Abstract
Aims
PECAM-1 is an abundant endothelial cell surface receptor that becomes highly enriched at endothelial cell-cell junctions, where it functions to mediate leukocyte transendothelial migration, sense changes in shear and flow, and maintain the vascular permeability barrier. Homophilic interactions mediated by the PECAM-1 extracellular domain are known to be required for PECAM-1 to perform these functions; however, much less is understood about the role of its cytoplasmic domain in these processes.
Main methods
CRISPR/Cas9 gene editing technology was employed to generate human endothelial cell lines that either lack PECAM-1 entirely, or express mutated PECAM-1 missing the majority of its cytoplasmic domain (ΔCD-PECAM-1). The endothelial barrier function was evaluated by Electric Cell-substrate Impedance Sensing, and molecular mobility was assessed by fluorescence recovery after photobleaching.
Key findings
We found that ΔCD-PECAM-1 concentrates normally at endothelial cell junctions, but has the unexpected property of conferring increased baseline barrier resistance, as well as a more rapid rate of recovery of vascular integrity following thrombin-induced disruption of the endothelial barrier. Fluorescence recovery after photobleaching analysis revealed that ΔCD-PECAM-1 exhibits increased mobility within the plane of the plasma membrane, thus allowing it to redistribute more rapidly back to endothelial cell-cell borders to reform the vascular permeability barrier.
Significance
The PECAM-1 cytoplasmic domain plays a novel role in regulating the rate and extent of vascular permeability following thrombotic or inflammatory challenge.
Keywords: PECAM-1, glycosylation, sialic acid, endothelial cell, adhesion, permeability, vascular biology
INTRODUCTION
Platelet endothelial cell adhesion molecule (PECAM-1, CD31) is a 130-kDa member of the immunoglobulin (Ig) superfamily that is expressed on the surface of hematopoietic progenitor cells, leukocytes, and platelets, and is highly enriched at the intercellular junctions of confluent endothelial cell monolayers (Albelda et al., 1990, Muller et al., 1989, Newman et al., 1990). PECAM-1 is comprised of a 118-residue cytoplasmic domain, a 19-residue transmembrane domain, and an extracellular domain containing six Ig homology domains, the amino terminal two of which mediate PECAM-1/PECAM-1 homophilic interactions(Paddock et al., 2016, Sun et al., 1996a, Sun et al., 1996b). Extracellular domain-mediated homophilic binding is critical for concentrating PECAM-1 at endothelial cell-cell junctions (Sun et al., 2000, Wong et al., 2000), where it plays an important role in maintaining endothelial barrier integrity following thrombotic or inflammatory challenge – a function that has been demonstrated both in vivo (Carrithers et al., 2005, Ferrero et al., 1995, Graesser et al., 2002, Maas et al., 2005, Mahooti et al., 2000) and in vitro (Lertkiatmongkol et al., 2016, Mei et al., 2014, Privratsky et al., 2011).
The PECAM-1 cytoplasmic domain is encoded by eight exons (Kirschbaum et al., 1994), is largely unstructured (Paddock et al., 2011), and carries out multiple functions in endothelial cells. Specifically, it is required for PECAM-1 to (1) function as part of a mechanosensory complex (Collins et al., 2012, Conway and Schwartz, 2015, Kaufman et al., 2004, Tzima et al., 2005) (2) confer cytoprotection in response to proapoptotic stimuli (Bergom et al., 2006, Evans et al., 2001, Gao et al., 2003), and (3) interact with other junctional adhesion proteins and cytoskeletal molecules (Biswas et al., 2006, Biswas et al., 2003, Biswas et al., 2005, Ilan et al., 2000, Ilan et al., 1999, Tzima et al., 2005). Studies of fusion proteins that contain the PECAM-1 extracellular Ig domains, but transmembrane and cytoplasmic domains of ICAM-1, have demonstrated that the PECAM-1 cytoplasmic domain is not required for its border localization (Sun et al., 2000, Wong et al., 2000). Little is known, however, about the influence of the PECAM-1 cytoplasmic domain on barrier integrity in endothelial cells.
We employed CRISPR/Cas9 gene editing technology to generate a series of novel human endothelial cell lines that either lack PECAM-1 entirely, or express a mutant form of PECAM-1 missing the majority of its cytoplasmic domain. These were then used to examine whether the PECAM-1 cytoplasmic domain regulates endothelial barrier function and, if so, how. Our results demonstrate that loss of the PECAM-1 cytoplasmic domain does not affect its ability to concentrate at the borders of confluent endothelial cells, but unexpectedly enhances its ability to maintain and restore endothelial junctional integrity after challenge. These results suggest that the ability of PECAM-1 to move freely within the plane of the plasma membrane is controlled by its cytoplasmic domain, which in turn determines the efficiency with which endothelial cells are able to establish and maintain their vascular permeability barrier.
RESULTS
Creation of PECAM-1-deficient and PECAM-1 cytoplasmic domain-deleted human immortalized endothelial cell lines
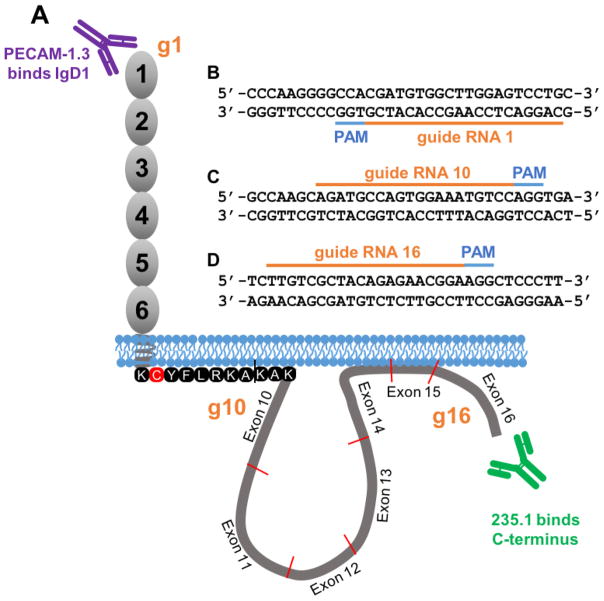
Previous studies examining the function of the PECAM-1 cytoplasmic domain have been carried out using murine NIH3T3 cells (Albelda et al., 1991, DeLisser et al., 1994), monkey Cos7 cells (Albelda et al., 1991), Chinese hamster ovary cells (Wong et al., 2000), murine L-cells (Albelda et al., 1991, Sun et al., 1996a), murine brain endothelioma cells (Tzima et al., 2005, Wong et al., 2000), bovine aortic endothelial cells (Tzima et al., 2005), and human mesothelioma cells (Sun et al., 2000). Although these cell lines grow as adherent monolayers that allow PECAM-1, via diffusion trapping, to concentrate at cell-cell junctions, potential cytoplasmic and/or plasma membrane partners likely vary widely between each of these cell lines and authentic human endothelial cells. Because such components may provide an important context for the function of the PECAM-1 cytoplasmic domain, we used CRISPR/Cas9 technology to edit the PECAM-1 gene in human endothelial cells in situ to produce two novel immortalized cell lines: one in which PECAM-1 is missing completely (KO-PECAM-1 iHUVECs), and one in which only the PECAM-1 cytoplasmic domain has been deleted (ΔCD-PECAM-1 iHUVECs). A schematic diagram depicting sequences of the guide RNAs (gRNAs) used to create these cell lines, and the approximate location of their corresponding target sites in the PECAM-1 gene, is shown in Fig. 1. KO-PECAM-1 iHUVECs were produced by transducing iHUVECs with a lentiviral vector encoding the Cas9 nuclease and gRNA 1 (Fig. 1B) to create an insertion/deletion mutation resulting in a premature stop codon within PECAM-1 exon 1. ΔCD-PECAM-1 iHUVECs were created using a lentiviral vector encoding Cas9 and gRNAs 10 (Fig. 1C) and 16 (Fig. 1D), resulting in deletion of the cytoplasmic domain bounded by exons 10 through 16. The cysteine residue that becomes palmitoylated (Sardjono et al., 2006), as well as positively charged R and K residues that constitute the stop transfer sequence immediately inside the inner face of the plasma membrane, were intentionally left in place to prevent slippage of the transmembrane domain into and out of the lipid bilayer.
Figure 1. Strategy used to generate PECAM-1 knockout and cytoplasmic domain-deleted iHUVEC cell lines.
(A) Schematic of PECAM-1 showing the locations of antibody binding sites for mAb PECAM-1.3, specific for PECAM-1 IgD1, and mAb 235.1, specific for the C-terminus of the PECAM-1 cytoplasmic domain. (B) Guide RNA (gRNA) sequence (orange bar) and the protospacer adjacent motif (PAM) sequences (blue) used to introduce an insertion/deletion in exon 1 of the PECAM-1 gene to generate a PECAM-1-deficient iHUVEC line (KO-PECAM-1). (C–D) Sequence of the gRNAs that frame the PECAM-1 cytoplasmic domain used to generate an iHUVEC line expressing PECAM-1 lacking its cytoplasmic domain (ΔCD-PECAM-1). The approximate location of the binding sites of the gRNA relative to their location in exons 1, 10 and 16 are shown schematically in orange in panel A.
Deletion of the PECAM-1 cytoplasmic domain does not affect the ability of PECAM-1 to localize at endothelial cell-cell borders
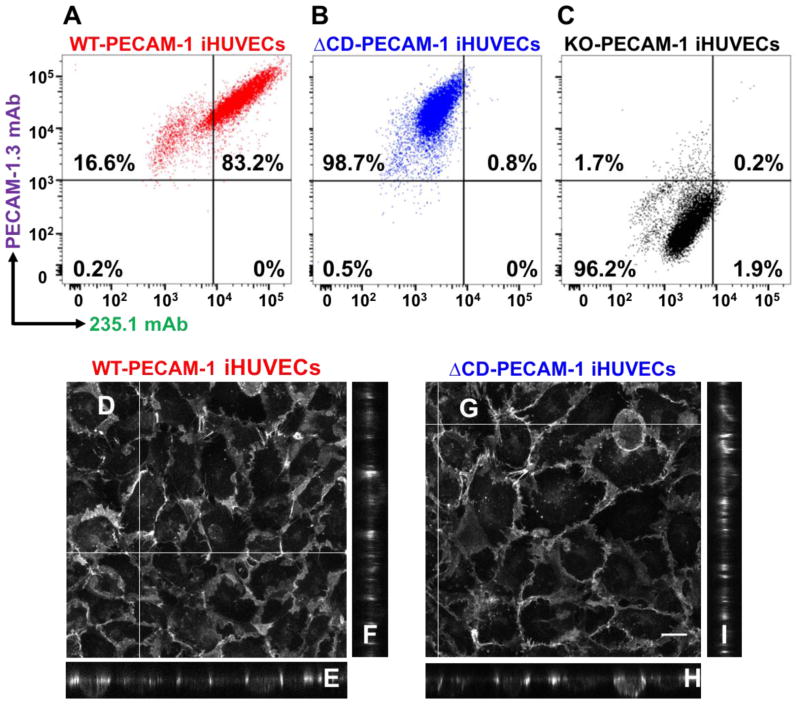
Flow cytometry, employing monoclonal antibodies (mAbs) PECAM-1.3 and 235.1, which are specific for amino and C-termini of the PECAM-1, respectively (depicted in Fig 1.), was used to verify that KO-PECAM-1 iHUVECs lacked PECAM-1 expression, while the ΔCD-PECAM-1 iHUVECs expressed the extracellular, but not cytoplasmic, domain of PECAM-1. As expected, wild-type iHUVECs bound both mAbs (Fig. 2A), ΔCD-PECAM-1 bound only mAb PECAM-1.3 (Fig. 2B), while KO-PECAM-1 iHUVECs bound neither (Fig. 2C). Confocal microscopy was then employed to assess the ability of wild-type PECAM-1 (Fig. 2D–F) and ΔCD-PECAM-1 (Fig. 2G–I) to become concentrated at endothelial cell-cell junctions. Reconstruction of the Z-axis in each of these micrographs demonstrates that ΔCD-PECAM-1 localizes to endothelial intercellular junctions to the same extent as does WT-PECAM-1, and both forms are largely absent from the apical surface in confluent endothelial cell monolayers.
Figure 2. Characterization of CRISPR-generated iHUVEC cell lines.
Flow cytometric data showing the binding of mAbs PECAM-1.3 and 235.1 to wild-type iHUVECs (panel A), ΔCD-PECAM-1 iHUVECs (panel B), and knockout PECAM-1 iHUVECs (panel C). Note the comparable surface expression levels of PECAM-1 in the WT and ΔCD iHUVEC cell lines, but absence of cytoplasmic tail in the ΔCD iHUVEC line. (D–I) Confocal fluorescence microscopy showing combined projection images (Panels D and G), as well as representative cross-sectional images (denoted by white lines) of representative z-planes (Panels E, F, H, and I) in iHUVEC cells expressing either WT-PECAM-1 or ΔCD-PECAM-1. Note that absence of the PECAM-1 cytoplasmic domain does not affect its ability to concentrate at endothelial cell-cell borders. Scale bar = 20 μm.
The PECAM-1 cytoplasmic domain regulates baseline barrier function and the rate of restoration of endothelial cell junctional integrity following disruption by thrombin
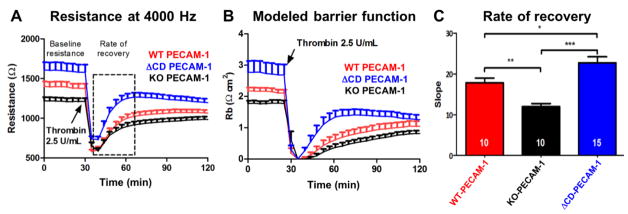
Previous studies have shown the importance of PECAM-1 extracellular domain-mediated homophilic binding in the establishment and maintenance of the vascular barrier (Lertkiatmongkol et al., 2016, Mei et al., 2014, Privratsky et al., 2011), but little is known about the contribution of the PECAM-1 cytoplasmic domain to endothelial cell barrier function. Electric Cell-substrate Impedance Sensing (ECIS) technology, which can monitor subtle changes in endothelial cell barrier function in real-time (Giaever and Keese, 1991), was used to determine whether the PECAM-1 cytoplasmic domain plays a role in regulating vascular permeability. iHUVEC cell lines expressing WT-, ΔCD-, or KO-PECAM-1 were plated on gold electrodes to form confluent monolayers, and thrombin was used to disrupt junctional integrity. As shown in Fig. 3, KO-PECAM-1 iHUVECs had poorer baseline barrier resistance, and exhibited a significantly slower rate of recovery of endothelial cell barrier function following thrombin challenge, than did endothelial cells expressing wild-type PECAM-1, as expected. In contrast, ΔCD-PECAM-1 iHUVECs exhibited tighter baseline resistance and a faster rate and extent of recovery of barrier restoration, suggesting that the cytoplasmic domain of PECAM-1 regulates its ability to contribute to the endothelial cell permeability barrier.
Figure 3. Absence of the PECAM-1 cytoplasmic domain confers enhanced baseline barrier function and faster restoration of endothelial cell junctional integrity following thrombin challenge.
(A) ECIS analysis of the endothelial cell permeability barrier under resting and stimulated conditions of iHUVECs expressing WT and ΔCD forms of PECAM-1. The lines display the mean ± s.d. of the resistance (Ω) over time. The dashed box indicates the time frame used to calculate the rate of recovery. N=10 for the WT and KO-PECAM-1 iHUVEC lines, and 15 for the ΔCD-PECAM-1 cell line. (B) Modeled barrier function (Rb) of data shown in panel A. (C) Linear regression analysis of the resistance curves showing the mean ± s.d. of the slope from the nadir immediately after thrombin challenge to a point near full recovery. Statistics were carried out using one-way ANOVA analysis. *P<0.05; **P<0.01; *** P<0.001. Note the increased baseline barrier resistance as well as the enhanced rate of barrier recovery following thrombin stimulation of iHUVECs expressing ΔCD-PECAM-1.
Deletion of PECAM-1 cytoplasmic domain enhances the mobility of PECAM-1 within the plane of the endothelial cell plasma membrane
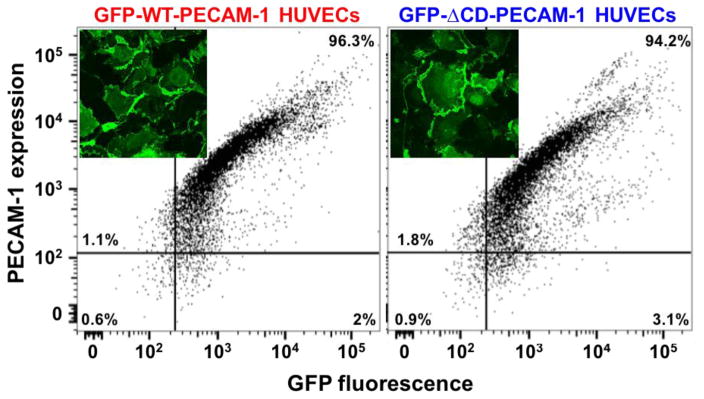
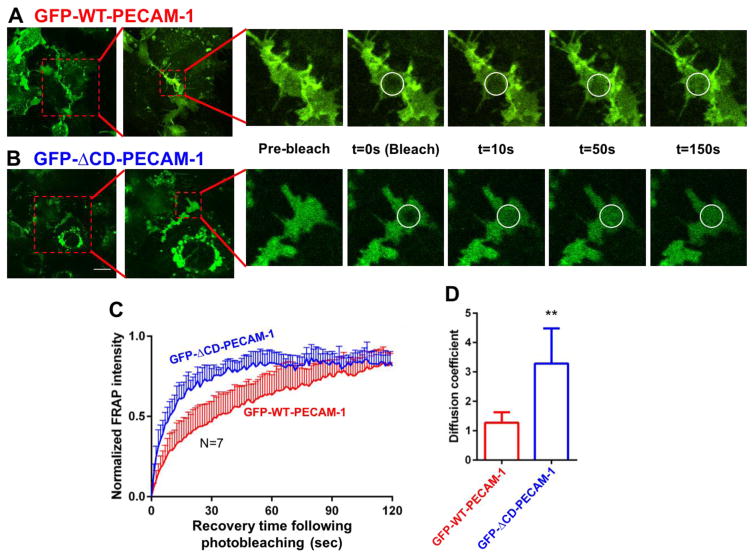
To account for the observation that cytoplasmic domain-deleted PECAM-1 forms tighter baseline barriers that are able to be restored more quickly following thrombotic or inflammatory challenge, we constructed lentiviruses encoding wild-type- and ΔCD-PECAM-1 fused to green fluorescent protein (GFP), and transduced them into PECAM-1-deficient iHUVECs. Flow cytometric and confocal microscopic analysis confirmed that both constructs were expressed to a similar degree on the cell surface (Fig. 4), and capable of concentrating at cell-cell borders (Fig. 4 inset). To examine the possibility that the cytoplasmic domain restricted the mobility of PECAM-1 within the plane of the endothelial cell plasma membrane, we subjected confluent monolayers of these cells to fluorescence recovery after photobleaching (FRAP) analysis. A high intensity laser was used to photobleach cell-cell junctional regions with similar intensities of PECAM-1 staining, after which the rate of recovery of fluorescence, which reports the migration of GFP-tagged molecules into the photobleached region, was quantified using confocal microscopy. As shown in Figure 5, GFP-ΔCD-PECAM-1 had a higher rate of diffusion within the plasma membrane compared to GFP-WT-PECAM-1.
Figure 4. Generation of iHUVEC cell lines expressing full-length and ΔCD forms of human PECAM-1 fused to GFP.
Lentiviral constructs encoding fusion proteins comprised of full-length wild-type PECAM-1 fused to GFP, or ΔCD-PECAM-1 fused to GFP, were transduced into CRISPR-generated PECAM-1-negative iHUVEC cells. Transduced cells expressing similar levels of PECAM-1 were selected by fluorescence-activated cell sorting, and expression additionally evaluated by confocal microscopy (insets).
Figure 5. Fluorescence recovery after photobleaching (FRAP) analysis of the lateral mobility of PECAM-1 within the plane of the plasma membrane.
GFP-positive cells that had formed well-defined cell-cell junctions were subjected to FRAP analysis as described in Materials and Methods. Representative images of iHUVECs expressing wild-type (panel A) and ΔCD-PECAM-1 (panel B) fused to GFP are shown at the indicated time points before and after laser-induced photobleaching. Photobleached areas are marked by white circles. (C) Normalized fluorescence intensity of GFP-WT-PECAM-1 (red) and GFP-ΔCD-PECAM-1 (blue) in the photobleached areas over time following photobleaching. (D) The diffusion coefficients for wild-type and ΔCD-PECAM-1 were calculated from the FRAP images using ImageJ. Data are expressed as the mean ± the standard deviation of seven independent experiments. Significant differences are indicated as ** P<0.01. Scale bar = 20 μm.
DISCUSSION
PECAM-1 is a cell adhesion and signaling receptor expressed on the surface of platelets and leukocytes, and is also the most abundant cell surface molecule on endothelial cells. PECAM-1 becomes highly concentrated at endothelial cell junctions via diffusion trapping – a passive process in which PECAM-1 molecules, diffusing laterally within the lipid bilayer of the plasma membrane, come into contact with PECAM-1 molecules on adjacent cells, interact homophilically in trans, and become “trapped” at the cell junction, where they embellish the permeability barrier (Sun et al., 2000). While the presence of PECAM-1 at endothelial cell-cell borders has been known to contribute to junctional integrity for more than 20 years (Ferrero et al., 1995), and that its absence results in increased vascular permeability under conditions of inflammatory, mechanical, or thrombotic stress (Carrithers et al., 2005, Graesser et al., 2002, Maas et al., 2005, Mahooti et al., 2000), the precise mechanism by which PECAM-1 contributes to junctional integrity, both under steady-state conditions and following barrier disruption, is incompletely understood.
Recent studies examining the barrier properties of endothelial cells, and other validated model cell systems that express mutant forms of PECAM-1 have begun to shed light on the molecular requirements for PECAM-1 to contribute to endothelial cell junctional integrity. Privratsky et al. knocked down expression of PECAM-1 in three different endothelial cell lines using siRNAs, and found, using ECIS technology, that loss of PECAM-1 resulted in both markedly reduced baseline barrier resistance, as well as a significantly decreased capacity to re-establish the endothelial cell permeability barrier following thrombin challenge (Privratsky et al., 2011). Cells expressing a mutant form of PECAM-1 containing a single K89A mutation in Ig domain 1 known to abolish homophilic binding (Newton et al., 1997) exhibited poor barrier resistance properties, similar to those of cells expressing no PECAM-1 at all, demonstrating the importance of PECAM-1-mediated homophilic binding to junctional integrity. Lertkiatmongkol et al. have recently shown that a sialic acid-containing glycan emanating from N25 reinforces dynamic endothelial cell-cell interactions by stabilizing the PECAM-1 homophilic binding interface (Lertkiatmongkol et al., 2016).
The importance of the PECAM-1 cytoplasmic domain in PECAM-1-mediated barrier functions has been less well studied. Cells expressing a mutant form of PECAM-1 in which functionally important immunoreceptor tyrosine-based inhibitory motif (ITIM) tyrosine residues had been mutated to phenylalanine exhibited normal baseline barrier resistance and restored vascular barrier integrity at a rate indistinguishable from cell expressing wild-type PECAM-1 (Privratsky et al., 2011), demonstrating that ITIM-mediated cellular signaling downstream of PECAM-1-mediated homophilic binding plays no discernable role in supporting the endothelial cell permeability barrier. Whether other structural or functional features of the cytoplasmic domain might play a role in PECAM-1-mediated contributions to barrier function, however, were not examined.
In the present investigation, we employed CRISPR/Cas9 gene editing technology to remove exons 10–16 of the PECAM-1 gene in human endothelial cells (Fig. 1) so that we could examine the effects of deleting the cytoplasmic domain on both baseline and dynamic cell permeability barrier function. Editing the PECAM-1 gene in situ has the advantage of not affecting gene expression levels (Fig. 2A–C), while retaining the cellular and molecular regulatory context in which PECAM-1 is normally expressed. Consistent with previous studies employing cell lines transfected with cDNAs encoding PECAM-1 isoforms either lacking the cytoplasmic domain (DeLisser et al., 1994) or containing an irrelevant cytoplasmic domain (Sun et al., 2000, Wong et al., 2000), absence of the PECAM-1 cytoplasmic domain had no effect on the ability of PECAM-1 to, with time, localize to cell-cell borders (Fig. 2D–I and Fig. 4 insets). When the kinetics of PECAM-1 receptor mobility were quantitatively examined using FRAP analysis of GFP-tagged forms of PECAM-1 with or without a cytoplasmic tail, however, we found that PECAM-1 receptors missing their cytoplasmic domain diffused more rapidly within the plane of the plasma membrane (Fig. 5) – a property that manifests itself functionally by conferring both improved baseline barrier resistance and a faster rate of re-establishing a permeability barrier following its disruption by thrombin (Fig. 3). Taken together, these data suggest that the PECAM-1 cytoplasmic domain, perhaps via ITIM-independent interactions with one or more as yet unidentified cytosolic binding partners, functions as a previously unrecognized point of regulation by restraining PECAM-1 receptor mobility within the plane of the plasma membrane and the subsequent homophilic interactions that are important for forming the endothelial cell permeability barrier.
Cytoplasmic domains have been shown to regulate the function and subcellular location of many cell adhesion molecules, including vascular endothelial cadherin (Kowalczyk et al., 1998), integrins (Chan et al., 1992, Marcantonio et al., 1990, O’Toole et al., 1991), selectins (Kansas et al., 1993), CD44 (Lesley et al., 1992), as well as other Ig superfamily CAMs (Carpen et al., 1992, Powell et al., 1991, Shin et al., 1991), often via their association with one or more elements of the actin or membrane cytoskeleton. The removal of the β-catenin–binding domain within the cytoplasmic tail of VE-cadherin has been reported to result in disorganization of adherens junctions and hyperpermeability of vascular endothelial cells (Miyazaki et al., 2011). Similarly, calpain-mediated cleavage of N-cadherin showed reduced cell-cell adhesion (Jang et al., 2009), while calpain cleavage of E-cadherin is involved in tumor progression (Trillsch et al., 2016, Ye et al., 2013). Thus, deletion of the cytoplasmic domain of cadherins results in distinctly different cell biological effects than does deletion of the PECAM-1 cytoplasmic domain. Su et al has shown that the decreased barrier function could be attributed to the increased degradation of VE-cadherin after the truncation (Su and Kowalczyk, 2017). Although, as important components at intercellular junctions, PECAM-1 and cadherins share some similarities in terms of structure and functions, cadherins and PECAM-1 are quite distinct in their lateral border localization and detergent extractability (Ayalon et al., 1994), reflecting differences in their mode of association with the cytoskeleton. As for PECAM-1, Ayalon et al. reported more than 20 years ago that a proportion of PECAM-1 is associated with the Triton-insoluble cytoskeleton in endothelial cells (Ayalon et al., 1994), and there are numerous reports that PECAM-1 may be linked to the cytoskeletal adapter molecules, β and γ catenin (Biswas et al., 2006, Biswas et al., 2003, Biswas et al., 2005, Ilan et al., 2000, Ilan et al., 1999), though this has not been universally observed (Lampugnani et al., 1995). Wong et al. reported that PECAM-1 can be associated in cis with the integrin αvβ3 (Wong et al., 2000), which is in turn associated with the actin cytoskeleton. In stimulated platelets, PECAM-1 may be linked to the actin cytoskeleton through the cytosolic adaptor protein, moesin (Gamulescu et al., 2003), and in endothelial cells under conditions of fluid shear stress with the intermediate filament protein vimentin (Conway et al., 2013). Whether these or other cytoskeletal proteins associate with PECAM-1 to restrict its mobility under resting or shear conditions, or in response to endothelial cell injury should be a fascinating topic for future investigations.
CONCLUSIONS
These results demonstrate that, in the absence of its cytoplasmic domain, PECAM-1 is freer to diffuse within the plane of the plasma membrane, migrate to, and become concentrated at endothelial cell-cell junctions, where it engages in homophilic interactions that establish and maintain barrier function. Whether other mechanisms may account for regulation of endothelial barrier function by the truncation of PECAM-1’s cytoplasmic domain remains an intriguing question, the elucidation of which will have important implications for understanding the pathological processes in vascular permeability disorders.
MATERIALS AND METHODS
Antibodies
Domain-specific mouse anti-human PECAM-1 monoclonal antibodies (mAbs) used in this study include: 235.1 (specific for the C-terminal 15 amino acids), and PECAM-1.3 (specific for Ig Domain 1), and have been previously described (Newman et al., 2002, Yan et al., 1995). Normal mouse IgG and secondary antibodies were purchased from Thermo Fisher Scientific (Waltham, MA). Alexa Fluor® 647-labeled mAb PECAM-1.3 and Alexa Fluor® 647-labeled mAb 235.1 were generated using a labeling kit purchased from Thermo Fisher Scientific (Waltham, MA).
Guide RNA plasmid constructs
Guide RNAs (gRNAs) were designed using the clustered regularly interspaced short palindromic repeats (CRISPR) Design Tool (http://crispr.mit.edu/) to minimize off-target effects and selected to precede a 5′-NGG protospacer-adjacent motif (PAM). gRNAs used in this study were: gRNA1 forward: 5′-CACCGCAGGACTCCAAGCCACATCG-3′, reverse: 5′-AAACCGATGTGGCTT-GGAGTCCTGC-3′; gRNA10 forward: 5′-CACCGAGATGCCAGTGGAAATGTCC-3′, reverse: 5′-AAACGGACATTTCCACTGGCATCTC-3′; gRNA16 forward: 5′-CACCGTTGTCGCTAC-AGAGAACGGA-3′, reverse: 5′-AAACTCCGTTCTCTGTAGCGACAAC-3′. Oligos were annealed and cloned into the BsmBI site of the Cas9 expression plasmid lentiCRISPR v2 (#52961, Addgene, Cambridge, MA) following a previously described protocol (Sanjana et al., 2014).
Cell lines and transduction
Cell culture reagents were obtained from Mediatech (Manassas, VA) unless otherwise specified. Immortalized human umbilical vein endothelial cells (iHUVEC, generated by transducing HUVECs with the recombinant retrovirus LXSN16 E6/E7) and PEC02 cells (generated by transducing iHUVECs with a lentivirus expressing a PECAM1-specific siRNA PEC02 - (Privratsky et al., 2011)) were maintained in RPMI1640 medium (Mediatech), 10% FBS (Sigma, St Louis, MO), 5% human AB serum (Gemini, West Sacramento, CA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.5 mg/ml endothelial cell growth supplement (Corning, Corning, NY) as previously described (Privratsky et al., 2011).
Generation of a PECAM-1-deficient immortalized HUVEC line
Lentiviruses were generated and titrated by the Viral Vector Core Facility at the Blood Research Institute. iHUVECs cells were plated on 24-well plates one day before transduction, and transduced with gRNA1 lentiviral particles at an MOI of 5 in iHUVECs media containing 0.8 μg/ml polybrene. After 48 hours, media containing puromycin (0.5 μg/ml) was added. Transduced cells were stained with 30 μg/ml Alexa Fluor 647-labeled mAb PECAM-1.3 and sorted by flow cytometry (ARIA-IIIu Cell Sorter, BD Biosciences, San Jose, CA) for PECAM-1 negative cells. KO-PECAM-1 endothelial cells were maintained in iHUVEC medium containing puromycin.
Generation of ΔCD-PECAM-1 iHUVEC cell line
iHUVECs were plated on 24-well plates one day before transduction, and then transduced with a 1:1 mixture of gRNA10 and gRNA16 lentiviral particles at an MOI of 5 in iHUVECs media containing 0.8 μg/ml polybrene. Forty-eight-hours post-transduction, puromycin (0.5 μg/ml) was added to the medium. Cells were sorted as single cells into individual wells of 96-well plates 15 to 18 days post-puromycin selection. HUVECs in which PECAM-1 had been knocked down using an siRNA (PEC02 cells (Privratsky et al., 2011)) were used as feeder cells. Three weeks later, clones were stained by 30 μg/ml Alexa Fluor 647-labeled mAb PECAM-1.3 and sorted by flow cytometry to obtain a PECAM-1-positive population, and puromycin was introduced into the medium for 48 hours to further remove the feeder cells.
Characterization of endothelial cell lines by flow cytometry
Flow cytometric analysis of WT-, ΔCD-, or KO-PECAM-1 iHUVECs was performed using a BD Cytofix/Cytoperm™ (BD Biosciences, San Jose, CA) according to the manufacturer’s directions. Briefly, Alexa Fluor® 647-labeled mAb PECAM-1.3 (30 μg/ml) was used in surface staining, and Alexa Fluor® 488-labeled mAb 235.1 (30 μg/ml) was used in intracellular staining. Flow cytometry was performed using a Becton-Dickenson LSRII (BD Biosciences, San Jose, CA). Flow cytometry data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
Confocal microscopy
Cells were plated at a density of 2 × 105/well on gelatin-coated slide chambers (BD Biosciences) at least 24 hours before staining. Wells were rinsed with DPBS, cells fixed with 2% paraformaldehyde for 20 minutes, and then permeabilized with ice-cold 0.5% Triton X-100 for 2 minutes, and blocked with PBS containing 3% BSA. Monolayers were then incubated with mAb PECAM-1.1 (10 μg/ml) at room temperature for 1 hour. Binding was detected using Alexa Fluor 647 anti-mouse IgG (Invitrogen) and images were obtained using a FluoView FV1000 multi-photon emission microscope (Olympus, Center Valley, PA). Cross sectional reconstruction was accomplished with MetaMorph (Molecular Devices, Inc., Nashville, TN) workstation to combine the entire Z series into a stacked projection.
ECIS measurements of endothelial barrier function
Cells were grown to confluence on gold electrodes that had been coated with 0.1% gelatin (Invitrogen, Carlsbad, CA) for an hour at 37°C and subjected to Electric Cell-substrate Impedance Sensing (ECIS) analysis using an ECIS Z-Theta Instrument (Applied Biophysics, Troy, NY). To measure PECAM-1-mediated endothelial cell barrier function, cells were grown in 400 μl of iHUVEC medium on 8W10E+ electrode arrays until forming a tight monolayer. After stimulating cells with 1 unit of human thrombin (Sigma-Aldrich, St. Louis, MO), endothelial barrier disruption and restoration were measured in real time and continuously recorded at multiple frequencies and modeled with ECIS software (Applied Biophysics, Troy, NY) to obtain the barrier function parameter, Rb, which is expressed as the average basal electrical resistances (in Ωcm2).
Generation and characterization of endothelial cell lines expressing GFP-PECAM-1 chimeric proteins
A silent mutation was introduced into the PAM sequence corresponding to guide 1 in PECAM-1 cDNA using QuikChange II site-directed mutagenesis (Agilent Technologies, Santa Clara, CA) in order to protect it from CRISPR/Cas9 using the following oligonucleotide primers: (1) Fwd, 5′-GGTGGGCCCAAGGGGCGACCATGTGGCTTGGAGTC-3′; (2) Rev, 5′-GACTCCAAGC-CACATGGTCGCCCCTTGGGCCCACC-3′ (silent mutation sites are underlined). Mutated full-length human PECAM-1 or ΔCD PECAM-1 was inserted into the backbone plasmid pLenti CMV GFP Neo (Addgene # 17447) using an In-Fusion® HD Cloning Kit (Clontech, Laboratories, Inc., Mountain View, CA) to generate a GFP-PECAM-1 chimeric protein. A linker sequence was inserted between GFP and the mutated WT-PECAM-1 or ΔCD-PECAM-1. The primers for GFP-WT-PE chimeric protein were: forward, 5′-GGTGGCGGAGGCTCTCAAGAAAACTCTTTCA-CAATCAACAG-TGTT-3′; reverse, 5′-GAGGTTGATTGTCGACCTAAGTTCCATCAAGGG-AGCCTT-3′. The primers for GFP-ΔCD-PECAM-1 chimeric protein were: forward, 5′-GGTGG-CGGAGGCTCTCAAGAAAACTCTTTCACAATCAACAGTGTT-3′; reverse, 5′-GAGGTTG-ATTGTCGACCTACTTGGCCTTGGCTTTCCTCA-3′. KO-PECAM-1 cells were seeded in 24-well plates and transduced with lentiviral particles containing GFP-WT-PECAM-1 or GFP-ΔCD-PECAM-1 at an MOI of 5 in 0.8 μg/ml polybrene-containing iHUVEC media. Transduced cells were further selected by addition of 0.5 mg/ml G418 48 hours post-transduction. Cell lines were sorted for Alexa Fluor 647-labeled mAb PECAM-1.3-positive expression.
Fluorescence Recovery after Photobleaching (FRAP)
Cells were plated on a 60 mm-diameter dish (Celltreat, Pepperell, MA) and cultured for at least 24 hours until cell monolayers had reached confluence. FRAP experiments were designed and performed as previously described (Day et al., 2012) using an FV1000 laser-scanning confocal microscope (Olympus, Tokyo, Japan) with a 100X objective lens. GFP fluorescence was imaged by excitation with a 488-nm laser at 37°C with 5% CO2 using an Air-Therm-H heater (World Precision Instrument, Sarasota, FL). The region of interest at cell–cell contacts was selected and bleached for 300 ms using a 405 nm laser. Fluorescence intensity was monitored continuously and images were acquired until recovery reached a plateau using the FluoView-ASW-10 version 04.02.02.09 software (Olympus, Tokyo, Japan). The fluorescence intensity of the bleached region was corrected for photobleaching and normalized to prebleaching fluorescence intensity by ImageJ (NIH, Bethesda, MD). This algorithm allows computation of the diffusion coefficient of the fluorescent probes (2D random walk). Prism 5 software (GraphPad Software, San Diego, CA) was used for plotting the data and statistical analysis.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA). Data were analyzed by one-way ANOVA followed by Holm-Sidak’s multiple-comparisons test. Multiple comparisons tests were only applied when a significant difference was determined in the ANOVA (p < 0.05). Results are expressed as mean ± S.D.
Acknowledgments
We thank Dr. William Muller, Northwestern University, for providing the iHUVEC cell line, Dr. Sid Rao (Blood Research Institute) for bioinformatics assistance in enacting CRISPR technology, the BRI Lentiviral Core Laboratory for lentiviral production, and Dr. Marie Schultz (BRI Imaging Core) for assistance in confocal FRAP technology and analysis.
Funding
This research was supported by grant HL40926 (to PJN) from the Heart, Lung, and Blood Institute of the National Institutes of Health, and by a grant (to DL) from the China Scholarship Council.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
D.L. performed the majority of the studies, analyzed data, and wrote the manuscript. H.M. and Y.H. supplied funding, and helped edit the manuscript. D.K.N designed experiments, analyzed data, and wrote the manuscript. P.J.N designed experiments, analyzed data, and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991;114:1059–68. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda SM, Oliver PD, Romer LH, Buck CA. EndoCAM: A novel endothelial cell-cell adhesion molecule. J Cell Biol. 1990;110:1227–37. doi: 10.1083/jcb.110.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalon O, Sabanai H, Lampugnani MG, Dejana E, Geiger B. Spatial and temporal relationships between cadherins and PECAM-1 in cell-cell junctions of human endothelial cells. J Cell Biol. 1994;126:247–58. doi: 10.1083/jcb.126.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergom C, Goel R, Paddock C, Gao C, Newman DK, Matsuyama S, et al. The cell-adhesion and signaling molecule PECAM-1 is a molecular mediator of resistance to genotoxic chemotherapy. Cancer Biol Ther. 2006;5:1699–707. doi: 10.4161/cbt.5.12.3467. [DOI] [PubMed] [Google Scholar]
- Biswas P, Canosa S, Schoenfeld D, Schoenfeld J, Li P, Cheas LC, et al. PECAM-1 affects GSK-3beta-mediated beta-catenin phosphorylation and degradation. Am J Pathol. 2006;169:314–24. doi: 10.2353/ajpath.2006.051112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas P, Canosa S, Schoenfeld J, Schoenfeld D, Tucker A, Madri JA. PECAM-1 promotes beta-catenin accumulation and stimulates endothelial cell proliferation. Biochem Biophys Res Commun. 2003;303:212–8. doi: 10.1016/s0006-291x(03)00313-9. [DOI] [PubMed] [Google Scholar]
- Biswas P, Zhang J, Schoenfeld JD, Schoenfeld D, Gratzinger D, Canosa S, et al. Identification of the regions of PECAM-1 involved in beta- and gamma-catenin associations. Biochem Biophys Res Commun. 2005;329:1225–33. doi: 10.1016/j.bbrc.2005.02.095. [DOI] [PubMed] [Google Scholar]
- Carpen O, Pallai P, Staunton DE, Springer TA. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and alpha-actinin. J Cell Biol. 1992;118:1223–34. doi: 10.1083/jcb.118.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol. 2005;166:185–96. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BMC, Kassner PD, Schiro JA, Byers HR, Kupper TS, Hemler ME. Distinct cellular functions mediated by different VLA integrin a subunit cytoplasmic domains. Cell. 1992;68:1051–60. doi: 10.1016/0092-8674(92)90077-p. [DOI] [PubMed] [Google Scholar]
- Collins C, Guilluy C, Welch C, O’Brien ET, Hahn K, Superfine R, et al. Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Current biology: CB. 2012;22:2087–94. doi: 10.1016/j.cub.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Current biology: CB. 2013;23:1024–30. doi: 10.1016/j.cub.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DE, Schwartz MA. Mechanotransduction of shear stress occurs through changes in VE-cadherin and PECAM-1 tension: Implications for cell migration. Cell adhesion & migration. 2015;9:335–9. doi: 10.4161/19336918.2014.968498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CA, Kraft LJ, Kang M, Kenworthy AK. Analysis of protein and lipid dynamics using confocal fluorescence recovery after photobleaching (FRAP) Curr Protoc Cytom. 2012;Chapter 2(Unit2):19. doi: 10.1002/0471142956.cy0219s62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisser HM, Chilkotowsky J, Yan H-C, Daise M, Buck CA, Albelda SM. Deletions in the cytoplasmic domain of Platelet-Endothelial Cell Adhesion Molecule-1 (PECAM-1, CD31) result in changes in ligand binding properties. J Cell Biol. 1994;124:195–203. doi: 10.1083/jcb.124.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PC, Taylor ER, Kilshaw PJ. Signaling through CD31 protects endothelial cells from apoptosis. Transplantation. 2001;71:457–60. doi: 10.1097/00007890-200102150-00020. [DOI] [PubMed] [Google Scholar]
- Ferrero E, Ferrero ME, Pardi R, Zocchi MR. The platelet endothelial cell adhesion molecule-1 (PECAM1) contributes to endothelial barrier function. Febs Lett. 1995;374:323–6. doi: 10.1016/0014-5793(95)01110-z. [DOI] [PubMed] [Google Scholar]
- Gamulescu MA, Seifert K, Tingart M, Falet H, Hoffmeister KM. Platelet moesin interacts with PECAM-1 (CD31) Platelets. 2003;14:211–7. doi: 10.1080/0953710031000118830. [DOI] [PubMed] [Google Scholar]
- Gao C, Sun W, Christofidou-Solomidou M, Sawada M, Newman DK, Bergom C, et al. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood. 2003;102:169–79. doi: 10.1182/blood-2003-01-0003. [DOI] [PubMed] [Google Scholar]
- Giaever I, Keese CR. Micromotion of mammalian cells measured electrically. Proc Natl Acad Sci USA. 1991;88:7896–900. doi: 10.1073/pnas.88.17.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, et al. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002;109:383–92. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan N, Cheung L, Pinter E, Madri JA. Platelet-endothelial cell adhesion molecule-1 (CD31), a scaffolding molecule for selected catenin family members whose binding is mediated by different tyrosine and serine/threonine phosphorylation. J Biol Chem. 2000;275:21435–43. doi: 10.1074/jbc.M001857200. [DOI] [PubMed] [Google Scholar]
- Ilan N, Mahooti S, Rimm DL, Madri JA. PECAM-1 (CD31) functions as a reservoir for and a modulator of tyrosine-phosphorylated b-catenin. J Cell Sci. 1999;112:3005–14. doi: 10.1242/jcs.112.18.3005. [DOI] [PubMed] [Google Scholar]
- Jang YN, Jung YS, Lee SH, Moon CH, Kim CH, Baik EJ. Calpain-mediated N-cadherin proteolytic processing in brain injury. J Neurosci. 2009;29:5974–84. doi: 10.1523/JNEUROSCI.6178-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansas GS, Ley K, Munro JM, Tedder TF. Regulation of leukocyte rolling and adhesion to high endothelial venules through the cytoplasmic domain of L-selectin. J Exp Med. 1993;177:833–8. doi: 10.1084/jem.177.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DA, Albelda SM, Sun J, Davies PF. Role of lateral cell-cell border location and extracellular/transmembrane domains in PECAM/CD31 mechanosensation. Biochem Biophys Res Commun. 2004;320:1076–81. doi: 10.1016/j.bbrc.2004.06.055. [DOI] [PubMed] [Google Scholar]
- Kirschbaum NE, Gumina RJ, Newman PJ. Organization of the gene for human Platelet/Endothelial Cell Adhesion Molecule-1 (PECAM-1) reveals alternatively spliced isoforms and a functionally complex cytoplasmic domain. Blood. 1994;84:4028–37. [PubMed] [Google Scholar]
- Kowalczyk AP, Navarro P, Dejana E, Bornslaeger EA, Green KJ, Kopp DS, et al. VE-cadherin and desmoplakin are assembled into dermal microvascular endothelial intercellular junctions: a pivotal role for plakoglobin in the recruitment of desmoplakin to intercellular junctions. J Cell Sci. 1998;111:3045–57. doi: 10.1242/jcs.111.20.3045. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, et al. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin) J Cell Biol. 1995;129:203–17. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertkiatmongkol P, Paddock C, Newman DK, Zhu J, Thomas MJ, Newman PJ. The Role of Sialylated Glycans in Human Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1)-mediated Trans Homophilic Interactions and Endothelial Cell Barrier Function. The Journal of biological chemistry. 2016;291:26216–25. doi: 10.1074/jbc.M116.756502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J, He Q, Miyake K, Hamann A, Hyman R, Kincade PW. Requirements for Hyaluronic acid binding by CD44: A role for the cytoplasmic domain and activation by antibody. J Exp Med. 1992;175:257–66. doi: 10.1084/jem.175.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol. 2005;288:H159–H64. doi: 10.1152/ajpheart.00500.2004. [DOI] [PubMed] [Google Scholar]
- Mahooti S, Graesser D, Patil S, Newman P, Duncan G, Mak T, et al. PECAM-1 (CD31) expression modulates bleeding time in vivo. Am J Pathol. 2000;157:75–81. doi: 10.1016/S0002-9440(10)64519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio EE, Guan JL, Trevithick JE, Hynes RO. Mapping of the functional determinants of the integrin beta 1 cytoplasmic domain by site-directed mutagenesis. Cell Regul. 1990;1:597–604. doi: 10.1091/mbc.1.8.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H, Campbell JM, Paddock CM, Lertkiatmongkol P, Mosesson MW, Albrecht R, et al. Regulation of endothelial cell barrier function by antibody-driven affinity modulation of platelet endothelial cell adhesion molecule-1 (PECAM-1) The Journal of biological chemistry. 2014;289:20836–44. doi: 10.1074/jbc.M114.557454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Taketomi Y, Takimoto M, Lei XF, Arita S, Kim-Kaneyama JR, et al. m-Calpain induction in vascular endothelial cells on human and mouse atheromas and its roles in VE-cadherin disorganization and atherosclerosis. Circulation. 2011;124:2522–32. doi: 10.1161/CIRCULATIONAHA.111.021675. [DOI] [PubMed] [Google Scholar]
- Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989;170:399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DK, Hoffman S, Kotamraju S, Zhao T, Wakim B, Kalyanaraman B, et al. Nitration of PECAM-1 ITIM tyrosines abrogates phosphorylation and SHP-2 binding. Biochem Biophys Res Commun. 2002;296:1171–9. doi: 10.1016/s0006-291x(02)02060-0. [DOI] [PubMed] [Google Scholar]
- Newman PJ, Berndt MC, Gorski J, White GC, Lyman S, Paddock C, et al. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–22. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Newton JP, Buckley CD, Jones EY, Simmons DL. Residues on both faces of the first immunoglobulin fold contribute to homophilic binding sites on PECAM-1/CD31. J Biol Chem. 1997;272:20555–63. doi: 10.1074/jbc.272.33.20555. [DOI] [PubMed] [Google Scholar]
- O’Toole TE, Mandelman D, Forsyth J, Shattil SJ, Plow EF, Ginsberg MH. Modulation of the affinity of integrin alpha IIb beta 3 (GPIIb-IIIa) by the cytoplasmic domain of alpha IIb. Science. 1991;254:845–7. doi: 10.1126/science.1948065. [DOI] [PubMed] [Google Scholar]
- Paddock C, Lytle BL, Peterson FC, Holyst T, Newman PJ, Volkman BF, et al. Residues within a lipid-associated segment of the PECAM-1 cytoplasmic domain are susceptible to inducible, sequential phosphorylation. Blood. 2011;117:6012–23. doi: 10.1182/blood-2010-11-317867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock C, Zhou D, Lertkiatmongkol P, Newman PJ, Zhu J. Structural basis for PECAM-1 homophilic binding. Blood. 2016;127:1052–61. doi: 10.1182/blood-2015-07-660092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SK, Cunningham BA, Edelman GM, Rodriguez-Boulan E. Targeting of transmembrane and GPI-anchored forms of N-CAM to opposite domains of a polarized epithelial cell. Nature. 1991;353:76–7. doi: 10.1038/353076a0. [DOI] [PubMed] [Google Scholar]
- Privratsky JR, Paddock CM, Florey O, Newman DK, Muller WA, Newman PJ. Relative contribution of PECAM-1 adhesion and signaling to the maintenance of vascular integrity. J Cell Sci. 2011;124:1477–85. doi: 10.1242/jcs.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nature methods. 2014;11:783–4. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardjono CT, Harbour SN, Yip JC, Paddock C, Tridandapani S, Newman PJ, et al. Palmitoylation at Cys595 is essential for PECAM-1 localisation into membrane microdomains and for efficient PECAM-1-mediated cytoprotection. Thromb Haemost. 2006;96:756–66. [PubMed] [Google Scholar]
- Shin J, Dunbrack RL, Jr, Lee S, Strominger JL. Signals for retention of transmembrane proteins in the endoplasmic reticulum studied with CD4 truncation mutants. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:1918–22. doi: 10.1073/pnas.88.5.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Kowalczyk AP. The VE-cadherin cytoplasmic domain undergoes proteolytic processing during endocytosis. Molecular biology of the cell. 2017;28:76–84. doi: 10.1091/mbc.E16-09-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Paddock C, Shubert J, Zhang H, Amin K, Newman PJ, et al. Contributions of the extracellular and cytoplasmic domains of platelet- endothelial cell adhesion molecule-1 (PECAM-1/CD31) in regulating cell- cell localization. J Cell Sci. 2000;113:1459–69. doi: 10.1242/jcs.113.8.1459. [DOI] [PubMed] [Google Scholar]
- Sun J, Williams J, Yan H-C, Amin KM, Albelda SM, DeLisser HM. Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem. 1996a;271:18561–70. doi: 10.1074/jbc.271.31.18561. [DOI] [PubMed] [Google Scholar]
- Sun Q-H, DeLisser HM, Zukowski MM, Paddock C, Albelda SM, Newman PJ. Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J Biol Chem. 1996b;271:11090–8. doi: 10.1074/jbc.271.19.11090. [DOI] [PubMed] [Google Scholar]
- Trillsch F, Kuerti S, Eulenburg C, Burandt E, Woelber L, Prieske K, et al. E-Cadherin fragments as potential mediators for peritoneal metastasis in advanced epithelial ovarian cancer. Br J Cancer. 2016;114:213–20. doi: 10.1038/bjc.2015.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–31. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- Wong CW, Wiedle G, Ballestrem C, Wehrle-Haller B, Etteldorf S, Bruckner M, et al. PECAM-1/CD31 trans-homophilic binding at the intercellular junctions is independent of its cytoplasmic domain; evidence for heterophilic interaction with integrin avb3 in cis. Mol Biol Cell. 2000;11:3109–21. doi: 10.1091/mbc.11.9.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H-C, Pilewski JM, Zhang Q, DeLisser HM, Romer L, Albelda SM. Localization of multiple functional domains on human PECAM-1 (CD31) by monoclonal antibody epitope mapping. Cell Adhesion and Communication. 1995;3:45–66. doi: 10.3109/15419069509081277. [DOI] [PubMed] [Google Scholar]
- Ye Y, Tian H, Lange AR, Yearsley K, Robertson FM, Barsky SH. The genesis and unique properties of the lymphovascular tumor embolus are because of calpain-regulated proteolysis of E-cadherin. Oncogene. 2013;32:1702–13. doi: 10.1038/onc.2012.180. [DOI] [PubMed] [Google Scholar]