Abstract
Allergic rhinitis (AR) and chronic rhinosinusitis with nasal polyps (CRSwNP) are inflammatory diseases of the upper airway, with a similar immunologic profile, characterized by aberrant and persistent type 2 inflammation. One cell population that has been identified as altered in both disease types is regulatory T cell (Treg). Tregs have the capacity to modulate T-effector function and suppress inflammatory cytokine production in a broad range of cell types. Given the ability of Tregs to control inflammation, the role of Tregs in respiratory diseases has attracted much attention. As discussed in this article, alterations in the Treg numbers and function, or both, have been identified in AR and CRSwNP, although much of the data is conflicting. Here, we explored what is known and, in many cases, unknown about the mechanisms by which Tregs differentiate and function, and how these functions can be controlled in the mucosal microenvironment. By gaining a greater understanding of these processes, it may be possible to harness the natural immunosuppressive activity of Tregs to ameliorate the chronic inflammation associated with AR and CRSwNP.
Keywords: Allergic rhinitis, chronic rhinosinusitis, nasal polyp, regulatory T cells, rhinitis, sinusitis, T cell
Allergic rhinitis (AR) and chronic rhinosinusitis (CRS) share a similar type 2 skewed immunologic profile. The long-term consequence of this elevation in type 2 cytokines is that they can drive many of the physical symptoms of AR and CRS, including tissue remodeling, rhinorrhea, and excessive mucus production.1 The question remains as to (1) how type 2 cytokines become altered in these diseases, and (2) how we can quell excessive type 2 inflammation? The answer to both of these questions likely centers on the role of regulatory T cells (Treg) in the airways. In this article, we examined what is known about alterations in Treg numbers and function in these diseases.
INCIDENCE AND HEALTH CARE BURDEN OF AR AND CRS
AR has consistently increased in prevalence and now affects up to 6% worldwide2 and up to 40% of adults in the United States.3 With symptoms regularly increasing or decreasing, its severity varies constantly, which makes it difficult to gauge.2 As such, AR can have a profound effect on quality of life, especially in children. Although it can be difficult to quantify, the annual cost of AR was estimated in 2004 to be between $2 billion and $5 billion/y in the United States.4 CRS affects up to 12% of the U.S. population, with direct and indirect costs that surpass $21 billion/y in the United States.5 CRS is a complex disease, with two different presentations, CRS with nasal polyps (CRSwNP) and without nasal polyps (CRSsNP).
CRSsNP can be caused by a broad range of disorders, including structural abnormalities (e.g., nasal septal deviation, septal perforation, nasal valve dysfunctions) and other pathologies (e.g., primary ciliary dyskinesia or gastroesophageal reflux).6 CRSsNP has historically been connected with T-helper (Th) type 1 skewing, although results of some studies indicated a wide variation in the immune profile expressed,7,8 with reports of tissue immunologic characteristics even seeming similar to those of controls.6 CRSwNP, in addition to the polyps themselves, presents with a characteristic type 2 eosinophil-skewed immune response, similar to that seen in AR. The close relationship between AR and CRSwNP may be, in part, due to the similar type 2 skewed immune profile and the fact that 82% of CRSwNP are atopic.9 Therefore, CRSwNP was the primary focus of this article.
One immune cell subtype, Treg, has experienced an increase in interest and relevance as technology has progressed to the point that we can accurately study it. Once known as suppressive T cells in the 1980s, Tregs have been established as a powerful force in the immunologic milieu, with far reaching and potent effects on self-tolerance, prevention of autoimmune disease, and allergy.10 The purpose of this review was to examine the current state of knowledge on the role of Tregs in AR and CRSwNP.
Treg DEVELOPMENT AND FUNCTION
One of the first hurdles to understanding the role of Tregs in disease was to establish a set of markers by which to define Tregs, which remains a problem today because no marker unique to Tregs alone has been found. However, as with other T-cell phenotypes, a combination of markers has been used to accurately identify Tregs as a unique population of cells. Tregs are commonly defined as CD4+, CD25+, CD69+, and express CD127low/−, and have been found to have a maximum suppressive ability with the CD45RA+CD45RO− phenotype.10,11 However, not all Tregs are CD4+. Subsets of highly suppressive CD8+CD25+ Forkhead Box P3+ (FoxP3+) Tregs in both humans and mice have been identified.12–14 Although CD8+ Tregs have been identified in the pathophysiology of other diseases, there is limited information about their role in respiratory diseases. As such, this review focused discussions primarily on CD4+ Tregs.
Tregs also express, on their surface, the regulatory mediator cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). One of the most important markers that has been used for their identification is the FoxP3 transcription factor, which is integral to suppressive activity, differentiation, and function.10 Although FoxP3 is one of the most widely used markers for Tregs, it is also expressed by non-Tregs when induced to do so,15 and evidence has even been found of FoxP3 expression at the double-positive stage of T-cell differentiation.16 Because this was not discovered until relatively recently, older studies on the function and presence of Tregs that solely used FoxP3+ to identify them may have been measuring activated effector T cells in addition to Tregs and, therefore, may be inaccurate. Further investigations have led to the discovery of two unique subgroups of Tregs, their thymic Tregs (tTreg) (in older literature, referred to as natural T regulatory cell [nTreg]) and Tregs induced in the periphery (pTreg) (in older literature, referred to as inducible T regulatory cell [iTreg]).17
Although tTreg formation is not yet fully understood, it is known that tTregs are differentiated within the thymus through a series of interactions with major histocompatibility complex (MHC) self peptides.18 However, pTregs are converted peripherally from other CD4+ T cells through expression of transforming growth factor beta (TGF) β and the CNS1 gene.19 Although not without controversy, there is some evidence that tTregs can be delineated from pTregs by the presence of Helios protein and, in murine models, the extracellular marker neuropilin-1.20,21 FoxP3+Helios+ cells can be identified specifically as tTregs, which do not produce as many cytokines but which are important to the creation of pTregs, whereas FoxP3+Helios− Tregs are pTregs and have greater cytokine expression, especially of interleukin (IL) 10.22 Although the role of Helios has been questioned due to its transient expression in all types of developing T cells,23 pTregs seem to consistently exert more of a regulatory effect through the use of IL-10 even while displaying subset heterogeneity, such as expressing more or less FoxP3.24 Treg lineages can be broken down even further, e.g., CD45RA Tregs tend to use TGF-β for their regulatory effects, whereas CD45RA− Tregs use CTLA-4 and are more dependent on IL-10.25
Functionality of Tregs can be defined as the ability to suppress T effector and helper cells through cytokine production, induction of apoptosis (via perforin and granzyme B secretion), and blockade of costimulation via CTLA-4. Their functionality is further mediated by the expression level of FoxP3 by the specific Treg.26 Expression of FoxP3 is augmented by Treg T cell receptor (TCR) interaction with MHC-II signals, and deficiency in FoxP3 results in an absence of CD4+CD25+ cells.27 In addition, suppressive functionality may be regulated by expression of Helios protein, which has been found to require intrinsic FoxP3 expression as well as TGF-β signaling, which further highlights the role of FoxP3 as the “master regulator.”21
Tregs secrete TGF-β, IL-10, and IL-35 as their main regulatory cytokines. These factors induce a number of functional alterations to other immune cells with which they interact. Typically, IL-10 is one of the primary regulatory cytokines of the immune system, and downregulates all T-cell cytokine expression and macrophage activity as well as enhances B-cell survival. TGF-β has a variety of effects systemically, but, in the context of local immune cell function, it functions similarly to IL-10 and, therefore, inhibits T- and B-cell proliferation. IL-35 is a recently identified cytokine that seems to have anti-inflammatory and immunosuppressive capabilities similar to TGF-β. It suppresses IL-4 and transcription factor GATA-3 to reduce Th2 proliferation.28 A discrepancy between the two cytokines lies in the fact that although TGF- β can be found in many tissues at all times, IL-35 (as well as IL-10) are not typically constitutively expressed, except in Tregs.28–30
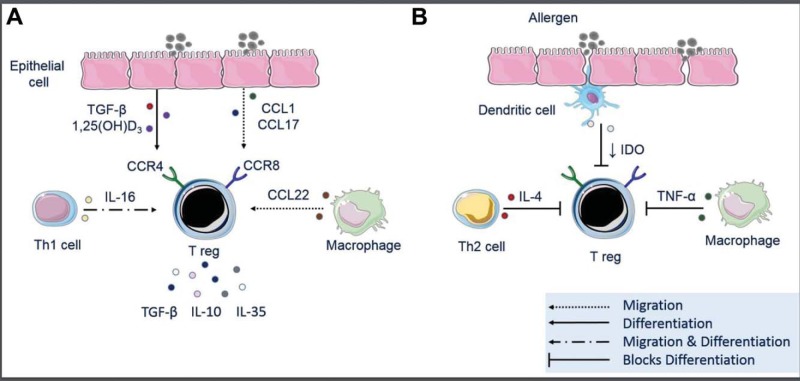
Treg effector functions are predominantly exerted in the local microenvironment, and thus the migration of Tregs is critical for their important functions. Treg migration is moderated by C-C chemokine receptors (CCR) 4 and CCR8, which respond to chemokine (C-C motif) ligand (CCL) 22, CCL17, CCL1, and viral macrophage inflammatory protein-1 (MIP-1).31 Tregs also express CD62L, CCR7, and CXCR4, which are indicators of secondary lymphoid homing capacity.32 As summarized in Fig. 1 A, epithelial cells serve as a source of CCL1 and CCL17, whereas CCL22 and IL-16 come from macrophages and Th1 cells, respectively.33–37 IL-16 seems to be a potent chemoattractant, which induces Tregs to migrate and express increased FoxP3 activity. IL-16 also seems to attract Th1 cells, with a net effect in the tissue of Treg-mediated Th2 suppression and normalization of the immunologic population of the tissue.38 Histamine 2 and 4 receptors have been shown to release IL-16, and ligation of the histamine 4 receptor (H4R) receptor results in recruitment of pTregs, which helps to resolve airway hyperresponsiveness and inflammation.39
Figure 1.
The influence of airway-derived factors in regulatory T cell (Treg) migration and differentiation. (A) The presence of Tregs in the airway can be influenced by a number of locally produced mediators. Airway epithelial cells can produce factors such as 1,25-dihydroxyvitamin D3 (1,25[OH]D3) and transforming growth factor (TGF) β both of which can induce the differentiation of naive T cells to become Tregs. Epithelial cells also produce chemotactic factors, such as chemokine (C-C motif) ligand (CCL) 1 and CCL17, which induce Treg migration through C-C Chemokine Receptor (CCR) 4 and CCR8 receptors signaling. Mediators made by local immune infiltrates, such as macrophage production of CCL22, and T-helper (Th) type 1 cell production of interleukin (IL) 16 can also induce Treg migration to the airway. (B) Airway-produced factors can also impair Treg functions. Th2 and proinflammatory cytokines, such as IL-4 and tumor necrosis factor (TNF) α, respectively, can skew naive T cells away from Treg differentiation. Impaired epithelial membrane and cell-cell junctional integrity in asthma, Allergic rhinitis (AR) and chronic rhinosinusitis with nasal polyps (CRSwNP) allow for the increased passage of antigen into the subepithelial space, which, in turn, can activate dendritic cells and drive them to promote a proinflammatory state characterized by reduced indoleamine 2,3-dioxygenase (IDO).
ALTERATIONS IN Treg NUMBERS AND FUNCTIONS IN AR AND CRSwNP
From studies performed with FoxP3-deficient scurfy mice and observations of immunodysregulation, polyendocrinopathy, enteropathy, and X-linked syndrome in humans with dysfunctional FoxP3, we know that a complete lack of Treg function will lead to significant autoimmune disorders and, ultimately, death.40,41 Use of depletion of Tregs mice has provided conflicting reports about the role of Tregs in lower airway allergic inflammation.42 In studies by Baru et al.,43 which used Balb/c and C57BL/6 mice, which are more resistant to allergy development, Treg depletion during allergen provocation failed to alter lung inflammation. Conversely, depletion of Tregs during the sensitization phase of allergy development resulted in a dramatic exacerbation of inflammation for both strains of mice, which suggests that Tregs may play an important role in the establishment of allergic airway disease.43 To our knowledge, no study has reported the use of depletion of Tregs mice or other Treg depletion methods to assess the role of Tregs in murine models of AR or CRS.
As summarized in Table 1, in patients with AR, circulating Treg numbers in the peripheral blood of patients were found to be either lower than or equivalent in control patients.44–46 Analysis of transcription factor expression by using immunohistochemical techniques conflict with some studies that demonstrated FoxP3, T-box transcription factor (T-bet), and TGF-β expression to be lower in patients with AR than in controls with increased GATA-3 expression,47,48 whereas another study found that FoxP3 and GATA-3 have elevated expression in patients with AR, at least during the preallergy season.49 These discrepancies further highlighted the importance of investigating the functional fitness of Tregs to more accurately denote the functional significance of Treg infiltrates locally within tissues.
Table 1.
Summary of human studies that examined Treg frequency and functionality in patients with AR or CRSwNP

Treg = Regulatory T cell; AR = allergic rhinitis; CRSwNP = chronic rhinosinusitis with nasal polyp; ↓ = decrease; PB = peripheral blood; FoxP3 = ; TGF = transforming growth factor; IL = interleukin; TNF = tumor necrosis factor; NB = nasal biopsy; T-bet = T-box transcription factor (T-bet); SM = sinus mucosa; IT = inferior turbinate; mRNA = messenger RNA; ↑ = increase; CRSsNP = chronic rhinosinusitis without nasal polyp.
In CRSwNP, CD4+ Tregs were found to be lower in the peripheral blood of affected patients than in controls.50 In the sinus mucosa and inferior turbinate, FoxP3 expression was also generally reduced in patients with CRSwNP versus controls, with higher T-bet and GATA-3 expression.51,52 However, one study found FoxP3 expression to be unchanged in these tissues.53 In a study that examined both CD4+ and CD8+ Tregs in peripheral blood and sinus tissue, no difference in circulating levels of either Tregs populations was observed. However, in sinonasal tissue CD4+CD25+FoxP3+ Tregs were increased compared with tissue from control subjects. Conversely, CD8+CD25+FoxP3+ Tregs were decreased compared with control subject–derived tissue. In comparisons of patients with CRSwNP who were atopic versus nonatopic, the presence of allergy was found to have no impact on the level of either Treg subset.54 Currently, there is no satisfactory explanation for the differences in findings between the groups. Discrepancies between the studies could be due to different sampling methods or different presentations of the disease; studies found significant variation in cytokine profiles between different countries of origin or ethnicities in CRS.55 Therefore, similar to AR, additional investigation is needed, focused more specifically on Treg function and less on expression of markers to determine their presence or absence.
POTENTIAL CAUSES OF Treg DYSFUNCTION
Changes in the local inflammatory milieu may contribute to altered Treg numbers and/or function in the airway. For example, local upregulation of IL-6 can transform naive T cells into Th17 cells and thus shift away from Treg induction.56 As highlighted in Fig. 1 B, the inflammatory cytokine tumor necrosis factor (TNF) α has also been shown to decrease FoxP3 expression and to suppress Treg functions.57 Furthermore, in a murine allergic airway inflammation model, it was demonstrated that TNF-α receptor antagonist reduces inflammation and increases the percentage of Tregs in circulation and their suppressive ability as measured by IL-10 production.58 These findings may have applicability to the upper airway because both patients with AR and patients with CRSwNP have been shown to have increased TNF-α levels.59–61 As mentioned previously, TGF-β promotes naive T cells to become Tregs. However, patients with AR have a lowered level of TGF-β compared with controls.62 Similarly, patients with CRSwNP have decreased TGF-β in their airways compared with controls.63–65 Loss of this well-known differentiator of naive T cells into Tregs may contribute to their loss in AR and CRSwNP. However, given that Tregs secrete TGF-β to induce suppression, it is also possible that the loss of TGF-β in AR and may impact the reduction in Treg directly CRSwNP. Therefore, additional studies are needed to extrapolate the exact interplay between TGF-β and Tregs in the upper airway.
Reduced Treg chemotaxis to the tissue from the periphery represents another potential mechanism that contributes to local Treg dysfunction. In patients with allergic asthma, a lack of responsiveness to chemokines is thought to be one potential mechanism that contributes to a loss of local tissue control of immune responses. In patients with allergic asthma, Tregs were found to have decreased chemotactic responses to the Treg chemotaxis factor CCL1.66 The mechanisms associated with the loss of chemotactic activity were not elucidated. Whether due to motility issues and/or the loss of chemokine receptor expression is not clear, but the mechanisms that drive these changes warrant a more thorough investigation. Similarly, it was found that chemotaxis of Tregs to mucosal tissues is impaired in CRSwNP31; however, the precise mechanism responsible for this reduction is yet to be determined. Treg migration, both in airway homeostasis and inflammation, represents an area in which much additional research is needed.
Airway epithelial cell barrier dysfunction is hypothesized to be another contributor to pathogenesis of AR and CRSwNP67–70 (Fig. 1 B). A breakdown in epithelial barrier junctional proteins has been shown to result in a leakiness that facilitates increased passage of antigen, which could hyperstimulate immune cells, particularly antigen-presenting dendritic cells (DC), in the subepithelial space. In AR and CRSwNP, DC numbers are elevated in the local tissues and, in some cases, systemically.71–74 After allergen exposure, these activated and matured DCs stimulate an inflammatory effector T-cell response instead of a tolerogenic phenotype. For example, DCs from patients with asthma who were exposed to house-dust mite antigen had reduced indoleamine 2, 3-dioxygenase expression, which shifted the T-cell response toward Th2 and away from Treg induction.75 Inhalation of Aspergillus fumagatis is another potential driver of Treg dysfunction in airway disease. A. fumagatis is a ubiquitous fungal antigen and the most common fungus in the airway, inhaled at a rate of several hundred conidia per day.76–78 Although studies in mice found that a single exposure to A. fumagatis induced increases in Treg numbers and function in the lung, repeated exposure models, more akin to the exposure humans experience, was found to shift T-cell responses toward a pathogenic Th2–Th17 phenotype.79
The secosteroid hormone 1,25-dihydroxyvitamin D3 (1,25[OH]D3) has been shown in multiple disease states to induce Tregs.80–85 However, patients with CRSwNP have reduced sinonasal levels of 1,25(OH)D3 compared with controls or those with CRSsNP.86 This deficiency is likely caused by an impaired ability of human sinonasal epithelial cells to metabolize 25(OH)D3 to its activate metabolite, 1,25(OH)D3.87 These local deficiencies are independent of atopic status, age, race, gender, or vitamin D3 sufficiency status. Therefore, it is possible that local 1,25(OH)D3 deficiencies may contribute to the reductions in Tregs found in CRSwNP. To date, the local airway impairment of vitamin D metabolism has only been described in CRSwNP, and, therefore, the applicability of these findings to AR remains to be determined.
HARNESSING Tregs FOR THE TREATMENT OF AR AND CRSwNP
Results of studies found that the use of corticosteroids increases both the number and the suppressive function of Tregs in the airways, via an IL-10–dependent mechanism, which suggests a possible mechanism for longer-term resolution of inflammation than just the corticosteroid itself.88 Allergen-specific immunotherapy has also been proven to increase the number of circulating Tregs; however, this increase is only seen while the immunotherapy is ongoing, with Treg levels dropping to pretreatment levels when allergen exposure ceases.89 In addition to standard therapy, novel therapeutic agents in humans have the potential to normalize the immunologic profile of the airway. Tregs can be isolated from the blood, expanded ex vivo, and reinfused into the bloodstream. The reinfused cells could be detected circulating for 2 weeks, with no extra risk of infection or early mortality.90 The results of the application of ex vivo Treg expansion have been promising, but work has primarily been performed in the realms of autoimmune disease, organ transplantation, and graft-versus-host disease.91
To our knowledge, there currently are no published studies that have looked at the effect of applying Tregs, especially pTregs, locally to the respiratory mucosa to determine whether it would resolve inflammatory symptoms. To our knowledge, there also are no studies on the effects of IL-2–mediated Treg induction in the respiratory tract. However, analysis of exciting new data indicates that antigen and rapamycin, a tolerogenic immunomodulator, delivered via synthetic polymeric nanoparticles into the respiratory tract and other areas of the body stimulates local Treg proliferation. This effect is seen even in the presence of agonists, which indicates a powerful tolerogenic affect. Although this experiment has thus far only been displayed in mouse models, it could prove a very compelling treatment option.92
CONCLUSION
AR and CRS displayed a similar type 2–skewed immunologic profile that was also characterized by the reduced presence of Tregs, especially pTregs. Whereas results of Toll like receptor (TLR) a number of studies in AR and CRSwNP indicated that Treg numbers are decreased, much remains to be understood about the function of Tregs or, in many cases, dysfunction. Current treatments have been found to be effective, at least in part through indirect modulation of Treg populations. However, these effects seem to be transient, and, therefore, given the body of research presented herein, the development of novel strategies to normalize Treg effector responses, numbers, and migration are needed.
Footnotes
C. Atkinson and J.K. Mulligan are supported by the South Carolina Clinical and Translational Research Institute, with an academic home at the Medical University of South Carolina, National Institute of Health/National Center for Advancing Translational Sciences grants KL2TR001452 and UL1TR001450
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: Linking innate and adaptive immunity in asthma. Nat Rev Immunol 8:193–204, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet 378:2112–2122, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Feng CH, Miller MD, Simon RA. The united allergic airway: Connections between allergic rhinitis, asthma, and chronic sinusitis. Am J Rhinol Allergy 26:187–190, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reed SD, Lee TA, McCrory DC. The economic burden of allergic rhinitis: A critical evaluation of the literature. Pharmacoeconomics 22:345–361, 2004. [DOI] [PubMed] [Google Scholar]
- 5. DeConde AS, Soler ZM. Chronic rhinosinusitis: Epidemiology and burden of disease. Am J Rhinol Allergy 30:134–139, 2016. [DOI] [PubMed] [Google Scholar]
- 6. Konig K, Klemens C, Haack M, et al. Cytokine patterns in nasal secretion of non-atopic patients distinguish between chronic rhinosinusitis with or without nasal polys. Allergy Asthma Clin Immunol 12:19, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang X, Zhang N, Bo M, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol 138:1344–1353, 2016. [DOI] [PubMed] [Google Scholar]
- 8. Tan BK, Klingler AI, Poposki JA, et al. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol 139:699–703.e7, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tan BK, Zirkle W, Chandra RK, et al. Atopic profile of patients failing medical therapy for chronic rhinosinusitis. Int Forum Allergy Rhinol 1:88–94, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 10:490–500, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Hoffmann P, Boeld TJ, Eder R, et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol 39:1088–1097, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Robb RJ, Lineburg KE, Kuns RD, et al. Identification and expansion of highly suppressive CD8(+)FoxP3(+) regulatory T cells after experimental allogeneic bone marrow transplantation. Blood 119:5898–5908, 2012. [DOI] [PubMed] [Google Scholar]
- 13. Peng LS, Zhuang Y, Shi Y, et al. Increased tumor-infiltrating CD8(+)Foxp3(+) T lymphocytes are associated with tumor progression in human gastric cancer. Cancer Immunol Immunother 61:2183–2192, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eusebio M, Kuna P, Kraszula L, et al. The relative values of CD8+CD25+Foxp3brigh Treg cells correlate with selected lung function parameters in asthma. Int J Immunopathol Pharmacol 28:218–226, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Gavin MA, Torgerson TR, Houston E, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A 103:6659–6664, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tuovinen H, Pekkarinen PT, Rossi LH, et al. The FOXP3+ subset of human CD4+CD8+ thymocytes is immature and subject to intrathymic selection. Immunol Cell Biol 86:523–529, 2008. [DOI] [PubMed] [Google Scholar]
- 17. Abbas AK, Benoist C, Bluestone JA, et al. Regulatory T cells: Recommendations to simplify the nomenclature. Nat Immunol 14:307–308, 2013. [DOI] [PubMed] [Google Scholar]
- 18. Caramalho Í, Nunes-Cabaço H, Foxall RB, Sousa AE. Regulatory T-cell development in the human thymus. Front Immunol 6:395, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Josefowicz SZ, Niec RE, Kim HY, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482:395–399, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin X, Chen M, Liu Y, et al. Advances in distinguishing natural from induced Foxp3(+) regulatory T cells. Int J Clin Exp Pathol 6:116–123, 2013. [PMC free article] [PubMed] [Google Scholar]
- 21. Takatori H, Kawashima H, Matsuki A, et al. Helios enhances Treg cell function in cooperation with FoxP3. Arthritis Rheumatol 67:1491–1502, 2015. [DOI] [PubMed] [Google Scholar]
- 22. Kim YC, Bhairavabhotla R, Yoon J, et al. Oligodeoxynucleotides stabilize Helios-expressing Foxp3(+) human T regulatory cells during in vitro expansion. Blood 119:2810–2818, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akimova T, Beier UH, Wang L, et al. Helios expression is a marker of T cell activation and proliferation. PLoS One 6:e24226, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ballke C, Gran E, Baekkevold ES, Jahnsen FL. Characterization of regulatory T-cell markers in CD4+ T cells of the upper airway mucosa. PLoS One 11:e0148826, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30:899–911, 2009. [DOI] [PubMed] [Google Scholar]
- 26. Zhang JG, Chen XJ, Liu T, Jiang SJ. FOXP3(+) associated with the pro-inflammatory regulatory T and T helper 17 effector cells in asthma patients. Exp Ther Med 12:2753–2758, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4:330–336, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Chen C, Deng Y, Chen H, et al. Decreased concentration of IL-35 in plasma of patients with asthma and COPD. Asian Pac J Allergy Immunol 32:211–217, 2014. [DOI] [PubMed] [Google Scholar]
- 29. Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450:566–569, 2007. [DOI] [PubMed] [Google Scholar]
- 30. Li X, Mai J, Virtue A, et al. IL-35 is a novel responsive anti-inflammatory cytokine—A new system of categorizing anti-inflammatory cytokines. PLoS One 7:e33628, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim YM, Munoz A, Hwang PH, Nadeau KC. Migration of regulatory T cells toward airway epithelial cells is impaired in chronic rhinosinusitis with nasal polyposis. Clin Immunol 137:111–121, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lim HW, Broxmeyer HE, Kim CH. Regulation of trafficking receptor expression in human forkhead box P3+ regulatory T cells. J Immunol 177:840–851, 2006. [DOI] [PubMed] [Google Scholar]
- 33. Hoelzinger DB, Smith SE, Mirza N, et al. Blockade of CCL1 inhibits T regulatory cell suppressive function enhancing tumor immunity without affecting T effector responses. J Immunol 184:6833–6842, 2010. [DOI] [PubMed] [Google Scholar]
- 34. Belperio JA, Dy M, Murray L, et al. The role of the Th2 CC chemokine ligand CCL17 in pulmonary fibrosis. J Immunol 173:4692–4698, 2004. [DOI] [PubMed] [Google Scholar]
- 35. Richter JR, Sutton JM, Belizaire RM, et al. Macrophage-derived chemokine (CCL22) is a novel mediator of lung inflammation following hemorrhage and resuscitation. Shock 42:525–531, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Skundric DS, Cruikshank WW, Drulovic J. Role of IL-16 in CD4+ T cell-mediated regulation of relapsing multiple sclerosis. J Neuroinflammation 12:78, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Montes-Vizuet R, Vega-Miranda A, Valencia-Maqueda E, et al. CC chemokine ligand 1 is released into the airways of atopic asthmatics. Eur Respir J 28:59–67, 2006. [DOI] [PubMed] [Google Scholar]
- 38. McFadden C, Morgan R, Rahangdale S, et al. Preferential migration of T regulatory cells induced by IL-16. J Immunol 179:6439–6445, 2007. [DOI] [PubMed] [Google Scholar]
- 39. Morgan RK, McAllister B, Cross L, et al. Histamine 4 receptor activation induces recruitment of FoxP3+ T cells and inhibits allergic asthma in a murine model. J Immunol 178:8081–8089, 2007. [DOI] [PubMed] [Google Scholar]
- 40. Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 27:18–20, 2001. [DOI] [PubMed] [Google Scholar]
- 41. Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27:20–21, 2001. [DOI] [PubMed] [Google Scholar]
- 42. Lahl K, Sparwasser T. In vivo depletion of FoxP3+ Tregs using the DEREG mouse model. In Regulatory T Cells: Methods and Protocols. Totowa, NJ: Humana Press, 157–172, 2011. [DOI] [PubMed] [Google Scholar]
- 43. Baru AM, Ganesh V, Krishnaswamy JK, et al. Absence of Foxp3+ regulatory T cells during allergen provocation does not exacerbate murine allergic airway inflammation. PLoS One 7:e47102, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang X, Chen Y, Zhang F, et al. Peripheral Th17/Treg cell-mediated immunity imbalance in allergic rhinitis patients. Braz J Otorhinolaryngol 80:152–155, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Han D, Wang C, Lou W, et al. Allergen-specific IL-10-secreting type I T regulatory cells, but not CD4(+)CD25(+)Foxp3(+) T cells, are decreased in peripheral blood of patients with persistent allergic rhinitis. Clin Immunol 136:292–301, 2010. [DOI] [PubMed] [Google Scholar]
- 46. Genc S, Eroglu H, Kucuksezer UC, et al. The decreased CD4+CD25+ FoxP3+ T cells in nonstimulated allergic rhinitis patients sensitized to house dust mites. J Asthma 49:569–574, 2012. [DOI] [PubMed] [Google Scholar]
- 47. Sogut A, Yilmaz O, Kirmaz C, et al. Regulatory-T, T-helper 1, and T-helper 2 cell differentiation in nasal mucosa of allergic rhinitis with olive pollen sensitivity. Int Arch Allergy Immunol 157:349–353, 2012. [DOI] [PubMed] [Google Scholar]
- 48. Sun R, Tang XY, Yang Y. Immune imbalance of regulatory T/type 2 helper cells in the pathogenesis of allergic rhinitis in children. J Laryngol Otol 130:89–94, 2016. [DOI] [PubMed] [Google Scholar]
- 49. Malmhäll C, Bossios A, Pullerits T, Lötvall J. Effects of pollen and nasal glucocorticoid on FOXP3+, GATA-3+ and T-bet+ cells in allergic rhinitis. Allergy 62:1007–1013, 2007. [DOI] [PubMed] [Google Scholar]
- 50. Sharma S, Watanabe S, Sivam A, et al. Peripheral blood and tissue T regulatory cells in chronic rhinosinusitis. Am J Rhinol Allergy 26:371–379, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shi J, Fan Y, Xu R, et al. Characterizing T-cell phenotypes in nasal polyposis in Chinese patients. J Investig Allergol Clin Immunol 19:276–282, 2009. [PubMed] [Google Scholar]
- 52. Van Bruaene N, Pérez-Novo CA, Basinski TM, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol 121:1435–1441, 1441.e1–e3, 2008. [DOI] [PubMed] [Google Scholar]
- 53. Liu T, Song CH, Liu AM, et al. Forkhead box P3+ T cells express interleukin-17 in nasal mucosa of patients with both allergic rhinitis and polyposis. Clin Exp Immunol 163:59–64, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pant H, Hughes A, Schembri M, et al. CD4(+) and CD8(+) regulatory T cells in chronic rhinosinusitis mucosa. Am J Rhinol Allergy 28:e83–e89, 2014. [DOI] [PubMed] [Google Scholar]
- 55. Wang X, Zhang N, Bo M, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol 138:1344–1353, 2016. [DOI] [PubMed] [Google Scholar]
- 56. Pilette C, Jacobson MR, Ratajczak C, et al. Aberrant dendritic cell function conditions Th2-cell polarization in allergic rhinitis. Allergy 68:312–321, 2013. [DOI] [PubMed] [Google Scholar]
- 57. Valencia X, Stephens G, Goldbach-Mansky R, et al. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood 108:253–261, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Elsakkar MG, Sharaki OA, Abdallah DM, et al. Adalimumab ameliorates OVA-induced airway inflammation in mice: Role of CD4+ CD25+ FOXP3+ regulatory T-cells. Eur J Pharmacol 786:100–108, 2016. [DOI] [PubMed] [Google Scholar]
- 59. Nonaka M, Nonaka R, Jordana M, Dolovich J. GM-CSF, IL-8, IL-1R, TNF-alpha R, HLA-DR in nasal epithelial cells in allergic rhinitis. Am J Respir Crit Care Med 153:1675–1681, 1996. [DOI] [PubMed] [Google Scholar]
- 60. Bradding P, Mediwake R, Feather IH, et al. TNF alpha is localized to nasal mucosal mast cells and is released in acute allergic rhinitis. Clin Exp Allergy 25:406–415, 1995. [DOI] [PubMed] [Google Scholar]
- 61. Oyer SL, Mulligan JK, Psaltis AJ, et al. Cytokine correlation between sinus tissue and nasal secretions among chronic rhinosinusitis and controls. Laryngoscope 123:E72–E78, 2013. [DOI] [PubMed] [Google Scholar]
- 62. Ni K, Zhao L, Wu J, et al. Th17/Treg balance in children with obstructive sleep apnea syndrome and the relationship with allergic rhinitis. Int J Pediatr Otorhinolaryngol 79:1448–1454, 2015. [DOI] [PubMed] [Google Scholar]
- 63. Van Bruaene N, Derycke L, Perez-Novo CA, et al. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol 124:253–259, 259.e1–e2, 2009. [DOI] [PubMed] [Google Scholar]
- 64. Balsalobre L, Pezato R, Perez-Novo C, et al. Epithelium and stroma from nasal polyp mucosa exhibits inverse expression of TGF-β1 as compared with healthy nasal mucosa. J Otolaryngol Head Neck Surg 42:29, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li X, Meng J, Qiao X, et al. Expression of TGF, matrix metalloproteinases, and tissue inhibitors in Chinese chronic rhinosinusitis. J Allergy Clin Immunol 125:1061–1068, 2010. [DOI] [PubMed] [Google Scholar]
- 66. Nguyen KD, Vanichsarn C, Fohner A, Nadeau KC. Selective deregulation in chemokine signaling pathways of CD4+CD25(hi)CD127(lo)/(-) regulatory T cells in human allergic asthma. J Allergy Clin Immunol 123:933–999.e10, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Den Beste KA, Hoddeson EK, Parkos CA, et al. Epithelial permeability alterations in an in vitro air-liquid interface model of allergic fungal rhinosinusitis. Int Forum Allergy Rhinol 3:19–25, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bleier BS, Mulligan RM, Schlosser RJ. Primary human sinonasal epithelial cell culture model for topical drug delivery in patients with chronic rhinosinusitis with nasal polyposis. J Pharm Pharmacol 64:449–456, 2012. [DOI] [PubMed] [Google Scholar]
- 69. Sweerus K, Lachowicz-Scroggins M, Gordon E, et al. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J Allergy Clin Immunol 139:72–78.e1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mattila P, Joenvaara S, Renkonen J, et al. Allergy as an epithelial barrier disease. Clin Transl Allergy 1:5, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity 43:29–40, 2015. [DOI] [PubMed] [Google Scholar]
- 72. Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: From protection to immunopathology. Annu Rev Immunol 30:243–270, 2012. [DOI] [PubMed] [Google Scholar]
- 73. O'Connell BP, Schlosser RJ, Wentzel JL, et al. Systemic monocyte-derived dendritic cells and associated Th2 skewing in chronic rhinosinusitis. Otolaryngol Head Neck Surg 150:312–320, 2014. [DOI] [PubMed] [Google Scholar]
- 74. Kleinjan A, Lambrecht BN. Dendritic cells in rhinitis. Handb Exp Pharmacol (188):115–136, 2009. [DOI] [PubMed] [Google Scholar]
- 75. Maneechotesuwan K, Wamanuttajinda V, Kasetsinsombat K, et al. Der p 1 suppresses indoleamine 2, 3-dioxygenase in dendritic cells from house dust mite–sensitive patients with asthma. J Allergy Clin Immunol 123:239–248, 2009. [DOI] [PubMed] [Google Scholar]
- 76. Denning DW, Park S, Lass-Florl C, et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis 52:1123–1129, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mullins J, Seaton A. Fungal spores in lung and sputum. Clin Allergy 8:525–533, 1978. [DOI] [PubMed] [Google Scholar]
- 78. Latgé JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 12:310–350, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Murdock BJ, Shreiner AB, McDonald RA, et al. Coevolution of TH1, TH2, and TH17 responses during repeated pulmonary exposure to Aspergillus fumigatus conidia. Infect Immun 79:125–135, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mattozzi C, Paolino G, Salvi M, et al. Peripheral blood regulatory T cell measurements correlate with serum vitamin D level in patients with psoriasis. Eur Rev Med Pharmacol Sci 20:1675–1679, 2016. [PubMed] [Google Scholar]
- 81. Vijayendra Chary A, Hemalatha R, Seshacharyulu M, et al. Vitamin D deficiency in pregnant women impairs regulatory T cell function. J Steroid Biochem Mol Biol 147:48–55, 2015. [DOI] [PubMed] [Google Scholar]
- 82. Zhang X, Wu X, Xiong L, et al. Role of vitamin D3 in regulation of T helper cell 17 and regulatory T-cell balance in rats with immunoglobulin a nephropathy. Iran J Kidney Dis 8:363–370, 2014. [PubMed] [Google Scholar]
- 83. Terrier B, Derian N, Schoindre Y, et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res Ther 14:R221, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Morales-Tirado V, Wichlan DG, Leimig TE, et al. 1α,25-dihydroxyvitamin D3 (vitamin D3) catalyzes suppressive activity on human natural regulatory T cells, uniquely modulates cell cycle progression, and augments FOXP3. Clin Immunol 138:212–221, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Smolders J, Thewissen M, Peelen E, et al. Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS One 4:e6635, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schlosser RJ, Carroll WW, Soler ZM, et al. Reduced sinonasal levels of 1α-hydroxylase are associated with worse quality of life in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol 6:58–65, 2016. [DOI] [PubMed] [Google Scholar]
- 87. Mulligan JK, Nagel W, O'Connell BP, et al. Cigarette smoke exposure is associated with vitamin D3 deficiencies in patients with chronic rhinosinusitis. J Allergy Clin Immunol 134:342–349, 2014. [DOI] [PubMed] [Google Scholar]
- 88. Robinson DS. Regulatory T cells and asthma. Clin Exp Allergy 39:1314–1323, 2009. [DOI] [PubMed] [Google Scholar]
- 89. Lloyd CM, Hessel EM. Functions of T cells in asthma: More than just T(H)2 cells. Nat Rev Immunol 10:838–848, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood 117:1061–1070, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xia Y, Cai W, Thomson AW, Hu X. Regulatory T cell therapy for ischemic stroke: How far from clinical translation? Transl Stroke Res 7:415–419, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Maldonado RA, LaMothe RA, Ferrari JD, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci U S A 112:E156–E165, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]