Abstract
Comparative effectiveness research trials in real-world settings may require participants to choose between preferred intervention options. A randomized clinical trial with parallel experimental and control arms is straightforward and regarded as a gold standard design, but by design it forces and anticipates the participants to comply with a randomly assigned intervention regardless of their preference. Therefore, the randomized clinical trial may impose impractical limitations when planning comparative effectiveness research trials. To accommodate participants’ preference if they are expressed, and to maintain randomization, we propose an alternative design that allows participants’ preference after randomization, which we call a “preference option randomized design (PORD)”. In contrast to other preference designs, which ask whether or not participants consent to the assigned intervention after randomization, the crucial feature of preference option randomized design is its unique informed consent process before randomization. Specifically, the preference option randomized design consent process informs participants that they can opt out and switch to the other intervention only if after randomization they actively express the desire to do so. Participants who do not independently express explicit alternate preference or assent to the randomly assigned intervention are considered to not have an alternate preference. In sum, preference option randomized design intends to maximize retention, minimize possibility of forced assignment for any participants, and to maintain randomization by allowing participants with no or equal preference to represent random assignments. This design scheme enables to define five effects that are interconnected with each other through common design parameters—comparative, preference, selection, intent-to-treat, and overall/as-treated—to collectively guide decision making between interventions. Statistical power functions for testing all these effects are derived, and simulations verified the validity of the power functions under normal and binomial distributions.
Keywords: Preference, comparative effectiveness research, power, decision making, randomization
1. Introduction
Translation to practice phase 3 translational, or T3, research is often conducted as comparative effectiveness research (CER) in real-world settings.1,2 CER studies usually involve treatments or interventions with proven efficacy in controlled research settings3 for which double-blind parallel-group randomized designs are a gold standard experimental design for establishing treatment efficacy. However, it may be difficult to carry out such rigorous controlled designs in real-world settings, where treatments are often unmasked and participants have strong preferences.4,5 The traditional parallel randomized design forces a randomly assigned intervention regardless of participants’ preference, and anticipates that the participants will comply with the assigned intervention. Therefore, this design may impose impractical limitations when planning CER trials where such compliance may not be realistic. Herein, we consider CER trial designs that primarily aims to test two interventions, denoted in general by A and B. Two examples of such CER trials that compare two efficacious interventions for delivering a medicine or a program in real-world settings are described. In these examples, the effectiveness of the medicine or program on clinical outcome depends on successful delivery for patients’ intake or uptake, thus accommodation of patient preference may be important.
One example is a CER trial that aims to test the effectiveness of two delivery models of a very powerful medication for treating hepatitis C virus (HCV) infection with >90% sustained viral response (SVR) rate.6 The treatment requires a three-month daily regimen to result in SVR, making patient adherence a critical issue.7 To this end, directly observed therapy (DOT)8 has been shown effective to both increase adherence to antiviral medications and improve viral outcomes in HIV-infected patients receiving medicines at a clinic.9,10 It is often very challenging to administer a classic DOT at a clinic in real-world settings where HCV medications may be taken multiple times each day, and when patients do not attend the clinic daily. Therefore, DOT has often been modified (mDOT) with a less intensive mode to meet such challenges.11 Another alternative way of delivering and monitoring the intake of the medicine to increase adherence is utilization of patient navigators (PN) who coordinate treatments, assist the participants to overcome logistical barriers, and provide health education and psychosocial support.12 To compare effectiveness of mDOT vs. PN on the SVR endpoint outcome, the CER may need to allow participants to choose their preferred delivery method.
Another example is a CER trial evaluating different ways to deliver the Diabetes Prevention Program (DPP). DPP has been shown to be effective in reducing the incidence of diabetes among high-risk individuals; when compared to placebo and metformin, the intensive lifestyle modification intervention reduced the incidence by 58% and 39%, respectively.13 The DPP trial informed the creation of the National DPP program, implemented by the Centers for Disease Control and Prevention (CDC). The CDC’s National DPP includes a 16-week core curriculum with follow-up maintenance classes thereafter. Evaluation criteria include reporting of attendance and reporting of physical activity, and weight loss (https://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf). Maintaining recognition from the CDC requires that programs observe an average weight loss greater than 5%. DPP has been implemented in community, faith-based, workplace and healthcare-settings.14,15 Similar to the DOT case, it is challenging for many participants to attend enough sessions to successfully reduce their body weight and subsequent risk of developing diabetes.16 There are a number of virtual DPP (vDPP) apps, implemented either online using a desktop computer or with a smart-phone based app17 that may help increase engagement. Therefore, allowing participants’ preference, a CER trial might want to compare the effectiveness of DDP vs. vDPP on attendance, weight change, and downstream outcomes such as glycated hemoglobin (HbA1C) reduction.
There have been a variety of designs that accommodate participants’ preference.18–20 For example, the Zelen’s double consent randomized trial design21,22 involves two stages to accommodate preference. First, it randomizes participants, and then it seeks participants’ consent to preferred or randomly assigned treatments by asking whether or not participants consent to the assigned intervention. If they do not, the design allows them to switch treatment. For this reason, Zelen’s design might raise ethical concerns because participants are not informed of treatments before randomization.23 Apart from this concern, we suspect that it is likely that inquiring directly about preference after randomization might cause unintended crossover perhaps influenced by the query itself.
Wennberg et al.’s24 or Rucker’s25 preference clinical trial design, on the other hand, also involves two or more stages but it seeks consent before initial randomization. First, consented participants are randomly assigned to a randomization arm or a choice arm. Then, participants assigned to the randomization arm will be randomly assigned to a treatment whereas those assigned to the choice arm will either choose their preferred treatment or, if they have no preference, further be randomized. Although this type of design has been widely implemented in diverse research areas,20,26 the participants in the randomization arm should comply with the randomly assigned treatments and, are neither queried as to nor allowed to switch over to their preferred treatments. In addition, variations of the two-stage designs such as fully or partially randomized preference design have also been adopted.20,27
To the best of our knowledge, however, there is no design which can collectively encompass all desirable features that we believe are pertinent to the CER paradigm that: (1) minimize forced assignment and influenced switch; (2) maintain and compare randomized groups; (3) administer single randomization; (4) accommodate preference; (5) and thus maximize retention, adherence or compliance. To this end, building upon existing preference designs,20,28 we propose a novel simpler study design, referred to herein as the “preference option randomized design” (PORD), which allows participants to switch to their preferred interventions only if they actively express preference after randomization. PORD design components including that process are introduced in Section 2. Under this design, the following diverse effects that are inter-connected through common design parameters can be defined, estimated, and tested: comparative effect; preference effect; selection effect; intent-to-treat (ITT) effect; overall/as-treated (AT) effect. Definitions of all of these effects are detailed in Section 3, and their sample estimates are derived in Section 4. Statistical power function for testing each effect is derived and verified by simulation examinations in Section 5. Followed is discussion in Section 6.
2. PORD design components
2.1. Study population
We do not attempt to make any inferences about the population that would not participate in trials even though strong preferences, expressed or not, for certain interventions might be present among this group. We do, however, attempt to make inferences about the population who would participate in trials that meet their needs and accommodate their preferences with appropriate interventions. We assume there are three mutually exclusive and exhaustive classes of participants in this population. They are participants who: (1) prefer and choose intervention A whichever intervention is assigned; (2) prefer and choose intervention B whichever intervention is assigned; or (3) prefer A or B equally or with no particular preference by taking whichever intervention is assigned. All of these three classes are assumed to have a sizable number of participants.
2.2. Informed consent process
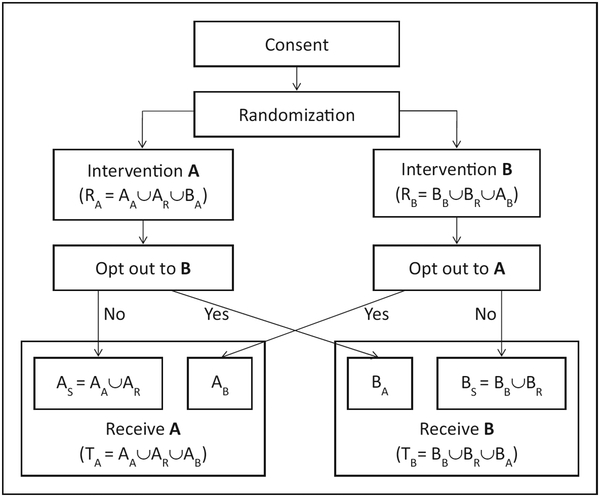
A key component of a PORD is its informing participants about their preference options during the consent process prior to randomization (Figure 1). As usual during a consent process, intervention properties are fully explained to the participants. However, the unique features of the PORD consent process are that participants will be informed and instructed that: (1) they will have the opportunity to express their intervention preference after randomization. Participants will be informed to clearly express their preference after randomization only if their randomly assigned intervention is not their preferred intervention; and (2) if they do not express an intervention preference after randomization they will be assumed to accept randomization.
Figure 1.
Illustration diagram of preference option randomized design.
Participants will not be asked about their preferred interventions. Rather, they will have to volunteer their preferences independently. This feature intends to prevent participants from influenced or unintended crossover if they are prompted to consider their preference when asked post-randomization. It also intends to avoid participant forced assignment and to keep participants who would refuse to take a randomly assigned intervention from dropping out because it is not their preferred intervention. To ensure that a participant fully understands the contents and intents of the interventions, the random assignment and the opt-out process, research staff may ask the participant to explain back those crucial elements. In sum, all of these instructions during the PORD informed consent process are intended to maximize retention, minimize possibility of forced assignment or to influence a switch among participants with no preference who otherwise represent those who comply with random assignment.
2.3. Randomization
Once it is clear that participants fully understand the interventions and the preference- or choice-based opt-out process, they will be randomly assigned. There will be only a single randomization process (Figure 1). The specific randomization strategy depends on the study settings. For example, unbalanced allocations might occur due to potentially non-symmetric preferences and might require implementation of adaptive randomization such as urn randomization.29 Discussion of various strategies is nevertheless beyond the scope of the present study.
2.4. Opt-out process
PORD will allow a participant to cross over to his/her preferred intervention only when the participant actively expresses the preference after randomization (Figure 1), as informed during the consent process. The length of time interval between randomization and opt-out based on choice should be flexible depending on the nature of interventions, study settings, resources, and characteristics of target populations. Especially when the time interval is relatively long, a reminder of the opt-out process might minimize possibility of participant crossing over later during the study period.
2.5. Participant compositions
We denote by AS (a group or set of) participants who tacitly opt in to stay in intervention arm A when A is assigned. The components of AS are those (AA) for whom A is their preference, and those (AR) who have no or equal preference but would be willing to comply with the assigned intervention A, that is, AS = AA∪AR. We further denote by AB participants who actively switch to A from B. Therefore, the combination of AA and AB represents participants who prefer A. In a similar fashion, when B is assigned, we denote by BS (a group or set of) participants who tacitly opt in to stay in intervention arm BS. The components of BS are those (BB) for whom B is their preference, and those (BR) who have no or equal preference but would be willing to comply with the assigned intervention B, that is, BS = BB∪BR. Group BA represents participants who actively switch to B from A. Therefore, the combination of BB and BA represents participants who prefer B. Collectively, we denote by RA and RB participants who are randomly assigned to interventions A and B, respectively, that is, RA=AA∪AR∪BA and RB=BB∪BR∪AB. We also denote by TA and TB participants who are treated with interventions A and B, respectively, that is, TA=AA∪AR∪AB and TB=BB∪BR∪BA. All these participant compositions are presented in Figure 1.
2.6. Identifiability of groups
When a PORD study is completed, although both As and Bs are identifiable by design, their components are unidentifiable. First, both AR and BR are unidentifiable but will represent participants who are randomly assigned interventions A and B, respectively with no or equal preference. Likewise, both AA and BB are unidentifiable but will represent participants who prefer the randomly assigned interventions A and B, respectively. All the other groups—AB, BA, RA, RB, TA, and TB—are identifiable by design.
2.7. Study outcome distribution
The outcome variable for the ith participant will be denoted by Yi, distributions of which we herein consider independent normal or binomial/Bernoulli. However, distributions of Yi can be flexible as long as a large sample theory could be applied for constructing asymptotically normally distributed test statistics such as a Wald test that we adopted below.
2.8. Group size
n(Ω) will denote the group or sample size of participants in set Ω, i.e. n(Ω)=#{i|Yi∈Ω}.
2.9. Assumptions
2.9.1. Assumption 1 (A1)
We assume exclusion restriction (ER)30 that the expected outcomes to be the same between those who actively cross over or opt out to an intervention arm of their preferences and those who stay in the randomly assigned intervention arm because the assigned intervention is their preference. That is
2.9.2. Assumption 2 (A2)
We also assume no selection bias from randomization (NSBR)19 that preference rate of a intervention in a stay group will be equal to that of the intervention in the other randomly intervention assigned arm, that is
And thus accordingly
2.9.3. Assumption 3 (A3)
We assume equal variances (EVs), that is, variance of Y does not depend on any subgroups Ω, i.e. Var(Y |Ω=Var(Y)=σ2 for all Ω For normal distributions, a residual variance or a pooled within-group variance may serve as an equal σ2. However, this assumption does not hold for a non-normal outcome Y whose variance depends on its expectation. Nevertheless, for a binary outcome, an EV could be estimated based on average of hypothesized probabilities of all groups. This approach is applied for simulation studies below.
3. Definitions of effects
3.1. Comparative effect
Let us first denote: ξA=E(Y|AR) and ξB=E(Y|BR), representing outcome expectations among participants with no or equal preferences to randomly assigned interventions. Then, the comparative effect ξ can be defined as
This effect represents the difference in expectations of outcome Y between participants who were randomly assigned to A or B. Therefore, this effect is equivalent to a potential effect that could be obtained from a parallel randomized clinical trial (RCT). For this reason, the randomization features in RCTs can be preserved in a PORD study.
Nonetheless, both ξA and ξB are unidentifiable because both the corresponding sets of participants AR and BR are unidentifiable by design. Owing to the ER (A1) and NSBR (A2) assumptions, however, both ξA and ξB can be derived from identifiable expectations such as E(Y|AS) and E(Y|AB). First, note that
| (1) |
| (2) |
| (3) |
Equation (1) holds due to a law of total expectation; the second equation (2) due to the ER (A1) assumption; and the last (3) due to the NSBR (A2) assumption. Therefore, the unidentifiable ξA=E(Y|AR) can be estimated as
Likewise, ξB=E(Y|BR) can be estimated in a similar fashion as
3.2. Preference effect
Extending the notions of the preference effect proposed by Turner et al.,28 we define the preference effect λ as
where λA=E(Y|AA∪AB)–E(Y|AR) and λB=E(Y|BB∪BA)–E(Y|BR), and w′s are the inverses of the variances of the corresponding estimates. λA and λB represent the effects of active preference to interventions A and B over equal or no preference, respectively, and λ is a weighted average of λA and λB representing an overall preference effect. We note that since P(AA∩AB)=0 and it is assumed that E(Y|AA)=E(Y|AB), it is immediate that E(Y|AA∩AB)=E(Y|AB) and E(Y|BB∪BA)=E(Y|BA). Thus, λA and λA can be reduced to: λA=E(Y|AB)–ξA and λB=E(Y|BA)–ξB.
3.3. Selection effect
A selection effect ς can be defined as
This quantity represents the effect of selecting intervention A over intervention B. That is, it quantifies the extent of benefit or harm for participants who prefer A over those who prefer B when compared to their respective counterparts with no or equal preference.
3.4. ITT effect
Participants randomly assigned to interventions A and B were intended to be treated with the assigned interventions. Therefore, ITT effect can be defined as
where ηA=E(Y|RA) and ηB=E(Y|RB). These expectations can be derived as follows
and
3.5. Overall/As-treated (AT) effect
Overall intervention effect τ is defined as difference in outcome expectations between those who are actually treated with A and B. Thus, it is the same as “as-treated” effect and can be quantified as
Where τA=E(Y|TA) and τB=E(Y|TB). These expectations can be derived as follows.
and
where
and
4. Sample estimates
In what follows, sample estimates are in general denoted by or , in the form of and where Ω1 ⊂ Ω2
4.1. Comparative effect estimate
Sample estimates of both unidentifiable ξA and ξB can be derived as follows. First, observe that
since , , , and n(RB) = n(AB)+n(BS). It can similarly be shown that
It is now straightforward to derive variance estimates based on the EV (A3) assumption as follows
and
Finally, we have and .
4.2. Preference effect estimate
First, a sample estimate of E(Y|AA∪AB) can be expressed as follows
since the unidentifiable n(AA) and can be estimated as
and
Similarly, . It follows that
and
And, thus , where , , with
and
due to the EV (A3) assumption. Finally, we have .
4.3. Selection effect estimate
It is immediate that , and .
4.4. ITT effect estimate
A sample estimate of the ITT effect can be obtained as follows: , and due to the EV (A3) assumption, .
4.5. Overall/AT effect
A sample estimate of the overall/AT effect can be obtained as follows: , and due to the EV (A3) assumption, , where n(TA) = n(AS) + n(AB) and n(TB) = n(BS) + n(BA).
5. Power functions
5.1. Test statistic
Once sample estimates and their variances are derived, a Wald test statistic Dθ for testing H0: θ=0 vs. Ha: θ ≠ 0, where θ represents any of the five effect parameters, can be constructed as follows
| (4) |
5.2. Statistical power
As the distributions of Dθ under both H0 and Ha are asymptotically normal due to a large sample theory, its statistical power φθ with a two-sided significance level α can be expressed as
| (5) |
where Φ−1 is the inverse of the cumulative distribution function Φ of a standard normal variable, and z1−α/2 = Φ−1 (1 − α/2). However, depending on the sign or direction of θ either term of the right hand side is often negligible for power analysis, and thus the statistical power φθ is usually expressed as
5.3. Required parameters
As all aforementioned effects are inter-connected with each other through the following parameter values, to compute power for cohesively testing the significance of their individual effects, one needs to pre-specify:
-
(1)
Stay-in (or equivalently opt-out) rates: P(AS|RA) (or P(BA|RA)=1–P(AS|RA)) and P(BS|RB) (or P(AB|RB)=1–P(BS|RB));
-
(2)
Four outcome expectations: E(Y|AS), E(Y|BS), E(Y|AB), and E(Y|BA);
-
(3)
Sample sizes: n(RA) and n(RB);
-
(4)
The variance σ2 of Y and a two-sided significance level α.
Once all of these values are specified, the assumption A2 enables one to compute expected sizes of all sets or groups as follows: n(AS) = (1–P(BA|RA))n(RA), n(AR)=(1–P(AB|RB))n(AS), n(AA)=P(AB|RB)n(AS), n(AB)=P(AB|RB)n(RB), n(TA)=n(AS)+n(AB), n(BS)=(1 P(AB|RB), n(BR)=(1–P(BA|RA))n(BS), n(BB)=P(BA|RA)n(BS), n(BA)=P(BA|RA)n(RA), and n(TB)=n(Bs)+n(BA). In addition, magnitudes of all the effects (i.e. comparative effect ξ = ξA − ξB, preference effect λ = (wAλA + wBλB)/(wA + wB), selection effect ς = (wAλA − wBλB)/(wA + wB), ITT effect η = ηA − ηB, and overall/AT effect τ = τA − τB) and their variances can be derived as shown above in Section 3 and 4.
5.4. Closed-form power functions
When all of those effects and sample sizes are determined, all corresponding power functions can be derived using the effect and variance estimates presented above in Section 4. For instance, with the determined ξ = ξA − ξB, the statistical power for testing a comparative effect, H0: ξ = 0 vs. H0: ξ ≠ 0, can be expressed as
Likewise, for testing a preference effect, H0: λ=0 vs. Ha: λ≠0, we have
for testing a selection effect, H0: ζ = 0 vs. H0: ζ ≠ 0, we have
for testing a ITT effect, H0: η = 0 vs. H0: η ≠ 0, we have
and finally for testing an AT/overall effect, H0: τ = 0 vs. H0: τ ≠ 0, we have
5.5. Example for power computation
Investigators who plan to conduct the hepatitis C treatment trial example comparing mDOT (intervention A) and PN (intervention B) in terms of continuous log10 viral load outcome (instead of a binary SVR outcome for the sake of example) standardized by a standard deviation (SD) might take into account for power analysis at the design stage the following considerations made based on clinical experiences, literature search, or pilot study results: (1) the stay-in rate will be lower, or the opt-out rate will be higher, in the mDOT arm than the PN arm since mDOT requires more frequent regular visit to a clinic, P(AS|RA)=0.65 and P(BS|RB)=0.80; (2) therefore, the randomization should be unbalanced with a heavier weight to mDOT arm, n(RA)=400 and n(RB)=200, to result in approximately equal numbers of participants treated with between mDOT and PN; (3) the standardized outcome of mDOT might be better than that of PN among participants who stay in their randomly assigned arms, E(Y|AS)=0.9 and E(Y|BS)=0.4; (4) the standardized outcome of participants who opt out to mDOT from PN might also be better than that of those who opt out to PN from mDOT, E(Y|AB)=1.2 and E(Y|BA)=0.7; (5) (residual) outcome variance will be σ2=1 across all groups as the outcome is standardized without loss of generality; and (6) test will be two-sided with α=0.05 significance level.
Under this hypothesized parameter setting, the investigators will expect that: (1) sample sizes of participants who opt out to A from B and to B from A will be n(AB)=40 and n(BA)=140, respectively; (2) sample sizes of participants who stay in A and B will be n(AS)=260 and n(BS)=160, respectively; and (3) sample sizes of participants who are treated with A and B will be n(TA)=(TB)=300. And, the following parameter values will be expected: (1) for comparative effect, ξA=0.83, ξB=0.24, ξ=0.59; (2) for preference effect, λA=0.38, λB =0.46, wA=22.2, wB=31.5, λ=0.43; (3) for selection effect, ς=−0.12; (4) for ITT effect, ηA=0.83,ηB=0.56, η=0.27; and (5) for AT effect, τA=0.94, τA=0.54, and τ=0.40. Finally, the statistical power for each effect under the specifications above is computed as follows: φξ=0.96, φλ=0.88, φς=0.14, φη=0.88, and φτ>0.99. An R code for computations of all of these quantitates along with theoretical power (5) and simulation-based empirical power in equation (6) below is provided in Supplementary Material.
5.6. Special case
For a special case in which n(RA)=n(RB)=n(R) and P(AS|RA)=P(BS|RB)=πS, we have: n(AS)=n(BS)=πSn(R), n(AB)=n(BA)=(1–πS)n(R), n(TA)=n(TB)=n(R), wA=wB, λ=(λA+λB)/2, and ς = (λA − λB)/2 Furthermore all power functions listed above can be simplified to
and
Note that, although it appears that all of these power functions are a monotone function of πS under the special case, they are not necessarily so since all of ξ, λ, ς, η, and τ are also an implicit function of πS, and so are their variance estimates.
Table 1 displays effects of πS on all effects and their variance under a special case of: n(RA) n(RB)=n(R)=300, E(Y|AS)=0.9, E(Y|BS)=0.4, E(Y|AB)=1.2, E(Y|BA) 0.6, and σ2=1 As can be seen, the comparative decreases effect ξ and increases but its variance with increasing πS. On the other hand, both preference effect λ and selection effect ς decrease with increasing πS but their variances are the same and non-monotonic over πS. The ITT effect η increases with increasing πS whereas the overall/AT effect τ decreases. Their variances are the same but remain constant over πS. Figure 2 presents impact of πS on all power functions under the same special case of the design parameters. The magnitude of power for comparative effect ξ increases πS whereas those for preference effect λ and selection effect ς are exactly symmetric about πS = 50%, and ITT effect η is roughly symmetric about πS ≈55%. On the other hand, overall/AT effect τ and selection effect ς are almost constant at > 0.99% and 5–10%, respectively. If πS≈80%, then all effects except for the preference and selection effects will be detected with > 90% statistical power.
Table 1.
Derived effects and their variances over varying stay-in rates πS = P(AS|RA) = P(BS|RS) under n(RA) = n(RB) = n(R) = 300, E(Y|AS) = 0.9, E(Y|BS) = 0.4, E(Y|AB) = 1.2, E(Y|BA) = 0.6, and σ2 = 1.
| Stay-in rate | Comparative effect | Preference effect | Selection effect | ITT effect | Overall/AT effect | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| πS | ξ | λ | ς | η | τ | |||||
| 1% | −9.40 | 6732.7 | 25.00 | 1683.2 | 5.00 | 1683.2 | −0.59 | 0.007 | 0.60 | 0.007 |
| 10% | −0.40 | 7.267 | 2.50 | 1.852 | 0.50 | 1.852 | −0.49 | 0.007 | 0.59 | 0.007 |
| 20% | 0.10 | 0.967 | 1.25 | 0.260 | 0.25 | 0.260 | −0.38 | 0.007 | 0.58 | 0.007 |
| 30% | 0.27 | 0.299 | 0.83 | 0.088 | 0.17 | 0.088 | −0.27 | 0.007 | 0.57 | 0.007 |
| 40% | 0.35 | 0.129 | 0.63 | 0.043 | 0.13 | 0.043 | −0.16 | 0.007 | 0.56 | 0.007 |
| 50% | 0.40 | 0.067 | 0.50 | 0.027 | 0.10 | 0.027 | −0.05 | 0.007 | 0.55 | 0.007 |
| 60% | 0.43 | 0.038 | 0.42 | 0.019 | 0.08 | 0.019 | 0.06 | 0.007 | 0.54 | 0.007 |
| 70% | 0.46 | 0.024 | 0.36 | 0.016 | 0.07 | 0.016 | 0.17 | 0.007 | 0.53 | 0.007 |
| 80% | 0.48 | 0.015 | 0.31 | 0.016 | 0.06 | 0.016 | 0.28 | 0.007 | 0.52 | 0.007 |
| 90% | 0.49 | 0.010 | 0.28 | 0.023 | 0.06 | 0.023 | 0.39 | 0.007 | 0.51 | 0.007 |
| 99% | 0.50 | 0.007 | 0.25 | 0.172 | 0.05 | 0.172 | 0.49 | 0.007 | 0.50 | 0.007 |
Figure 2.
Comparisons of statistical power across five effects with varying stay-in rates πS=P(AS|RA)=P(BS|RS) under a special case of: n(RA)=n(RB)=n(R)=300, E(Y|AS)=0.9, E(Y|BS)=0.4, E(Y|AB)=1.2, E(Y|BA) 0.6, and σ2=1. A: theoretical statistical power: B: empirical statistical power based on 10,000 simulations. Black and gray lines represent theoretical and empirical power, respectively.
5.7. Simulation verification
Simulations were conducted with normal and binomial distributions of outcome Y. Simulation-based empirical power is defined as
| (6) |
where 1{.} is an indicator function; is the p value from testing significance of for the kth simulation with estimated sample variance of Y; and K is the total number of simulations. For the same specifications used for Table 1 and Figure 2, the magnitudes of empirical power (presented in gray lines in Figure 2) based on K=10,000 simulations for each specification are virtually identical to those of the theoretical power. In addition, under unbalanced sample sizes and various differential preference rates, Table 2 shows that both theoretical and empirical power, under normal distributions are again virtually identical to each other in view of in equations (5) and (6). Table 3 shows that simulation results even under binomial distributions are also very close to each other in view of , even if this quantity is in general slightly greater than that under the normal distributions. The asymptotic distributions of the test statistic Dθ (4) based on large sample theory are shown to be useful for binomial outcomes when an EV is estimated based on average of given probabilities under the parameter specification considered in Table 3. Furthermore, the type I error rates of Dθ (4) under both normal and binomial distributions appear to be preserved since the empirical power is close to the theoretical power even when it is near 0.05 meaning a null effect. With all the other parameters held fixed, effects of unbalanced stay-in rates between arms on statistical power appear to be greater for comparative and preference effects compared to that for the other effects for both normal and binomial outcomes (Tables 2 and 3).
Table 2.
Comparisons between theoretical and empirical power under normal distributions over varying sample sizes and sty-in rates with fixed E(Y|AS) = 0.9, E(Y|BS) = 0.4, E(Y|AB) = 1.2, E(Y|BA) = 0.6, σ2 = 1, two-sided α = 0.05, and number of simulations = 10,000.
| Sample size | Stay-in rate | Comparative effect | Preference effect | Selection effect | ITT effect | Overall/AT effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n(RA) | n(RB) | P(AS|RA) | P(BS|RB) | φξ | φλ | φζ | φη | φτ | |||||
| 250 | 150 | 20% | 40% | 0.185 | 0.186 | 0.578 | 0.571 | 0.118 | 0.125 | 0.568 | 0.567 | 0.999 | 0.999 |
| 30% | 30% | 0.067 | 0.070 | 0.623 | 0.614 | 0.103 | 0.103 | 0.744 | 0.736 | 0.999 | 1.00 | ||
| 40% | 20% | 0.081 | 0.090 | 0.564 | 0.558 | 0.081 | 0.078 | 0.873 | 0.863 | 0.999 | 0.999 | ||
| 200 | 200 | 20% | 40% | 0.198 | 0.196 | 0.586 | 0.581 | 0.086 | 0.087 | 0.595 | 0.595 | 1.00 | 1.00 |
| 30% | 30% | 0.068 | 0.074 | 0.630 | 0.626 | 0.074 | 0.075 | 0.770 | 0.764 | 1.00 | 1.00 | ||
| 40% | 20% | 0.082 | 0.091 | 0.566 | 0.561 | 0.061 | 0.062 | 0.893 | 0.883 | 1.00 | 1.00 | ||
| 150 | 250 | 20% | 40% | 0.194 | 0.189 | 0.549 | 0.542 | 0.062 | 0.062 | 0.568 | 0.574 | 1.00 | 1.00 |
| 30% | 30% | 0.067 | 0.074 | 0.589 | 0.581 | 0.056 | 0.051 | 0.744 | 0.735 | 1.00 | 1.00 | ||
| 40% | 20% | 0.079 | 0.088 | 0.524 | 0.520 | 0.051 | 0.054 | 0.873 | 0.862 | 1.00 | 1.00 | ||
| 250 | 150 | 60% | 80% | 0.796 | 0.795 | 0.556 | 0.557 | 0.051 | 0.049 | 0.568 | 0.563 | 0.995 | 0.995 |
| 70% | 70% | 0.654 | 0.657 | 0.589 | 0.587 | 0.056 | 0.057 | 0.377 | 0.386 | 0.997 | 0.998 | ||
| 80% | 60% | 0.418 | 0.420 | 0.597 | 0.590 | 0.081 | 0.089 | 0.213 | 0.228 | 0.997 | 0.997 | ||
| 200 | 200 | 60% | 80% | 0.822 | 0.823 | 0.594 | 0.595 | 0.052 | 0.051 | 0.595 | 0.598 | 0.999 | 0.998 |
| 70% | 70% | 0.682 | 0.680 | 0.630 | 0.627 | 0.074 | 0.078 | 0.398 | 0.404 | 1.00 | 1.00 | ||
| 80% | 60% | 0.439 | 0.447 | 0.642 | 0.633 | 0.121 | 0.125 | 0.224 | 0.242 | 1.00 | 1.00 | ||
| 150 | 250 | 60% | 80% | 0.798 | 0.797 | 0.584 | 0.586 | 0.065 | 0.064 | 0.568 | 0.564 | 1.00 | 0.999 |
| 70% | 70% | 0.654 | 0.647 | 0.623 | 0.617 | 0.103 | 0.108 | 0.377 | 0.382 | 1.00 | 1.00 | ||
| 80% | 60% | 0.416 | 0.423 | 0.639 | 0.635 | 0.164 | 0.171 | 0.213 | 0.222 | 1.00 | 1.00 | ||
| 0.009 | 0.008 | 0.008 | 0.018 | 0.001 | |||||||||
Table 3.
Comparisons between theoretical and empirical power under binomial distributions over varying sample sizes and sty-in rates with fixed E(Y|AS) = 0.7, E(Y|BS) = 0.5, E(Y|AB) = 0.8, E(Y|BA) = 0.6, two-sided α = 0.05, and number of simulations = 10,000.
| Sample size | Stay-in rate | Comparative effect | Preference effect | Selection effect | ITT effect | Overall/AT effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n(RA) | n(RB) | P(AS|RA) | P(BS|RB) | φξ | φλ | φζ | φη | φτ | |||||
| 250 | 150 | 20% | 40% | 0.217 | 0.227 | 0.418 | 0.415 | 0.060 | 0.050 | 0.230 | 0.229 | 0.963 | 0.971 |
| 30% | 30% | 0.093 | 0.094 | 0.464 | 0.462 | 0.054 | 0.050 | 0.369 | 0.378 | 0.962 | 0.964 | ||
| 40% | 20% | 0.052 | 0.057 | 0.422 | 0.416 | 0.051 | 0.060 | 0.528 | 0.533 | 0.946 | 0.951 | ||
| 200 | 200 | 20% | 40% | 0.234 | 0.242 | 0.438 | 0.437 | 0.051 | 0.042 | 0.242 | 0.246 | 0.990 | 0.993 |
| 30% | 30% | 0.096 | 0.096 | 0.485 | 0.473 | 0.050 | 0.047 | 0.380 | 0.404 | 0.987 | 0.990 | ||
| 40% | 20% | 0.052 | 0.049 | 0.438 | 0.434 | 0.051 | 0.052 | 0.554 | 0.565 | 0.976 | 0.980 | ||
| 150 | 250 | 20% | 40% | 0.228 | 0.235 | 0.422 | 0.413 | 0.051 | 0.045 | 0.230 | 0.232 | 0.998 | 0.998 |
| 30% | 30% | 0.093 | 0.090 | 0.464 | 0.466 | 0.054 | 0.054 | 0.369 | 0.375 | 0.996 | 0.998 | ||
| 40% | 20% | 0.052 | 0.045 | 0.418 | 0.414 | 0.060 | 0.057 | 0.528 | 0.535 | 0.989 | 0.991 | ||
| 250 | 150 | 60% | 80% | 0.716 | 0.710 | 0.457 | 0.462 | 0.073 | 0.064 | 0.528 | 0.536 | 0.946 | 0.949 |
| 70% | 70% | 0.580 | 0.587 | 0.464 | 0.463 | 0.054 | 0.044 | 0.369 | 0.366 | 0.962 | 0.964 | ||
| 80% | 60% | 0.383 | 0.385 | 0.451 | 0.454 | 0.051 | 0.043 | 0.230 | 0.233 | 0.963 | 0.965 | ||
| 200 | 200 | 60% | 80% | 0.744 | 0.749 | 0.474 | 0.474 | 0.058 | 0.049 | 0.554 | 0.568 | 0.976 | 0.979 |
| 70% | 70% | 0.607 | 0.605 | 0.485 | 0.484 | 0.050 | 0.039 | 0.389 | 0.384 | 0.987 | 0.987 | ||
| 80% | 60% | 0.403 | 0.413 | 0.474 | 0.475 | 0.058 | 0.052 | 0.242 | 0.254 | 0.990 | 0.991 | ||
| 150 | 250 | 60% | 80% | 0.718 | 0.731 | 0.451 | 0.454 | 0.051 | 0.039 | 0.528 | 0.541 | 0.989 | 0.990 |
| 70% | 70% | 0.580 | 0.576 | 0.464 | 0.466 | 0.054 | 0.045 | 0.369 | 0.373 | 0.996 | 0.995 | ||
| 80% | 60% | 0.381 | 0.383 | 0.457 | 0.463 | 0.073 | 0.062 | 0.230 | 0.235 | 0.998 | 0.998 | ||
| 0.013 | 0.012 | 0.012 | 0.024 | .008 | |||||||||
6. Discussion
The PORD design is a simplified version of existing preference trial designs which involve multiple steps of randomizations or consent processes. However, it has many aforementioned desirable features including those which other designs might not have. For example, although it does not have a randomization arm, a PORD includes by design randomized groups who willingly, as opposed to potentially reluctantly, accept randomly assigned intervention. In addition, it enables CER investigators to estimate and test diverse effects. It might be convenient to determine sample sizes, n(RA) and n(RA), for a primary effect in a routine iterative fashion since inversions of power functions to sample size formulas are complicated, albeit possible, especially when opt-out rates and randomizations are unbalanced. We recommend the simulation approach (6) for binary outcomes in particular since the asymptotic normality of Dθ (4) might not always be reliable especially for small sample sizes and very low or high success probabilities. Definite thresholds of sample sizes and probabilities for reliable normal approximations are unknown although the conventional “np > 5” rule for all identifiable and unidentifiable groups may serve as a criterion for the purpose of using the theoretical power function φθ (5) for binary outcomes. Nonetheless, it takes only a few seconds to estimate simulation-based empirical power by running 10,000 simulations for a combination of pre-specified parameters using the R code provided in Supplementary Material.
We stress that power calculations for all five effects need to be conducted through the common design parameters since they will all be inter-connected. In other words, if one computes power for one effect independently from the other effects, then the power calculations may not be cohesive under a PORD design. Since the PORD involves five effects, proper adjustment of the significance level for multiple testing would be an issue for computing power or sample sizes. If a PORD study is aimed to test a single primary effect, then any adjustment may not be necessary for testing the primary effect. However, if a PORD aims to find any significant effect among the five effects, then a multiplicity adjustment (e.g. Bonferroni correction) to the significance level should be applied for computing power.
Use of PORD or any other types of preference design might not be suitable when a vast majority of participants are expected to have clear preference. In this case, willingness to be randomized might be diminished.31 If a PORD study was implemented in such a case, the NSBR (A2) assumption in particular would not consequently hold since it is highly likely that almost all participants in the stay-in groups would be those who preferred the assigned interventions. It follows that there would be disproportionately very few AR(BR) participants in AS(Bs). For this reason, estimates of the comparative effect, preference effect, and selective effect following the PORD analytic strategies would be biased. Furthermore, even if the NSBR assumption could be relaxed so that AS(Bs) is treated as the same as AB(BA), AT effect would be confounded by the preference effect. Therefore, application of PORD would be useful when an adequate size of participants is anticipated to have equal or no preferences.
When a PORD study is successfully implemented and completed, all five effect estimates could collectively or specifically guide treatment or intervention decision making at the individual patient or clinician level, and at the organization level such as hospitals and community-based organizations. For example, if the preference effect is significant but comparative effect is not, then clinicians would ask for patient’s preferences and prescribe accordingly. If the preference effect is not significant but comparative or overall effect is, then clinicians would recommend the more effective intervention to patients even if patients might prefer the less effective intervention. Per the selection effect, patients who choose more convenient delivery methods such as app-aided DPP might have less motivation than those who choose more demanding conventional delivery methods. Therefore, it is likely that their outcome might be even worse on average than those who do not have preference and thus that the selection effect of choosing conventional methods would be significant. In this case, the recommendation of convenient delivery methods might be discarded at an organizational level. If all effects are not significant and the outcomes are equivalently effective between interventions, then an optimal intervention approach can be implemented considering individual patient-level and clinician preferences, considering also practical constraints such as cost and insurance reimbursement.
The following limitations should be taken into account for implementing a PORD. First, especially when the opt-out rate is unbalanced, it is likely that the covariate profiles balance among participants between AS and BS may be compromised. For the comparisons of identifiable groups such as ITT or AT effect, these effects can be adjusted for differential characteristics applying multivariable regression analysis. However, for comparisons between unidentifiable groups, post-hoc adjustment to the comparative, preference and selection estimates will be difficult and we believe that additional assumptions on covariate profiles for unidentifiable groups would be necessary. Examination of this potential post-randomization selection bias would be a valuable future research undertaking. Second, if the opt-out rate is anticipated to be high, then the power for testing for the comparative effect in particular will be small. Therefore, if the comparative effect is of the primary interest, then a large sample size would be required. In such case, it might be more reasonable to have preference or selection effect as the primary aim. Third, the effect of violations of assumptions (A1)–(A3) on the statistical analysis is not examined in the present paper. These assumptions, albeit well-accepted, are rather ideal and may not hold in the real world situations. At the analysis stage, however, it will be possible to test the EV assumption (A3) between identifiable groups and if rejected, an appropriate test with unequal variances can be applied to testing significance of effects. For comparison of unidentifiable groups, testing EV is not possible, and thus a pooled variance from identifiable groups (e.g. As, AB, Bs, and BA) can be used for constructing test statistics Dθ (4) as demonstrated in the simulation R code in Supplementary Material.
In conclusion, we believe that the PORD is an ideal design to address many challenges arising from CER to accommodate participants’ preferences in real-world settings while effectively estimating and testing diverse effects without compromising randomization.
Supplementary Material
Acknowledgements
We are grateful to the two anonymous reviewers whose comments and suggestions were helpful for improving the contents of the manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research described was supported in part by NIH/NCATS UL1TR001073, NIH/NHLBI K01HL125466, NIH/NIDA R01DA034086, PCORI HPC-1503–28122 and NIH/NIDDK P30DK111022.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material
Supplementary material is available for this article online.
References
- 1.Westfall JM, Mold J and Fagnan L. Practice-based research – “Blue Highways” on the NIH roadmap. JAMA 2007; 297: 403–406. [DOI] [PubMed] [Google Scholar]
- 2.Waldman SA and Terzic A. Clinical and translational science: from bench-bedside to global village. Clin Transl Sci 2010; 3: 254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Concato J, Peduzzi P, Huang GD, et al. Comparative effectiveness research: what kind of studies do we need? J Investig Med 2010; 58: 764–769. [DOI] [PubMed] [Google Scholar]
- 4.Corbett MS, Watson J and Eastwood A. Randomised trials comparing different healthcare settings: an exploratory review of the impact of pre-trial preferences on participation, and discussion of other methodological challenges. BMC Health Serv Res 2016; 16: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidani S, Fox M, Streiner DL, et al. Examining the influence of treatment preferences on attrition, adherence and outcomes: a protocol for a two-stage partially randomized trial. BMC Nurs 2015; 14: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dore GJ, Altice F, Litwin AH, et al. Elbasvir-Grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med 2016; 165: 625. [DOI] [PubMed] [Google Scholar]
- 7.Grebely J, Robaeys G, Bruggmann P, et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy 2015; 26: 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volmink J, Matchaba P and Garner P. Directly observed therapy and treatment adherence. Lancet 2000; 355: 1345–1350. [DOI] [PubMed] [Google Scholar]
- 9.Arnsten JH, Litwin AH and Berg KM. Effect of directly observed therapy for highly active antiretroviral therapy on virologic, immunologic, and adherence outcomes: a meta-analysis and systematic review. Jaids 2011; 56: E33–E34. [DOI] [PubMed] [Google Scholar]
- 10.Berg KM, Litwin A, Li XA, et al. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: a randomized controlled trial. Drug Alcohol Depend 2011; 113: 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litwin AH, Berg KM, Li X, et al. Rationale and design of a randomized controlled trial of directly observed hepatitis C treatment delivered in methadone clinics. BMC Infect Dis 2011; 11: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruggmann P and Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis 2013; 57: S56–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ackermann RT, Finch EA, Brizendine E, et al. Translating the Diabetes Prevention Program into the community – the DEPLOY pilot study. Am J Prev Med 2008; 35: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackermann RT and Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community – the YMCA model. Diabetes Educ 2007; 33: 69. [DOI] [PubMed] [Google Scholar]
- 16.Yeh MC, Heo M, Suchday S, et al. Translation of the Diabetes Prevention Program for diabetes risk reduction in Chinese immigrants in New York City. Diabet Med 2016; 33: 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen F, Su W, Becker SH, et al. Clinical and economic impact of a digital, remotely-delivered intensive behavioral counseling program on medicare beneficiaries at risk for diabetes and cardiovascular disease. PLoS One 2016; 11: e0163627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Younge JO, Kouwenhoven-Pasmooij TA, Freak-Poli R, et al. Randomized study designs for lifestyle interventions: a tutorial. Int J Epidemiol 2015; 44: 2006–2019. [DOI] [PubMed] [Google Scholar]
- 19.Long Q, Little RJ and Lin XH. Causal inference in hybrid intervention trials involving treatment choice. J Am Stat Assoc 2008; 103: 474–484. [Google Scholar]
- 20.Walter SD, Turner R, Macaskill P, et al. Beyond the treatment effect: evaluating the effects of patient preferences in randomised trials. Stat Methods Med Res 2017; 26: 489–507. [DOI] [PubMed] [Google Scholar]
- 21.Zelen M. Strategy and alternate randomized designs in cancer clinical trials. Cancer Treat Rep 1982; 66: 1095–1100. [PubMed] [Google Scholar]
- 22.Zelen M. Randomized consent designs for clinical trials: an update. Stat Med 1990; 9: 645–656. [DOI] [PubMed] [Google Scholar]
- 23.Fost N. Sounding Board. Consent as a barrier to research. N Engl J Med 1979; 300: 1272–1273. [DOI] [PubMed] [Google Scholar]
- 24.Wennberg JE, Barry MJ, Fowler FJ, et al. Outcomes research, PORTs, and health care reform. Ann N Y Acad Sci 1993; 703: 52–62. [DOI] [PubMed] [Google Scholar]
- 25.Rucker G. A two-stage trial design for testing treatment, self-selection and treatment preference effects. Stat Med 1989; 8: 477–485. [DOI] [PubMed] [Google Scholar]
- 26.King M, Nazareth I, Lampe F, et al. Impact of participant and physician intervention preferences on randomized trials – a systematic review. JAMA 2005; 293: 1089–1099. [DOI] [PubMed] [Google Scholar]
- 27.Adamson SJ, Bland JM, Hay EM, et al. Patients’ preferences within randomised trials: systematic review and patient level meta-analysis. Br Med J 2008; 337: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner RM, Walter SD, Macaskill P, et al. Sample size and power when designing a randomized trial for the estimation of treatment, selection, and preference effects. Med Decis Making 2014; 34: 711–719. [DOI] [PubMed] [Google Scholar]
- 29.Wei LJ and Lachin JM. Properties of the urn randomization in clinical trials. Control Clin Trials 1988; 9: 345–364. [DOI] [PubMed] [Google Scholar]
- 30.Angrist JD, Imbens GW and Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc 1996; 91: 444–455. [Google Scholar]
- 31.Sidani S, Fox M and Epstein DR. Contribution of treatment acceptability to acceptance of randomization: an exploration. J Eval Clin Pract 2017; 23: 14–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.