Abstract
Background
Current medicines do not provide sufficient seizure control for nearly one third of patients with epilepsy. New options are needed to address this treatment gap. We recently found that the atypical amino acid D-leucine protected against acutely-induced seizures in mice but its effect in chronic seizures has not been explored. We hypothesized that D-leucine would protect against spontaneous recurrent seizures. We also investigated whether mice lacking a previously-described D-leucine receptor (Tas1R2/R3) would be protected against acutely-induced seizures.
Methods
Male FVB/NJ mice were subjected to kainic acid-induced status epilepticus and monitored by video EEG (surgically implanted electrodes) for 4 weeks before, during, and after treatment with D-leucine. Tas1R2/R3 knockout mice and controls underwent the maximal electroshock threshold (MES-T) and 6 Hz tests.
Results
There was no difference in number of calendar days with seizures or seizure frequency with D-leucine treatment. In an exploratory analysis, mice treated with D-leucine had a lower number of dark cycles with seizures. Tas1R2/R3 knockout mice had elevated seizure thresholds in the MES-T test but not the 6 Hz test.
Conclusions
D-leucine treatment was ineffective against chronic seizures after kainic acid-induced status epilepticus but there was some efficacy during the dark cycle. Because D-leucine is highly concentrated in the pineal gland, these data suggest that D-leucine may be useful as a tool for studying circadian patterns in epilepsy. Deletion of the Tas1R2/R3 receptor protected against seizures in the MES-T test and therefore may be a novel target for treating seizures.
Keywords: D-amino acid, kainic acid, epilepsy, taste receptors, sleep
1. Introduction
Novel treatments are needed for the nearly one-third of patients with epilepsy whose seizures are not controlled adequately by current medicines [1–3]. More recent data indicate that failure to achieve sustained seizure freedom for more than one year occurs in half of adults with epilepsy and furthermore, many children and adults have repeated periods of seizure recurrence, alternating with periods of remission [4,5]. Thus, current treatments are not associated with long-term seizure control for a substantial proportion of patients. Nutrient-based therapies (including ketogenic diets, the modified Atkins diet, and the low glycemic index treatment) represent an established clinical alternative to currently used medications and leads to durable seizure control in some patients [6,7]. Although most nutrient-based epilepsy treatments have focused on fats and carbohydrates, amino acids also may control seizures [8–10]. We showed recently that L-leucine protected against seizures in mice when injected prior to the excitotoxin kainic acid but surprisingly, only its D-enantiomer was effective in terminating ongoing seizure activity after the onset of seizures [11]. The importance of this finding was that the latter scenario resembles how antiseizure medicines are used in the clinic (most seizure medicines are tested in preclinical models prior to delivery of seizure-inducing stimuli). The atypical amino acid D-leucine is a component of bacterial cell walls and although it is not incorporated de novo into mammalian proteins, it is found in trace amounts in the mammalian brain, including rat and mouse hippocampus, mouse cerebral cortex, and other regions [12,13]. D-leucine accounts for 16–20% of total leucine in perfused mouse cortex and hippocampus (midrange amongst brain amino acids) but it has one of the lowest concentrations in blood [14]. No leucine isomerases have been reported and only one enzyme (D-amino acid oxidase) accounts for nearly all its metabolism into an imino acid, ammonia, and hydrogen peroxide [15–17]. Together, these data suggest that D-leucine concentrations in brain tissue are highly regulated and may modulate neuronal function, although its exact physiological role is unclear.
D-leucine is abundant in the pineal gland, an organ that transduces signals from light stimulation and produces melatonin, thereby partly regulating sleep-wake cycles [13,15,18]. Temporal lobe seizure activity alters the duration of different sleep stages in humans and rodents [19,20]. Therefore, the antiseizure effects of D-leucine might differ between light and dark cycles.
Based on our prior work showing that D-leucine terminated ongoing kainic acid-induced seizures, we hypothesized that D-leucine would protect against spontaneous recurrent seizures (SRS) in a rodent model of epilepsy. We also examined a possible role in seizure protection for Tas1R2/R3, the only receptor reported to date to bind D-leucine.
2. Methods
2.1 Animals
FVB/NJ mice were obtained from Jackson Labs (Bar Harbor, ME) and bred locally. Tas1R2/R3 double knockout mice (on a C57BL/6 background) and wildtype C57BL/6 mice (controls) were from a colony at Henry Ford Health System (Detroit, MI) that was created at University of California, San Diego [21]. These mice were bred locally for three generations prior to seizure testing (genotype was confirmed with PCR). All mice were given free access to water and fed chow ad lib (Teklad Global 2018SX, Madison, WI, USA). Mice were housed 3–4 per cage, except those that had undergone surgery, who were housed singly. The light/dark cycles were 14 hr and10 hr in duration, respectively. All animal protocols were approved by the Johns Hopkins Animal Care and Use Committee and were in compliance with National Institutes of Health Guide for the Care and Use of Laboratory animals (NIH Publications No. 8023, revised 1978).
2.2 Electrode implantation
At six weeks of age, mice were anesthetized in an induction chamber with isoflurane (5%), then transferred quickly to a stereotactic device (Stoelting Co., Chicago, IL, USA) with anesthesia administered by nose cone (2–3% isoflurane, Baxter, Deerfield, IL, USA). Depth of anesthesia was judged by lack of response to gentle paw pinch; respirations were monitored visually while mice were anesthetized. The surgical site was shaved and then prepared with betadine and hydrogen peroxide. A midline incision was made to expose the skull. Screw holes were manually tapped with a sterile 0.025″ drill bit, located 1.5 mm lateral to the sagittal suture and 2.0 mm anterior or 4 mm posterior to bregma. At the coordinates listed, recording electrodes were therefore located over the left frontal, right frontal, and right parietal neocortex regions (confirmed by visual inspection). The ground electrode was located over the left parietal neocortex. The prefabricated headmount (Pinnacle Technology, Lawrence, KS, USA) was positioned over the screw holes after cyanoacrylate (Elmers Products, High Point, NC, USA) was applied and secured with steel screw electrodes, including one ground electrode (0.10″ and 0.12″; Pinnacle Technology, Lawrence, KS, USA). A small midline incision was made over the trapezius to insert EMG electrodes into the muscle. Silver epoxy (Pinnacle Technologies, Lawrence, KS, USA) was applied to improve contact between the screw electrodes and the headmount. Dental acrylic coating (Lang Dental Manufacturing Co., Wheeling, IL) was applied to insulate and protect the EEG leads. Chromic gut 4-0 sutures (Med-Vet International, Mettawa, IL, USA) were used to close the skin layer around the implant and antibiotic ointment (bacitracin/neomycin/polymixin B, Johnson & Johnson, New Brunswick, NJ, USA) was applied. Ketoprofen (5 mg/kg, s.c., Zoetis, Parsippany, NJ, US) was injected after surgery. Post-operatively, the mouse was moved to a cage that was pre-warmed with a heating pad and monitored until ambulating to food and water sources. Mice were treated with either topical lidocaine 5% (Amneal, Bridgewater, NJ) or ketoprofen in the first 18 hours following surgery.
2.3 EEG monitoring
Continuous video EEG monitoring was performed 5–7 days per week for 8–24 hr per day (typically, 12–18 hr, 6 days per week). Mice were housed in a clear cylinder with ad lib access to food and water during monitoring (Pinnacle Technology, Inc., Lawrence, KS, USA). Electrodes were connected to the data collection system via an amplifier and low-torque swivel; dark cycle illumination was provided by an infrared camera (Pinnacle Technology, Inc., Lawrence, KS, USA). Raw data were collected and analyzed using Sirenia Acquisition and Seizure Basic software (version 1.7.5, Pinnacle Technology, Inc., Lawrence, KS, USA). The raw EEG tracing was inspected manually for evidence of seizures and then rescreened using a power level of 100 uV2. When seizures were noted on EEG, the video was used to clinically stage the seizure [22]. Because this was our initial cohort, assessments were unblinded. Video of each mouse was reviewed (with corresponding inspection of raw EEG data) to determine the amount of sleep (quantified in minutes) over 15 min in three hourly epochs on four different days during the pretreatment, treatment, and posttreatment periods (i.e., 12 epochs per treatment period). Sleep was defined as immobility, lying in a curled-up posture with eyes closed and a consistent change in EEG activity from the awake state. Specific days and epochs of time were selected using a random number generator. None of the epochs included seizures.
2.4 Induction of status epilepticus
One week after surgery, kainic acid (5.3 mg/ml PBS; Cayman Chemical, Ann Arbor, MI, USA) was injected intraperitoneally at a dose of 25–100 mg kainic acid/kg mouse body weight (in increments of 10–25 mg/kg) until the mouse went into status epilepticus, defined as persistent epileptiform activity on the EEG accompanied by recurrent clinical seizures for at least 12 hours (the minimum time we determined in pilot experiments to induce epilepsy). Some mice received diazepam i.p. (5 mg/kg) (Hospira, Lake Forest, IL, USA) if clinically evident convulsions were persistent and severe. Mice were injected with PBS (i.p.) 4–8 hr after induction of status epilepticus to maintain adequate hydration.
2.5 D-leucine treatment
D-leucine (Oakwood Chemicals, Estill, SC, USA) was administered ad lib in drinking water (1.5% w/v, using sterile water as a diluent) from a standard water bottle (Pinnacle Technology, Inc., Lawrence, KS, USA) continuously for 28 days during the treatment period.
2.6 Maximal electroshock threshold (MES-T) test
The maximal electroshock threshold test was performed as previously described [23]. Tas1R2/R3 knockout mice (4 males, 3 females) or C57BL/6 controls (5 males, 1 female), all 10 weeks of age at the beginning of testing, were used. Briefly, tetracaine was applied (as in the 6 Hz test) before corneal stimulation using a Rodent Shocker 221 (Harvard Apparatus, Holliston, MA, USA). Settings included shock duration 0.2 s, 500 v, 150 mA; current settings were adjusted in a staircase-like manner, adjusted based on responses in serial testing (i.e., if no seizure occurred, then current was increased to the next level). Scoring was based on the presence or absence of tonic hindlimb extension (i.e., extension of the hindlimbs to 180 degrees in the rostral-caudal plane). The person performing testing was blinded to genotype and mice were selected for testing in random order by an assistant.
2.7 6 Hz test
The 6 Hz electroshock test was performed as described [23]. Tas1R2/R3 knockout mice (4 males, 3 females) or C57BL/6 controls (5 males, 1 female), all 6 weeks of age at the beginning of testing, were used (note that this test was performed in this cohort prior to the MES-T test). Briefly, tetracaine 0.5% ophthalmic solution (Bausch & Lomb, Tampa, FL, USA.) was administered to the corneas before current was delivered (ECT Unit #57800, Ugo Basile North America, Collegeville, PA, USA) at a frequency of 6 Hz, pulse width 0.2 ms, and shock duration 3 s. Current was adjusted in a staircase paradigm, similar to the MES-T test. Seizures were defined as any typical seizure activity including clonus, immobility, facial muscle twitching, automatisms including chewing and unilateral pawing, and a Straub tail. The person performing testing was blinded to genotype and mice were selected for testing in random order by an assistant. Mice were tested in this paradigm before the MES-T.
2.8 Statistical analysis
For EEG analyses, two analyses were performed. In the first, a logistic regression was used to consider the difference in odds of having a seizure or not in each period, adjusting the standard error of the odds ratios for the multiple day measurements and multiple time periods per mouse. The second analysis examined only periods that had at least one seizure, using a negative binomial analysis between the periods, again adjusting the standard error of the incidence rate ratios for the multiple day measurements and multiple time periods per mouse. The incidence rate ratios indicate the average ratio of seizures per day if the mouse had at least one seizure on that day. Statistical analyses were performed using Stata 13.1 (Stata Corporation, College Station, TX, USA).
In the 6 Hz test and MES-T tests, probit analyses were used to calculate the convulsive current 50 (CC50), current where 50% of mice experienced any seizure behavior (Minitab 16, State College, PA, USA). The number of trials used in these analyses was based on our prior work showing nonoverlapping 95% confidence intervals in both the 6 Hz and MES-T tests [11,23]. Sleep duration was analyzed using a paired t-test (GraphPad Prism 7, La Jolla, CA, USA). The level of significance for all tests was P ≤ 0.05 for two comparisons, with Bonferroni corrections for multiple comparisons.
2.9 Role of the funding source
NIH and the Pakula Family provided financial support for the conduct of the research but played no role in study design; collection, analysis, and interpretation of data; manuscript preparation; or decision to submit the manuscript for publication.
3. Results
3.1 D-leucine protects against recurrent seizures in a light cycle-dependent pattern
The short-term effects of D-leucine on acute kainic acid-induced seizures have been described but it is unknown whether it can control SRS [11]. SRS were induced using the post-kainic acid status epilepticus model of temporal lobe epilepsy [24]. FVB/NJ mice develop SRS after i.p. injection of kainic acid and were selected for this reason in this study (NIH Swiss mice used in our prior work do not develop SRS after i.p. injection of kainic acid) [24]. Video EEG commenced one hr prior to injection of kainic acid and continued intermittently until 28 days after treatment stopped, as described in Section 2.3. Mice were under surveillance for 60–168 hr per week, encompassing both dark and light cycles. SRS were noted within the first 2–11 days after the onset of status epilepticus (Fig. 1). Seizures were characterized by the sudden onset of behavior arrest, followed by unilateral pawing and/or rearing, then loss of righting reflex and recurrent clonic activity in all four limbs. Each of these behaviors was noted in varying combinations but the EEG pattern was similar in all, with the onset of recurrent fast high-voltage spike activity in all electrodes that rapidly increased in amplitude and frequency, followed by a decrease in amplitude and frequency, then relative attenuation of amplitude, before a return to baseline EEG (Fig. 1). The typical duration of these events was 10 to 60 seconds. Some mice also showed a pattern of sudden onset of behavior arrest and forceful loss of righting reflex (i.e., appearing to be “thrown” to the bottom of the cage), sometimes accompanied by brief clonic twitching, followed by a rapid return of righting reflex and a brief behavior arrest (total duration 1–2 sec). The EEG consistently showed the sudden onset of high-voltage spiking, similar to the middle of the more prolonged seizures, with a sudden return of the EEG to baseline (1–2 sec total duration). Despite their short duration, these events had a very clear interruption in both ongoing behavior and EEG and therefore were counted as seizures.
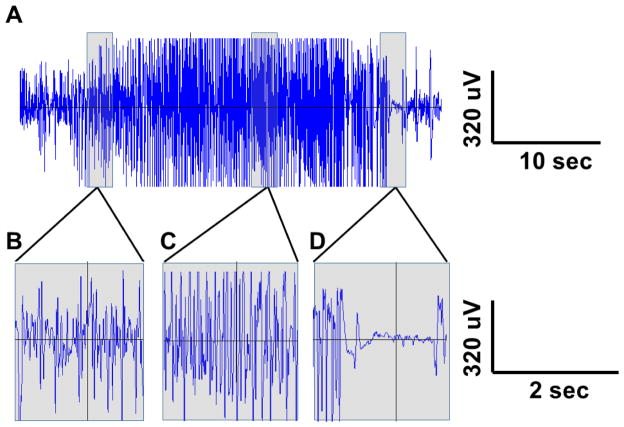
Figure 1. EEG tracing from a typical kainic acid-induced seizure.
(A) EEG was recorded in a male FVB/NJ mouse during a seizure using epidural screw electrodes (cross-hemisphere derivation shown; high pass filter 0.5 Hz, low pass filter 40 Hz). Sections of EEG tracing in (A) are shown on a longer time scale to demonstrate further EEG detail after seizure onset (B), mid-seizure (C), and during periods of brief suppression at the end of the seizure (D).
Baseline EEG was recorded for 30–40 days, without treatment. All mice had recurrent SRS. Seizure counting (for the purpose of our analyses, for 28 days) started once a recurrent seizure pattern was established. D-leucine treatment was then performed for the next 28 days to assess the impact of the amino acid on epilepsy. To determine whether D-leucine had disease modifying properties, the amino acid was then discontinued and EEGs were recorded for another 28 days.
There was no overall impact of D-leucine treatment on either number of days with seizures (adjusted for hours recorded) or seizure frequency on the days when seizures occurred when the pretreatment and treatment periods were compared (Table 1). There also was no difference between the treatment and posttreatment periods, indicating the absence of a sustained disease modifying effect. Seizure frequency increased slightly between the pretreatment and posttreatment periods but there was no difference between the two nontreatment periods in terms of number of days when seizures occurred.
Table 1.
| Combined time (light + dark cycles) | |||
|---|---|---|---|
| Likelihood of days with seizures | |||
| Period | odds ratio | 95% CI | P |
| Pre vs Tx | 0.84 | 0.32–2.18 | 0.72 |
| Pre vs Post | 0.68 | 0.30–1.53 | 0.35 |
| Tx vs Post | 0.8 | 0.19–3.47 | 0.77 |
| Rate of seizures (only on days with seizures) | |||
| Period | odds ratio | 95% CI | P |
| Pre vs Tx | 1.07 | 0.91–1.27 | 0.41 |
| Pre vs Post | 1.6 | 1.10–2.33 | 0.013 |
| Tx vs Post | 1.49 | 1.07–2.09 | 0.019 |
| Dark cycle | |||
| Likelihood of days with seizures | |||
| Period | odds ratio | 95% CI | P |
| Pre vs Tx | 0.4 | 0.24–0.65 | <0.001 |
| Pre vs Post | 0.77 | 0.37–1.61 | 0.49 |
| Tx vs Post | 1.94 | 0.84–4.5 | 0.12 |
| Rate of seizures (only on days with seizures) | |||
| Period | odds ratio | 95% CI | P |
| Pre vs Tx | 1.42 | 0.83–2.43 | 0.2 |
| Pre vs Post | 1.64 | 0.76–3.56 | 0.21 |
| Tx vs Post | 1.16 | 0.41–3.24 | 0.78 |
| Light cycle | |||
| Likelihood of days with seizures | |||
| Period | odds ratio | 95% CI | P |
| Pre vs Tx | 1.12 | 0.40–3.11 | 0.83 |
| Pre vs Post | 0.72 | 0.31–1.66 | 0.44 |
| Tx vs Post | 0.64 | 0.14–3.00 | 0.57 |
| Rate of seizures (only on days with seizures) | |||
| Period | odds ratio | 95% CI | P |
| Pre vs Tx | 1.04 | 0.82–1.34 | 0.7 |
| Pre vs Post | 1.55 | 1.21–1.98 | <0.001 |
| Tx vs Post | 1.48 | 1.23–1.78 | <0.001 |
Pre = pretreatment; Tx = treatment with D-leucine; Post = post-treatment
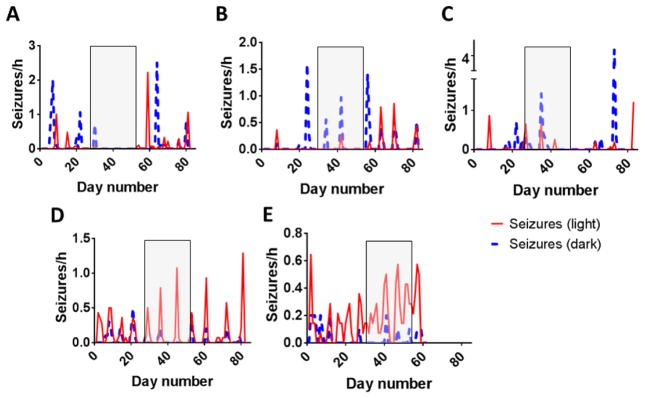
D-leucine is found in the pineal gland (one of the major organs that regulates response to light and dark cycles) [13,15], raising the possibility that it might have a light cycle-dependent effect on seizures. During the dark cycle, the number of days with seizures decreased from pretreatment to the treatment period but the number of seizures returned to baseline once treatment was stopped (Table 1 & Fig. 2). There was no difference in seizure frequency between treatment periods. During the dark cycle, there was no substantial difference between the pretreatment and post treatment periods for either number of days with seizures or seizure frequency. These data raise the possibility that the impact of D-leucine on seizures, which is expressed in high concentrations in the pineal gland, may depend on dark-light cycles.
Figure 2. Seizures per hour in mice before, during, and after treatment with D-leucine.
Mice were monitored by continuous video EEG (A–E). Seizures per hour are indicated during light and dark cycles. Shaded boxes represent the treatment period with D-leucine (1.5% w/v in drinking water). Mouse #5 died after the treatment period ended and therefore, the posttreatment period was truncated. Note that seizure frequency scales differ between panels.
During the light cycle, mice treated with D-leucine had a lower seizure frequency on days when seizures occurred during the treatment period compared to the posttreatment period (there was no difference between the pre-treatment and treatment periods) (Table 1 & Fig. 2). Once again, during the light cycle, there was an increase in seizure frequency between the pretreatment and posttreatment periods (Table 1 & Fig. 2). The magnitude of difference between the former and latter comparisons was similar, leading to the conclusion that the major difference was in the seizure frequency in the posttreatment period, not with D-leucine treatment. During the light cycle, there was no difference in the number of days that mice had seizures between treatment periods. Therefore, there was no impact of D-leucine on seizures during the light cycle.
Temporal lobe seizures alter the duration of different parts of sleep-wake cycles and one potential explanation for the dark cycle findings shown here is that D-leucine affects the duration of sleep cycles [19,20]. Therefore, we examined duration of sleep during different light and dark cycles to determine if amino acid treatment altered the duration of this inactivity. D-leucine treatment did not alter group total sleep time when pretreatment and treatment periods were compared, although most individual mice showed a slight increase (Fig. 3A). When dark and light cycles were analyzed separately, no differences were noted in sleep duration (Fig. 3B & C). Thus, D-leucine does not appear to alter sleep duration in mice with epilepsy and therefore, the effect of the amino acid on dark cycle seizures does not appear to be due to altered sleep patterns.
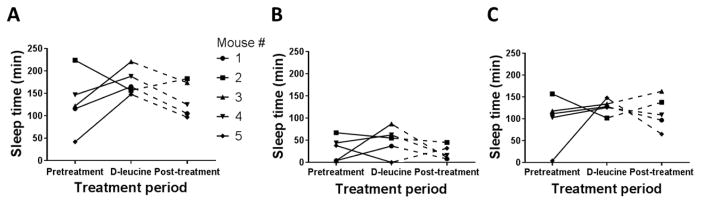
Figure 3. Duration of sleep activity during light and dark cycles.
(A) Total sleep time during pretreatment, treatment, and posttreatment phases (P = 0.22 for pretreatment vs. treatment periods, paired t-test). (B) Sleep time during light cycle hours during each of the treatment phases (P = 0.46 for pretreatment vs. treatment periods, paired t-test). (C) Sleep time during dark cycle hours during each of the treatment phases (P = 0.42 for pretreatment vs. treatment periods, paired t-test). In all panels, the solid line indicates the period used for statistical comparison; data from the dashed lines were not included in the comparison because mouse #5 did not complete the full posttreatment period.
3.2 Deletion of a putative D-amino acid receptor has antiseizure effects
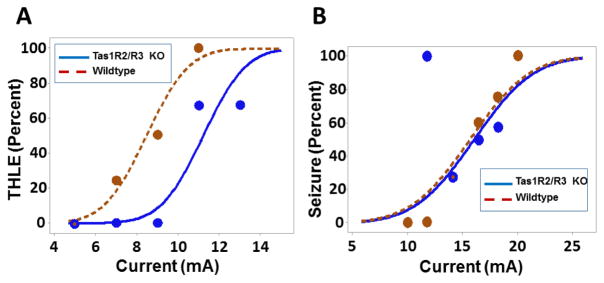
We sought to identify a receptor responsible for the antiseizure effects of D-leucine noted in our prior work (kainic acid and 6 Hz tests) and during the dark cycle in the current EEG experiments. We were unable to identify a receptor for D-leucine in our prior work [11]. In the routine monitoring of mice, we noted a slight increase in water consumption during D-leucine treatment (Fig. S1 shows water measured volumes over time but because of spillage issues, they should be considered relative rather than absolute). D-leucine has been shown to be a ligand for Tas1R2/R3 G-protein coupled taste receptors, which were not assayed in our initial screen [25,26]. Ligands for the Tas1R2/R3 receptor have been shown to have an antiseizure effect in the maximal electroshock test but the impact of the receptor complex (vs. ligands) in seizures has not been reported [27]. Tas1R2/R3 knockout mice had an increased seizure threshold in the maximal electroshock threshold test (MES-T) (Fig. 4A). In contrast, Tas1R2/R3 knockout mice showed no difference from controls in the 6 Hz electroshock threshold test (Fig. 4B), where the ketogenic diet has shown efficacy in NIH Swiss mice [23,28,29].
Figure 4. Acute seizure testing in Tas1R2/R3 double knockout mice.
(A) Probability of mice having a tonic hindlimb extension seizure (THLE) at the indicated MES-T stimulus currents for Tas1R2/R3 knockout mice or C57BL/6 controls. Data are from the same cohort used in Fig. 4B, retested in 3 sessions separated by one week; each mouse received a given current stimulus only once. Data points represent the percentage of mice from all cohorts that had seizures at a given current. Probit analysis curves represent a probability function, therefore not all points lie on the curve (N = 19 trials for 7 Tas1R2/R3 knockout mice, with 3 fatalities over the course of testing; N = 12 trials for 6 wildtype mice, with 4 fatalities over the course of testing; CC50 = 11.2 mA (95% CI = 10.0–12.4 mA) for Tas1R2/R3 knockout, CC50 = 8.5 mA for wildtype mA (95% CI = 7.1 – 9.9 mA) (P = 0.03, probit analysis). (B) Probability of mice having a seizure at the indicated 6 Hz stimulus currents for Tas1R2/R3 knockout mice or C57BL/6 controls (this test was performed prior to the MES-T test). Data are from the same cohort used in Fig. 4A, retested in 3 sessions separated by one week; each mouse received a given current stimulus only once. Data points represent the percentage of mice from all cohorts that had seizures at a given current. Probit analysis curves represent a probability function, therefore not all points lie on the curve (N = 21 trials including 7 Tas1R2/knockout mice and 6 wildtype mice, repeated multiple times; CC50 = 16.1 mA (95% CI = 13.7–18.4 mA) for Tas1R2/R3 knockout, CC50 = 15.7 mA for wildtype mA (95% CI = 13.2 – 18.2 mA) (P = 0.83, probit analysis). All mice survived this testing.
4. Discussion
4.1 D-leucine in seizure protection
Most currently used seizure medicines protect against acutely-induced seizures after a single dose [30]. We showed previously that D-leucine inhibits acute kainic acid-induced seizures, even when administered after seizure onset; D-leucine also elevated seizure threshold in the 6 Hz test after it was administered for 2 weeks via drinking water [11]. However, a more stringent test and clinically useful question is whether a compound can prevent the SRS that define epilepsy [30]. Prolonged recordings performed in this study are likely more helpful in determining the efficacy of treatment when compared to shorter periods used in other studies because of the longer sampling time and inclusion of multiple periods of intermittent seizure exacerbations (Fig. 2). Statistical analyses employed here also accounted for interindividual variation in seizure frequency, which also has been noted in the intrahippocampal kainic acid test [31].
Our data (designed as a limited hypothesis-generating, not confirmatory, study) showed that D-leucine failed the primary endpoint of stopping SRS after kainic acid-induced status epilepticus. This suggests that its clinical utility as a general antiseizure medicine is limited. Interestingly, in an exploratory analysis, D-leucine conferred seizure protection during the dark cycle.
Another major goal in current epilepsy therapeutics is to find treatments that alter the natural history of a disease (i.e., a disease modifier). Practically speaking, a disease-modifying effect should lead to a long-term change after the treatment is removed [32]. We found that even when D-leucine afforded protection during the dark cycle, seizures recurred after treatment was discontinued. Thus, our data are consistent with an antiseizure (i.e., acute), not antiepileptic (i.e., chronic or possibly disease-modifying), effect. It is unclear whether D-leucine would have different outcomes if treatment were started earlier (i.e., during a latent period between the initial status epilepticus and development of SRS) or in different chemical, kindling, or genetic models of epilepsy. It also is possible that D-leucine did not work because FVB/NJ mice might not be as sensitive to this amino acid’s antiseizure effects, compared to NIH Swiss mice used in our previous work [11]. Because of complications that appeared to be related to a combination of seizures, treatment, and mouse sex, we were unable to test our primary hypothesis in female mice, where the response might differ from males.
4.2 Circadian seizure patterns
The relationship between sleep cycles and seizures has been noted for over 130 years [33]. Our exploratory analysis showed that D-leucine improved the number of dark cycles with seizures. In humans, seizure frequency detected on scalp recordings may rely partly on the anatomical location of the seizure onset zone during different times of day (e.g., seizures emanating from frontal regions are more common during sleep while those from the mesial temporal structures tend to be more common during the awake state), although long-term (84-day) intracranial recordings have shown that the circadian difference was between mesial temporal onset vs. neocortical (temporal or frontal) [34–37]. These findings may be age-dependent, with infants being an exception [38]. In preclinical models, long-term (30 week) recording in a rat model of focal-onset seizures after kainic acid-induced status epilepticus showed an increased seizure frequency (detected with EEG depth electrodes) during the light cycle, similar to humans and a mouse model of pilocarpine-induced epilepsy [39,40]. In contrast, a rat model of kainic acid-induced epilepsy showed that activity level (specifically, inactivity) was more closely correlated with recurrent motor seizures than timing in the light-dark cycle; the correlation with activity level was confirmed in a rat model of electrical stimulation-induced temporal lobe epilepsy with seizures monitored using intracranial electrodes [41,42]. However, one study showed no circadian relationship to seizure frequency after pilocarpine-induced seizures [43]. Differences between these models may have been attributable to differences in seizure induction paradigm, monitoring techniques, and/or the rodents (differences in source, breeding, etc.). In our mouse cohort, seizures were more frequent in the dark cycle in three mice but more frequent during the light cycle in two mice. Because we did not use depth electrodes, the exact seizure onset zone was not defined in our mice, limiting a direct comparison to other cohorts.
The mechanism for differences between light and dark cycle seizures reported by others likely is related to connections between the suprachiasmatic nucleus and other limbic, cortical, and subcortical structures; suprachiasmatic nucleus gene expression (e.g., transcriptional modulators including Period 1 and BMAL1) also may play a role [44–46]. The exact role played by specific neurotransmitters and their receptors (e.g., GABA, norepinephrine, and serotonin, among others) is under investigation but hormonal influences by melatonin, cortisol, and feeding also are involved (summarized in [47]). Some fundamental questions remain, such as whether melatonin is proconvulsant or anticonvulsant, which may depend on its concentration in specific brain regions; data from human studies are inconclusive [48,49]. Preclinical work also has shown that the pineal gland also may prevent seizure spread from one hemisphere to the other (the role of melatonin in this context has not been defined) [50].
Data from our exploratory analysis showing differences during the dark cycle suggest that D-leucine may be useful as a probe for studying seizure patterns based on light/dark exposure. Because D-leucine treatment did not appear to induce a substantial clinical change in the duration of sleep time across our cohort, the antiseizure effect of D-leucine appears to be independent of a direct effect on sleep. Endogenous D-leucine is present in high concentrations in the pineal gland, which is involved in the transduction of light signals into hormonal output that affects sleep cycles [13,15,51]. It is unknown whether exogenous D-leucine exerts its antiseizure effects via the pineal gland. The pharmacology of D-leucine in seizure control could be explored by measuring pineal concentrations of D-leucine during light or dark cycles on/off treatment and correlating these concentrations with seizure control. Our data also point to the need for further investigation into whether certain types of medications may be more efficacious for discrete seizure outcomes during different phases of the sleep cycle. In turn, this might have implications for specific types of epilepsy (e.g., electrical status epilepticus of sleep, continuous spike waves of sleep) or its complications in patients with diverse types of epilepsy (e.g., SUDEP or sudden unexpected death of someone with epilepsy).
4.3 Tas1R2/R3 in seizure control
Our prior screen failed to identify a receptor for D-leucine but that panel did not include the sweet taste receptor Tas1R2/R3 [25,52]. This receptor, which is expressed in tongue taste buds, also has been detected (gene and protein) in the hippocampus and neocortex, two major seizure sources in the brain, raising the question of whether Tas1R2/R3 in modulates seizure activity [53,54]. Interestingly, melatonin receptor (MT1 and MT2) mRNAs have been detected in rat circumvallate papilla taste buds (although colocalization studies with taste receptors have not been reported) and nonsensory epithelium [55]. A link between the pineal gland, melatonin, and taste receptors also has not been reported. Based on prior work showing binding of D-leucine to the receptor (in cultured non-neuronal cells overexpressing the protein) and our data suggesting a slight increase in water consumption during D-leucine exposure (consistent with other work showing taste preference of D-leucine over most other D-amino acids, [56]), we investigated whether deletion of the Tas1R2/R3 receptor led to seizure protection or susceptibility [25].
Prior work using the maximal electroshock test showed that known substrates of the Tas1R2/R3 receptor modestly protect against tonic hindlimb extension [27]. Somewhat paradoxically, we found that mice lacking this receptor required more current to achieve tonic hindlimb extension (i.e., required more current to reach the endpoint) in the threshold version of the maximal electroshock test (Fig. 4A). This raises the possibility that these ligands have a net inhibitory action through mechanisms that remain to be elucidated (possibly involving other signaling pathways). One possible resolution to the difference between ligands and receptors in the MES and MES-T tests is that different versions of specific seizure tests may identify different antiseizure properties in the same drug, as noted for pentylenetetrazol (but not reported for the maximal electroshock test) [57,58]. The MES-T test differs from the standard maximal electroshock test in that the main variable in the former is current delivered to the mouse (yielding a threshold current), while in the latter, only a limited number of currents is delivered to mice that have received a wide range of test compound doses (yielding an effective dose). We found previously that D-leucine acutely protected against new-onset seizures in the kainic acid status epilepticus test and after a chronic exposure, in the 6 Hz electroshock threshold test, both in NIH Swiss mice [11]. However, this is not a generalized effect, given the lack of response of D-leucine in the MES and 6 Hz tests performed by the NIH Epilepsy Therapy Screening Program in CF1 mice (R. Raeissi, B. Klein, J. Kehne, personal communication, 2017). More definitive proof that D-leucine exerts antiseizure effects via the Tas1R2/R3 receptor (vs. another receptor) was not tested here (e.g., via administration of D-leucine to mice lacking the Tas1R2/R3 receptor). Therefore, the mechanism by which D-leucine exerts its antiseizure effects remains unknown and results may be rodent strain-dependent.
Conclusions
D-leucine failed the primary endpoint of controlling spontaneous seizures.
In an exploratory analysis, D-leucine protected against seizures during the dark cycle.
D-leucine did not appear to have a disease modifying effect after one month of untreated seizure activity.
Deleting the Tas1R2R3 receptor protects against seizures in the maximal electroshock threshold test.
Supplementary Material
Relative amount of cumulative water consumption for each mouse during pretreatment, treatment, and posttreatment periods. There was substantial spillage, so comparisons should be considered relative, not absolute. Solid line, pretreatment period; dashed line, posttreatment period. Note that there is no posttreatment data point for mouse #5 (deceased).
Highlights.
-
5
D-leucine failed the primary endpoint of controlling spontaneous seizures.
-
6
D-leucine protected against seizures in the dark cycle.
-
7
D-leucine did not appear to have a disease-modifying effect.
-
8
Deleting Tas1R2R3 protects against maximal electroshock threshold seizures.
Acknowledgments
This study was supported by K08NS070931 and the Pakula Family (ALH). For assistance with EEG statistical analyses and the idea of analyzing light and dark cycles separately, we thank Carol B. Thompson (Johns Hopkins Bloomberg School of Public Health Biostatistics Center), supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health grant 1UL1TR001079. Tas1R2/R3 knockout mice and C57BL6 controls were a kind gift from Pablo Ortiz, PhD (Henry Ford Health System, Detroit, MI, USA), created in a colony at University of California, San Diego (used under a Uniform Biological Material Transfer Agreement with University of California, San Diego, PI: Charles Zuker, PhD HHMI/Columbia University). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders And Stroke, National Center for Research Resources, National Center for Advancing Translational Sciences, or the National Institutes of Health. The authors gratefully acknowledge Dr. Keri Martinowich and Dennisse Jimenez (Lieber Institute, Baltimore, MD) for training the authors to perform mouse EEG. This work was performed while ALH was a full-time employee of Johns Hopkins University. Johns Hopkins University filed a provisional patent application for the use of D-leucine in seizures (serial number 61/940,615) but the authors no longer have an intellectual property or financial interest in this application.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 2.Kobau R, Zahran H, Thurman DJ, Zack MM, Henry TR, Schachter SC, et al. Epilepsy surveillance among adults--19 States, Behavioral Risk Factor Surveillance System, 2005. MMWR Surveill Summ. 2008;57:1–20. [PubMed] [Google Scholar]
- 3.Brodie MJ, Barry SJE, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–1554. doi: 10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi H, Hayat MJ, Zhang R, Hirsch LJ, Bazil CW, Mendiratta A, et al. Drug-resistant epilepsy in adults: Outcome trajectories after failure of two medications. Epilepsia. 2016;57:1152–1160. doi: 10.1111/epi.13406. [DOI] [PubMed] [Google Scholar]
- 5.Berg AT, Rychlik K. The course of childhood-onset epilepsy over the first two decades: a prospective, longitudinal study. Epilepsia. 2015;56:40–48. doi: 10.1111/epi.12862. [DOI] [PubMed] [Google Scholar]
- 6.Masino SA, Rho JM. Mechanisms of Ketogenic Diet Action. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- 7.Hartman AL, Stafstrom CE. Harnessing the power of metabolism for seizure prevention: focus on dietary treatments. Epilepsy Behav. 2013;26:266–272. doi: 10.1016/j.yebeh.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dufour F, Nalecz KA, Nalecz MJ, Nehlig A. Modulation of pentylenetetrazol-induced seizure activity by branched-chain amino acids and alpha-ketoisocaproate. Brain Res. 1999;815:400–404. doi: 10.1016/s0006-8993(98)01188-3. [DOI] [PubMed] [Google Scholar]
- 9.Dufour F, Nalecz KA, Nalecz MJ, Nehlig A. Modulation of absence seizures by branched-chain amino acids: correlation with brain amino acid concentrations. Neurosci Res. 2001;40:255–263. doi: 10.1016/s0168-0102(01)00232-2. [DOI] [PubMed] [Google Scholar]
- 10.Skeie B, Petersen AJ, Manner T, Askanazi J, Steen PA. Effects of valine, leucine, isoleucine, and a balanced amino acid solution on the seizure threshold to picrotoxin in rats. Pharmacol Biochem Behav. 1994;48:101–103. doi: 10.1016/0091-3057(94)90504-5. [DOI] [PubMed] [Google Scholar]
- 11.Hartman AL, Santos P, O’Riordan KJ, Stafstrom CE, Hardwick JM. Potent anti-seizure effects of D-leucine. Neurobiol Dis. 2015;82:46–53. doi: 10.1016/j.nbd.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekborg-Ott KH, Armstrong DW. Evaluation of the concentration and enantiomeric purity of selected free amino acids in fermented malt beverages (beers) Chirality. 1996;8:49–57. doi: 10.1002/(SICI)1520-636X(1996)8:1<49::AID-CHIR10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Hamase K, Homma H, Takigawa Y, Fukushima T, Santa T, Imai K. Regional distribution and postnatal changes of D-amino acids in rat brain. Biochim Biophys Acta. 1997;1334:214–222. doi: 10.1016/s0304-4165(96)00095-5. [DOI] [PubMed] [Google Scholar]
- 14.Weatherly CA, Du S, Parpia C, Santos PT, Hartman AL, Armstrong DW. d-Amino Acid Levels in Perfused Mouse Brain Tissue and Blood: A Comparative Study. ACS Chem Neurosci. 2017 doi: 10.1021/acschemneuro.6b00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamase K, Inoue T, Morikawa A, Konno R, Zaitsu K. Determination of free D-proline and D-leucine in the brains of mutant mice lacking D-amino acid oxidase activity. Anal Biochem. 2001;298:253–258. doi: 10.1006/abio.2001.5382. [DOI] [PubMed] [Google Scholar]
- 16.Tishkov VI, Khoronenkova SV. D-Amino acid oxidase: structure, catalytic mechanism, and practical application. Biochemistry Mosc. 2005;70:40–54. [PubMed] [Google Scholar]
- 17.Morikawa A, Hamase K, Inoue T, Konno R, Zaitsu K. Alterations in D-amino acid levels in the brains of mice and rats after the administration of D-amino acids. Amino Acids. 2007;32:13–20. doi: 10.1007/s00726-005-0357-8. [DOI] [PubMed] [Google Scholar]
- 18.Borjigin J, Zhang LS, Calinescu A-A. Circadian regulation of pineal gland rhythmicity. Mol Cell Endocrinol. 2012;349:13–19. doi: 10.1016/j.mce.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bazil CW, Castro LH, Walczak TS. Reduction of rapid eye movement sleep by diurnal and nocturnal seizures in temporal lobe epilepsy. Arch Neurol. 2000;57:363–368. doi: 10.1001/archneur.57.3.363. [DOI] [PubMed] [Google Scholar]
- 20.Matos G, Tsai R, Baldo MV, de Castro I, Sameshima K, Valle AC. The sleep-wake cycle in adult rats following pilocarpine-induced temporal lobe epilepsy. Epilepsy Behav. 2010;17:324–331. doi: 10.1016/j.yebeh.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 22.Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 23.Hartman AL, Zheng X, Bergbower E, Kennedy M, Hardwick JM. Seizure tests distinguish intermittent fasting from the ketogenic diet. Epilepsia. 2010;51:1395–1402. doi: 10.1111/j.1528-1167.2010.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Houk B, Shah J, Hauser KF, Luo Y, Smith G, et al. Genetic background regulates semaphorin gene expression and epileptogenesis in mouse brain after kainic acid status epilepticus. Neuroscience. 2005;131:853–869. doi: 10.1016/j.neuroscience.2004.09.064. [DOI] [PubMed] [Google Scholar]
- 25.Bassoli A, Borgonovo G, Caremoli F, Mancuso G. The taste of D- and L-amino acids: In vitro binding assays with cloned human bitter (TAS2Rs) and sweet (TAS1R2/TAS1R3) receptors. Food Chem. 2014;150:27–33. doi: 10.1016/j.foodchem.2013.10.106. [DOI] [PubMed] [Google Scholar]
- 26.Yarmolinsky DA, Zuker CS, Ryba NJP. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talevi A, Enrique AV, Bruno-Blanch LE. Anticonvulsant activity of artificial sweeteners: a structural link between sweet-taste receptor T1R3 and brain glutamate receptors. Bioorg Med Chem Lett. 2012;22:4072–4074. doi: 10.1016/j.bmcl.2012.04.076. [DOI] [PubMed] [Google Scholar]
- 28.Hartman AL, Lyle M, Rogawski MA, Gasior M. Efficacy of the ketogenic diet in the 6-Hz seizure test. Epilepsia. 2008;49:334–339. doi: 10.1111/j.1528-1167.2007.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samala R, Willis S, Borges K. Anticonvulsant profile of a balanced ketogenic diet in acute mouse seizure models. Epilepsy Res. 2008;81:119–127. doi: 10.1016/j.eplepsyres.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Barker-Haliski ML, Johnson K, Billingsley P, Huff J, Handy LJ, Khaleel R, et al. Validation of a preclinical drug screening platform for pharmacoresistant epilepsy. Neurochem Res. 2017 doi: 10.1007/s11064-017-2227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu G, Gu B, He X-P, Joshi RB, Wackerle HD, Rodriguiz RM, et al. Transient inhibition of TrkB kinase after status epilepticus prevents development of temporal lobe epilepsy. Neuron. 2013;79:31–38. doi: 10.1016/j.neuron.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varvel NH, Jiang J, Dingledine R. Candidate drug targets for prevention or modification of epilepsy. Annu Rev Pharmacol Toxicol. 2015;55:229–247. doi: 10.1146/annurev-pharmtox-010814-124607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gowers WR. Epilepsy and other chronic convulsive diseases: Their causes, symptoms & treatment. 1885 [Google Scholar]
- 34.Pavlova MK, Shea SA, Bromfield EB. Day/night patterns of focal seizures. Epilepsy Behav. 2004;5:44–49. doi: 10.1016/j.yebeh.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Herman ST, Walczak TS, Bazil CW. Distribution of partial seizures during the sleep--wake cycle: differences by seizure onset site. Neurology. 2001;56:1453–1459. doi: 10.1212/wnl.56.11.1453. [DOI] [PubMed] [Google Scholar]
- 36.Spencer DC, Sun FT, Brown SN, Jobst BC, Fountain NB, Wong VSS, et al. Circadian and ultradian patterns of epileptiform discharges differ by seizure-onset location during long-term ambulatory intracranial monitoring. Epilepsia. 2016;57:1495–1502. doi: 10.1111/epi.13455. [DOI] [PubMed] [Google Scholar]
- 37.Kaleyias J, Loddenkemper T, Vendrame M, Das R, Syed TU, Alexopoulos AV, et al. Sleep-wake patterns of seizures in children with lesional epilepsy. Pediatr Neurol. 2011;45:109–113. doi: 10.1016/j.pediatrneurol.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Ramgopal S, Powell C, Zarowski M, Alexopoulos AV, Kothare SV, Loddenkemper T. Predicting diurnal and sleep/wake seizure patterns in paediatric patients of different ages. Epileptic Disord. 2014;16:56–66. doi: 10.1684/epd.2014.0644. [DOI] [PubMed] [Google Scholar]
- 39.Van Nieuwenhuyse B, Raedt R, Sprengers M, Dauwe I, Gadeyne S, Carrette E, et al. The systemic kainic acid rat model of temporal lobe epilepsy: Long-term EEG monitoring. Brain Res. 2015;1627:1–11. doi: 10.1016/j.brainres.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Pitsch J, Becker AJ, Schoch S, Müller JA, de Curtis M, Gnatkovsky V. Circadian clustering of spontaneous epileptic seizures emerges after pilocarpine-induced status epilepticus. Epilepsia. 2017 doi: 10.1111/epi.13795. [DOI] [PubMed] [Google Scholar]
- 41.Hellier JL, Dudek FE. Spontaneous motor seizures of rats with kainate-induced epilepsy: effect of time of day and activity state. Epilepsy Res. 1999;35:47–57. doi: 10.1016/s0920-1211(98)00127-2. [DOI] [PubMed] [Google Scholar]
- 42.Quigg M, Clayburn H, Straume M, Menaker M, Bertram EH. Effects of circadian regulation and rest-activity state on spontaneous seizures in a rat model of limbic epilepsy. Epilepsia. 2000;41:502–509. doi: 10.1111/j.1528-1157.2000.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 43.Bajorat R, Wilde M, Sellmann T, Kirschstein T, Köhling R. Seizure frequency in pilocarpine-treated rats is independent of circadian rhythm. Epilepsia. 2011;52:e118–22. doi: 10.1111/j.1528-1167.2011.03200.x. [DOI] [PubMed] [Google Scholar]
- 44.Eun B, Kim HJ, Kim SY, Kim TW, Hong ST, Choi KM, et al. Induction of Per1 expression following an experimentally induced epilepsy in the mouse hippocampus. Neurosci Lett. 2011;498:110–113. doi: 10.1016/j.neulet.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 45.Gerstner JR, Smith GG, Lenz O, Perron IJ, Buono RJ, Ferraro TN. BMAL1 controls the diurnal rhythm and set point for electrical seizure threshold in mice. Front Syst Neurosci. 2014;8:121. doi: 10.3389/fnsys.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sedigh-Sarvestani M, Blumenfeld H, Loddenkemper T, Bateman LM. Seizures and brain regulatory systems: consciousness, sleep, and autonomic systems. J Clin Neurophysiol. 2015;32:188–193. doi: 10.1097/WNP.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loddenkemper T, Lockley SW, Kaleyias J, Kothare SV. Chronobiology of epilepsy: diagnostic and therapeutic implications of chrono-epileptology. J Clin Neurophysiol. 2011;28:146–153. doi: 10.1097/WNP.0b013e31821213d4. [DOI] [PubMed] [Google Scholar]
- 48.Banach M, Gurdziel E, Jędrych M, Borowicz KK. Melatonin in experimental seizures and epilepsy. Pharmacol Rep. 2011;63:1–11. doi: 10.1016/s1734-1140(11)70393-0. [DOI] [PubMed] [Google Scholar]
- 49.Brigo F, Igwe SC, Del Felice A. Melatonin as add-on treatment for epilepsy. Cochrane Database Syst Rev. 2016:CD006967. doi: 10.1002/14651858.CD006967.pub4. [DOI] [PubMed] [Google Scholar]
- 50.Ninchoji T, Uemura K, Shimoyama I. Effect of pinealectomy on cortically kindled rats. Epilepsy Res. 1990;7:240–244. doi: 10.1016/0920-1211(90)90021-m. [DOI] [PubMed] [Google Scholar]
- 51.Hamase K, Homma H, Takigawa Y, Imai K. Alteration in the D-amino acid content of the rat pineal gland under anesthesia. Amino Acids. 1999;17:277–283. doi: 10.1007/BF01366926. [DOI] [PubMed] [Google Scholar]
- 52.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci. 2009;3:12. doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 55.Zubare-Samuelov M, Peri I, Tal M, Tarshish M, Spielman AI, Naim M. Some sweet and bitter tastants stimulate inhibitory pathway of adenylyl cyclase via melatonin and alpha 2-adrenergic receptors in Xenopus laevis melanophores. Am J Physiol, Cell Physiol. 2003;285:C1255–62. doi: 10.1152/ajpcell.00149.2003. [DOI] [PubMed] [Google Scholar]
- 56.Kasahara T, Iwasaki K, Sato M. Taste effectiveness of some D- and L-amino acids in mice. Physiol Behav. 1987;39:619–624. doi: 10.1016/0031-9384(87)90162-4. [DOI] [PubMed] [Google Scholar]
- 57.Mandhane SN, Aavula K, Rajamannar T. Timed pentylenetetrazol infusion test: a comparative analysis with s.c.PTZ and MES models of anticonvulsant screening in mice. Seizure. 2007;16:636–644. doi: 10.1016/j.seizure.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 58.Coppola G, Arcieri S, D’Aniello A, Messana T, Verrotti A, Signoriello G, et al. Levetiracetam in submaximal subcutaneous pentylentetrazol-induced seizures in rats. Seizure. 2010;19:296–299. doi: 10.1016/j.seizure.2010.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative amount of cumulative water consumption for each mouse during pretreatment, treatment, and posttreatment periods. There was substantial spillage, so comparisons should be considered relative, not absolute. Solid line, pretreatment period; dashed line, posttreatment period. Note that there is no posttreatment data point for mouse #5 (deceased).