Abstract
We investigated the effects of isolated meniscectomy on tibiofemoral skeletal kinematics and cartilage contact arthrokinematics in vivo. We recruited nine patients who had undergone isolated medial or lateral meniscectomy, and used a dynamic stereo-radiography (DSX) system to image the patients’ knee motion during decline walking. A volumetric model-based tracking process determined 3D tibiofemoral kinematics from the recorded DSX images. Cartilage contact arthrokinematics was derived from the intersection between tibial and femoral cartilage models co-registered to the bones. The kinematics and arthrokinematics were analyzed for early stance and loading response phase (30% of a gait cycle), comparing the affected and intact knees. Results showed that four patients with medial meniscectomy had significantly greater contact centroid excursions in the meniscectomized medial compartments while five patients with lateral meniscectomy had significantly greater cartilage contact area and lateral shift of contact centroid path in the meniscectomized lateral compartments, comparing to those of the same compartments in the contralateral intact knees. No consistent difference however was identified in the skeletal kinematics. The current study demonstrated that cartilage-based intra-articlular arthrokinemtics is more sensitive and insightful than the skeletal kinematics in assessing the meniscectomy effects.
Keywords: in vivo, tibiofemoral kinematics, cartilage contact, meniscectomy, dynamic stereo-radiography
INTRODUCTION
Meniscectomy—the surgical removal of a portion or the entirety of an injured meniscus—is one of the most frequently performed orthopaedic procedures [1]. It is known, however, to have deleterious consequences such as degenerative joint changes and accelerated onset of osteoarthritis (OA) [2, 3]. Accurate assessment of the effects of meniscectomy on joint motion and contact congruity is an initial but critical step in understanding how patho-mechanics instigates the development of OA [4]. Data resulting from such assessment would also serve as baselines for evaluating the efficacy of alternative repair strategies and post-meniscectomy interventions such as meniscus transplantation [5].
The effects of meniscectomy on tibiofemoral joint function or mechanics have been examined by in vitro cadaveric studies and in vivo motion analysis studies [5–8]. With an in vitro experimental model, Spang et al. discovered that total medial meniscectomy increased anterior cruciate ligament (ACL) strain and anterior tibial translation [5]. The latter effect on anterior tibial translation was contrary to the conclusion from an earlier in vitro study [6]. This disparity, as well as the methodological inconsistency speculated to have caused it, reflects the limitations of in vitro studies: testing conditions, including combinations of kinematics and loading, and types of simulated meniscectomy, cannot be made physiological variable. It is a formidable challenge for cadaveric studies to replicate the complex combination of and interplay between the gravitational, inertial and active muscular forces. Sturnieks et al. performed the first in vivo gait analysis of pain-free meniscectomy patients and reported reduced range of motion and lower peak moments in the sagittal plane on the operated limb comparing to the nonoperated limb [7]. Netravali et al. conducted in vivo biomechanical study of patients with partial medial meniscectomy and identified significant kinematic and kinetic differences between the meniscectomized and contralateral intact knees [8]. The study employed surface-based measurement of tibiofemoral kinematics and a point cluster method [9, 10] to mitigate skin motion artifacts that otherwise could be substantial enough to obscure the effect or difference of interest [11, 12]. However, no study has yet to attain measures that delineate intra-articular cartilage contact or interactions which are more pertinent to the fundamental patho-mechanics of meniscectomy. Previous studies that investigated the knee cartilage contact have typically involved magnetic resonance imaging (MRI) and most of studies were for in vitro conditions or in vivo low-speed movements [13–17]. A newly validated approach at our center combining accurate bone kinematics data from biplane radiography with cartilage models from MRI is ready for noninvasively assessing in vivo cartilage contact during functional activities—the accuracy of cartilage contact estimation has been comprehensively validated in vitro against a laser scanning gold standard under multiple body weight loading and over a range of knee flexion angles (with root mean square errors in contact area averaged 8.4% and 4.4% of the medial and lateral compartmental areas, respectively) [18]. One challenge associated with experimental study of the biomechanical effects of meniscectomy is the variability of meniscectomy presented. The difficulty of accurately estimating and resecting the desired percentage of the meniscus intra-operatively, even with a meniscal measuring device, has been reported [19]. This, in addition to the natural variability of the types of meniscus injury that necessitate the meniscectomy, makes it particularly challenging to achieve a study cohort with well controlled clinical or surgical variables. On the other hand, coping with rather than controlling the variability may afford a valuable opportunity to elicit insight into what is common or remains invariant across patients or cohorts.
In this study, we investigated three-dimensional (3D) in vivo tibiofemoral skeletal kinematics and arthrokinematics (cartilage contact kinematics) of meniscectomized knees using a state-of-art dynamic stereo-radiographic (DSX) imaging system in our facility, where the similar approach has been validated [18]. We sought to take advantage of the system’s sub-millimeter accuracy in translation and sub-degree accuracy in rotation [20] in detecting the following hypothesized effects of meniscectomy: (1) altered three-dimensional tibiofemoral kinematics, (2) increased cartilage contact area and deformation, and (3) altered tibiofemoral cartilage contact locations and trajectories.
MATERIALS AND METHODS
We recruited nine patients (six females and three males)—age: 25 ± 11 (18–53) years old [average ± standard deviation (range)]; weight: 80.2 ± 22.0 (46.2–112.0) kg; Height: 177.8 ± 9.1 (162.0–187.0) cm; BMI: 25.0 ± 5.2 (17.6–33.0), who had undergone unilateral subtotal or total meniscectomy (four medial and five lateral meniscectomy; Table 1) to participate in this study. These patients were identified from candidates who were scheduled for meniscal allograft transplantation surgery. The condition of patients’ meniscectomized meniscus (such as the location and portion of removed tissue) was arthroscopically examined and documented (Table 1) by the operating surgeon (CHD), and further confirmed by processed MRI data (pixel size=0.365 × 0.365 mm2, slice thickness =0.7 mm, pixel resolution=384 × 384 pixels, field of view =14.0 cm, number of slices=160). The participants had no other knee injuries (such as deficient anterior or/and posterior cruciate ligaments), total joint arthroplasty, cardiovascular disease or neurological disorders that affected lower extremity function. Pregnancy tests were performed prior to the experiment to exclude pregnant participants. This study was approved by the University of Pittsburgh Institutional Review Board (IRB) and informed consent was obtained from all participants.
Table 1.
Arthroscopic assessment of the meniscal condition for the patients with medial or lateral meniscectomy.
| (a) Four patients with medial meniscectomy | ||
| Injured Knee | Description of Injured Meniscus | |
| S2 | Left | 66% of posterior horn, 0% mid portion and 66% anterior horn remaining. |
| S3 | Right | 66% of posterior horn, 0% mid portion and 100% anterior horn remaining. |
| S5 | Right | 66% of anterior horn, 33% of mid portion and 100% of posterior horn remaining. |
| S6 | Right | lost most of the posterior and medial portion of the medial meniscus with only 20% of rim remaining, 66% of anterior horn remaining |
| (b) Five patients with lateral meniscectomy | ||
| Injured Knee | Description of Injured Meniscus | |
| S1 | Left | 33% of posterior horn, less than 33% of mid body, 50% of anterior horn remaining. |
| S4 | Left | 33% in all posterior, mid, and anterior regions remaining; early arthritis of left knee; grade 3 change in weight-bearing area of posterior horn. |
| S7 | Right | 0% of posterior horn, 33% (or less) of mid portion, and 100% of anterior horn remaining. |
| S8 | Right | balanced and stable rim with 50% of meniscus remaining. |
| S9 | Left | total lateral meniscectomy. |
During the experiment, the participants performed decline-walking trials (15 degrees tilted with respect to the ground) on a dual-belt instrumented treadmill at 1.0 m/s (Fig. 1). The DSX system imaged both knees in motion (one-second duration including heel strike) at a frame rate of 100 Hz (X-ray parameters: 80 KV, 125 mA, 1 ms pulse width). The ground reaction forces (GRFs) were measured at 1000 Hz by two force plates (Bertec Corporation, Columbus, OH) embedded in the treadmill. CT scans (slice spacing: 0.625 mm; in-plane resolution: 0.3125 mm) of both knees were also collected prior to the DSX testing. The 3D femoral and tibial bone models were reconstructed from the CT images using a combination of commercial software (Mimics, Materialise, Leuven, Belgium) and manual segmentation.
Figure 1.
A subject performs decline walking (15 degrees, 1 m/s) while her knee joints were being imaged by a dynamic stereo-radiographic system.
A volumetric model-based tracking process determined 3D tibiofemoral kinematics using recorded DSX images and CT-acquired bone models [20]. All collected frames (100 frames per second) were processed in the tracking process. A 10Hz low-pass filter (8th-order Butterworth) was employed to reduce noise from the 3D tracking results before calculating the kinematics. The six-degree-of-freedom (6-DOF) tibiofemoral kinematics including anterior-posterior (AP), proximal-distal (PD) and lateral-medial (LM) translations and internal-external rotation, abduction-adduction and flexion-extension were expressed in the tibial and femoral anatomical coordinate systems defined based on CT-acquired bone models [21]. The translations were expressed in the tibial anatomical coordinate system, while the rotations of the tibia relative to the femur were defined with respect to the femoral anatomical coordinate system. A gait cycle was defined to begin at heel strike and end at the same heel hitting the ground again, consistently identifiable from the vertical GRF profile.
The arthrokinematics measures of cartilage contact were derived using a validated in situ analysis [18, 22]. The cartilage and bone models of tibia and femur were segmented from MRI data and reconstructed in Mimics software. The tibial and femoral cartilage was mapped onto the respective CT-based bone models by co-registering the MRI-based bone models to the CT-based ones (reported root mean square error of alignment: 0.63 mm) [18]. The time-varying positions of the bone models along with the cartilage models were determined by the DSX-measured tibiofemoral bone kinematics, and the intersection volume between tibial and femoral cartilage was calculated at each time interval (Fig. 2 & 3). Three measures were assessed based on the intersection volume between tibial and femoral cartilage at each frame (Fig. 3): (1) the contact area as the area of intersection, (2) the average penetration depth as the intersection depth, and (3) the location of contact centroid as the depth-weighted geometric center of the contact area. The contact area and location of the contact centroid were measured on the tibial plateau plane—a plane determined through Principle Component Analysis (PCA) of the tibial cartilage outer surface (The X and Y axes were designated as the first and second principal component axes passing the centroid of cartilage surface; Fig. 3), whereas the penetration depth was measured orthogonally to the plane. The medial and lateral tibial cartilage outlines (pink and blue boundaries in Figure 3, respectively) are the 2D representations of cartilage boundaries projected onto the plateau plane. The location of contact centroid was expressed in the AP and ML directions on the tibial plateau plane. In addition, AP and ML excursions of the contact centroids on the tibial plateau were assessed by subtracting the minimum coordinates of the contact position from the maximum in respective directions across all the frames, and the total contact length was assessed as the total distance travelled along the trajectory.
Figure 2.
Determination of cartilage contact arthrokinematics from MRI-acquired tibial and femoral cartilage models co-registered with the CT-acquired bone models, driven by DSX-measured skeletal kinematics.
Figure 3.
The representative cartilage intersection projected on the tibial plateau plane (tibial cartilage outlines—thin-line boundaries; lateral: blue; medial: pink). The intersection depth is color-mapped (the depth increases from blue to red); the black solid dots indicate the depth-weighted contact centroids.
Tibiofemoral kinematics and arthrokinematics measures were analyzed for loading response phase and mid stance—from heel strike to the 30% point of a gait cycle, where the greatest forces and rate of change of forces occur [23]. Because crossing of the contralateral limb interferes with knee imaging, obtaining two unobstructed views required for high-accuracy motion assessment through the entire stance phase would require a second set of trials, increasing both the testing time and radiation exposure multiplicatively. The 6-DOF kinematics and arthrokinematics as time-varying responses were sampled at every 1% increment of the 30% of gait cycle.
For all the time-varying variables (such as tibiofemeral kinematics, the location of contact centroid, contact area and penetration), the differences on the corresponding compartments between the menisecetomized and intact knees were computed at each percent increment during the first 30% of gait cycle. Statistical significance regarding to the time-varying differences between the menisecetomized and intact knees could be visually inspected on the 95% confidence interval (CI95) band of the difference values across all participants: if the CI95 band of the difference values does not include zero, the difference is statistically significant (p<0.5). Paired t-tests were used to compare the AP and ML contact point excursions and contact path lengths on the medial and lateral compartments of meniscectomized knees against those on the corresponding compartments of the contralateral intact knees. A significance level of 0.05 and a marginal significance level of 0.1 were used in the present study.
RESULTS
Tibiofemoral kinematics
No consistent significant differences in tibiofemoral kinematics were found between the meniscectomized and contralateral intact knees (p > 0.5) in four patients with medial meniscectomy (Fig. 4a), nor in five patients with lateral meniscectomy (Fig. 4b).
Figure 4.
The 6-DOF tibiofemoral kinematics for (a) four patients with medial meniscectomy and (b) five patients with lateral meniscectomy.
Cartilage contact arthrokinematics
In four medial meniscectomy patients, significant differences were only identified in the medial compartments between the meniscectomized and contralateral intact knees: the ML excursions were significantly greater (p = 0.03, Fig. 5) and the AP excursions were marginally-significantly greater (p = 0.09, Fig. 5) in the medical compartments of the meniscectomized knees. There was no significant difference in the centroid location (in either ML or AP direction), the total contact length of contact centroids, the contact area, or the average penetration depth (Table 2a).
Figure 5.
The average centroid paths of four medial meniscectomy patients on a representative tibial plateau. ** and * denote significant (p<0.5) and marginally significant difference (p<0.1) on ML and AP excursion on the medial compartments between medially meniscectomized and intact knees, respectively.
Table 2.
Differences (meniscectomized – intact) in cartilage contact arthrokinemtics measures during the early stance phase (0–30% of the gait cycle). ** denotes significant difference (p<0.05) and * indicates marginally significant difference (p<0.1) between the meniscectomized and the contralateral intact knees.
| (a) Four patients with medial meniscectomy | ||||
| Measures (unit: mm or mm2) | S2 | S3 | S5 | S6 |
| Medial Compartment | ||||
| Time-averaged contact area | −6 ± 209 | −55 ± 80 | 146 ± 23 | 358 ± 35 |
| Time-averaged penetration | 0.05 ± 0.5 | 0.7 ± 1.1 | 0.2 ± 0.2 | 0.02 ± 0.2 |
| AP coordinates | −1.9 ± 3.9 | 12.1 ± 3.4 | −2.3 ± 2.0 | −4.7 ± 3.0 |
| ML coordinates | −6.7 ± 7.2 | 1.6 ± 5.0 | −2.2 ± 1.9 | −1.4 ± 1.5 |
| AP excursion* | 5.4 | −0.3 | 7.7 | 12.1 |
| ML excursion** | 7.7 | 7.6 | 4.5 | 2.2 |
| Total contact length | −5.4 | 6.4 | 6.4 | −2.2 |
| Lateral Compartment | ||||
| Time-averaged contact area | −34 ± 54 | −361 ± 52 | −20 ± 16 | 61 ± 59 |
| Time-averaged penetration | 0.05 ± 0.4 | −1.1 ± 0.5 | −0.3 ± 0.1 | 0.1 ± 0.3 |
| AP coordinates | −3.2 ± 2.2 | 10.2 ± 2.1 | −0.5 ± 2.6 | 2.7 ± 2.5 |
| ML coordinates | −3.4 ± 2.5 | 5.0 ± 0.8 | −0.8 ± 2.7 | −1.9 ± 1.7 |
| AP excursion | 1.6 | −3.3 | 5.7 | 6.0 |
| ML excursion | 4.6 | −1.0 | 6.2 | 4.7 |
| Total contact length | −8.0 | −13.2 | 2.6 | 23.4 |
| (b) Five patients with lateral meniscectomy | |||||
| Measures (unit: mm or mm2) | S1 | S4 | S7 | S8 | S9 |
| Medial Compartment | |||||
| Time-averaged contact area | 145 ± 39 | 219 ± 26 | −74 ± 41 | −114 ± 24 | 100 ± 27 |
| Time-averaged penetration | 0.5 ± 0.1 | 0.4 ± 0.1 | −0.2 ± 0.1 | −0.3 ± 0.1 | 0.4 ± 0.04 |
| AP coordinates | −2.2 ± 2.3 | 6.1 ± 1.8 | 1.1 ± 3.4 | 0.2 ± 2.2 | −3.2 ± 2.3 |
| ML coordinates | −2.2 ± 3.2 | 6.8 ± 2.3 | 1.1 ± 1.7 | −3.1 ± 1.6 | 2.8 ± 1.6 |
| AP excursion | 4.3 | 5.7 | 0.2 | −1.9 | 0.03 |
| ML excursion | 4.8 | −4.0 | 1.1 | −1.7 | 1.7 |
| Total contact length | −0.9 | 22.1 | −5.1 | −6.2 | −7.8 |
| Lateral Compartment | |||||
| Time-averaged contact area** | 229 ± 23 | 120 ± 61 | 178 ± 58 | 145 ± 37 | 200 ± 34 |
| Time-averaged penetration | 0.6 ± 0.2 | 0.3 ± 0.2 | 0.2 ± 0.1 | 1.2 ± 0.2 | 1.2 ± 0.1 |
| AP coordinates | −1.4 ± 3.0 | −1.8 ± 4.0 | 7.1 ± 2.5 | 4.7 ± 2.7 | −1.0 ± 1.6 |
| ML coordinates** | 6.7 ± 1.5 | 4.7 ± 2.3 | 4.9 ± 1.4 | 2.0 ± 3.8 | 4.7 ± 1.0 |
| AP excursion | −7.3 | 1.4 | −1.2 | −8.5 | −2.7 |
| ML excursion | −2.9 | 1.5 | 2.5 | −7.9 | −3.6 |
| Total contact length | −13.6 | 8.0 | 2.6 | −10.3 | −6.7 |
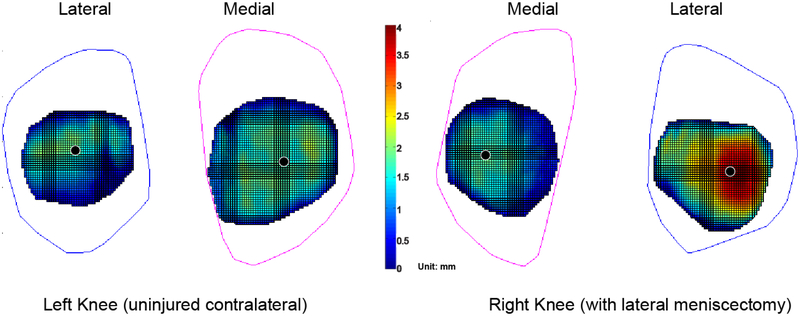
In five lateral meniscectomy patients, significant differences were only identified in the lateral compartments between the meniscectomized and contralateral intact knees: the contact areas were significantly larger (p < 0.5; Table 2b) and the lateral shift of the centroid paths was significantly greater (p < 0.5; Fig. 6, Table 2b) in the lateral components of the meniscectomized knees.
Figure 6.
The average centroid paths of five lateral meniscectomy patients on a representative tibial plateau. ** denotes the centroid path in the lateral compartment on the meniscectomized knees was significantly shifted laterally, comparing to that of the corresponding compartment on the intact knees (p<0.5).
DISCUSSION
The present study quantified the differences in in vivo tibiofemoral kinematics and cartilage contact arthrokinematics between meniscectomized and contralateral intact knees during a functional activity. The results confirmed our speculation that kinematical differences due to the subtotal or total meniscectomy would be pronounced only in the more discriminating arthrokinematics measures at the intra-articular level, and the lateral and medial meniscectomy had both between- and within-type varied effects. The results also rendered some evidence supporting previous clinical and modeling studies in that lateral meniscectomy was considered more detrimental than medial meniscectomy [24–27]. The observed larger cartilage contact area in the lateral compartment of the lateral meniscectomized knees—agreed with a subject-specific finite element modeling study of a patient with lateral meniscectomy [22]—indicated increased loading region on the cartilage, which provides a logical explanation for the higher cartilage degeneration risk after a lateral meniscectomy [24]. The lateral shift in the contact centroid path also may cause normally unloaded regions to withstand abnormally high loading [28], leading to poorer clinical outcome following lateral meniscectomy than medial meniscectomy [29, 30].
In patients who underwent medial meniscectomy, a shift of cartilage contact centroid was not seen in the meniscectomized knees. This may be attributable to the anatomy of medial compartment—specifically, the convex femoral condyle and concave medial tibial plateau, which provides some degree of congruity naturally, even in the absence of part of medial meniscus [25]. It is also consistent with finite element simulation results [31] that showed the removal of various portions of the medial meniscus did not alter the location of maximum contact pressure. Subtle yet significant changes in the medial meniscectomized knees were identified in the trajectories of the contact centroids (i.e., excursion changes in the medial compartment), indicating increased joint instability [32].
This study demonstrated that categorically a medial and a lateral meniscectomy manifest the effects on the knee cartilage contact and interactions differently. In general, a uni-lateral unicompartmental meniscectomized knee only exhibited significant arthrokinematic changes in the corresponding compartment. The contact path shift and increased cartilage contact area in the lateral meniscectomized knees are likely to be related to the greater mobility of the lateral meniscus and greater fraction of the load transmitted through it [33]. As to the medial meniscus, it is known that its posterior portion, when intact, plays a crucial role in restraining AP translation and maintaining knee stability [34–37]. This may also help explain why the medial meniscectomy patients seemed relatively more stable as compared to the lateral meniscectomy patients, since most of them had major portion of posterior corner of the medial meniscus intact (S2, S3, and S5; see Table 1a), in addition to the aforementioned relatively greater congruency of the medial compartment. The destructive effects of posterior-corner medial meniscectomy were notable in S6 who only had about 20% of rim remaining for the posterior and medial portions of the medial meniscus: in addition to larger ML excursion in the meniscectomized knee as other medial meniscectomy patients, S6 also showed markedly greater cartilage contact area and AP excursion changes in the medial compartment along with the greatest total contact length change in the lateral compartment (358 mm2, 12.1 mm and 23.4 mm, respectively; see Table 2a). Nevertheless, the limited patient sample in the present study did not permit a more in-depth inspection of how joint contact characteristics vary depending on the location and extent of meniscal resection.
Contrary to prior studies of tibiofemoral kinematics following meniscectomy [5, 6, 8, 38, 39], the current study did not identify any consistent skeletal kinematic difference between the meniscectomized and contralateral intact knees during early stance phase (no significance was detected even at selected discrete time points (e.g., heel strike) or averaged over the early stance phase). It is difficult to compare our study with the previous studies, given the different experimental methodologies (e.g., in vitro vs. in vivo, choices of task and task parameters) and patient pools (e.g., unknown specific meniscectomy conditions). Specifically, the patient pool in the present study did not seem to be the most representative, especially for our patients with medial meniscectomy—three out of four patients had major resection on the mid-portion (Table 1), while a large prospective study reported that 98% medial meniscal tears were on the posterior portion, 28% on the mid-portion and only 1% involved the anterior horn [40]. This might directly contribute to the conflicting findings against previous in vivo studies of patients with medial meniscectomy [8, 38], where they found greater tibial rotation in the meniscectomized knees and assumed (no documentation of the location and extent of meniscal debridement available) most of their patients involved resection on the posterior horn of the medial meniscus based on the same prospective study [40]. Regardless, the arthrokinematics measures devised in this study allowed us to successfully discern significant differences for five patients with lateral meniscectomy and four with medial meniscectomy respectively, and thus proved to be a more sensitive and insightful approach.
Decline walking was selected as the movement activity for the study. This activity is considered to be modestly stressful and demanding on the knee and can elicit higher levels of shear and compressive forces and activations/co-activations of some muscles than walking on flat ground [41]. A recent study of the meniscal root tear effects on joint kinematics [42] confirmed that more pronounced differences between injured and intact knees were observed during decline walking as compared to level walking.
We recognize the limitation of using non-deformable models for assessing the cartilage contact arthrokinematics and the fact that accurate time-dependent cartilage geometry or deformation information in vivo is currently not achievable. The use of non-deformity models tends to overestimate the areas in contact [18, 22]. We however feel this overestimation posed chiefly a systematic bias with minimal effects on differences between meniscectomized and intact states, particularly of measures such as contact centroid locations or paths. We are also aware that both cartilage and bone can be modeled using MRI without exposing patients to radiation (the current radiation exposure was well below the IRB limit and the exposed area—knee—was far away from other vital organs), however, MRI-derived bone models are subject to geometric distortion—leads to decreased kinematic accuracy, comparing to the present CT-derived bone models that have low distortion and can utilize full volumetric radiodensity information in the model-based tacking procedure [20, 43]. It is also acknowledged that the current study with a limited patient sample was exploratory in nature. Patients with isolated meniscectomy are difficult to recruit since other injuries (e.g., deficient anterior cruciate ligament) are associated with meniscal tears frequently [44–46]. There was variability in the types of meniscectomy (different locations and portions of removed meniscus) in the participating patients, as well as in the time and thus healing since the surgery—there is evidence that gait mechanics can change up to two years post-operation [47, 48]. For instance, S4, who showed evidence of early arthritis of the laterally meniscectomized knee (grade three change in the weight-bearing area of the posterior horn, Table 1b), had a relatively greater difference in the internal/external rotation between the injured and intact knees as compared to other patients with lateral meniscectomy (averaged difference: 5.9 ± 1.8 degree; also shown in Fig. 4b), which might slightly skew the group data. In future studies, it would be sensible to incorporate the change in muscle function of the lower extremity and whole-body dynamic analysis [12], thus gaining more insights into the patients’ coping or adaptation strategies. Further sophisticate subject-specific finite element models with task-specific kinematic and kinetic inputs would provide more valuable information of tissue-level response during in vivo functional activities [22]. The current study encourages us to further pursue this direction and to start addressing issues such as why not all meniscectomy patients have significant cartilage loss as shown by a longitudinal clinical study [49].
HIGHLIGHTS.
In vivo cartilage contact arthrokinemtics are examined noninvasively, comparing the meniscectomized to the contralateral intact knees.
Isolated meniscectomy effects are assessed without other confounding injuries.
Cartilage-based arthrokinemtics is more sensitive than skeletal kinematics.
ACKNOWLEDGMENTS
This work was supported by the NIH (R03-AR059939) and a Musculoskeletal Transplant Foundation (MTF) Established Investigator Grant. The authors thank Tom Gale, Jennifer D’Auria, Ryan Byrne, and Andrew Smith for their assistance in data acquisition and processing. Ethical approval was obtained from University of Pittsburgh Institutional Review Board (Protocol No. 10040479).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: DISCLAIMER
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Conflict of interest: None
REFERENCES
- [1].Montgomery SR, Zhang A, Ngo SS, Wang JC, Hame SL. Cross-sectional Analysis of Trends in Meniscectomy and Meniscus Repair. Orthopedics. 2013;36:e1007–13. [DOI] [PubMed] [Google Scholar]
- [2].Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003;48:2178–87. [DOI] [PubMed] [Google Scholar]
- [3].Scheller G, Sobau C, Bulow JU. Arthroscopic partial lateral meniscectomy in an otherwise normal knee: Clinical, functional, and radiographic results of a long-term follow-up study. Arthroscopy. 2001;17:946–52. [DOI] [PubMed] [Google Scholar]
- [4].Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447–57. [DOI] [PubMed] [Google Scholar]
- [5].Spang JT, Dang AB, Mazzocca A, Rincon L, Obopilwe E, Beynnon B, et al. The effect of medial meniscectomy and meniscal allograft transplantation on knee and anterior cruciate ligament biomechanics. Arthroscopy. 2010;26:192–201. [DOI] [PubMed] [Google Scholar]
- [6].Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. The Journal of bone and joint surgery American volume. 1982;64:883–8. [PubMed] [Google Scholar]
- [7].Sturnieks DL, Besier TF, Mills PM, Ackland TR, Maguire KF, Stachowiak GW, et al. Knee joint biomechanics following arthroscopic partial meniscectomy. J Orthop Res. 2008;26:1075–80. [DOI] [PubMed] [Google Scholar]
- [8].Netravali NA, Giori NJ, Andriacchi TP. Partial medial meniscectomy and rotational differences at the knee during walking. J Biomech. 2010;43:2948–53. [DOI] [PubMed] [Google Scholar]
- [9].Andriacchi TP, Alexander EJ, Toney MK, Dyrby C, Sum J. A point cluster method for in vivo motion analysis: applied to a study of knee kinematics. Journal of biomechanical engineering. 1998;120:743–9. [DOI] [PubMed] [Google Scholar]
- [10].Alexander EJ, Andriacchi TP. Correcting for deformation in skin-based marker systems. J Biomech. 2001;34:355–61. [DOI] [PubMed] [Google Scholar]
- [11].Li K, Zheng L, Tashman S, Zhang X. The inaccuracy of surface-measured model-derived tibiofemoral kinematics. J Biomech. 2012;45:2719–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zheng L, Li K, Shetye S, Zhang X. Integrating dynamic stereo-radiography and surface-based motion data for subject-specific musculoskeletal dynamic modeling. J Biomech. 2014;47:3217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Emken JL, Benitez R, Sideris A, Bobrow JE, Reinkensmeyer DJ. Motor adaptation as a greedy optimization of error and effort. Journal of neurophysiology. 2007;97:3997–4006. [DOI] [PubMed] [Google Scholar]
- [14].Zhou Y, Gu, Zhang H-J. Bayesian Tangent Shape Model: Estimating Shape and Pose Parameters via Bayesian Inference. IEEE International Conference on Computer Vision and Pattern Recognition. [Google Scholar]
- [15].Hirashima M, Oya T. How does the brain solve muscle redundancy? Filling the gap between optimization and muscle synergy hypotheses. Neurosci Res. 2015. [DOI] [PubMed] [Google Scholar]
- [16].Boonstra TW, Danna-Dos-Santos A, Xie H-BB, Roerdink M, Stins JF, Breakspear M. Muscle networks: Connectivity analysis of EMG activity during postural control. Scientific reports. 2015;5:17830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hosseini A, Van de Velde SK, Kozanek M, Gill TJ, Grodzinsky AJ, Rubash HE, et al. In vivo time-dependent articular cartilage contact behavior of the tibiofemoral joint. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Thorhauer E, Tashman S. Validation of a method for combining biplanar radiography and magnetic resonance imaging to estimate knee cartilage contact. Med Eng Phys. 2015;37:937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Arno S, Hadley S, Campbell KA, Bell CP, Hall M, Beltran LS, et al. The effect of arthroscopic partial medial meniscectomy on tibiofemoral stability. Am J Sports Med. 2013;41:73–9. [DOI] [PubMed] [Google Scholar]
- [20].Anderst W, Zauel R, Bishop J, Demps E, Tashman S. Validation of three-dimensional model-based tibio-femoral tracking during running. Med Eng Phys. 2009;31:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975–83. [DOI] [PubMed] [Google Scholar]
- [22].Carey RE, Zheng L, Aiyangar AK, Harner CD, Zhang X. Subject-specific finite element modeling of the tibiofemoral joint based on CT, magnetic resonance imaging and dynamic stereo-radiography data in vivo. Journal of biomechanical engineering. 2014;136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Perry J, Burnfield J. Gait Analysis: Normal and Pathological Function. New Jersey: SLACK Incorporated; 1992. [Google Scholar]
- [24].Pena E, Calvo B, Martinez MA, Palanca D, Doblare M. Why lateral meniscectomy is more dangerous than medial meniscectomy. A finite element study. J Orthop Res. 2006;24:1001–10. [DOI] [PubMed] [Google Scholar]
- [25].McDermott ID, Amis AA. The consequences of meniscectomy. The Journal of bone and joint surgery British volume. 2006;88:1549–56. [DOI] [PubMed] [Google Scholar]
- [26].Chatain F, Adeleine P, Chambat P, Neyret P, Societe Francaise dA. A comparative study of medial versus lateral arthroscopic partial meniscectomy on stable knees: 10-year minimum follow-up. Arthroscopy. 2003;19:842–9. [DOI] [PubMed] [Google Scholar]
- [27].McNicholas MJ, Rowley DI, McGurty D, Adalberth T, Abdon P, Lindstrand A, et al. Total meniscectomy in adolescence. A thirty-year follow-up. The Journal of bone and joint surgery British volume. 2000;82:217–21. [PubMed] [Google Scholar]
- [28].Stergiou N, Ristanis S, Moraiti C, Georgoulis AD. Tibial rotation in anterior cruciate ligament (ACL)-deficient and ACL-reconstructed knees: a theoretical proposition for the development of osteoarthritis. Sports medicine. 2007;37:601–13. [DOI] [PubMed] [Google Scholar]
- [29].Covall DJ, Wasilewski SA. Roentgenographic changes after arthroscopic meniscectomy: five-year follow-up in patients more than 45 years old. Arthroscopy. 1992;8:242–6. [DOI] [PubMed] [Google Scholar]
- [30].Macnicol MF, Thomas NP. The knee after meniscectomy. The Journal of bone and joint surgery British volume. 2000;82:157–9. [PubMed] [Google Scholar]
- [31].Zielinska B, Donahue TL. 3D finite element model of meniscectomy: changes in joint contact behavior. Journal of biomechanical engineering. 2006;128:115–23. [DOI] [PubMed] [Google Scholar]
- [32].Farrokhi S, Voycheck CA, Klatt BA, Gustafson JA, Tashman S, Fitzgerald GK. Altered tibiofemoral joint contact mechanics and kinematics in patients with knee osteoarthritis and episodic complaints of joint instability. Clin Biomech (Bristol, Avon). 2014;29:629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clinical orthopaedics and related research. 1975:184–92. [DOI] [PubMed] [Google Scholar]
- [34].Musahl V, Citak M, O’Loughlin PF, Choi D, Bedi A, Pearle AD. The effect of medial versus lateral meniscectomy on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med. 2010;38:1591–7. [DOI] [PubMed] [Google Scholar]
- [35].Thompson WO, Thaete FL, Fu FH, Dye SF. Tibial meniscal dynamics using three-dimensional reconstruction of magnetic resonance images. Am J Sports Med. 1991;19:210–5; discussion 5–6. [DOI] [PubMed] [Google Scholar]
- [36].Levy IM, Torzilli PA, Gould JD, Warren RF. The effect of lateral meniscectomy on motion of the knee. The Journal of bone and joint surgery American volume. 1989;71:401–6. [PubMed] [Google Scholar]
- [37].Fox AJ, Bedi A, Rodeo SA. The basic science of human knee menisci: structure, composition, and function. Sports health. 2012;4:340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Edd SN, Netravali NA, Favre J, Giori NJ, Andriacchi TP. Alterations in knee kinematics after partial medial meniscectomy are activity dependent. Am J Sports Med. 2015;43:1399–407. [DOI] [PubMed] [Google Scholar]
- [39].Sturnieks DL, Besier TF, Hamer PW, Ackland TR, Mills PM, Stachowiak GW, et al. Knee strength and knee adduction moments following arthroscopic partial meniscectomy. Med Sci Sports Exerc. 2008;40:991–7. [DOI] [PubMed] [Google Scholar]
- [40].Metcalf MH, Barrett GR. Prospective evaluation of 1485 meniscal tear patterns in patients with stable knees. Am J Sports Med. 2004;32:675–80. [DOI] [PubMed] [Google Scholar]
- [41].Kuster M, Wood GA, Sakurai S, Blatter G. 1994 Nicola Cerulli Young Researchers Award. Downhill walking: a stressful task for the anterior cruciate ligament? A biomechanical study with clinical implications. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 1994;2:2–7. [DOI] [PubMed] [Google Scholar]
- [42].Marsh CA, Martin DE, Harner C, Tashman S. Effect of Posterior Horn Medial Meniscus Root Tear on In Vivo Knee Kinematics. Orthopaedic Journal of Sports Medicine 2014;2:2325967114541220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Moro-oka TA, Hamai S, Miura H, Shimoto T, Higaki H, Fregly BJ, et al. Can magnetic resonance imaging-derived bone models be used for accurate motion measurement with single-plane three-dimensional shape registration? J Orthop Res. 2007;25:867–72. [DOI] [PubMed] [Google Scholar]
- [44].Lento PH, Akuthota V. Meniscal injuries: A critical review. Journal of back and musculoskeletal rehabilitation. 2000;15:55–62. [DOI] [PubMed] [Google Scholar]
- [45].Cipolla M, Scala A, Gianni E, Puddu G. Different patterns of meniscal tears in acute anterior cruciate ligament (ACL) ruptures and in chronic ACL-deficient knees. Classification, staging and timing of treatment. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 1995;3:130–4. [DOI] [PubMed] [Google Scholar]
- [46].Bellabarba C, Bush-Joseph CA, Bach BR Jr. Patterns of meniscal injury in the anterior cruciate-deficient knee: a review of the literature. American journal of orthopedics. 1997;26:18–23. [PubMed] [Google Scholar]
- [47].Bulgheroni P, Bulgheroni MV, Ronga M, Manelli A. Gait analysis of pre- and post-meniscectomy knee: a prospective study. The Knee. 2007;14:472–7. [DOI] [PubMed] [Google Scholar]
- [48].Hall M, Wrigley TV, Metcalf BR, Hinman RS, Dempsey AR, Mills PM, et al. A longitudinal study of strength and gait after arthroscopic partial meniscectomy. Med Sci Sports Exerc. 2013;45:2036–43. [DOI] [PubMed] [Google Scholar]
- [49].Cicuttini FM, Forbes A, Yuanyuan W, Rush G, Stuckey SL. Rate of knee cartilage loss after partial meniscectomy. J Rheumatol. 2002;29:1954–6. [PubMed] [Google Scholar]