Abstract
Background
Vascular endothelial fibrinolytic function is impaired in adults with prehypertension and hypertension and plays a mechanistic role in the development of atherothrombotic events. The influence of β‐blockers on endothelial fibrinolysis is unknown. This study compared the effects of chronic nebivolol and metoprolol treatment on endothelial tissue‐type plasminogen activator (t‐PA) release in adults with elevated blood pressure (BP).
Methods and Results
Forty‐four middle‐aged adults (36% women) with elevated BP completed a 3‐month, double‐blind, randomized, placebo‐controlled trial comparing nebivolol (5 mg/d), metoprolol succinate (100 mg/d), and placebo. Net endothelial t‐PA release was determined in vivo in response to intrabrachial infusions of bradykinin and sodium nitroprusside before and after each intervention. In a subset, the dose‐response curves to bradykinin and sodium nitroprusside were repeated with a coinfusion of the antioxidant vitamin C. At baseline, resting BP and endothelial t‐PA release were comparable between the 3 groups. BP decreased to a similar extent (≈10 mm Hg) in the nebivolol‐ and metoprolol‐treated groups. There was a substantial increase (≈30%; P<0.05) in the capacity of the endothelium to release t‐PA following chronic treatment with nebivolol but not metoprolol or placebo. Mitigating oxidant stress with vitamin C coinfusion potentiated t‐PA release (90%; P<0.05) at baseline in all groups. However, after the intervention, t‐PA release was unchanged by vitamin C coinfusion in the nebivolol group only.
Conclusions
Nebivolol but not metoprolol improves endothelial t‐PA release in adults with elevated BP. This may be an important vascular benefit of nebivolol.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01595516.
Keywords: hypertension, metoprolol, nebivolol, oxidative stress, tissue‐type plasminogen activator, vitamin C
Subject Categories: Vascular Disease, Thrombosis, Atherosclerosis
Clinical Perspective
What Is New?
In spite of lowering blood pressure (BP), metoprolol therapy was no different from placebo on vascular endothelial fibrinolysis.
Nebivolol improves endothelial fibrinolysis in adults with elevated BP, at least in part, through mitigating oxidative stress.
What Are the Clinical Implications?
The use of antihypertensives with unique characteristics may provide personalized benefits to patients.
Nebivolol has benefits on vascular health that are independent of its BP‐lowering effect and may have a clinical advantage in the treatment of elevated BP.
Additional randomized clinical trial data will be necessary to translate these finding into clinical outcomes and determine whether nebivolol prevents clinical events in patients with elevated BP.
Introduction
Adults with elevated blood pressure (BP) are at increased risk of atherothrombotic events such as myocardial infarction and stroke.1 Elevated oxidative stress in adults with hypertension likely contributes to alterations in vascular endothelial function leading to a prothrombotic state.2, 3 Impairments in endogenous fibrinolysis play a mechanistic role in the development of atherothrombotic events in this high‐risk population.1 The capacity of the vascular endothelium to release tissue‐type plasminogen activator (t‐PA) is a sensitive indicator of endogenous fibrinolytic potential. Endothelial t‐PA release has been mechanistically linked to the prevention of arterial thrombosis and clinical cardiovascular events and is impaired in humans with elevated BP.4, 5, 6
Mitigation of clinical risk in adults with elevated BP has been generally attributed to the benefit of BP lowering independent of method. However, since the specific mechanisms underlying this benefit remain incompletely defined, it has been proposed that some BP‐lowering medications have unique end organ effects that might impart additional benefit. Indeed, in vivo studies have demonstrated that several agents improve endothelial t‐PA release in adults with hypertension while others show no benefit.7, 8 However, there are no data regarding the influence of chronic β‐blocker therapy on endogenous fibrinolysis.
Nebivolol, a third‐generation β‐blocker with high selectivity for β1‐adrenergic receptors and unique antioxidant effects, has proven to be highly effective in treating hypertension.9, 10, 11 Nebivolol exhibits unique properties that distinguish it from other β‐blockers. For example, nebivolol has been shown to enhance both basal and stimulated nitric oxide (NO) release resulting in lower oxidative stress,12, 13 peripheral vasodilation, and improved endothelial vasomotor function.4, 14, 15 There are also data to suggest that nebivolol may have favorable effects on the fibrinolytic system,16, 17 but there is currently no in vivo clinical evidence that treatment with nebivolol improves vascular endothelial t‐PA release. Enhancing endothelial fibrinolytic capacity may represent an important thromboprotective effect of nebivolol.
We hypothesized that nebivolol, but not metoprolol, treatment would improve the capacity of the endothelium to release t‐PA in adults with elevated BP. In addition, we postulated that this improvement in t‐PA release would be through mitigation of oxidative stress. The current study used a 3‐month, double‐blind, randomized, placebo‐controlled trial (NCT01595516) to compare the effect of chronic nebivolol and metoprolol treatment on vascular endothelial fibrinolytic function in vivo in adult humans with elevated BP.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patients
Forty‐four middle‐aged adults with elevated BP (systolic BP >130 mm Hg and/or diastolic BP >85 mm Hg) completed a 3‐month, double‐blind, randomized, placebo‐controlled trial: 16 received nebivolol (10 men/6 women; 5 mg/d [Forest Laboratories, Inc]); 16 received metoprolol succinate (10 men/6 women; 100 mg/d [AstraZeneca LP]); and 12 received placebo (8 men/4 women; 1 gelatin capsule per day [Forest Laboratories, Inc]). Although each tablet had a different appearance, patients only had access to the specific tablet supplied by the pharmacy to assist with blinding. The doses of nebivolol and metoprolol were chosen to elicit similar reductions in BP. Resting BP was determined by the average of ≥2 seated BP readings from two separate visits per American Heart Association guidelines.18 All patients were free of overt coronary and metabolic disease as assessed by medical history, physical examination, fasting blood chemistries, and ECGs and BP at rest and during incremental exercise performed to exhaustion. In addition, all patients presented with a resting heart rate >50 beats per minute. None of the patients smoked, were taking medications (including vitamins), or performed regular physical exercise for at least 1 year before the start of the study. All of the women were at least 1 year postmenopausal and had never taken or discontinued use of hormone replacement therapy at least 1 year before the start of the study. After baseline testing, patients were randomly assigned to 1 of the 3 experimental groups. All participants returned biweekly for pill counts and a new set of pills throughout the intervention. Measurements following the intervention were performed during a single visit. Before participation, all of the patients had the research study and its potential risks and benefits explained fully before providing written informed consent according to the guidelines of the University of Colorado at Boulder. The study was approved by the institutional review board of University of Colorado, Boulder.
Measurements
Blood pressure
Resting BP measurements were performed with the patients in the sitting position on at least two separate days at least 1 week apart. Participants were instructed not to ingest caffeine‐containing beverages before all BP measurements. The recordings were made under quiet, comfortable ambient (≈24°C) laboratory conditions. To avoid the possibility of investigator bias, measurements were performed with a semiautomated device (Dinamap) that uses an oscillometric technique over the brachial artery. Recordings were made in triplicate with patients in the upright sitting position. All measurements conformed to American Heart Association guidelines as established by the Council for High Blood Pressure Research.18
Body composition
Body mass was measured to the nearest 0.1 kg using a medical beam balance (Detecto). Percentage of body fat was determined by dual energy x‐ray absorptiometry (Lunar Radiation Corporation). Body mass index was calculated as weight (kilograms) divided by height (meters) squared. Minimal waist circumference was measured according to previously published guidelines.19
Metabolic measurements
Fasting plasma lipid, lipoprotein, glucose, and insulin concentrations were determined using standard techniques by the clinical laboratory affiliated with the Clinical Translational Research Center at the University of Colorado at Boulder.
Intra‐arterial fibrinolytic protocol
All measurements were performed in a temperature‐controlled room between 7 am and 10 am after a 12‐hour overnight fast as previously described by our laboratory.20 Briefly, an intravenous catheter was placed in a deep antecubital vein of the nondominant arm. Thereafter, a 5‐cm, 20‐gauge catheter was introduced into the brachial artery of the same arm under local anesthesia (1% lidocaine). Forearm blood flow (FBF) was measured using strain‐gauge venous occlusion plethysmography (D.E. Hokanson) and presented as mL (100 mL forearm volume)−1 min−1. Following the measurement of resting blood flow for 5 minutes, bradykinin was infused intra‐arterially at rates of 12.5, 25, and 50 ng (100 mL tissue)−1 min−1 and sodium nitroprusside at 1.0, 2.0, 4.0 μg (100 mL tissue)−1 min−1 for 5 minutes at each dose as previously described.20 To avoid an order effect, the sequence of drug administration was randomized. Forearm volume was determined by the water displacement method.
Net endothelial release of t‐PA antigen and plasminogen activator inhibitor‐1 (PAI‐1) antigen in response to bradykinin and sodium nitroprusside was calculated according to Jern et al21 using the following equation:
where CV and CA represent the concentration in the vein and artery, respectively. For both t‐PA and PAI‐1, a positive difference indicated a net release and a negative difference, net uptake. Arterial and venous blood samples were collected simultaneously at baseline and at the end of each drug dose. Enzyme immunoassay was used to determine t‐PA and PAI‐1 antigen concentrations. Hematocrit was measured in triplicate using the standard microhematocrit technique and corrected for trapped plasma volume within the erythrocytes.22 The total amount of t‐PA antigen released across the forearm in response to bradykinin was calculated as the incremental area under each curve using a trapezoidal model. In order to avoid confounding effects from potential infection or acute inflammation on fibrinolytic function, all patients were free of recent infection/inflammation (>2 weeks) as determined by questionnaire.23
Acute vitamin C administration
The acute effects of intra‐arterial vitamin C on the capacity of the endothelium to release t‐PA was determined before and after each intervention in 10 (7 men/3 women) of the 16 patients who received nebivolol and 10 (7 men/3 women) of the 16 patients who received metoprolol. None of the patients who were randomized to the placebo group received the co‐infusion of bradykinin+vitamin C in order to reduce patient burden on a group assumed not to receive any treatment benefit. After allowing sufficient time (≈20 minutes) for FBF and plasma fibrinolytic concentrations to return to baseline following the initial infusions of bradykinin and sodium nitroprusside described above, vitamin C (24 mg/min) was infused at a constant rate. This dose of vitamin C has been shown to increase the local forearm plasma concentration to levels that protect plasma from free radical–mediated lipid peroxidation24 and to improve endothelial function in obese adults.25 After 20 minutes, the acute vitamin C infusion was maintained at the same rate while the bradykinin and sodium nitroprusside dose‐response curves were repeated in the same order as performed earlier. Net endothelial release rates of t‐PA antigen and PAI‐1 antigen were determined at time 0, after 20 minutes of vitamin C infusion, and after each dose of bradykinin and sodium nitroprusside.
Statistical Analysis
All authors had full access to the data and take responsibility for its integrity and the data analysis. Differences in patient baseline characteristics were determined by between‐groups ANOVA. Differences in FBF and endothelial t‐PA and PAI‐1 antigen release in response to bradykinin, sodium nitroprusside, and bradykinin+vitamin C involving both main effects and interactions (group×intervention) were determined by repeated‐measures ANOVA. Post hoc comparisons were performed using the Tukey procedure. There were no significant sex interactions, therefore the data were pooled and presented together. All data are expressed as mean±SEM. Statistical significance was set a priori at P<0.05.
Results
Selected patient characteristics are presented in Table 1. There were no baseline differences in age or anthropometric, metabolic, or hemodynamic variables between the groups. In response to the interventions, there were clinically modest, albeit significant, reductions in total cholesterol and low‐density lipoprotein cholesterol in the nebivolol group and high‐density lipoprotein cholesterol in the placebo group. Table 2 shows the heart rate and BP responses among the groups. There were no differences in resting heart rate or BP between the groups. Both nebivolol and metoprolol treatment resulted in similar and significant reductions in heart rate and systolic (≈15 mm Hg), diastolic (≈10 mm Hg), and mean arterial (≈10 mm Hg) BP. There were no significant changes in heart rate and BP in the placebo group.
Table 1.
Selected Patient Characteristics
| Variable | Nebivolol | Metoprolol | Placebo | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Men/women | 10/6 | 10/6 | 10/6 | 10/6 | 8/4 | 8/4 |
| Age, y | 58±2 | 58±2 | 58±2 | 58±2 | 57±2 | 57±2 |
| Body mass, kg | 89.9±4.3 | 90.7±4.3 | 94.5±5.3 | 95.9±5.3 | 91.1±6.5 | 91.9±6.4 |
| BMI, kg/m2 | 29.9±1.5 | 30.1±1.6 | 31.5±1.5 | 32.0±1.5 | 29.0±1.6 | 29.2±1.6 |
| Body fat, % | 35.9±1.9 | 36.4±1.8 | 36.1±2.3 | 37.9±2.2 | 34.4±2.8 | 34.9±2.8 |
| Waist circumference, cm | 96.6±3.3 | 97.4±3.3 | 102.0±4.3 | 100.7±4.8 | 97.1±3.9 | 96.1±3.4 |
| Total cholesterol, mg/dL | 199.3±6.3 | 178.8±6.3a | 216.0±10.4 | 192.1±9.8 | 216.4±12.2 | 192.4±11.9 |
| LDL‐C, mg/dL | 122.5±5.2 | 104.6±5.3a | 133.1±9.3 | 124.3±8.7 | 145.0±8.4 | 129.7±10.2 |
| HDL‐C, mg/dL | 51.1±4.4 | 43.5±3.0 | 56.6±5.0 | 44.9±3.8 | 48.7±2.7 | 39.7±2.3a |
| Triglycerides, mg/dL | 128.4±12.4 | 142.6±12.5 | 134.0±21.8 | 114.8±17.5 | 109.5±11.2 | 96.8±4.7 |
| Glucose, mg/dL | 95.1±2.4 | 95.1±3.0 | 94.1±1.9 | 94.8±3.0 | 94.9±3.3 | 91.2±2.3 |
| Insulin, μU/mL | 13.4±1.4 | 13.6±1.6 | 10.8±1.4 | 10.7±1.4 | 12.9±1.0 | 10.9±1.0 |
Values are mean±SEM. BMI indicates body mass index; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol.
P<0.05 vs before intervention.
Table 2.
Patient Blood Pressure
| Variable | Nebivolol | Metoprolol | Placebo | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Heart rate, bpm | 64±1 | 58±2a | 71±2 | 64±3a | 69±2 | 72±2 |
| SBP, mm Hg | 140±2 | 125±2a | 138±2 | 125±3a | 138±2 | 135±3 |
| DBP, mm Hg | 85±2 | 78±2a | 87±2 | 79±2a | 85±3 | 81±2 |
| MAP, mm Hg | 104±1 | 93±2a | 104±2 | 94±2a | 103±2 | 99±3 |
Values are mean±SEM. bpm indicates beats per minute; DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure.
P<0.05 vs before intervention.
FBF responses to bradykinin (nebivolol: from 4.8±0.3 to 15.1±0.8 mL/100 mL tissue; metoprolol: from 4.5±0.3 to 13.9±0.8 mL/100 mL tissue per minute; and placebo: from 4.7±0.2 to 15.1±0.7 mL/100 mL tissue per minute) and sodium nitroprusside (nebivolol: from 5.1±0.3 to 15.9±0.8 mL/100 mL tissue per minute; metoprolol: from 4.8±0.3 to 14.7±0.9 mL/100 mL tissue per minute; and placebo: from 5.1±0.3 to 15.9±1.1 mL/100 mL tissue per minute) were not significantly different between the three groups before intervention. After each intervention, there was no change in the FBF responses to either bradykinin or sodium nitroprusside in the nebivolol, metoprolol, or placebo groups (data not shown).
Basal and stimulated endothelial t‐PA release in response to bradykinin (nebivolol: from −1.2±0.8 to 47.2±4.3 ng/100 mL tissue per minute; metoprolol: from −1.2±1.2 to 48.2±5.9 ng/100 mL tissue per minute; and placebo: from −0.2±1.2 to 51.1±4.9 ng/100 mL tissue per minute) were not significantly different between the 3 groups before intervention. Nebivolol treatment resulted in a significant increase (≈55%) in t‐PA release in response to bradykinin (from −1.8±0.9 to 72.8±5.7 ng/100 mL tissue per minute) (Figure 1). Consequently, the total amount of t‐PA antigen released (area under the bradykinin curve) increased by ≈60% (P<0.05) with nebivolol therapy (450±46 versus 286±32 ng/100 mL tissue). In marked contrast, there was no significant effect of either metoprolol therapy or placebo on the capacity of the endothelium to release t‐PA (Figure 1). Sodium nitroprusside had no significant effect on endothelial t‐PA release either at baseline (nebivolol: from −1.3±0.9 to 2.4±2.2 ng/100 mL tissue per minute; metoprolol: from 0.7±2.3 to 0.1±3.1 ng/100 mL tissue per minute; and placebo: from −0.8±1.2 to −0.1±3.9 ng/100 mL tissue per minute) or after each intervention. Neither bradykinin nor sodium nitroprusside evoked significant changes in PAI‐1 antigen release in any group at baseline or after intervention (data not shown).
Figure 1.
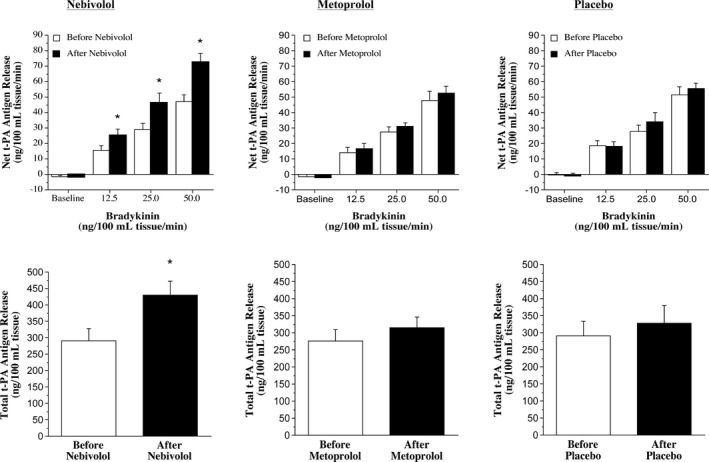
Nebivolol, but not metoprolol, treatment improves tissue‐type plasminogen activator (t‐PA) release. Net endothelial release rate (top panels) and total amount (bottom panels) of t‐PA antigen released across the forearm in response to bradykinin before and after 3 months of treatment with nebivolol, metoprolol, or placebo. *P<0.05 vs before nebivolol.
Infusion of vitamin C significantly increased forearm plasma vitamin C concentrations similarly in the nebivolol and metoprolol groups in the protocols performed before (nebivolol: from 0.7±0.1 to 8.9±0.8 mg/dL; metoprolol: from 0.7±0.1 to 11.7±0.9 mg/dL) and after (nebivolol: from 0.8±0.1 to 9.6±1.6 mg/dL; metoprolol: from 0.6±0.1 to 10.6±1.6 mg/dL) each intervention. FBF responses to bradykinin were not significantly affected by vitamin C administration at baseline or after nebivolol or metoprolol treatment (Figure 2). Before each intervention, the co‐infusion of vitamin C significantly increased (≈90%) net endothelial t‐PA release in both the nebivolol and metoprolol groups (Figures 3 and 4). However, after nebivolol, but not metoprolol, the co‐infusion of vitamin C did not augment endothelial t‐PA in response to bradykinin. Indeed, after nebivolol treatment, there was no significant difference in net endothelial release of t‐PA between the presence (from −3.8±2.6 to 79.9±7.6 ng/100 mL tissue per minute) or absence (from −1.5±1.3 to 67.5±7.1 ng/100 mL tissue per minute) of vitamin C (Figure 3). In contrast, the co‐infusion of vitamin C resulted in a similar increase in endothelial t‐PA after (from −2.7±1.1 to 73.8±7.4 ng/100 mL tissue per minute) compared with before (from −2.5±0.8 to 82.4±10.1 ng/100 mL tissue per minute) metoprolol treatment (Figure 4). There was no significant intervention effect of vitamin C on the t‐PA response to sodium nitroprusside or on the PAI‐1 antigen response to either bradykinin or sodium nitroprusside in either group (data not shown).
Figure 2.
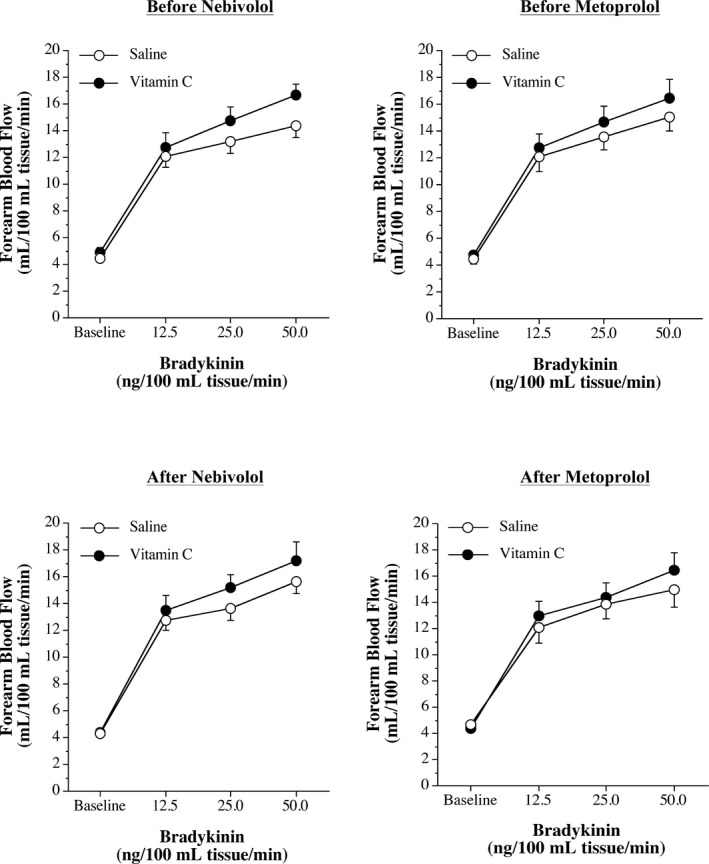
Forearm blood flow responses to bradykinin and vitamin C before and after blood pressure treatment. Forearm blood flow responses to bradykinin in the absence and presence of vitamin C before (top panels) and after (bottom panels) 3 months of treatment with nebivolol or metoprolol.
Figure 3.
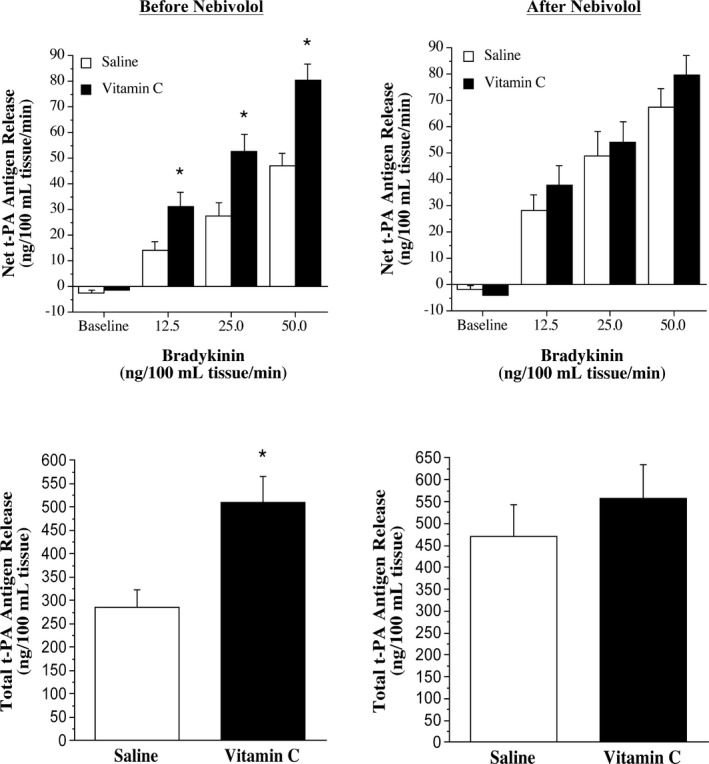
Tissue‐type plasminogen activator (t‐PA) release to bradykinin and vitamin C before and after nebivolol treatment. Net endothelial release rate (top panels) and total amount (bottom panels) of t‐PA antigen released across the forearm in response to bradykinin in the absence and presence of vitamin C before and after 3 months of nebivolol treatment. *P<0.05 vs saline.
Figure 4.
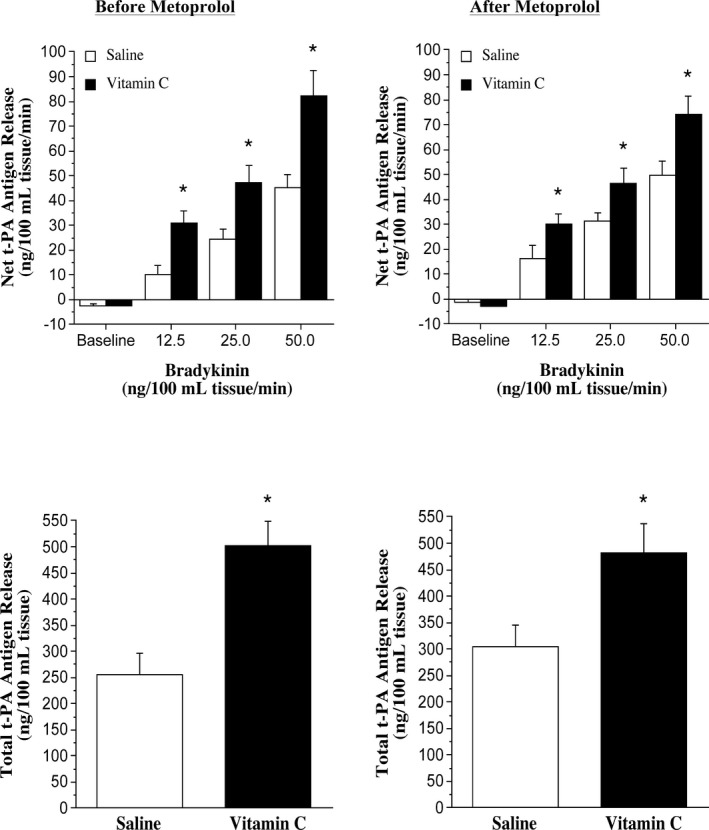
Tissue‐type plasminogen activator (t‐PA) release to bradykinin and vitamin C before and after metoprolol treatment. Net endothelial release rate (top panels) and total amount (bottom panels) of t‐PA antigen released across the forearm in response to bradykinin in the absence and presence of vitamin C before and after 3 months of metoprolol treatment. *P<0.05 vs saline.
Discussion
The current study is the first to determine the effects of β‐blockers on endothelial fibrinolytic regulation. We demonstrate that nebivolol, but not metoprolol, improves the capacity of the endothelium to release t‐PA in adults with elevated BP. Furthermore, the nebivolol‐induced improvement in endothelial t‐PA release occurs, at least in part, through the mitigation of oxidative stress. These data indicate a novel pleiomorphic effect of nebivolol, independent of BP lowering, on vascular endothelial function and, in turn, thrombogenic risk.
The ability of the endothelium to locally and rapidly release t‐PA is critical for efficient thrombolysis. Indeed, endothelial t‐PA release is an important endogenous defense mechanism against intravascular fibrin deposition and thrombosis.26 Studies in both animal models and humans have demonstrated that diminished endothelial t‐PA release is associated with accelerated atherosclerotic vascular disease.27, 28 For example, t‐PA–deficient mice demonstrate augmented rates of atherosclerotic fibrin deposition and extensive myocardial tissue necrosis.28 Whereas in humans, impaired capacity of the endothelium to release t‐PA has been linked to atheromatous plaque development and higher rates of myocardial infarction.29 We4 and others30 have previously demonstrated that endothelial t‐PA release is markedly blunted in adults with elevated BP and that the magnitude of impairment is similar between adults with prehypertension and stage 1 hypertension.4 Impaired endothelial fibrinolytic function is considered to be an important factor contributing to the increased risk of atherothrombotic vascular disease associated with elevated BP.31
Very little is known regarding the effects of BP‐lowering agents on endothelial control of the fibrinolytic system.30, 32 While acutely lowering BP in adults with hypertension does not have beneficial effects on endothelial t‐PA release,33 chronic BP control with angiotensin‐converting enzyme inhibition or calcium channel blockade,8 but not angiotensin receptor blockade,7 improves arterial t‐PA release.31 The results of the present study significantly extend these findings by demonstrating that chronic nebivolol, but not metoprolol, treatment improves the capacity of the endothelium to release t‐PA. It is important to emphasize that the magnitude of the BP‐lowering effect (≈10 mm Hg) was similar between nebivolol and metoprolol, suggesting that the improvement in endothelial t‐PA release with nebivolol was independent of the reduction in BP and was a specific effect of the compound. Enhanced endothelial fibrinolytic potential may underlie the report that nebivolol prevents ischemic events in patients with heart failure.34
Excessive oxidant stress has been demonstrated in humans with elevated BP2, 3 and has been shown to play an important role in BP‐related endothelial dysfunction. For example, Taddei and colleagues3 demonstrated that intra‐arterial infusion of vitamin C (24 ng/min) improves acetylcholine‐mediated endothelium‐dependent vasodilation in adults with hypertension, supporting the concept that oxidative stress contributes to BP‐related endothelial vasodilator dysfunction. The results of the present study complement and significantly extend these findings by demonstrating, for the first time, that intra‐arterial infusion of vitamin C potentiates bradykinin‐stimulated endothelial t‐PA release in adults with elevated BP. Indeed, before each intervention, the rate and total amount of t‐PA released in response to bradykinin markedly increased (≈90%) with concomitant vitamin C administration. These results indicate that oxidative stress is a major contributor to the impairment in the capacity of the endothelium to release t‐PA in adults with elevated BP. Our findings are supported by cellular studies demonstrating that oxidative stress inhibits t‐PA release from endothelial cells.35, 36 Moreover, we have previously demonstrated that reducing oxidative stress, either acutely or chronically, enhances t‐PA release in overweight and obese adults.37 A key and seminal finding of the present study is that the augmentation in endothelial t‐PA release with intra‐arterial vitamin C observed before the interventions was blunted following nebivolol but not metoprolol therapy, suggesting that suppression of oxidative stress is an important mechanism underlying the beneficial effects of nebivolol on endothelial fibrinolytic function. These clinical data bolster compelling in vitro evidence of the antioxidant‐related effects of nebivolol on the fibrinolytic system.38 Indeed, Garbin et al38 compared the effects of nebivolol and atenolol on gene expression of fibrinolytic proteins in human umbilical vein endothelial cells in response to an oxidative, prothrombotic stimulus (oxidized low‐density lipoprotein). In contrast to atenolol, nebivolol significantly reduced the oxidized low‐density lipoprotein–induced increase in gene expression of the primary t‐PA inhibitor, PAI‐1. Considering endogenous fibrinolytic potential is determined at the vascular wall, understanding the modulatory effects of nebivolol on endothelial release of t‐PA provides further insight into the unique vascular, and potentially thromboprotective, effects of nebivolol.
Although not the primary objective of the study, it is interesting to note that there were no changes in the FBF response to bradykinin in any treatment arm. Nebivolol increases NO bioavailability through several mechanisms. We39 and others40 have demonstrated that nebivolol treatment improves acetylcholine‐stimulated endothelium‐dependent vasodilation. The vasodilatory response to acetylcholine is caused by activation of endothelial NO synthase and production and release of NO. Vasodilation in response to bradykinin has an NO component in adults with hypertension41 but bradykinin also stimulates the release of prostanoids and endothelium‐derived hyperpolarizing factor in addition to NO. It is possible that nebivolol does not significantly affect these other vasodilator mediators, thus the overall effect of nebivolol on bradykinin‐stimulated endothelium dependent vasodilation is minimal.
Study Limitations
There are a few experimental considerations regarding the present study. First, although we observed significant improvement in endovascular fibrinolytic function with chronic nebivolol treatment, and endothelial t‐PA release has been inversely linked with cardiovascular disease,27, 29 we are unable to conclude that nebivolol treatment will decrease cardiovascular events in patients with elevated BP. From a clinical and public health perspective, it is important to note that the Joint National Commission 8 guidelines removed β‐blocker therapy as an initial treatment for systemic hypertension.42 This recommendation was based on several clinical trials before the development and use of vasodilating β‐blockers.43, 44, 45 Nebivolol has been shown to have excellent efficacy for lowering BP,46 particularly in populations historically resistant to β‐blockade.47 Unfortunately, there have been no studies evaluating the effect of nebivolol on morbidity and mortality in adults with hypertension.48 However, the results of the present study coupled with other studies demonstrating nebivolol‐induced improvement in endothelial vasodilator capacity and reduction vasoconstrictor tone39, 49 are encouraging and provide additional support for ancillary cardiovascular benefits of nebivolol in adults with elevated BP.50 Second, our results were obtained in the brachial artery and not a coronary artery. As such, we can only assume that similar impairments in endothelial t‐PA release with elevated BP and improvements with nebivolol treatment occurred in the coronary vasculature. However, the isolated forearm model used to assess endothelial t‐PA in vivo in the present study correlates strongly and positively with t‐PA release in the coronary circulation.27, 29 Moreover, given that the brachial artery is largely devoid of disease, it is possible that we are underestimating the degree of endovascular impairment with elevated BP and vascular enhancement with nebivolol therapy.
Conclusions
The results of this study indicate that nebivolol but not metoprolol treatment improves endothelial t‐PA release in adults with elevated BP. Moreover, the nebivolol‐induced improvements in endothelial t‐PA release were associated with a reduction in oxidative stress. Importantly, these findings offer greater insight into the postulated antithrombotic effects of nebivolol34 and provide additional rationale for clinical trials to determine the effect of nebivolol on cardiovascular events in adults with elevated BP.
Sources of Funding
This study was supported by funding from Forest Research Institute, Inc (BYS‐MD‐72) and by National Institutes of Health/National Center for Advancing Translational Sciences Colorado Clinical Translational Science Award grant No. UL1 TR001082‐05. The contents are the authors’ sole responsibility and do not necessarily represent the official National Institutes of Health.
Disclosures
Drs Stauffer and DeSouza received significant other research support from Forest Research Institute, Inc and Dr Stauffer received significant other research support from Stealth Biotherapeutics, Inc. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
Acknowledgments
We thank all of the patients who participated in the study, as well as the clinical staff at the Clinical Translational Research Center, University of Colorado, Boulder, for their assistance.
(J Am Heart Assoc. 2017;6:e007437 DOI: 10.1161/JAHA.117.007437.)29122812
References
- 1. Lip GY, Blann AD. Endothelium and fibrinolysis in hypertension: important facets of a prothrombotic state? Hypertension. 2008;52:218–219. [DOI] [PubMed] [Google Scholar]
- 2. Ferroni P, Basili S, Paoletti V, Davi G. Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr Metab Cardiovasc Dis. 2006;16:222–233. [DOI] [PubMed] [Google Scholar]
- 3. Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin c improves endothelium‐dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–2229. [DOI] [PubMed] [Google Scholar]
- 4. Diehl KJ, Weil BR, Greiner JJ, Wright KP, Stauffer BL, DeSouza CA. Impaired endogenous fibrinolytic capacity in prehypertensive men. J Hum Hypertens. 2015;29:468–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hrafnkelsdottir T, Ottosson P, Gudnason T, Samuelsson O, Jern S. Impaired endothelial release of tissue‐type plasminogen activator in patients with chronic kidney disease and hypertension. Hypertension. 2004;44:300–304. [DOI] [PubMed] [Google Scholar]
- 6. Hrafnkelsdottir T, Wall U, Jern C, Jern S. Impaired capacity for endogenous fibrinolysis in essential hypertension. Lancet. 1998;352:1597–1598. [DOI] [PubMed] [Google Scholar]
- 7. Matsumoto T, Minai K, Horie H, Ohira N, Takashima H, Tarutani Y, Yasuda Y, Ozawa T, Matsuo S, Kinoshita M, Horie M. Angiotensin‐converting enzyme inhibition but not angiotensin II type 1 receptor antagonism augments coronary release of tissue plasminogen activator in hypertensive patients. J Am Coll Cardiol. 2003;41:1373–1379. [DOI] [PubMed] [Google Scholar]
- 8. Ridderstrale W, Ulfhammer E, Jern S, Hrafnkelsdottir T. Impaired capacity for stimulated fibrinolysis in primary hypertension is restored by antihypertensive therapy. Hypertension. 2006;47:686–691. [DOI] [PubMed] [Google Scholar]
- 9. Baldwin CM, Keam SJ. Nebivolol: in the treatment of hypertension in the US. Am J Cardiovasc Drugs. 2009;9:253–260. [DOI] [PubMed] [Google Scholar]
- 10. Cheng JW. Nebivolol: a third‐generation beta‐blocker for hypertension. Clin Ther. 2009;31:447–462. [DOI] [PubMed] [Google Scholar]
- 11. Kuroedov A, Cosentino F, Luscher TF. Pharmacological mechanisms of clinically favorable properties of a selective beta1‐adrenoceptor antagonist, nebivolol. Cardiovasc Drug Rev. 2004;22:155–168. [DOI] [PubMed] [Google Scholar]
- 12. de Groot AA, Mathy MJ, van Zwieten PA, Peters SL. Antioxidant activity of nebivolol in the rat aorta. J Cardiovasc Pharmacol. 2004;43:148–153. [DOI] [PubMed] [Google Scholar]
- 13. Khan MU, Zhao W, Zhao T, Al Darazi F, Ahokas RA, Sun Y, Bhattacharya SK, Gerling IC, Weber KT. Nebivolol: a multifaceted antioxidant and cardioprotectant in hypertensive heart disease. J Cardiovasc Pharmacol. 2013;62:445–451. [DOI] [PubMed] [Google Scholar]
- 14. Czuriga I, Riecansky I, Bodnar J, Fulop T, Kruzsicz V, Kristof E, Edes I; Investigators N, Group NI . Comparison of the new cardioselective beta‐blocker nebivolol with bisoprolol in hypertension: the Nebivolol, Bisoprolol Multicenter Study (NEBIS). Cardiovasc Drugs Ther. 2003;17:257–263. [DOI] [PubMed] [Google Scholar]
- 15. Kamp O, Metra M, Bugatti S, Bettari L, Dei Cas A, Petrini N, Dei Cas L. Nebivolol: haemodynamic effects and clinical significance of combined beta‐blockade and nitric oxide release. Drugs. 2010;70:41–56. [DOI] [PubMed] [Google Scholar]
- 16. Liappas G, Gonzalez‐Mateo G, Aguirre AR, Abensur H, Albar‐Vizcaino P, Parra EG, Sandoval P, Ramirez LG, Del Peso G, Acedo JM, Bajo MA, Selgas R, Sanchez Tomero JA, Lopez‐Cabrera M, Aguilera A. Nebivolol, a beta1‐adrenergic blocker, protects from peritoneal membrane damage induced during peritoneal dialysis. Oncotarget. 2016;7:30133–30146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ayers K, Byrne LM, DeMatteo A, Brown NJ. Differential effects of nebivolol and metoprolol on insulin sensitivity and plasminogen activator inhibitor in the metabolic syndrome. Hypertension. 2012;59:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. [DOI] [PubMed] [Google Scholar]
- 19. Lohman T, Roche AH, Mortorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 20. Van Guilder GP, Hoetzer GL, Smith DT, Irmiger HM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelial t‐PA release is impaired in overweight and obese adults but can be improved with regular aerobic exercise. Am J Physiol Endocrinol Metab. 2005;289:E807–E813. [DOI] [PubMed] [Google Scholar]
- 21. Jern S, Wall U, Bergbrant A, Selin‐Sjogren L, Jern C. Endothelium‐dependent vasodilation and tissue‐type plasminogen activator release in borderline hypertension. Arterioscler Thromb Vasc Biol. 1997;17:3376–3383. [DOI] [PubMed] [Google Scholar]
- 22. Chaplin H Jr, Mollison PL. Correction for plasma trapped in the red cell column of the hematocrit. Blood. 1952;7:1227–1238. [PubMed] [Google Scholar]
- 23. Macko RF, Ameriso SF, Gruber A, Griffin JH, Fernandez JA, Barndt R, Quismorio FP Jr, Weiner JM, Fisher M. Impairments of the protein C system and fibrinolysis in infection‐associated stroke. Stroke. 1996;27:2005–2011. [DOI] [PubMed] [Google Scholar]
- 24. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA. 1989;86:6377–6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50:159–165. [DOI] [PubMed] [Google Scholar]
- 26. Muldowney JA III, Vaughan DE. Tissue‐type plasminogen activator release: new frontiers in endothelial function. J Am Coll Cardiol. 2002;40:967–969. [DOI] [PubMed] [Google Scholar]
- 27. Newby DE, McLeod AL, Uren NG, Flint L, Ludlam CA, Webb DJ, Fox KA, Boon NA. Impaired coronary tissue plasminogen activator release is associated with coronary atherosclerosis and cigarette smoking: direct link between endothelial dysfunction and atherothrombosis. Circulation. 2001;103:1936–1941. [DOI] [PubMed] [Google Scholar]
- 28. Christie PD, Edelberg JM, Picard MH, Foulkes AS, Mamuya W, Weiler‐Guettler H, Rubin RH, Gilbert P, Rosenberg RD. A murine model of myocardial microvascular thrombosis. J Clin Invest. 1999;104:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Newby DE, Wright RA, Labinjoh C, Ludlam CA, Fox KA, Boon NA, Webb DJ. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation. 1999;99:1411–1415. [DOI] [PubMed] [Google Scholar]
- 30. Giannarelli C, Virdis A, De Negri F, Duranti E, Magagna A, Ghiadoni L, Salvetti A, Taddei S. Tissue‐type plasminogen activator release in healthy subjects and hypertensive patients: relationship with beta‐adrenergic receptors and the nitric oxide pathway. Hypertension. 2008;52:314–321. [DOI] [PubMed] [Google Scholar]
- 31. Fogari R, Zoppi A. Antihypertensive drugs and fibrinolytic function. Am J Hypertens. 2006;19:1293–1299. [DOI] [PubMed] [Google Scholar]
- 32. Giannarelli C, Virdis A, De Negri F, Magagna A, Duranti E, Salvetti A, Taddei S. Effect of sulfaphenazole on tissue plasminogen activator release in normotensive subjects and hypertensive patients. Circulation. 2009;119:1625–1633. [DOI] [PubMed] [Google Scholar]
- 33. Ridderstrale W, Saluveer O, Carlstrom M, Jern S, Hrafnkelsdottir TJ. The impaired fibrinolytic capacity in hypertension is unaffected by acute blood pressure lowering. J Thromb Thrombolysis. 2011;32:399–404. [DOI] [PubMed] [Google Scholar]
- 34. Ambrosio G, Flather MD, Bohm M, Cohen‐Solal A, Murrone A, Mascagni F, Spinucci G, Conti MG, van Veldhuisen DJ, Tavazzi L, Coats AJ. Beta‐blockade with nebivolol for prevention of acute ischaemic events in elderly patients with heart failure. Heart. 2011;97:209–214. [DOI] [PubMed] [Google Scholar]
- 35. Shatos MA, Doherty JM, Stump DC, Thompson EA, Collen D. Oxygen radicals generated during anoxia followed by reoxygenation reduce the synthesis of tissue‐type plasminogen activator and plasminogen activator inhibitor‐1 in human endothelial cell culture. J Biol Chem. 1990;265:20443–20448. [PubMed] [Google Scholar]
- 36. Zhang J, Ren S, Sun D, Shen GX. Influence of glycation on LDL‐induced generation of fibrinolytic regulators in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18:1140–1148. [DOI] [PubMed] [Google Scholar]
- 37. Van Guilder G, Hoetzer G, Greiner J, Stauffer B, Desouza C. Acute and chronic effects of vitamin C on endothelial fibrinolytic capacity in overweight and obese adult humans. J Physiol. 2008;586:3525–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garbin U, Fratta Pasini A, Stranieri C, Manfro S, Mozzini C, Boccioletti V, Pasini A, Cominacini M, Evangelista S, Cominacini L. Effects of nebivolol on endothelial gene expression during oxidative stress in human umbilical vein endothelial cells. Mediators Inflamm. 2008;2008:367590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Diehl KJ, Stauffer BL, Dow CA, Bammert TD, Brunjes DL, Greiner JJ, DeSouza CA. Chronic nebivolol treatment suppresses endothelin‐1‐mediated vasoconstrictor tone in adults with elevated blood pressure. Hypertension. 2016;67:1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zanchetti A. Clinical pharmacodynamics of nebivolol: new evidence of nitric oxide‐mediated vasodilating activity and peculiar haemodynamic properties in hypertensive patients. Blood Press Suppl. 2004;1:17–32. [DOI] [PubMed] [Google Scholar]
- 41. Panza JA, Garcia CE, Kilcoyne CM, Quyyumi AA, Cannon RO III. Impaired endothelium‐dependent vasodilation in patients with essential hypertension. Evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation. 1995;91:1732–1738. [DOI] [PubMed] [Google Scholar]
- 42. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 43. MRC trial of treatment of mild hypertension: principal results. Medical research council working party. Br Med J (Clin Res Ed). 1985;291:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cardiovascular risk and risk factors in a randomized trial of treatment based on the beta‐blocker oxprenolol: the International Prospective Primary Prevention Study in Hypertension (IPPPSH). The IPPPSH Collaborative Group. J Hypertens. 1985;3:379–392. [DOI] [PubMed] [Google Scholar]
- 45. Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe‐Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H; Group LS . Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 46. Van Bortel LM, Fici F, Mascagni F. Efficacy and tolerability of nebivolol compared with other antihypertensive drugs: a meta‐analysis. Am J Cardiovasc Drugs. 2008;8:35–44. [DOI] [PubMed] [Google Scholar]
- 47. Saunders E, Smith WB, DeSalvo KB, Sullivan WA. The efficacy and tolerability of nebivolol in hypertensive African American patients. J Clin Hypertens (Greenwich). 2007;9:866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta‐blockers for hypertension. Cochrane Database Syst Rev. 2017;1:CD002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cockcroft JR, Chowienczyk PJ, Brett SE, Chen CP, Dupont AG, Van Nueten L, Wooding SJ, Ritter JM. Nebivolol vasodilates human forearm vasculature: evidence for an L‐arginine/NO‐dependent mechanism. J Pharmacol Exp Ther. 1995;274:1067–1071. [PubMed] [Google Scholar]
- 50. Pedersen ME, Cockcroft JR. What is the role, if any, for beta‐blockers as initial therapy for uncomplicated hypertension? Curr Opin Cardiol. 2009;24:325–332. [DOI] [PubMed] [Google Scholar]


