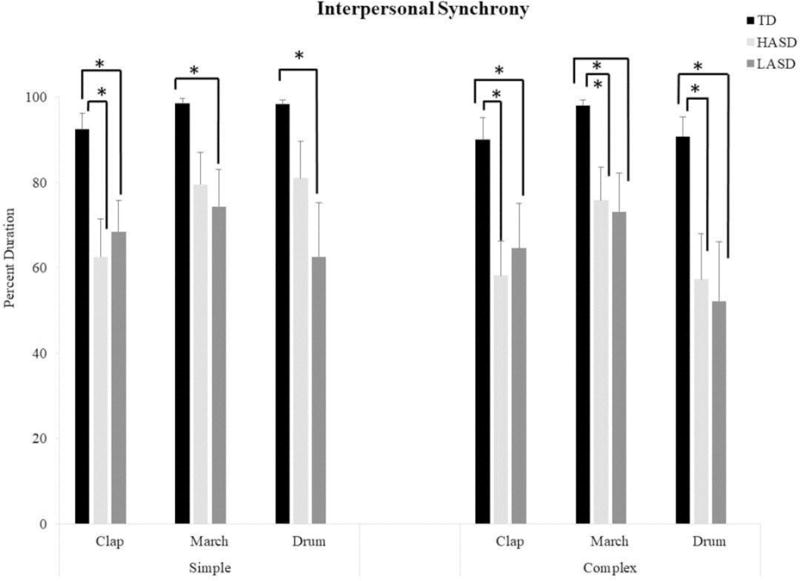
Interpersonal synchrony during the social context of rhythmic actions in the TD, HASD, and LASD groups.
3.6. Correlating IQ and ADOS versus motor measures in ASD
IQ significantly correlated with BOT-2 measures (Table 5, r=0.45–0.65, p values = 0.03 to 0.0009). Note that IQ did not correlate with any other measures of praxis, movement variability, movement rate, or IPS. ADOS scores significantly correlated with praxis errors including total errors (Table 5, r=0.54 to 0.61, p values = 0.002 to 0.009), overflow errors (Table 5, r= 0.59 to 0.69, p values = 0.004 to 0.0004), and rhythmicity errors (Table 5, r=0.39–0.49, p values = 0.02 to 0.05). ADOS scores also correlated with the time taken to complete the action sequences in the SIPT-BMC test (r=0.46, p=0.03). Additionally, ADOS scores also correlated with movement variability during solo march-clap and social simple drum motions (Table 5, r=0.44–0.51, p values = 0.02 to 0.04). No other significant correlations were found between motor skills, cognitive skills, and measures of autism severity.
Table 5.
Results of pairwise correlations between IQ, ADOS, and motor measures
| Motor vs IQ, ADOS | Pearson’s correlation | P-value (s) |
|---|---|---|
| BOT-BC (GM) v IQ | 0.57 | 0.005* |
| BOT-FM v IQ | 0.54 | 0.009* |
| BOT-SF v IQ | 0.66 | 0.0009* |
| BOT-MD v IQ | 0.45 | 0.03* |
| BOT v ADOS | Ranges from −0.01 to −0.38 | all p-values ns |
| SIPT Total v ADOS Social Affect | 0.56 | 0.006* |
| SIPT Total v ADOS RBB | 0.54 | 0.009* |
| SIPT Total v ADOS Total | 0.61 | 0.002* |
| SIPT Total v ADOS comparison | 0.4 | 0.06| |
| SIPT Time v ADOS RBB | 0.46 | 0.03* |
| SIPT Overflow v ADOS Social Affect | 0.59 | 0.004* |
| SIPT Overflow v ADOS RBB | 0.66 | 0.0009* |
| SIPT Overflowv ADOS Total | 0.69 | 0.0004* |
| SIPT Overflow v ADOS Comparison | 0.44 | 0.03* |
| SIPT Rhythmicity v ADOS Social Affect | 0.48 | 0.02* |
| SIPTRhythmicity v ADOS RBB | 0.39 | 0.06| |
| SIPT Rhythmicity v ADOS Total | 0.49 | 0.02* |
| SIPT Rhythmicity v ADOS Comparison | 0.3 | ns |
| SIPT Mirror v ADOS Social Affect | 0.38 | 0.07| |
| SIPT Mirror v ADOS RBB | 0.35 | 0.1| |
| SIPT Mirror v ADOS Total | 0.4 | 0.05| |
| SIPT Mirror v ADOS Comparison | 0.2 | ns |
| SIPT scores v IQ | Ranges from −0.2 to −0.3 | ns |
| SIPT Time v IQ | −0.4 | 0.05| |
| CV Solo March-Clap v ADOS Social Affect | 0.44 | 0.04* |
| CV Social Simple Drum v ADOS Comparison | 0.51 | 0.02* |
= significant difference < 0.05
= statistical trend 0.05 – 0.1
ns = not significant p > 0.1
4. Discussion
Our study suggests that regardless of the cognitive status of children, motor dysfunction is pervasive in children with ASD. Compared to TD children, children with ASD have universal difficulties in several aspects of motor function including – (1) gross and fine motor performance, (2) certain aspects of praxis during performance of sequential, imitation-based tasks, (3) simultaneous coordination of the two sides of the body during rhythmic upper and lower-limb tasks, as well as (4) social-motor coordination and interpersonal synchrony. Still, standardized gross and fine motor measures correlated significantly with IQ but not with ADOS scores. In contrast, praxis errors and time taken to complete action sequences strongly correlated with ADOS scores but not IQ. Certain movement variability measures correlated with the ADOS scores but none of the other motor measures correlated with ADOS or IQ. In the next few sections, we discuss the possible reasons underlying our findings and their implications with respect to our current understanding of ASD and potential targets for intervention in this disorder.
4.1. Gross and fine motor performance
On the standardized BOT-2 measure, both the HASD and LASD groups had significantly lower scores in terms of their gross and fine motor skills compared to the TD group. Our findings fit with previous work in this area by other research groups (Ament et al., 2015; Green et al., 2009; Barbeau et al., 2015; Provost et al., 2007; Liu et al., 2013; Whyatt & Craig, 2012, 2013; Jansiewicz et al., 2006). In general, the LASD group received lower scores than the HASD group on the BOT-2. We also observed a strong relationship between IQ and gross/fine motor performance measures. However, group differences between the HASD and LASD group were only significant for the body coordination composite and not for the fine manual composite or the manual dexterity subtests of the BOT-2. While we think these results should further replicated in future studies, this difference could be explained by the extensive neuropathology in the LASD group and/or the possible gross motor gains with development specifically occurring in the HASD group. Nevertheless, we want to emphasize that motor dysfunction existed across the autism spectrum (i.e., for all motor scores, both HASD & LASD < TD) regardless of children’s cognitive abilities. Our findings align with those of Smits-Englesman and colleagues who report that although cognitive and motor skills are inter-related possibly through common underlying neural substrates, however, cognitive skills only account for 19% of the variance in motor skills in TD children and children with developmental motor difficulties (Smits-Engleman & Hill, 2012).
4.2. Praxis and imitation skills
Compared to the TD group, we found greater praxis errors and longer completion times in both HASD and LASD groups. Our findings replicate the results of other studies that also report praxis impairments in children with ASD (Dewey et al., 2007; Dowell et al., 2009; Gizzonio et al., 2015). From a neuroscience perspective, as discussed in the introduction, deficits in the bilaterally distributed praxis network and poor long-range connectivity between the visual and motor areas of the brain have been thought to underlie the imitation and praxis impairments in ASD (Nebel et al., 2016; Mahajan et al., 2016; Mostofsky & Ewen, 2011; Haswell et al., 2009). We also found a strong correlation between ADOS scores and both praxis errors (total, overflow, rhythmicity) and time taken to complete actions in the ASD group, although no such relationships existed between praxis measures and IQ scores. Several other groups have also demonstrated that praxis performance of children with ASD is correlated with overall autism severity but not with intelligence (Dowell et al., 2009; Dziuk et al., 2007; Gizzonio et al., 2015; Bhat et al., 2016). Overall, given this consistent evidence for the specificity of praxis impairments in ASD, we recommend that dyspraxia be considered a defining feature of ASD.
Similar to past studies, we found that children with ASD universally took longer to complete rhythmic action sequences compared to the TD group (Green et al., 2009; Biscaldi et al., 2014; Isenhower et al., 2012; Jansiewicz et al., 2006). Children may have intentionally slowed their actions within the action sequence. This might have been a trade-off used by children to maintain accuracy in complex praxis tasks that require both speed and accuracy (Whyatt et al., 2012; von Hofsten, 2007). Furthermore, there was also a trend for slower movement times in the LASD group compared to the HASD group. On a related note, we found that the LASD group made greater rhythmicity errors compared to the HASD and TD groups. Perhaps the movement slowing helped the HASD group reduce their rhythmicity errors and improve accuracy of imitation of the movement pattern, but the LASD group continued to show these errors despite movement slowing. In fact, movement slowing is a common compensatory strategy shown by patients with incoordination following cerebellar disease (Bastian et al., 1996). In individuals with ASD, Mari and colleagues have similarly reported significant slowing of reach-to-grasp movements in a ‘low-ability’ (IQ: 70–79) group compared to the ‘average-ability’ and ‘high-ability’ groups (IQ: 80–109) (Mari et al., 2003).
Both ASD groups demonstrated significant overflow errors compared to the TD group. Similarly, Dewey and colleagues found that even after controlling for IQ, children with ASD made greater overflow errors compared to TD children and children with developmental coordination disorder (DCD) (Dewey et al., 2007). Overflow errors have been attributed to poor inhibitory control of neurons within the cerebral cortex as well as a delayed functional segregation of upper and lower limb areas within the primary motor cortex in individuals with ASD (Mostofsky, Newschaffer, & Denckla, 2003; Barber et al., 2012; Nebel et al., 2014). Lastly, consistent with the limited literature on this topic, we found that the HASD group did not demonstrate any significant differences in mirroring compared to the TD group (Vanvuchelen et al., 2007; Smith & Bryson, 1998; Carmo et al., 2013). However, our findings of significant mirroring errors in the LASD group compared to the TD group need to be replicated and investigated further.
4.3. Bilateral coordination and social synchrony skills
We found that compared to the TD group, the LASD group had more variable and slower movements for all types of rhythmic actions (except drumming) performed alone and with a social partner. Although the HASD group in general had more variable movements (except drumming) compared to the TD group, they had slower movement rates only for few actions (complex clap in the solo context and the simple and complex march actions in the social context). The lack of any group differences for drumming actions could be possibly because even TD children found the drumming actions hard; in fact, the complex drumming action was the most variable across all three groups (see Figure 4). Past studies have also reported greater movement variability during rhythmic upper- and lower-limb tasks such as drumming, maraca shaking, and walking (Fleury et al., 2013; Isenhower et al., 2012; Kaur et al., 2013; Nayate et al., 2012) as well as during discrete discontinuous actions (Fleury et al., 2013). Interestingly, Fleury et al., (2013) found that children with ASD demonstrated greater movement variability during discontinuous/discrete circle drawing but not during continuous circle drawing. In contrast, we found significant movement impairments during continuous rhythmic actions. These differences in findings may be due to differences in task-specific demands across the two studies. We asked children to perform whole body gross motor movements to a metronome beat, whereas Fleury and colleagues assessed a fine motor action without additional temporal demands of synchronization with an external beat (Fleury et al., 2013). In terms of movement rates, in line with our findings, slower movement rates have been reported in children and adolescents with ASD during motor tasks such as drumming and repetitive hand movements (Biscaldi et al., 2014; Isenhower et al., 2012; Jansiewicz et al., 2006).
We also found that both HASD and LASD groups were equally impaired in synchronizing with their social partners during rhythmic actions compared to the TD group. The limited literature on interpersonal synchrony lends overall support to our findings (Marsh et al., 2013; Fitzpatrick et al., 2016, 2017). For instance, Marsh and colleagues reported weaker “in-phase synchrony” between pairs of ASD children and their caregivers compared to pairs of TD children and their caregivers during a rocking chair experiment (Marsh et al., 2013). Overall, the findings of this study advance our current understanding of social synchrony in ASD by providing systematic evidence for the pervasive presence of such deficits in both high- and low-functioning children with ASD.
From a perception-action perspective, children with ASD demonstrate deficits in perception, action, and perception-action coupling. Specifically, children with ASD have poor social attention skills (Dawson et al., 2012; Marsh et al., 2013), poor fundamental movement control skills (Green et al., 2009; Fournier et al., 2010; Kaur et al., 2013), as well as poor visuomotor coordination skills (Barbeau et al., 2015; Nebel et al., 2016), all of which could contribute to poor bilateral coordination and social synchrony in ASD. Atypical functional segregation in the primary motor cortex and reduced corpus callosal size may contribute to poor bilateral coordination skills in ASD (Nebel et al., 2014; Casanova et al., 2009; Casanova et al., 2011). In terms of social synchrony, as discussed earlier, poor long-range connectivity between the frontal and occipital lobes could contribute to the visuomotor integration difficulties in ASD (Mostofsky et al., 2007; Courchesne, Campbell & Solso, 2010). Moreover, impaired activation in the “mirror neuron” system may also contribute to the difficulties faced by individuals in synchronizing their actions with their social partners (Oberman et al., 2005; Theoret et al., 2005; Dapretto et al., 2006, Williams et al., 2006).
4.4. Clinical Implications
Our study findings reinforce and add to the decades of research evidence on the presence of motor impairments across the autism spectrum. Both high- and low-functioning children with ASD in our study demonstrated consistent impairments in several aspects of motor function including gross and fine motor performance, praxis/imitation, bilateral coordination, and interpersonal synchrony skills compared to age-matched TD peers. Moreover, for almost all variables included in this study, we found that both HASD and LASD groups were equally impaired, with no significant differences between the two ASD groups. Despite irrefutable evidence to date documenting movement dysfunction, motor symptoms are largely under-recognized in ASD. Our study calls for the recognition of motor dysfunction as an integral part of the core difficulties faced by individuals with ASD. Furthermore, certain motor impairments such as dyspraxia must be considered a defining feature of ASD given the repeated evidence for a strong relationship with autism severity. Children with ASD demonstrate deficits in different aspects of movement control; therefore, from an assessment point-of-view, we recommend that clinicians evaluate all aspects of motor function in ASD using a comprehensive battery of standardized tests and behavioral tasks similar to the ones used in this study. Moreover, given the links between motor, social communication, and cognitive development (Bhat et al., 2011; Fitzpatrick et al., 2016; Nebel et al., 2016; Dziuk et al., 2007), our study emphasizes the need for inclusion of movement-based interventions into the standard of care for children with ASD with various motor axes as treatment targets. In addition to the existing focus on promoting fine motor skills important for academic achievement of children with ASD, clinicians should also encourage gross motor play, imitation-based activities, and cooperative games within structured and semi-structured contexts. Recently, few studies have documented positive effects on balance, coordination, and flexibility following engagement in motor activities in children with ASD (Hilton et al., 2014; MacDonald et al., 2012; Pan, Chu, Tsai, Sung, Huang, & Ma, 2017). Moreover, to ensure that children find training activities fun and engaging, clinicians could encourage children to participate in creative, alternative therapies such as whole-body yoga or music-based rhythmic activities that effectively promote motor and social communication skills in children with ASD (Srinivasan et al., 2016a &b, Srinivasan et al., 2015a & b; Kaur et al., 2013).
4.5. Limitations & Future Directions
Our study had a relatively small sample size; therefore, we did not have the power to systematically assess the effects of confounding variables such as age, gender, and socioeconomic status of children on their motor skills. Another limitation of the current study was the lack of IQ scores for the TD group. However, we confirmed that the TD children in this sample were at or above their grade level in schools and that their parents did not report any atypical birth/family history or any developmental delays/diagnoses during the screening interview. Moreover, we only have abbreviated IQ scores from our ASD sample with no further decomposition of cognitive abilities into verbal and non-verbal IQ scores. In the current study, we did not control for order effects of actions during the experimental motor coordination paradigm and instead asked all children to perform actions in a fixed order. Moreover, although we tried to comprehensively evaluate motor performance in ASD using a battery of standardized tests and experimental tasks, we acknowledge the lack of objective kinematic measures of motor performance in this study. We recommend that future studies use more robust objective tools such as kinematic analyses and dynamical methods to identify subtle impairments in movement control and quality in children with ASD. Further, given the limited current knowledge regarding the neural substrates underlying different movement parameters in children with ASD, future studies could employ neuroimaging tools such as functional Magnetic Resonance Imaging (fMRI) and functional Near-infrared spectroscopy (fNIRS) to identify impairments in structural, functional, and effective connectivity in individuals with ASD (Mostofsky et al., 2011; Bhat et al., 2017).
Conclusion
We compared motor performance using a comprehensive battery of tests and behavioral paradigms in three groups of children between 5 and 12 years of age: HASD, LASD, and TD. Regardless of IQ scores, children with ASD had significant impairments in gross and fine motor performance, praxis, bilateral coordination, and interpersonal synchrony compared to their TD peers. Moreover, on the majority of our motor variables both, HASD and LASD groups were equally impaired further ascertaining the pervasiveness of motor dysfunction across the entire autism spectrum. Overall gross and fine motor performance significantly correlated with intelligence but not with autism severity. Praxis errors and time to completion strongly correlated with autism severity but not intelligence. Lastly, our findings emphasize the need for motor assessments and interventions to promote both motor and social communication development in children with ASD and to include dyspraxia among the defining features of ASD.
Highlights.
Our study used a comprehensive set of assessments to evaluate motor and imitation skills of children with Autism Spectrum Disorder (ASD). We used standardized tests and experimental tasks to examine gross and fine motor skills, praxis, coordination, and interpersonal synchrony of children with ASD with low and high IQ scores. Overall, this study adds to the existing evidence for motor impairments in ASD by providing a comprehensive profiling of different axes of motor ability in children with ASD. Our study highlights the need for clinicians and families to advocate for motor evaluations and interventions in children with ASD.
Acknowledgments
We would like to thank all the children and families who participated in the study. We thank Dr. Deborah Fein and her students Lauren Haisley and Dasal Jashar at the University of Connecticut for completing the ADOS and IQ assessments for children with ASD. We thank Kerry Marsh at the University of Connecticut for sharing her ideas on the assessment of interpersonal synchrony, and graduate students, Isabel Park and Prajakta Nair at the University of Connecticut for helping with data collections and analyses. Lastly, we would like to thank the National Institute of Mental Health for the R21MH089441 and R33MH089441 awards, Autism Speaks for the pilot treatment award # 8137, and the UConn Foundation for their large grant awarded to the last author. The last author’s writing efforts were supported by a pilot grant (Project PI: Bhat) from an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number U54-GM104941 (Site PI: Binder-Macleod).
Glossary
- ASD
Autism Spectrum Disorder
- IQ
Intelligent Quotient
- LASD
children with ASD with low IQ
- HASD
children with ASD with high IQ
- TD
Typically Developing
- BOT-2
Bruininks-Oseretsky Test of Motor Proficiency- 2nd Edition
- SIPT-BMC
Bilateral Motor Coordination subtest of Sensory Integration and Praxis Tests
- CV
Coefficient of Variation
- IPS
Interpersonal Synchrony
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest regarding the presented work.
References
- Ament K, Mejia A, Buhlman R, Erklin S, Caffo B, Mostofsky S, Wodka E. Evidence for specificity of motor impairments in catching and balance in children with autism. Journal of Autism and Developmental disorders. 2015;45:742–751. doi: 10.1007/s10803-014-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Ayres J. Sensory Integration and Praxis Tests (SIPT) Los Angeles, CA: Western Psychological Services; 1988. [Google Scholar]
- Bastian AJ, Martin TA, Keating JG, Thach WT. Cerebellar ataxia: Abnormal control of interaction torques across multiple joints. Journal of Neurophysiology. 1996;76(1):492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- Barbeau EB, Meilleur AAS, Zeffiro TA, Mottron L. Comparing motor skills in autism spectrum individuals with and without speech delay. Autism Research. 2015;8(6):682–693. doi: 10.1002/aur.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AB, Wetherby AM, Chambers NW. Brief report: Repetitive behaviors in young children with autism spectrum disorder and developmentally similar peers: A follow up to Watt et al.(2008) Journal of autism and developmental disorders. 2012;42(9):2006–2012. doi: 10.1007/s10803-011-1434-3. [DOI] [PubMed] [Google Scholar]
- Bauman ML. Brief report: neuroanatomic observations of the brain in pervasive developmental disorders. Journal of autism and developmental disorders. 1996;26(2):199–203. doi: 10.1007/BF02172012. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: A review and future directions. International Journal of Developmental Neuroscience. 2005;23(2–3):183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Becker KA. History of the Stanford-Binet intelligence scales: Content and psychometrics. Fifth. Itasca, IL: Riverside Publishing; 2003. [Google Scholar]
- Bedford R, Pickles A, Lord C. Early gross motor skills predict the subsequent development of language in children with autism spectrum disorder. Autism Research. 2016 doi: 10.1002/aur.1587. In press. http://dx.doi.org/10.1002/aur.1587. [DOI] [PMC free article] [PubMed]
- Berkeley SL, Zittel LL, Pitney LV, Nichols SE. Locomotor and object control skills of children diagnosed with autism. Adapted Physical Activity Quarterly. 2001;18(4):405–416. [Google Scholar]
- Bhat AN, Landa RJ, Galloway JC. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Physical Therapy. 2011;91:1116–1129. doi: 10.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, Landa RJ. Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behavior and Development. 2012;35(4):838–846. doi: 10.1016/j.infbeh.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN, Srinivasan S, Woxholdt C, Shield A. Differences in praxis performance and receptive language during fingerspelling between deaf children with and without Autism Spectrum Disorder. Autism. 2016:1–12. doi: 10.1177/1362361316672179. [DOI] [PubMed] [Google Scholar]
- Bhat AN, Hoffman MH, Trost SL, Culotta ML, Eilbott J, Tsuzuki D, Pelphrey KA. Cortical activation during action observation, action execution, and interpersonal synchrony in adults: A functional near-infrared spectroscopy (fNIRS) study. Frontiers in Human Neuroscience. 2017;11:431. doi: 10.3389/fnhum.2017.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscaldi M, Rauh R, Irion L, Jung NH, Mall V, Fleischhaker C, Klein C. Deficits in motor abilities and developmental fractionation of imitation performance in high-functioning autism spectrum disorders. European child & adolescent psychiatry. 2014;23(7):599–610. doi: 10.1007/s00787-013-0475-x. [DOI] [PubMed] [Google Scholar]
- Boas D, Elwell C, Ferrari M, Taga G. Twenty years of functional near-infrared spectroscopy (fNIRS): introduction for the special issue. Neuroimage. 2014;85:1–5. doi: 10.1016/j.neuroimage.2013.11.033. [DOI] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Prinz W. Movement observation affects movement execution in a simple response task. Acta psychologica. 2001;106(1):3–22. doi: 10.1016/s0001-6918(00)00024-x. [DOI] [PubMed] [Google Scholar]
- Bruininks RH, Bruininks BD. Bruininks-Oseretsky Test of Motor Proficiency. 2nd. Minneapolis, MN: NCS Pearson; 2005. [Google Scholar]
- Bryson SE, Smith IM. Epidemiology of autism: Prevalence, associated characteristics, and implications for research and service delivery. Developmental Disabilities Research Reviews. 1998;4(2):97–103. [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, Rizzolatti G. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42(2):323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- Carmo JC, Rumiati RI, Siugzdaite R, Brambilla P. Preserved imitation of known gestures in children with high-functioning autism. ISRN Neurology. 2013:1–8. doi: 10.1155/2013/751516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case-Smith J, Miller H. Occupational therapy with children with pervasive developmental disorders. American Journal of Occupational Therapy. 1999;53(5):506–513. doi: 10.5014/ajot.53.5.506. [DOI] [PubMed] [Google Scholar]
- Casanova MF, El-Baz A, Mott M, Mannheim G, Hassan H, Fahmi R, Farag A. Reduced gyral window and corpus callosum size in autism: possible macroscopic correlates of a minicolumnopathy. Journal of autism and developmental disorders. 2009;39(5):751–764. doi: 10.1007/s10803-008-0681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, El-Baz A, Elnakib A, Switala AE, Williams EL, Williams DL, Conturo TE. Quantitative analysis of the shape of the corpus callosum in patients with autism and comparison individuals. Autism. 2011;15(2):223–238. doi: 10.1177/1362361310386506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiello U. Understanding other people’s actions: intention and attention. Journal of Experimental Psychology: Human Perception and Performance. 2003;29(2):416. doi: 10.1037/0096-1523.29.2.416. [DOI] [PubMed] [Google Scholar]
- Charman T, Pickles A, Simonoff E, Chandler S, Loucas T, Baird G. IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP) Psychological medicine. 2011;41(03):619–627. doi: 10.1017/S0033291710000991. [DOI] [PubMed] [Google Scholar]
- Christensen DL, Baio J, van Naarden Braun K, Bilder D, Charles J, Constantino JN, Yeargin-Allsopp M. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. Surveillance Summaries. 2016;65(3):1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukoskie L, Townsend J, Westerfield M. Motor skill in autism spectrum disorders: A subcortical view. International review of neurobiology. 2013;113(7):207–249. doi: 10.1016/B978-0-12-418700-9.00007-1. [DOI] [PubMed] [Google Scholar]
- Colombi C, Liebal K, Tomasello M, Young G, Warneken F, Rogers SJ. Examining correlates of cooperation in autism Imitation, joint attention, and understanding intentions. Autism. 2009;13(2):143–163. doi: 10.1177/1362361308098514. [DOI] [PubMed] [Google Scholar]
- Courchesne E. New evidence of cerebellar and brainstem hypoplasia in autistic infants, children and adolescents: the MR imaging study by Hashimoto and colleagues. Journal of autism and developmental disorders. 1995;25(1):19–22. doi: 10.1007/BF02178164. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Current opinion in neurobiology. 1997;7(2):269–278. doi: 10.1016/s0959-4388(97)80016-5. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Research. 2011;1380:138–145. doi: 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Lincoln AJ. Unusual brain growth patterns in early life in patients with autistic disorder an MRI study. Neurology. 2001;57(2):245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature neuroscience. 2006;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Bernier R, Ring RH. Social attention: a possible early indicator of efficacy in autism clinical trials. Journal of Neurodevelopmental Disorders. 2012;4(1):11. doi: 10.1186/1866-1955-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Sommerville JA. Shared representations between self and other: a social cognitive neuroscience view. Trends in cognitive sciences. 2003;7(12):527–533. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Dewey D. Error analysis of limb and orofacial praxis in children with developmental motor deficits. Brain and Cognition. 1993;23(2):203–221. doi: 10.1006/brcg.1993.1055. [DOI] [PubMed] [Google Scholar]
- Dewey D, Cantell M, Crawford SG. Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society. 2007;13(02):246–256. doi: 10.1017/S1355617707070270. [DOI] [PubMed] [Google Scholar]
- Dowell LR, Mahone EM, Mostofsky SH. Associations of postural knowledge and basic motor skill with dyspraxia in autism: implication for abnormalities in distributed connectivity and motor learning. Neuropsychology. 2009;23(5):563. doi: 10.1037/a0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey R, Rapport MJK. Motor activity in children with autism: a review of current literature. Pediatric Physical Therapy. 2012;24(1):2–20. doi: 10.1097/PEP.0b013e31823db95f. [DOI] [PubMed] [Google Scholar]
- Ewen JB, Lakshmanan BM, Hallett M, Mostofsky SH, Crone NE, Korzeniewska A. Dynamics of functional and effective connectivity within human cortical motor control networks. Clinical Neurophysiology. 2015;126(5):987–996. doi: 10.1016/j.clinph.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick P, Diorio R, Richardson MJ, Schmidt RC. Dynamical methods for evaluating the time-dependent unfolding of social coordination in children with autism. Frontiers in integrative neuroscience. 2013;7 doi: 10.3389/fnint.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick P, Frazier JA, Cochran DM, Mitchell T, Coleman C, Schmidt RC. Impairments of social motor synchrony evident in autism spectrum disorder. Frontiers in Psychology. 2016;7 doi: 10.3389/fpsyg.2016.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick P, Romero V, Amaral JL, Duncan A, Barnard H, Richardson MJ, Schmidt RC. Social Motor Synchronization: Insights for Understanding Social Behavior in Autism. Journal of Autism and Developmental Disorders. 2017:1–16. doi: 10.1007/s10803-017-3124-2. [DOI] [PubMed] [Google Scholar]
- Flanagan JE, Landa R, Bhat A, Bauman M. Head lag in infants at risk for autism: a preliminary study. American Journal of Occupational Therapy. 2012;66(5):577–585. doi: 10.5014/ajot.2012.004192. [DOI] [PubMed] [Google Scholar]
- Fleury A, Kushki A, Tanel N, Anagnostou E, Chau T. Statistical persistence and timing characteristics of repetitive circle drawing in children with ASD. Developmental neurorehabilitation. 2013;16(4):245–254. doi: 10.3109/17518423.2012.758184. [DOI] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. Journal of autism and developmental disorders. 2010;40(10):1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds–a biological basis. Science. 1999;286(5445):1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Freitag CM, Kleser C, Schneider M, von Gontard A. Quantitative assessment of neuromotor function in adolescents with high functioning autism and Asperger syndrome. Journal of autism and developmental disorders. 2007;37(5):948–959. doi: 10.1007/s10803-006-0235-6. [DOI] [PubMed] [Google Scholar]
- Fuentes CT, Mostofsky SH, Bastian AJ. Children with autism show specific handwriting impairments. Neurology. 2009;73(19):1532–1537. doi: 10.1212/WNL.0b013e3181c0d48c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V. Before and below ‘theory of mind’: embodied simulation and the neural correlates of social cognition. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2007;362(1480):659–669. doi: 10.1098/rstb.2006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V. Mirror neurons and the social nature of language: The neural exploitation hypothesis. Social neuroscience. 2008;3(3–4):317–333. doi: 10.1080/17470910701563608. [DOI] [PubMed] [Google Scholar]
- Gallese V, Eagle MN, Migone P. Intentional attunement: Mirror neurons and the neural underpinnings of interpersonal relations. Journal of the American psychoanalytic Association. 2007;55(1):131–175. doi: 10.1177/00030651070550010601. [DOI] [PubMed] [Google Scholar]
- Getchell N. Age and task-related differences in timing stability, consistency, and natural frequency of children’s rhythmic, motor coordination. Developmental psychobiology. 2006;48(8):675–685. doi: 10.1002/dev.20186. [DOI] [PubMed] [Google Scholar]
- Getchell N. Developmental aspects of perception-action coupling in multi-limb coordination: rhythmic sensorimotor synchronization. Motor control. 2007;11(1):1. [PubMed] [Google Scholar]
- Getchell N, Whitall J. How do children coordinate simultaneous upper and lower extremity tasks? The development of dual motor task coordination. Journal of Experimental Child Psychology. 2003;85(2):120–140. doi: 10.1016/s0022-0965(03)00059-6. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin M, Butler E. Clumsiness in autism and Asperger syndrome: A further report. Journal of Intellectual Disability Research. 1998;42(1):43–48. doi: 10.1046/j.1365-2788.1998.00065.x. [DOI] [PubMed] [Google Scholar]
- Gizzonio V, Avanzini P, Campi C, Orivoli S, Piccolo B, Cantalupo G, Fabbri-Destro M. Failure in pantomime action execution correlates with the severity of social behavior deficits in children with autism: a praxis study. Journal of autism and developmental disorders. 2015;45(10):3085–3097. doi: 10.1007/s10803-015-2461-2. [DOI] [PubMed] [Google Scholar]
- Glazebrook C, Gonzalez D, Hansen S, Elliott D. The role of vision for online control of manual aiming movements in persons with autism spectrum disorders. Autism. 2009;13(4):411–433. doi: 10.1177/1362361309105659. [DOI] [PubMed] [Google Scholar]
- Green D, Baird G, Barnett AL, Henderson L, Huber J, Henderson SE. The severity and nature of motor impairment in Asperger’s syndrome: a comparison with specific developmental disorder of motor function. Journal of child psychology and psychiatry. 2002;43(5):655–668. doi: 10.1111/1469-7610.00054. [DOI] [PubMed] [Google Scholar]
- Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, Baird G. Impairment in movement skills of children with autistic spectrum disorders. Developmental Medicine & Child Neurology. 2009;51(4):311–316. doi: 10.1111/j.1469-8749.2008.03242.x. [DOI] [PubMed] [Google Scholar]
- Gowen E, Stanley J, Miall RC. Movement interference in autism-spectrum disorder. Neuropsychologia. 2008;46(4):1060–1068. doi: 10.1016/j.neuropsychologia.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadders-Algra M. Reduced variability in motor behaviour: an indicator of impaired cerebral connectivity? Early human development. 2008;84(12):787–789. doi: 10.1016/j.earlhumdev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Hallett M, Lebiedowska MK, Thomas SL, Stanhope SJ, Denckla MB, Rumsey J. Locomotion of autistic adults. Archives of Neurology. 1993;50(12):1304–1308. doi: 10.1001/archneur.1993.00540120019007. [DOI] [PubMed] [Google Scholar]
- Ham HS, Bartolo A, Corley M, Rajendran G, Szabo A, Swanson S. Exploring the relationship between gestural recognition and imitation: Evidence of dyspraxia in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2011;41(1):1–12. doi: 10.1007/s10803-010-1011-1. [DOI] [PubMed] [Google Scholar]
- Ham HS, Bartolo A, Corley M, Swanson S, Rajendran G. Case report: Selective deficit in the production of intransitive gestures in an individual with autism. Cortex. 2010;46(3):407–409. doi: 10.1016/j.cortex.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Hamilton AFDC, Grafton ST. Goal representation in human anterior intraparietal sulcus. Journal of Neuroscience. 2006;26(4):1133–1137. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. Journal of autism and developmental disorders. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Haswell CC, Izawa J, Dowell LR, Mostofsky SH, Shadmehr R. Representation of internal models of action in the autistic brain. Nature neuroscience. 2009;12(8):970–972. doi: 10.1038/nn.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton C, Graver K, LaVesser P. Relationship between social competence and sensory processing in children with high functioning autism spectrum disorders. Research in Autism Spectrum Disorders. 2007;1(2):164–173. [Google Scholar]
- Hilton CL, Zhang Y, Whilte MR, Klohr CL, Constantino J. Motor impairment in sibling pairs concordant and discordant for autism spectrum disorders. Autism. 2012;16(4):430–441. doi: 10.1177/1362361311423018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton CL, Cumpata K, Klohr C, Gaetke S, Artner A, Johnson H, Dobbs S. Effects of exergaming on executive function and motor skills in children with autism spectrum disorder: a pilot study. American Journal of Occupational Therapy. 2014;68(1):57–65. doi: 10.5014/ajot.2014.008664. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four factor index of social status. New Haven, CT: Yale University; 1975. Unpublished Manuscript. [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nature Reviews Neuroscience. 2006;7(12):942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one’s own mirror neuron system. PLoS biology. 2005;3(3):e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. science. 1999;286(5449):2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Isenhower RW, Marsh KL, Richardson MJ, Helt M, Schmidt RC, Fein D. Rhythmic bimanual coordination is impaired in young children with autism spectrum disorder. Research in Autism Spectrum Disorders. 2012;6(1):25–31. [Google Scholar]
- Ivry RB, Spencer RM. Evaluating the role of the cerebellum in temporal processing: beware of the null hypothesis. Brain. 2004;127(8):e13–e13. doi: 10.1093/brain/awh226. [DOI] [PubMed] [Google Scholar]
- Ivry R. Cerebellar timing systems. International review of neurobiology. 1997:555–573. [PubMed] [Google Scholar]
- Jackson PL, Decety J. Motor cognition: A new paradigm to study self–other interactions. Current opinion in neurobiology. 2004;14(2):259–263. doi: 10.1016/j.conb.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH. Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. Journal of autism and developmental disorders. 2006;36(5):613–621. doi: 10.1007/s10803-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Jeste SS. The neurology of autism spectrum disorders. Current opinion in neurology. 2011;24(2):132. doi: 10.1097/WCO.0b013e3283446450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones V, Prior M. Motor imitation abilities and neurological signs in autistic children. Journal of autism and developmental disorders. 1985;15(1):37–46. doi: 10.1007/BF01837897. [DOI] [PubMed] [Google Scholar]
- Just M, Keller T, Malave V, Kana R, Varma S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience and Biobehavioral Reviews. 2012;36(4):1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Gifford T, Marsh KL, Bhat A. Effect of robot-child interactions on bilateral coordination skills of typically developing children and a child with autism spectrum disorder: A preliminary study. Journal of Motor Learning and Development. 2013;1(2):31–37. [Google Scholar]
- Kaur M. Doctoral dissertation. University of Delaware; 2016. Creative yoga intervention for children with Autism Spectrum Disorder. [Google Scholar]
- Kinsbourne M, Helt M. Entrainment, mimicry, and interpersonal synchrony. The neuropsychology of autism. 2011:339–365. [Google Scholar]
- Kushki A, Chau T, Anagnostou E. Handwriting difficulties in children with autism spectrum disorders: A scoping review. Journal of autism and developmental disorders. 2011;41(12):1706–1716. doi: 10.1007/s10803-011-1206-0. [DOI] [PubMed] [Google Scholar]
- Koehne S, Hatri A, Cacioppo JT, Dziobek I. Perceived interpersonal synchrony increases empathy: insights from autism spectrum disorder. Cognition. 2016;146:8–15. doi: 10.1016/j.cognition.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of general psychiatry. 2007;64(7):853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Leary MR, Hill DA. Moving on: autism and movement disturbance. Mental retardation. 1996;34(1):39. [PubMed] [Google Scholar]
- Liu T, Breslin CM. Fine and gross motor performance of the MABC-2 by children with autism spectrum disorder and typically developing children. Research in Autism Spectrum Disorders. 2013;7(10):1244–1249. [Google Scholar]
- Lloyd M, MacDonald M, Lord C. Motor skills of toddlers with autism spectrum disorders. Autism. 2013;17(2):133–146. doi: 10.1177/1362361311402230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism diagnostic observation schedule, (ADOS-2) manual (part 1): modules 1–4. second. Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- MacDonald M, Esposito P, Hauck J, Jeong I, Hornyak J, Argento A, Ulrich DA. Bicycle training for youth with Down syndrome and autism spectrum disorders. Focus on Autism and Other Developmental Disabilities. 2012;27(1):12–21. [Google Scholar]
- MacNeil LK, Mostofsky SH. Specificity of dyspraxia in children with autism. Neuropsychology. 2012;26(2):165. doi: 10.1037/a0026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan R, Dirlikov B, Crocetti D, Mostofsky SH. Motor circuit anatomy in children with autism spectrum disorder with or without attention deficit hyperactivity disorder. Autism Research. 2016;9(1):67–81. doi: 10.1002/aur.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari M, Castiello U, Marks D, Marraffa C, Prior M. The reach–to–grasp movement in children with autism spectrum disorder. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2003;358(1430):393–403. doi: 10.1098/rstb.2002.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau J, Cochin S, Magne R, Barthelemy C. Impaired cortical activation in autistic children: is the mirror neuron system involved? International journal of psychophysiology. 2008;68(1):35–40. doi: 10.1016/j.ijpsycho.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Manjiviona J, Prior M. Comparison of Asperger syndrome and high-functioning autistic children on a test of motor impairment. Journal of autism and developmental disorders. 1995;25(1):23–39. doi: 10.1007/BF02178165. [DOI] [PubMed] [Google Scholar]
- Marsh KL, Isenhower RW, Richardson MJ, Helt M, Verbalis AD, Schmidt RC, Fein D. Autism and social disconnection in interpersonal rocking. Frontiers in integrative neuroscience. 2013;7 doi: 10.3389/fnint.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL. Ability profiles in children with autism: Influence of age and IQ. Autism. 2003;7(1):65–80. doi: 10.1177/1362361303007001006. [DOI] [PubMed] [Google Scholar]
- McPhillips M, Finlay J, Bejerot S, Hanley M. Motor Deficits in Children with Autism Spectrum Disorder: A Cross-Syndrome Study. Autism Research. 2014;7(6):664–676. doi: 10.1002/aur.1408. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: Profile of a complex information processing disorder. Journal of the International Neuropsychological Society. 1997;3(04):303–316. [PubMed] [Google Scholar]
- Minshew NJ, Sung K, Jones BL, Furman JM. Underdevelopment of the postural control system in autism. Neurology. 2004;63(11):2056–2061. doi: 10.1212/01.wnl.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- Miyahara M, Tsujii M, Hori M, Nakanishi K, Kageyama H, Sugiyama T. Brief report: motor incoordination in children with Asperger syndrome and learning disabilities. Journal of autism and developmental disorders. 1997;27(5):595–603. doi: 10.1023/a:1025834211548. [DOI] [PubMed] [Google Scholar]
- Montes G, Halterman JS. Association of childhood autism spectrum disorders and loss of family income. Pediatrics. 2008;121(4):821–826. doi: 10.1542/peds.2007-1594. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, Denckla MB. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. Journal of the International Neuropsychological Society. 2006;12(03):314–326. doi: 10.1017/s1355617706060437. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Newschaffer CJ, Denckla MB. Overflow movements predict impaired response inhibition in children with ADHD. Perceptual and motor skills. 2003;97(3 suppl):1315–1331. doi: 10.2466/pms.2003.97.3f.1315. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132(9):2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Ewen JB. Altered connectivity and action model formation in autism is autism. The Neuroscientist. 2011;17(4):437–448. doi: 10.1177/1073858410392381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayate A, Tonge BJ, Bradshaw JL, McGinley JL, Iansek R, Rinehart NJ. Differentiation of high-functioning autism and Asperger’s disorder based on neuromotor behaviour. Journal of autism and developmental disorders. 2012;42(5):707–717. doi: 10.1007/s10803-011-1299-5. [DOI] [PubMed] [Google Scholar]
- Nebel MB, Eloyan A, Nettles CA, Sweeney KL, Ament K, Ward RE, Mostofsky SH. Intrinsic visual-motor synchrony correlates with social deficits in autism. Biological psychiatry. 2016;79(8):633–641. doi: 10.1016/j.biopsych.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel MB, Joel SE, Muschelli J, Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Disruption of functional organization within the primary motor cortex in children with autism. Human brain mapping. 2014;35(2):567–580. doi: 10.1002/hbm.22188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noterdaeme M, Mildenberger K, Minow F, Amorosa H. Evaluation of neuromotor deficits in children with autism and children with a specific speech and language disorder. European child & adolescent psychiatry. 2002;11(5):219–225. doi: 10.1007/s00787-002-0285-z. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cognitive brain research. 2005;24(2):190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Ramachandran VS. The simulating social mind: the role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychological bulletin. 2007;133(2):310. doi: 10.1037/0033-2909.133.2.310. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Goldring S, Greiss-Hess L, Herrera AM, Steele J, Rogers SJ. Gross motor development, movement abnormalities, and early identification of autism. Journal of autism and developmental disorders. 2008;38(4):644–656. doi: 10.1007/s10803-007-0430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CY, Chu CH, Tsai CL, Sung MC, Huang CY, Ma WY. The impacts of physical activity intervention on physical and cognitive outcomes in children with autism spectrum disorder. Autism. 2017;21(2):190–202. doi: 10.1177/1362361316633562. [DOI] [PubMed] [Google Scholar]
- Piek JP, Dyck MJ. Sensory-motor deficits in children with developmental coordination disorder, attention deficit hyperactivity disorder and autistic disorder. Human movement science. 2004;23(3):475–488. doi: 10.1016/j.humov.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Provost B, Heimerl S, Lopez BR. Levels of gross and fine motor development in young children with autism spectrum disorder. Physical & Occupational Therapy in Pediatrics. 2007;27(3):21–36. [PubMed] [Google Scholar]
- Provost B, Lopez BR, Heimerl S. A comparison of motor delays in young children: autism spectrum disorder, developmental delay, and developmental concerns. Journal of autism and developmental disorders. 2007;37(2):321–328. doi: 10.1007/s10803-006-0170-6. [DOI] [PubMed] [Google Scholar]
- Rinehart NJ, Tonge BJ, Iansek R, McGinley J, Brereton AV, Enticott PG, Bradshaw JL. Gait function in newly diagnosed children with autism: cerebellar and basal ganglia related motor disorder. Developmental Medicine & Child Neurology. 2006;48(10):819–824. doi: 10.1017/S0012162206001769. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nature reviews neuroscience. 2001;2(9):661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Bennetto L, McEvoy R, Pennington BF. Imitation and pantomime in high-functioning adolescents with autism spectrum disorders. Child development. 1996;67(5):2060–2073. [PubMed] [Google Scholar]
- Sacrey LAR, Germani T, Bryson SE, Zwaigenbaum L. Reaching and grasping in autism spectrum disorder: a review of recent literature. Frontiers in neurology. 2014;5 doi: 10.3389/fneur.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salowitz NM, Eccarius P, Karst J, Carson A, Schohl K, Stevens S, Scheidt RA. Brief report: visuo-spatial guidance of movement during gesture imitation and mirror drawing in children with autism spectrum disorders. Journal of autism and developmental disorders. 2013;43(4):985–995. doi: 10.1007/s10803-012-1631-8. [DOI] [PubMed] [Google Scholar]
- Smith IM, Bryson SE. Imitation and action in autism: a critical review. Psychological bulletin. 1994;116(2):259–273. doi: 10.1037/0033-2909.116.2.259. [DOI] [PubMed] [Google Scholar]
- Srinivasan SM, Lynch KA, Bubela DJ, Gifford TD, Bhat AN. Effect of interactions between a child and a robot on the imitation and praxis performance of typically developing children and a child with autism: A preliminary study. Perceptual and motor skills. 2013;116(3):885–904. doi: 10.2466/15.10.PMS.116.3.885-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples KL, Reid G. Fundamental movement skills and autism spectrum disorders. Journal of autism and developmental disorders. 2010;40(2):209–217. doi: 10.1007/s10803-009-0854-9. [DOI] [PubMed] [Google Scholar]
- Srinivasan SM, Kaur M, Park IK, Gifford TD, Marsh KL, Bhat AN. The effects of rhythm and robotic interventions on the imitation/praxis, interpersonal synchrony, and motor performance of children with autism spectrum disorder (ASD): a pilot randomized controlled trial. Autism research and treatment. 2015 doi: 10.1155/2015/736516. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, Park IK, Neelly LB, Bhat AN. A comparison of the effects of rhythm and robotic interventions on repetitive behaviors and affective states of children with Autism Spectrum Disorder (ASD) Research in autism spectrum disorders. 2015;18:51–63. doi: 10.1016/j.rasd.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, Eigsti IM, Gifford T, Bhat AN. The effects of embodied rhythm and robotic interventions on the spontaneous and responsive verbal communication skills of children with Autism Spectrum Disorder (ASD): A further outcome of a pilot randomized controlled trial. Research in autism spectrum disorders. 2016;27:73–87. doi: 10.1016/j.rasd.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan SM, Eigsti IM, Neelly L, Bhat AN. The effects of embodied rhythm and robotic interventions on the spontaneous and responsive social attention patterns of children with autism spectrum disorder (ASD): A pilot randomized controlled trial. Research in autism spectrum disorders. 2016;27:54–72. doi: 10.1016/j.rasd.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutera S, Pandey J, Esser EL, Rosenthal MA, Wilson LB, Barton M, Fein D. Predictors of optimal outcome in toddlers diagnosed with autism spectrum disorders. Journal of autism and developmental disorders. 2007;37(1):98–107. doi: 10.1007/s10803-006-0340-6. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Teitelbaum O, Nye J, Fryman J, Maurer RG. Movement analysis in infancy may be useful for early diagnosis of autism. Proceedings of the National Academy of Sciences. 1998;95(23):13982–13987. doi: 10.1073/pnas.95.23.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum O, Benton T, Shah PK, Prince A, Kelly JL, Teitelbaum P. Eshkol–Wachman movement notation in diagnosis: The early detection of Asperger’s syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(32):11909–11914. doi: 10.1073/pnas.0403919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théoret H, Halligan E, Kobayashi M, Fregni F, Tager-Flusberg H, Pascual-Leone A. Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Current Biology. 2005;15(3):R84–R85. doi: 10.1016/j.cub.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Turner K, Frost L, Linsenbardt D, McIlroy J, Müller R. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behavioral and Brain Functions. 2006;2(34) doi: 10.1186/1744-9081-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanvuchelen M, Roeyers H, De Weerdt W. Nature of motor imitation problems in school-aged boys with autism A motor or a cognitive problem? Autism. 2007;11(3):225–240. doi: 10.1177/1362361307076846. [DOI] [PubMed] [Google Scholar]
- Vilensky JA, Damasio AR, Maurer RG. Gait disturbances in patients with autistic behavior: a preliminary study. Archives of neurology. 1981;38(10):646–649. doi: 10.1001/archneur.1981.00510100074013. [DOI] [PubMed] [Google Scholar]
- Von Hofsten C. Action in development. Developmental science. 2007;10(1):54–60. doi: 10.1111/j.1467-7687.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- Wapner S, Cirillo L. Imitation of a model’s hand movements: Age changes in transposition of left-right relations. Child development. 1968;39(3):887–894. [PubMed] [Google Scholar]
- Wheaton LA, Hallett M. Ideomotor apraxia: a review. Journal of the neurological sciences. 2007;260(1):1–10. doi: 10.1016/j.jns.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Whyatt CP, Craig CM. Motor skills in children aged 7–10 years, diagnosed with autism spectrum disorder. Journal of autism and developmental disorders. 2012;42(9):1799–1809. doi: 10.1007/s10803-011-1421-8. [DOI] [PubMed] [Google Scholar]
- Whyatt C, Craig C. Sensory-motor problems in Autism. Frontiers in integrative neuroscience. 2013;7 doi: 10.3389/fnint.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt C, Craig CM. Interceptive skills in children aged 9–11 years, diagnosed with Autism Spectrum Disorder. Research in Autism Spectrum Disorders. 2013;7(5):613–623. doi: 10.1007/s10803-011-1421-8. [DOI] [PubMed] [Google Scholar]
- Williams JH, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and ‘mirror neuron’functioning in autistic spectrum disorder. Neuropsychologia. 2006;44(4):610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]


