Abstract
Objective
Gene therapy that expresses apolipoprotein (apo) A-I from vascular wall cells has promise for preventing and reversing atherosclerosis. Previously, we reported that transduction of carotid artery endothelial cells with a helper-dependent adenoviral vector (HDAd) expressing apo A-I reduced early (4 weeks) fatty streak development in fat-fed rabbits. Here we tested whether the same HDAd could provide long-term protection against development of more-complex lesions.
Approach and Results
Fat-fed rabbits (n=25) underwent bilateral carotid artery gene transfer, with their left and right common carotids randomized to receive either a control vector (HDAdNull) or anapo A-I-expressing vector (HDAdApoAI). Twenty-four additional weeks of high-fat diet yielded complex intimal lesions containing lipid-rich macrophages as well as smooth muscle cells, often in a lesion cap. Twenty-four weeks after gene transfer, high levels of apo A-I mRNA (median ≥250-fold above background) were present in all HDAdApoAI-treated arteries. Compared to paired control HDAdNull-treated arteries in the same rabbit, HDAdApoAI-treated arteries had 30% less median intimal lesion volume (P=0.03), with concomitant reductions (23–32%) in intimal lipid, macrophage, and smooth muscle cell content (P≤0.05 for all). HDAdApoAI-treated arteries also had decreased intimal inflammatory markers. VCAM-1-stained area was reduced by 36% (P=0.03), with trends towards lower expression of ICAM-1, MCP-1, and TNF-α (13–39% less; P=0.06–0.1).
Conclusion
In rabbits with severe hyperlipidemia, transduction of vascular endothelial cells with an apo AI-expressing HDAd yields at least 24 weeks of local apo A-I expression that durably reduces atherosclerotic lesion growth and intimal inflammation.
Keywords: apolipoprotein, atherosclerosis, carotid artery, gene therapy, rabbits
Subject Terms: animal models of human disease, atherosclerosis, gene therapy, translational studies
Despite major advances in cardiovascular prevention and care,1 heart disease—primarily due to atherosclerosis—remains the leading cause of death in the United States2 and is increasing throughout the world.3, 4 Because of the central role of plasma-derived lipids (especially low-density lipoprotein cholesterol; LDL-C) in atherosclerosis, current medical therapies for atherosclerosis (e.g., statins, ezetemibe, and evolucumab) are aimed primarily at lowering plasma LDL-C. These therapies reduce major adverse cardiovascular events;5 however, they have not eliminated them. For example, lowering plasma LDL-C below 70 mg/dL in post-infarction patients only marginally (∼25%) decreased event rates during 30 months of follow-up.6 Addition of evolucumab to statin therapy reduced LDL-C from a median of 92 mg/dL to 30 mg/dL, but lowered cardiovascular events by only 14% compared to placebo.7 The limits of LDL-C-lowering are at hand; however, atherosclerosis-associated morbidity and mortality remain high.
In addition to therapies that lower plasma LDL-C, an alternative strategy aims to treat atherosclerosis by removing cholesterol from the blood vessel wall. This strategy was initially aimed at increasing plasma high-density lipoprotein cholesterol (HDL-C) because HDL-C was thought to be the primary mediator of reverse cholesterol transport, the endogenous process through which cholesterol is removed from tissues and transported to the liver for excretion.8 However, when added to statin therapy, pharmacologic agents that increase plasma HDL in humans (including niacin and CETP inhibitors) have not reduced cardiovascular events.9-11 Related therapeutic approaches include efforts to increase plasma levels of apolipoprotein (apo) A-I (the most abundant protein in HDL-C)12 by: 1) infusion of apo A-I protein, complexed with lipid to extend plasma half-life; 2) delivery of small molecules that mimic the function of apo A-I; or 3) use of drugs that enhance the function of plasma HDL. These approaches have shown promise in animal models and small human trials,13-17 but have not yet shown clinical benefit in larger human trials.18-20 Moreover, lifetime injections of apo A-I protein or apo A-I-mimetic peptides would be both expensive and impractical.
Several years ago, we proposed delivery of apo A-I to the blood vessel wall via vascular wall-targeted gene therapy as a novel treatment for atherosclerosis.21, 22 Compared to other apo A-I-based therapies—including systemic delivery of apo A-I protein and apo A-I gene therapy delivered to the liver—vascular wall-targeted apo A-I gene therapy has several theoretical advantages: 1) apo A-I is delivered precisely where it is needed: adjacent to a lipid-rich atherosclerotic lesion; 2) compared to HDL, free apo A-I is a superior promoter of cholesterol efflux;23 and 3) gene therapy has the potential to persist indefinitely,24-27 meaning that one treatment could provide lifetime protection. We previously showed that apo A-I vascular gene therapy retarded development of intimal fatty streaks in carotid arteries of fat-fed rabbits.22 However, the small size and self-limited nature of the lesions as well as the brief duration of the therapeutic effect (4 weeks) left it uncertain whether apo A-I gene therapy could have durable effects and whether it could prevent development of more-complex lesions. It was also unknown whether HDAd would durably express a transgene in an atherosclerotic milieu, in which endothelial injury and increased endothelial cell turnover28, 29 could lead to loss of vector genomes and transgene expression. To be clinically useful, apo A-I vascular gene therapy will need to be effective far longer than 4 weeks and will need to prevent growth of complex lesions, not only fatty streaks. Here we test whether HDAdApoAI, delivered to carotid arteries of hyperlipidemic rabbits, can continue to express apo A-I and prevent atherosclerotic lesion growth 24 weeks after treatment.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Rabbit Response to Atherogenic Diet
Four weeks of high-cholesterol diet elevated plasma cholesterol levels in all of the 27 rabbits initially enrolled. As expected,30, 31 the rabbits' response was highly variable, with plasma cholesterols ranging from 200 mg/dL to over 2,700 mg/dL (Figure I in the online-only Data Supplement). After vector infusion, each rabbit's diet was adjusted every 2 weeks based on measurements of plasma cholesterol, with a goal of maintaining plasma cholesterol levels within a range of 200–800 mg/dL. This range promotes robust atherosclerotic lesion development within 24 weeks, is well tolerated,30, 31 and avoids toxicity associated with an invariant high-cholesterol diet.32 In this study, only 2 rabbits developed signs of dietary toxicity, evidenced by high plasma cholesterol levels and inappetance. Both rabbits regained their appetites after switching to normal chow, and their plasma cholesterol levels dropped into the target range. At the time of vector infusion (HDAdApoAI in one common carotid; HDAdNull in the contralateral carotid), plasma cholesterol was 1,273±537 mg/dL. At harvest, 24 weeks later, plasma cholesterol was 565±162 mg/dL. 25 of 27 rabbits completed the study (see Materials and Methods in the online-only Data Supplement). Common carotid arteries were removed and processed as illustrated in Figure II in the online-only Data Supplement.
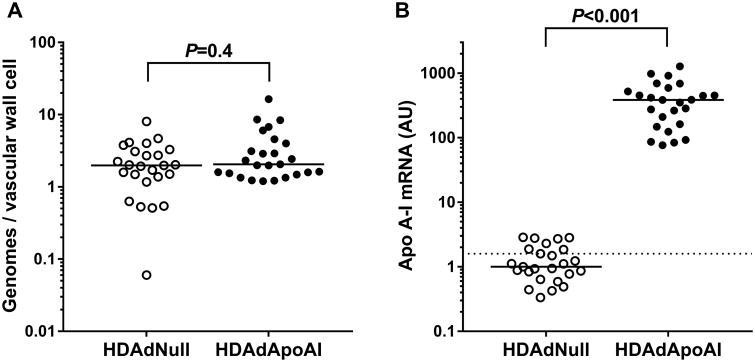
Persistence of HDAd Genomes and Apo A-I Expression in HDAdApoAI-Transduced Arteries
We measured vector genomes in 50 arteries, harvested 24 weeks after vector infusion. Vector genomes were detected in all arteries, with no difference between HDAdNull and HDAdApoAI-infused arteries (P=0.4). Artery extracts contained a median of 2.0 (1.5–3.2) vector genomes per vascular wall cell (Figure 1A). Our gene transfer protocol, using adenoviral vectors incubated in the carotid lumen, limits transduction essentially to a single layer of luminal endothelial cells (EC),33-35 which constitute ∼9% of total vascular cells (Figure 2 and data not shown). Therefore, 24 weeks after transduction, each EC appears to retain ∼22 copies of the HDAd vector genome. ApoA-I mRNA was near background in all HDAdNull-transduced carotid arteries (Figure 1B). In contrast, apo A-I mRNA was easily detectable in 25 of 25 (100%) of HDAdApoAI-transduced carotids, with a median level of apo A-I mRNA over 380-fold above the median PCR signal in HDAdNull-transduced carotids (P<0.001; Figure 1B). To detect vector-derived apo A-I protein, transduced artery segments were washed (with a goal of removing plasma-derived apo A-I), placed in explant culture, and the conditioned medium assessed by western blot. Low levels of apo A-I protein were detected in conditioned medium from only a few of the artery segments: 3 of 25 HDAdApoAI-infused arteries and 3 of 25 HDAdNull-infused arteries. We also measured plasma apo A-I protein by western blotting of plasma of 8 rabbits, using paired samples (i.e., both plasma samples from the same rabbit) obtained immediately before gene transfer and during artery harvest 24 weeks later. During this interval, plasma apo A-I levels declined ∼40% (P=0.007, Figure III in the online-only Data Supplement).
Figure 1.
Vector genome persistence and apo A-I mRNA expression. Rabbit carotid arteries were transduced with HDAdNull or HDAdApoAI and harvested 24 weeks later. (A) HDAd vector genomes were measured in carotid artery extracts and normalized to the number of vascular wall cells as determined by measurement of total DNA in extracts. P value from rank-sum test. (B) Apo A-I mRNA was measured in carotid artery extracts, normalized to GAPDH mRNA, and expressed as arbitrary units (AU, with the median value in the HDAdNull group defined as 1). Dotted line is assay background (see Materials and Methods). Data points represent individual arteries; bars are group medians. P value from rank-sum test.
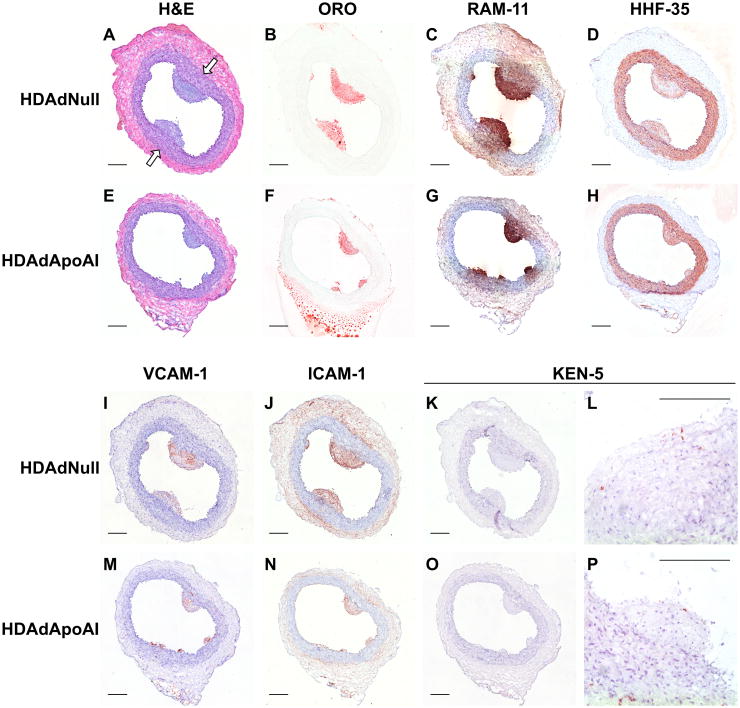
Figure 2.
Carotid atherosclerotic lesions from rabbits fed a high-fat diet for 24 weeks after gene therapy. Sections are from arteries treated either with HDAdNull (A–D, I–L) or HDAdApoAI (E–H, M–P). (A, E) hematoxylin and eosin (H&E) stain; (B, F) Oil Red O (ORO) stain; (C, G) immunostaining with RAM-11 antibody to detect macrophages; (D, H) immunostaining with HHF-35 antibody to detect muscle actin; (I, M) immunostaining to detect VCAM-1 or (J, N) ICAM-1; (K, L, O, P) immunostaining with KEN-5 antibody to detect T-cells. Sections A–D, and I–K are from the same artery, as are sections E–H and M–O. Sections L and P are higher magnification images of different arteries showing characteristic KEN-5 staining of T-cells. All panels except B and F: hematoxylin counterstain. (A) Arrows indicate medial thickening below large intimal lesions. Scale bars = 200 μm.
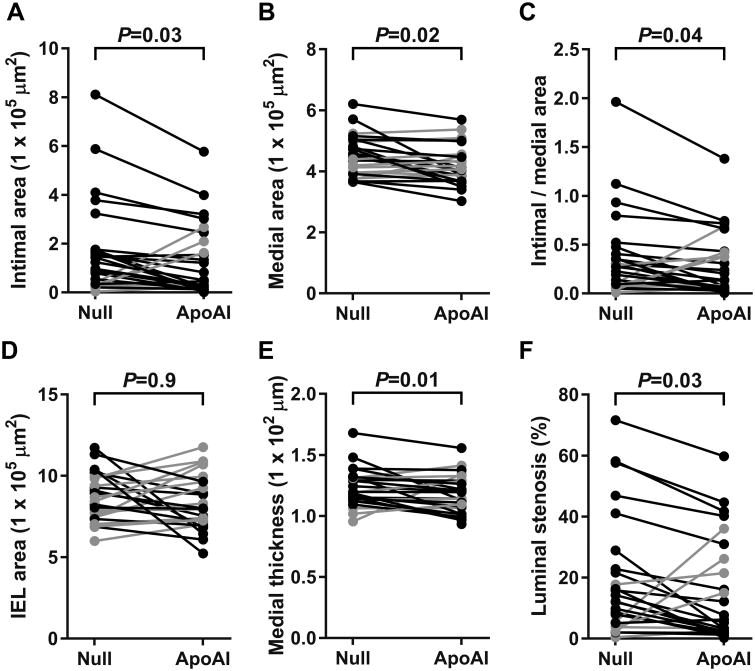
HDAdApoAI Decreases Intimal Area, Medial Area, Medial Thickness, Intimal: Medial Area Ratio, and Percent Luminal Stenosis
All arteries had intimal lesions (Figure 2). Intimal lesion size was highly variable, ranging from 1,300 μm2 to 810,000 μm2 (Figure 3A). As in our other rabbit studies,22, 30, 31, 36 intimal area was highly correlated between left and right arteries of the same rabbit (Figure IV in the online-only Data Supplement; r=0.85; P<0.0001). Because we expected this high correlation, we had decided a priori to use a paired statistical analysis to compare each HDAdApoAI-treated carotid to the HDAdNull-treated contralateral carotid in the same rabbit. However, for completeness, we also compared the overall group medians of HDAdApoAI-treated and HDAdNull-treated carotids and we include these results herein. The paired study design and accompanying paired statistical analyses (all P values from this point onwards are from paired tests) eliminate systemic factors (such as plasma cholesterol and apo A-I levels) as uncontrolled variables because both carotids of each rabbit are exposed to the same systemic factors.
Figure 3.
Impact of apo A-I expression on arterial morphology. Arteries were removed 24 weeks after treatment with either HDAdNull (Null) or HDAdApoAI (ApoAI), sectioned, stained, and analyzed with computer-assisted planimetry. (A) Intimal areas measured on HHF-35-stained sections. (B) Medial areas measured on H&E-stained sections. (C) Medial thickness calculated from measuring the medial area and the internal elastic lamina (IEL) perimeter, both on H&E-stained sections. (D) Ratio of intimal area to medial area. (E) Area within the IEL, calculated from the IEL perimeter, measured on H&E-stained sections. (F) Luminal stenosis calculated as intimal area divided by area within the IEL. Data points are means for each artery; points from arteries in the same rabbit are connected by bars. Rabbits in which the HDAdApoAI-treated artery had a lower value than the contralateral HDAdNull-treated artery are indicated in black; rabbits in which the HDAdApoAI-treated artery had a higher value than the contralateral HDAdNull-treated artery are indicated in grey. P values are from Wilcoxon signed-rank test comparing HDAdApoAI- and HDAdNull-treated carotids in the same rabbit.
Median intimal area was reduced by 58% in the group of 25 HDAdApoAI-treated carotid arteries compared to the 25 HDAdNull-treated arteries (Figure 3A and Table). Paired analyses showed a median 30% smaller intimal area in HDAdApoAI-treated carotids compared to their contralateral HDAdNull-treated carotids (P=0.03; Table). Median medial area was also reduced by a small amount (9%) in the group of HDAdApoAI-treated carotid arteries, with the paired analyses also showing a slightbut statistically significant decrease in medial area in the HDAdApoAI-treated carotids (3% reduction, P=0.02; Figure 3B). Intimal:medial area ratios were reduced for both the group of HDAdApoAI-treated carotids (48% reduction) and in the paired analyses (29% reduction; P=0.04; Figure 3C).
Table. Histologic and Immunohistochemical Analyses of All Arteries.
| HDAdNull* (n = 25) | HDAdApoAI* (n = 25) | Treatment Effect† | |||
|---|---|---|---|---|---|
|
| |||||
| Median (25% to 75%) | Median (25% to 75%) | Reduction (−) or Increase (+) in ApoAI versus Null Median (25% to 75%) | # Reduced with ApoAI (of 25 pairs) | P | |
| Intimal Area (μm2 × 103) | 120 (30 to 170) | 50 (22 to 209) | -30 (-74 to -16)% | 18 | 0.03 |
| Medial Area (μm2 × 103) | 450 (400 to 500) | 410 (370 to 450) | -3 (-13 to +3)% | 16 | 0.02 |
| Medial Thickness (μm) | 119 (114 to 131) | 114 (107 to 128) | -4 (-10 to 0)% | 18 | 0.01 |
| Intimal/Medial Ratio | 0.27 (0.09-0.40) | 0.14 (0.06-0.42) | -29 (-69 to +9)% | 18 | 0.04 |
| IEL Area (μm2 × 103) | 840 (740 to 980) | 800 (720 to 960) | +1 (-12 to +9)% | 12 | 0.9 |
| Luminal Stenosis (%) | 12 (5 to 23) | 6 (3 to 26) | -25 (-72 to -9)% | 19 | 0.03 |
| ORO Area (μm2 × 103) | 35 (3 to 59) | 10 (3 to 51) | -23 (-87 to +273)% | 15 | 0.05 |
| ORO Area (% of intima) | 28 (8 to 35) | 24 (13 to 37) | +8 (-26 to +40)% | 8 | 0.5 |
| RAM-11 Area (μm2 × 103) | 29 (3 to 63) | 7 (3 to 61) | -32 (-74 to +256)% | 17 | 0.03 |
| RAM-11 Area (% of intima) | 24 (10 to 42) | 22 (13 to 36) | -3 (-33 to +23)% | 13 | 0.7 |
| HHF-35 Area (μm2 × 103) | 25 (8 to 34) | 10 (4 to 24) | -31 (-70 to +13)% | 18 | 0.02 |
| HHF-35 Area (% of intima) | 18 (11 to 26) | 15 (12 to 18) | -3 (-24 to +13)% | 15 | 0.2 |
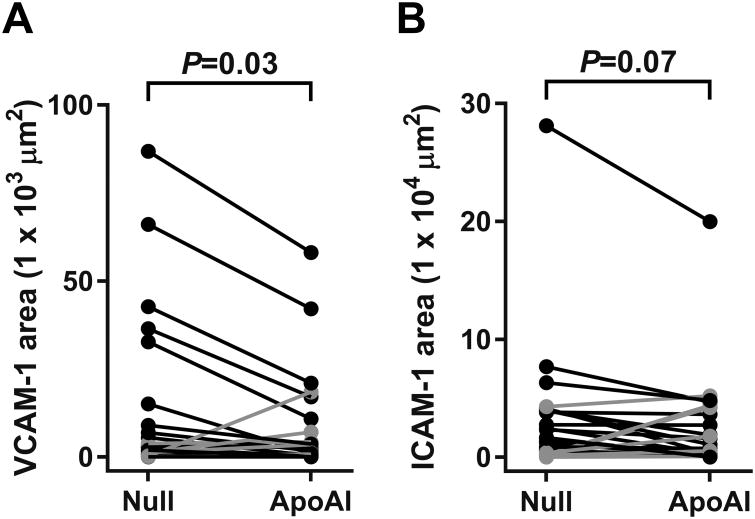
| VCAM-1 Area (μm2 × 103) | 2.0 (0.5 to 9.0) | 1.2 (0.3 to 7.1) | -36 (-77 to +181)% | 17 | 0.03 |
| VCAM-1 Area (% of intima) | 2.6 (1.2 to 8.7) | 3.5 (0.9 to 6.9) | -6 (-51 to +26)% | 14 | 0.5 |
| ICAM-1 Area (μm2 × 103) | 16 (4 to 40) | 7 (1 to 36) | -29 (-89 to +26)% | 17 | 0.07 |
| ICAM-1 Area (% of intima) | 16 (7 to 24) | 13 (7 to 17) | -10 (-42 to +64)% | 16 | 0.3 |
| KEN-5 Area (μm2 × 10) | 7.0 (3.1 to 17.8) | 3.3 (0.9 to 26.7) | +5 (-88 to +129)% | 12 | 0.9 |
| KEN-5 Area (% of intima) | 0.08 (0.03 to 0.15) | 0.07 (0.04 to 0.14) | -24 (-56 to +163)% | 14 | 0.9 |
| ABCA1 Area (μm2 × 103) | 9.7 (4.7 to 38.9) | 7.1 (2.3 to 44.7) | -20 (-58 to +17)% | 17 | 0.04 |
| ABCA1 Area (% of intima) | 22 (8 to 22) | 17 (11 to 23) | +10 (-20 to +43)% | 10 | 0.4 |
Data are from the group of HDAdNull-infused arteries and the group of HDAdApoAI-infused arteries.
Data are from paired analyses of HDAdNull- and HDAdApoAI-infused arteries in the same rabbit (25 pairs). A reduction indicates a lower value in the paired HDAdApoAI artery; an increase indicates a higher value in the paired HDAdApoAI artery. P values are for the paired comparisons.
Medial area could be reduced by thinning, potentially accompanied by inward or outward remodeling. There was only a small difference in area within the internal elastic lamina between the two groups (5%) and essentially no difference in the paired analyses (1% difference; P=0.9; Figure 3D and Table). Therefore, the small (3%; P=0.02) reduction in medial area in HDAdApoAI-treated carotids is not associated with arterial remodeling. Rather, the lower medial area in HDAdApoAI-treated carotids is due only to decreased medial thickness (P=0.01; Figure 3E and Table). Relevant to this, examination of histologic sections revealed modestly increased medial thickness adjacent to large intimal lesions (for example, Figure 2A), suggesting that thinner medias in HDAdApoAI-treated carotids were a consequence of smaller intimal lesions. Because area within the internal elastic lamina was unchanged between the groups (and medial area was not), in order to most appropriately normalize intimal area to vessel size we calculated percent luminal stenosis (luminal stenosis = intimal area/area within internal elastic lamina), in addition to calculating the intimal:medial area ratios. The median percent luminal stenosis was reduced by 50% in the group of HDAdApoAI-treated carotid arteries compared to the group of HDAdNull-treated arteries. There was a median 29% decrease in the HDAdApoAI-treated carotids compared to their contralateral controls (P=0.03; Figure 3F and Table).
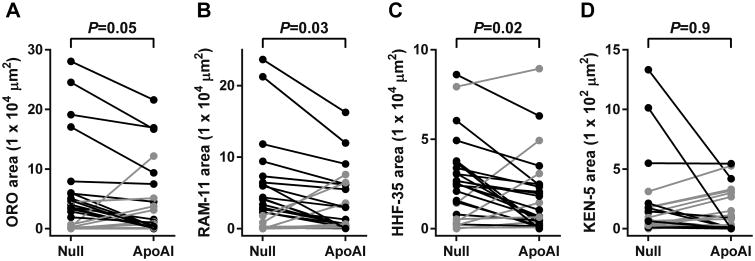
HDAdApoAI Decreases Intimal Lipid, Macrophage, and Smooth Muscle Area
The intimal lesions were rich in lipid and macrophages (Figure 2B, 2C, 2F, and 2G), but also contained significant numbers of smooth muscle cells (SMC), as judged by spindle-shaped cell morphology and staining for muscle actin (Figure 2D and 2H). In the group of HDAdApoAI-treated arteries, median intimal lipid content (Oil Red O) decreased 71% relative to the group of HDAdNull arteries (Table). Paired analyses also showed lower intimal lipid in HDAdApoAI-treated carotids compared to their contralateral HDAdNull-treated controls in 15 of 25 rabbits, with a median decrease of 23% (P=0.05; Figure 4A and Table). The reduction in lipid content was accompanied by a 76% reduction in median intimal macrophage area (RAM-11-stained area) in the group of HDAdApoAI-treated carotids (Table). Paired analyses showed lower intimal macrophage content in HDAdApoAI-treated carotids compared to contralateral HDAdNull-treated controlsin 17 of 25 rabbits, with a median decrease of 32% (P=0.03; Figure 4B and Table). SMC content of the intima was also decreased in the group of HDAdApoAI-treated carotids versus the group of HDAdNull-treated controls (60%; Table). In the paired analyses, HDAdApoAI treatment decreased SMC content in 18 of the 25 rabbits, with a median decrease of 31% (P=0.02; Figure 4C and Table). Rare T cells were present in the intimal lesions in both groups (Figure 2K, 2L, 2O, and 2P). Although there was a large difference in median intimal content of T cells between the 2 groups (53% lower in the HDAdApoAI group; Table), there was only a small, statistically insignificant difference between paired HDAdApoAI- and HDAdNull-treated arteries (5%; P=0.9; Figure 4D and Table). Finally, although HDAdApoAI-treated arteries had significantly lower amounts of intimal lipid, macrophages, and SMC, the percentage of intimal area occupied by each of these lesion components (and by T cells) did not differ significantly between paired HDAdApoAI-treated and HDAdNull-treated arteries (P=0.2–0.9; Table).
Figure 4.
Intimal lesion composition. Arteries were removed 24 weeks after treatment with either HDAdNull (Null) or HDAdApoAI (ApoAI), sectioned, stained, and analyzed with computer-assisted color thresholding and planimetry. Intimal areas staining with: (A) Oil Red O (ORO); (B) RAM-11 antibody (detects macrophages); (C) HHF-35 antibody (detects muscle actin); or (D) the KEN-5 antibody (detects T-cells). Data points are means for each artery; points from arteries in the same rabbit are connected by bars. Rabbits in which the HDAdApoAI-treated artery had a lower value than the contralateral HDAdNull-treated artery are indicated in black; rabbits in which the HDAdApoAI-treated artery had a higher value than the contralateral HDAdNull-treated artery are indicated in grey. P values are from Wilcoxon signed-rank test comparing HDAdApoAI- and HDAdNull-treated carotids in the same rabbit.
We also used mass spectrometry to measure free and esterified cholesterol in extracts of full-thickness artery segments (Figure V and Table I in the online-only Data Supplement). Free cholesterol content was 25% lower in the group of HDAdApoAI arteries than the group of HDAdNull arteries, with essentially no difference between paired arteries in the same rabbits (1% difference; P=0.5). Median cholesteryl ester and total cholesterol content were ∼60% lower in the group of HDAdApoAI arteries, but the difference between paired arteries was much less (6% and 9% decreases in paired HDAdApoAI arteries, respectively; P=0.4 for both). The median percentage of esterified cholesterol was almost identical between the 2 groups, with a median 7% reduction in paired HDAdApoAI arteries (P=0.2).
HDAdApoAI Alters Lesion Inflammatory Markers and ABCAI Expression
Compared to the vascular media and adventitia, intimal lesions contained relatively high levels of expression of vascular cell adhesion protein-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) (Figure 2I, 2J, 2M, and 2N). VCAM-1 and ICAM-1 were expressed both in luminal EC and deeper in the intima. Compared to the group of HDAdNull-infused arteries, HDAdApoAI reduced median intimal VCAM-1 staining by 40%, while median intimal ICAM-1 staining was reduced by 56%. Within-rabbit paired analyses showed reductions in both intimal VCAM-1 and ICAM-1 staining in HDAdApoAI-treated carotids compared to their contralateral HDAdNull-treated controls, with a median decrease of 36% for VCAM-1 and 29% for ICAM-1 (P=0.03 and P=0.07, respectively; Figure 5A, 5B and Table). Neither the percentage of intimal area stained for VCAM-1 nor the percentage of intimal area stained for ICAM-1 differed significantly between HDAdApoAI-treated carotid arteries and their paired HDAdNull-treated arteries (P=0.5 and P=0.3, respectively; Table).
Figure 5.
Intimal adhesion molecule expression. Arteries were removed 24 weeks after treatment with either HDAdNull (Null) or HDAdApoAI (ApoAI) sectioned, stained, and analyzed with computer-assisted color thresholding and planimetry. Intimal areas staining with: (A) antibody to VCAM-1; or (B) antibody to ICAM-1. Data points are means for each artery; points from arteries in the same rabbit are connected by bars. Rabbits in which the HDAdApoAI-treated artery had a lower value than the contralateral HDAdNull-treated artery are indicated in black; rabbits in which the HDAdApoAI-treated artery had a higher value than the contralateral HDAdNull-treated artery are indicated in grey. P values are from Wilcoxon signed-rank test comparing HDAdApoAI- and HDAdNull-treated carotids in the same rabbit.
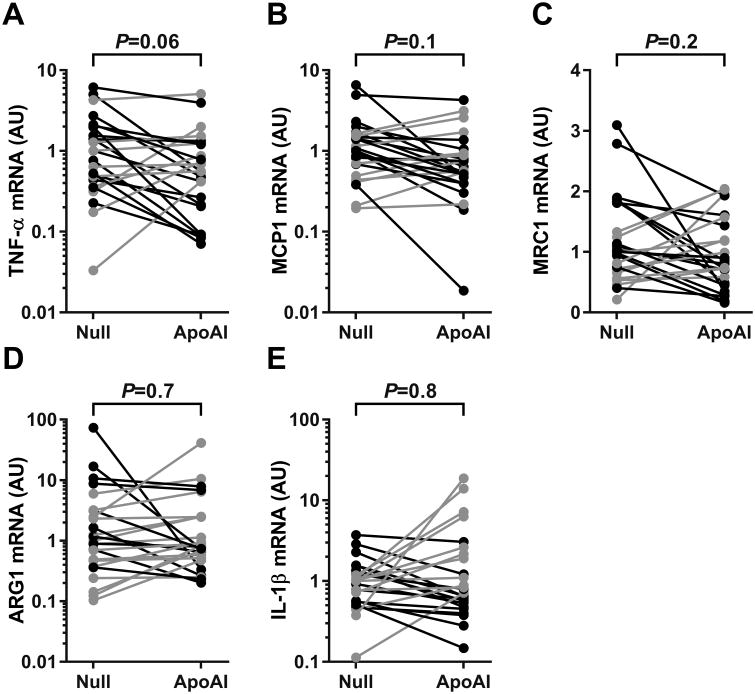
Using mRNA extracted from full-thickness artery segments (Figure II in the online-only Data Supplement), we measured levels of mRNA for 2 atherogenic cytokines: tumor necrosis factor-α (TNF-α) and monocyte chemoattractant protein-1 (MCP-1). Median mRNA levels of TNF-α and MCP-1 were reduced by 44% and 36%, respectively, in the group of HDAdApoAI-treated carotids versus the group of HDAdNull-treated carotids. Paired analyses showed lower TNF-α levels in HDAdApoAI-treated carotids compared to their contralateral controls, with a median reduction of 39% (P=0.06; Figure 6A and Table II in the online-only Data Supplement). The paired analyses also showed lower MCP-1 expression in HDAdApoAI-treated carotids, with a median decrease of 13% (P=0.1; Figure 6B and Table II in the online-only Data Supplement). We also attempted to measure mRNA for the atheroprotective cytokine interleukin (IL)-10; however, IL-10 mRNA was detectable in only a few of the arteries, precluding a meaningful comparison.
Figure 6.
Expression of inflammation-related markers in carotid arteries. Rabbit carotid arteries were transduced with HDAdNull (Null) or HDAdApoAI (ApoAI) and harvested 24 weeks later. mRNA was measured, normalized to GAPDH mRNA measured in the same extract, and expressed as arbitrary units (AU). (A) TNF‐α; (B) MCP-1; (C) MRC-1 (a M2 macrophage marker); (D) ARG-1 (a M2 macrophage marker); and (E) IL-1β (a M1 macrophage marker). Data points are values for each artery; points from arteries in the same rabbit are connected by bars. Rabbits in which the HDAdApoAI-treated artery had a lower value than the contralateral HDAdNull-treated artery are indicated in black; rabbits in which the HDAdApoAI-treated artery had a higher value than the contralateral HDAdNull-treated artery are indicated in grey. P values are from Wilcoxon signed-rank test comparing HDAdApoAI- and HDAdNull-treated carotids in the same rabbit.
Because macrophage phenotype is affected by apo A-I protein injection,37, 38 and because changes in macrophage phenotype can alter atherosclerosis progression,39, 40 we quantified mRNA encoding markers of rabbit M1 and M2 macrophages.41 The M2 markers mannose receptor C-type 1 (MRC1) and arginase 1 (ARG1) were both 20% lower in the group of HDAdApoAI-treated arteries compared to the group of HDAdNull-treated arteries. Paired analyses revealed a median decrease in MRC1 and an increase in ARG1 (22% and 11%; P=0.2 and 0.7, respectively; Figure 6C and 6D and Table II in the online-only Data Supplement). The M1 macrophage marker IL-1β was decreased by 20% in the group of HDAdApoAI-treated arteries, with an 18% reduction in the paired analyses (Figure 6E and Table II in the online-only Data Supplement; P=0.8). Attempts to measure mRNA for other proposed rabbit M1 [IL-12β, N-formyl peptide receptor 2 (FPR2)] and M2 [C-type lectin domain family 7 member A (CLEC7A)] markers by qRT-PCR were not successful because quantitative amplification of these mRNA could not be achieved in artery extracts.
Finally, because lipid-free apo A-I initiates reverse cholesterol transport largely via interaction with ATP-binding cassette subfamily A, member 1 (ABCA1), we tested whether ABCA1 is expressed in the rabbit carotids. We measured ABCA1 mRNA in extracts of 48 of the 50 experimental arteries. ABCA1 mRNA was detected in all arteries; with 60% lower ABCA1 mRNA levels in the group of HDAdApoAI-treated carotids compared to the group of HDAdNull-treated carotids (Figure VIA in the online-only Data Supplement). Compared to their paired controls, HDAdApoAI-treated carotids had a median 40% reduction in ABCA1 RNA (P=0.03; Table II in the online-only Data Supplement). ABCA1 protein was present throughout the intima and media, but was nearly absent in the adventitia (Figure VI E in the online-only Data Supplement). Intimal ABCA1 protein was reduced by a median 20% in HDAdApoAI-treated carotids compared to their paired controls (P=0.04; Table and FigureVI B in the online-only Data Supplement). However, when the amount of ABCA1 immunostaining was normalized to intimal area, there was no significant difference between HDAdApoAI-treated carotids and their paired controls (P=0.4; Table and Figure VI C in the online-only Data Supplement).
Discussion
We transduced carotid artery EC of hyperlipidemic rabbits with an apo A-I-expressing HDAd vector and tested whether expression of apo A-I could retard development of atherosclerosis over a 24-week period, despite persistent hyperlipidemia. Our major findings were: 1) a single infusion of HDAdApoAI yielded apo A-I expression that persisted for at least 24 weeks in hyperlipidemic rabbits; 2) apo A-I gene therapy reduced intimal lesion size by ∼30%; 3) apo A-I gene therapy reduced intimal lipid, macrophages, and smooth muscle cells; and 4) apo A-I gene therapy had local anti-inflammatory effects, significantly reducing intimal VCAM-1 protein and nominally decreasing intimal ICAM-1 protein as well as vascular wall TNF-α and MCP-1 mRNA.
Because of the central role of apo A-I in mediating cholesterol efflux, several groups have developed therapies aimed at increasing plasma apo A-I levels or augmenting plasma apo A-I-like activity. These therapies, which include small molecules that increase apo A-I synthesis, apo A-I-mimetic peptides, and infusion of apo A-I protein,17 have yielded promising results in animals and in small clinical trials.13, 42, 43 However, none has shown benefit in a large human trial. Moreover, clinical implementation of these apo A-I-directed therapies is hindered by the short half-life of plasma apo A-I, a parenteral delivery requirement for most of the protein and peptide formulations, high cost, toxicity, and limited understanding of the mode of action of apo A-I-mimetic peptides.17, 44
Apo A-I gene therapy has 2 major advantages over other apo A-I-directed therapies. First, a single dose of apo A-I gene therapy could potentially deliver apo A-I for years,24-27 obviating a need both for repeat dosing and for producing and purifying large amounts of apo A-I. Second, apo A-I gene therapy directs the synthesis and release of native apo A-I protein, eliminating the need to reconstitute apo A-I function with synthetic components.44 Because of these advantages, several groups have developed apo A-I gene therapy and reported therapeutic effects in preclinical models. For example, adenovirus-mediated apo A-I gene therapy targeted to mouse liver increases plasma apo A-I levels, reduces atherosclerosis progression, and causes atherosclerotic regression.45-47 Another approach to apo A-I gene therapy includes ex vivo transduction of bone marrow cells with integrating apo A-I-expressing viral vectors such as retrovirus or lentivirus. The transduced bone marrow is then used to reconstitute lethally irradiated atherosclerosis-prone mice.48-50 Using this approach, Fazio's group showed that macrophage-mediated apo A-I gene therapy reduced atherosclerosis without affecting plasma HDL cholesterol levels, suggesting that local vessel wall delivery of apo A-I is sufficient to achieve therapeutic effects.48, 49 Unfortunately, neither of these gene therapy approaches is easily translated into a human therapy. Liver-directed gene therapy typically requires infusion of large amounts of vector, which can be toxic.51 Because apo A-I is one of the most abundant native plasma proteins,12 large amounts of vector would be required for liver-targeted gene therapy aimed at increasing plasma apo A-I. Moreover, vector-mediated expression of apo A-I in the liver downregulates endogenous apo A-I expression, potentially resulting in no net increase in plasma apo A-I.46 In addition, expression of transgenes in the liver typically declines over time (likely due to cell turnover), requiring re-treatment.24, 45 In contrast, bone marrow-targeted gene therapy, in which stem cells are transduced with integrating vectors, could provide life-long apo A-I delivery.48 However, bone marrow-mediated gene therapy would require myeloablative conditioning that—for humans—carries unacceptable costs and risks.
Because of these limitations of liver- and bone marrow-targeted apo A-I gene therapy, we developed EC-targeted apo A-I gene therapy as an alternative approach. Delivery of apo A-I to the vessel wall from overlying EC has several theoretical advantages. First, apo A-I is delivered at high concentrations precisely where it is needed: adjacent to macrophages and smooth muscle cells in which lipid is accumulating. Basolateral secretion would deliver apo A-I directly to the intima; others have reported that endothelial cells secrete apo A-I predominantly from their basolateral surface.52 Apically secreted apo A-I would likely be transported across the endothelial monolayer via the same apical-to-basolateral transcytotic pathway through which plasma apo A-I is delivered to the subendothelium.53 Second, when produced by EC, apo A-I is secreted in a lipid-free state, in which it is a superior stimulator of cholesterol efflux.23 In contrast, apo A-I secreted by the liver is lipidated upon secretion, decreasing its cholesterol-efflux capacity.23, 54 Third, HDAd-mediated transgene expression in EC is inherently stable, lasting at least 48 weeks and likely far longer.22, 55 Fourth, continuous apo A-I delivery from EC eliminates a need for vector-encoded apo A-I to circulate in plasma, in which it can be damaged by systemic inflammatory processes.56, 57 Data presented herein supports the efficacy of vessel wall-targeted apo A-I gene therapy. HDAdApoAI-treated arteries had smaller lesions, less intimal lipid, and fewer intimal macrophages. The small number of HDAdApoAI-transduced cells (confined to a short segment of a single artery) and the paired study design exclude the possibility that these therapeutic effects are mediated by vector-mediated increases in plasma apo A-I (levels of which decreased during the course of the study).
We reported previously that transduction of rabbit carotid EC with HDAdApoAI significantly reduced atherosclerosis progression over a 4-week period.22 In more recent work, we found that this same gene therapy approach tended to promote regression of established atherosclerotic lesions.31 In relation to these earlier studies, results reported herein extend our work in important ways. Compared to the first study, in which treated lesions were limited to early fatty streaks that regressed spontaneously by 8 weeks,22 we now show that apo A-I gene therapy durably (24 weeks) retards lesion growth. Moreover, the present study establishes that apo A-I gene therapy can limit growth of more complex atherosclerotic lesions that contain smooth muscle cells as well as lipid and macrophages.
Compared to the second study, in which expression from HDAdApoAI in hyperlipidemic rabbits was measured for only 7 weeks,31 we now show that apo A-I expression continues at a high level for at least 24 weeks, despite persistent hyperlipidemia. This is important because hyperlipidemia and atherosclerosis can increase EC loss and replication,28, 29 and until now we had shown durable HDAdApoAI expression only in chow-fed rabbits.22, 58 In addition, in the second study HDAdApoAI appeared ineffective in treating large intimal lesions that develop in a subgroup of highly atherosclerosis-prone rabbits.31 Incontrast, in the present study apo A-I gene therapy uniformly prevented lesion growth in the most atherosclerosis-prone rabbits (Supplemental Figure VII in the online-only Data Supplement). We attribute the superior performance of apo A-I gene therapy in the present study to delivery of HDAdApoAI early in lesion development, versus delivery to existing lesions in the earlier study.31 ApoA-I secreted from a single layer of EC may not sufficiently raise apo A-I levels deep within large pre-existing intimal lesions to achieve a therapeutic effect. Another possible explanation for efficacy in the present versus the former study is the difference in duration of treatment (24 weeks versus 7 weeks); achievement of lesion regression may require more than 7 weeks of gene therapy. Taken together, our new results continue to show promise for HDAdApoAI-mediated gene therapy and support its further development as a therapy for human atherosclerosis.
In the present study, HDAdApoAI reduced intimal lesion size as well as intimal lipid and macrophages. These results are expected based on the ability of apo A-Ito remove cholesterol from macrophages and incorporate it into particles that can remove cholesterol from the vessel wall.59 HDAdApoAI also reduced the amount of intimal area occupied by SMC. This was unanticipated; however, it may reflect apo A-I-mediated removal of cholesterol from SMC that reduces SMC mass.60 This explanation would be consistent with a growing recognition that many intimal foam cells are of SMC lineage.61 Alternatively, decreased SMC content in the intima could reflect a lower rate of SMC migration or proliferation in apo A-I-treated arteries.62, 63 As in our previous study,31 the percentage of the intima occupied either by lipid, by total cells, or by specific cell types was unchanged in HDAdApoAI-treated arteries. This is likely because apo A-I removes lipid from both macrophages and SMC, thereby reducing intimal volumes of lipid, macrophages, and SMC in equal proportions.
In addition to stimulating removal of cellular cholesterol, lipid-poor apoA-I produced by HDAdApoAI may reduce atherosclerosis vialocal anti-inflammatory actions.64-66 These actions could include:blocking cytokine-mediated upregulation of ICAM-1 and VCAM-1;67 blocking production of inflammatory cytokines (e.g., MCP-1, IL-6, and TNF-α) and increasing production of anti-inflammatory cytokines (e.g., IL-10);38 stimulating an anti-inflammatory M2 phenotype in lesion macrophages;37 and inhibiting M1 macrophage polarization.68 Lipid-poor apo A-I can also reduce lipid raft content of inflammatory and other vascular cells, leading to redistribution of cytokine receptors out of lipid rafts and impaired transduction of inflammatory signals.69, 70 Consistent with these models, we found large reductions in intimal VCAM-1 and ICAM-1 staining in HDAdApoAI-treated arteries. Reduced VCAM-1 staining is reassuring because apo A-I reliably reduces VCAM-1 expression in other settings.21, 46, 71, 72 HDAdApoAI-transduced arteries also had lower levels of MCP-1 and TNF-α mRNA, although these reductions were of only borderline significance. Lack of statistical significance could be due to measurement of cytokine mRNA in whole vessel extracts; whereas, most of the measurements that differed significantly between HDAdApoAI and control arteries were limited to the intima, a site where EC-secreted apo A-I would be expected to have its maximal effect. Dilution of a therapeutic intimal signal by noise from the media and (especially) the adventitia could also explain why Oil Red O staining of the intima showed that apo A-I therapy significantly reduced lipid whereas mass-spectrometry-based measurements of lipid in whole artery extracts showed only a trend towards reduced cholesterol in HDAdApoAI arteries. These hypotheses could be tested by measuring mRNA in intimal tissue isolated by laser capture microdissection, by removing the adventitia before extracting tissue lipids (see Figure 2F for an example of adventitial lipid), or by isolating intimal cells ex vivo and measuring their cholesterol content.70
Removal of cellular cholesterol by apo A-I depends on—and could be limited by—ABCA1 expression.73 Becausecellular cholesterol loading upregulates ABCA1 expression,74, 75 we assumed that ABCA1 would be abundant in the carotid intima. Accordingly, ABCA1 immunoreactivity was present throughout the intima (including in foam cells) of both HDAdApoAI- and HDAdNull-transduced arteries. However, ABCA1 mRNA was significantly lower in HDAdApoAI-transduced versus HDAdNull-transduced arteries. Lower expression of ABCA1 in HDAdApoAI-transduced arteries could be a consequence of elevated apo A-I expression, because both apo A-I and HDL3 (generated by apoA-I-mediated cholesterol efflux) can downregulate ABCA1 expression.74-76 We found a smaller—but still significant—reduction in intimal ABCA1 protein levels in HDAdApoAI-transduced versus HDAdNull-transduced arteries. The smaller reduction in ABCA1 protein versus mRNA might result from stabilization of ABCA1 protein by apo A-I.77, 78 Downregulation of ABCA1 mRNA and protein in HDAdApoAI arteries could limit the efficacy of apo A-I gene therapy if ABCA1 levels fall sufficiently that cholesterol efflux is impaired. However, the observed decreases in ABCA1 mRNA and protein in HDAdApoAI arteries were small, and the decrease in intimal ABCA1 protein disappeared when protein levels were normalized to intimal area. Moreover, this potential limitation has not prevented efficacy of apo A-I in several animal models of apo A-I gene therapy and germ line transgenic overexpression.45-50, 79, 80
We were surprised that we did not find apo A-I protein in medium conditioned by explanted HDAdApoAI-transduced arteries. This was unanticipated because artery apo A-I mRNA levels in the present study were at least as high as in a recent study in which we detected apo A-I protein in explant culture medium of HDAdApoAI-transduced arteries (data not shown).31 In the earlier study we used DMEM with phenol red for the explant cultures; whereas in the present study we omitted phenol red, but this seems unlikely to explain the difference. It is possible that apo A-I protein is present in explant culture media in the current study but is below the level of detection. A drop in apo A-I protein despite stable apo A-I mRNA could result from translational downregulation;81, 82 however, complete cessation of apo A-I translation seems unlikely. Detection of low levels of rabbit apo A-I protein in experimental samples by western blotting has been a challenge for us, which we surmounted only recently by generation of new antibodies.31 To develop a more sensitive assay for apo A-I protein, we recently used these antibodies to develop an ELISA. The ELISA detected purified rabbit apo A-I with excellent sensitivity, but was much less sensitive for detection of apo A-I in rabbit plasma or artery explant culture medium (data not shown). Nevertheless, although we did not detect increased apo A-I protein in HDAdApoAI-infused arteries, numerous biologically plausible phenotypic changes in these arteries (decreases in lesion area, ORO area, macrophage area, SMC area, expression of VCAM-1, TNF-α, and ABCA1) strongly suggest that apo A-I protein is produced by HDAdApoAI.
In conclusion, we demonstrate for the first time that local vascular delivery of apo A-I can durably reduce development of atherosclerosis, even in a setting of severe hyperlipidemia. Challenges of pre-existing human immunity to viral vectors and lack of efficient percutaneous methods for delivering vectors to EC must be overcome before apo A-I vascular gene therapy can be applied clinically, but progress is evident in both of these areas.83, 84 We are optimistic that these challenges will be overcome and that local vascular delivery of apo A-I will become a complementary therapy to LDL lowering, leading to a further reduction in human atherosclerotic cardiovascular disease.
Supplementary Material
Highlights.
We used a rabbit model of carotid atherosclerosis to test whether local transduction of endothelial cells with an apo A-I-expressing helper-dependent adenoviral vector could durably reduce atherosclerotic lesion growth, despite persistent hyperlipidemia.
Helper-dependent adenoviral expression of apo A-I in hyperlipidemic rabbits was durable for at least 24 weeks.
Local apo A-I gene therapy significantly reduced intimal lesion area.
Intimal lipid, macrophage, and smooth muscle cell content were all decreased by apo A-I gene therapy.
Local apo A-I gene therapy uniformly decreased expression of intimal lesion inflammatory markers; these decreases in expression were either significant (VCAM-1) or borderline significant (ICAM-1, MCP-1, TNF-α).
Acknowledgments
We thank AdVec, Inc. for permission to use the HDAd reagents, Jingwan Zhang and Ervin Ham for technical assistance, Julia Feyk for administrative assistance, and the Department of Comparative Medicine veterinary services for surgical advice and support.
Sources of Funding: This work was supported by HL114541 and the John L. Locke, Jr. Charitable Trust. Alexis Stamatikos was supported by T32HL007828.
Abbreviations
- ABCA1
ATP-binding cassette subfamily A, member 1
- apo A-I
apolipoprotein A-I
- ARG1
arginase-1
- CLEC7A
C-type lectin domain family 7 member A
- EC
endothelial cells
- FPR2
formyl peptide receptor 2
- HDAd
helper-dependent adenovirus
- HDL-C
high-density lipoprotein cholesterol
- ICAM-1
intercellular adhesion molecule-1
- IL
interleukin
- LDL-C
low-density lipoprotein cholesterol
- MCP-1
monocyte chemoattractant protein 1
- MRC1
mannose receptor C-type 1
- SMC
smooth muscle cells
- TNF-α
tumor necrosis factor alpha
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Disclosures: None
References
- 1.Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, Mussolino ME, Hsu LL, Addou E, Engelgau MM, Gordon D. Decline in cardiovascular mortality: Possible causes and implications. Circ Res. 2017;120:366–380. doi: 10.1161/CIRCRESAHA.116.309115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weir HK, Anderson RN, Coleman King SM, Soman A, Thompson TD, Hong Y, Moller B, Leadbetter S. Heart disease and cancer deaths - trends and projections in the united states, 1969-2020. Prev Chronic Dis. 2016;13:E157. doi: 10.5888/pcd13.160211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAloon CJ, Boylan LM, Hamborg T, Stallard N, Osman F, Lim PB, Hayat SA. The changing face of cardiovascular disease 2000-2012: An analysis of the world health organisation global health estimates data. Int J Cardiol. 2016;224:256–264. doi: 10.1016/j.ijcard.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Shepard D, Vander Zanden A, Moran A, Naghavi M, Murray C, Roth G. Ischemic heart disease worldwide, 1990 to 2013: Estimates from the global burden of disease study 2013. Circ Cardiovasc Qual Outcomes. 2015;8:455–456. doi: 10.1161/CIRCOUTCOMES.115.002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cholesterol Treatment Trialists C. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of ldl cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 7.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR Committee FS, Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017 doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 8.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50 Suppl:S189–194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Investigators A-H. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low hdl cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 10.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 12.Schonfeld G, Pfleger B. The structure of human high density lipoprotein and the levels of apolipoprotein a-i in plasma as determined by radioimmunoassay. J Clin Invest. 1974;54:236–246. doi: 10.1172/JCI107758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant apoa-i milano on coronary atherosclerosis in patients with acute coronary syndromes: A randomized controlled trial. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 14.Tardif JC, Heinonen T, Noble S. High-density lipoprotein/apolipoprotein a-i infusion therapy. Curr Atheroscler Rep. 2009;11:58–63. doi: 10.1007/s11883-009-0009-7. [DOI] [PubMed] [Google Scholar]
- 15.Singh JP, Kauffman R, Bensch W, Wang G, McClelland P, Bean J, Montrose C, Mantlo N, Wagle A. Identification of a novel selective peroxisome proliferator-activated receptor alpha agonist, 2-methyl-2-(4-{3-[1-(4-methylbenzyl)-5-oxo-4,5-dihydro-1h-1,2,4-triazol-3-yl]prop yl}phenoxy)propanoic acid (ly518674), that produces marked changes in serum lipids and apolipoprotein a-1 expression. Mol Pharmacol. 2005;68:763–768. doi: 10.1124/mol.105.010991. [DOI] [PubMed] [Google Scholar]
- 16.Khera AV, Millar JS, Ruotolo G, Wang MD, Rader DJ. Potent peroxisome proliferator-activated receptor-alpha agonist treatment increases cholesterol efflux capacity in humans with the metabolic syndrome. Eur Heart J. 2015;36:3020–3022. doi: 10.1093/eurheartj/ehv291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millar JS, Cuchel M. Apoa-i-directed therapies for the management of atherosclerosis. Curr Atheroscler Rep. 2015;17:60. doi: 10.1007/s11883-015-0539-0. [DOI] [PubMed] [Google Scholar]
- 18.Michael Gibson C, Korjian S, Tricoci P, et al. Safety and tolerability of csl112, a reconstituted, infusible, plasma-derived apolipoprotein a-i, after acute myocardial infarction: The aegis-i trial (apoa-i event reducing in ischemic syndromes i) Circulation. 2016;134:1918–1930. doi: 10.1161/CIRCULATIONAHA.116.025687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews J, Janssan A, Nguyen T, et al. Effect of serial infusions of reconstituted high-density lipoprotein (cer-001) on coronary atherosclerosis: Rationale and design of the carat study. Cardiovasc Diagn Ther. 2017;7:45–51. doi: 10.21037/cdt.2017.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kempen HJ, Asztalos BF, Moerland M, Jeyarajah E, Otvos J, Kallend DG, Bellibas SE, Wijngaard PL. High-density lipoprotein subfractions and cholesterol efflux capacities after infusion of mdco-216 (apolipoprotein a-imilano/palmitoyl-oleoyl-phosphatidylcholine) in healthy volunteers and stable coronary artery disease patients. Arterioscler Thromb Vasc Biol. 2016;36:736–742. doi: 10.1161/ATVBAHA.115.307052. [DOI] [PubMed] [Google Scholar]
- 21.Flynn R, Buckler JM, Tang C, Kim F, Dichek D. Helper-dependent adenoviral vectors are superior in vitro to first-generation vectors for endothelial cell-targeted gene therapy. Mol Ther. 2010;18:2121–2129. doi: 10.1038/mt.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flynn R, Qian K, Tang C, Dronadula N, Buckler J, Jiang B, Wen S, Dichek H, Dichek D. Expression of apolipoprotein a-i in rabbit carotid endothelium protects against atherosclerosis. Mol Ther. 2011;19:1833–1841. doi: 10.1038/mt.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du XM, Kim MJ, Hou L, Le Goff W, Chapman MJ, Van Eck M, Curtiss LK, Burnett JR, Cartland SP, Quinn CM, Kockx M, Kontush A, Rye KA, Kritharides L, Jessup W. Hdl particle size is a critical determinant of abca1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116:1133–1142. doi: 10.1161/CIRCRESAHA.116.305485. [DOI] [PubMed] [Google Scholar]
- 24.Brunetti-Pierri N, Ng T, Iannitti D, Cioffi W, Stapleton G, Law M, Breinholt J, Palmer D, Grove N, Rice K, Bauer C, Finegold M, Beaudet A, Mullins C, Ng P. Transgene expression up to 7 years in nonhuman primates following hepatic transduction with helper-dependent adenoviral vectors. Hum Gene Ther. 2013;24:761–765. doi: 10.1089/hum.2013.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hacein-Bey-Abina S, Hauer J, Lim A, et al. Efficacy of gene therapy for x-linked severe combined immunodeficiency. N Engl J Med. 2010;363:355–364. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor ix gene therapy in hemophilia b. N Engl J Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bainbridge JW, Mehat MS, Sundaram V, et al. Long-term effect of gene therapy on leber's congenital amaurosis. N Engl J Med. 2015;372:1887–1897. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Florentin RA, Nam SC, Lee KT, Thomas WA. Increased 3h-thymidine incorporation into endothelial cells of swine fed cholesterol for 3 days. Exp Mol Pathol. 1969;10:250–255. doi: 10.1016/0014-4800(69)90055-0. [DOI] [PubMed] [Google Scholar]
- 29.Faggiotto A, Ross R, Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984;4:323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- 30.Du L, Zhang J, De Meyer GR, Flynn R, Dichek DA. Improved animal models for testing gene therapy for atherosclerosis. Hum Gene Ther Methods. 2014;25:106–114. doi: 10.1089/hgtb.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wacker BK, Dronadula N, Zhang J, Dichek DA. Local vascular gene therapy with apolipoprotein a-i to promote regression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37:316–327. doi: 10.1161/ATVBAHA.116.308258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolodgie FD, Katocs AS, Jr, Largis EE, Wrenn SM, Cornhill JF, Herderick EE, Lee SJ, Virmani R. Hypercholesterolemia in the rabbit induced by feeding graded amounts of low-level cholesterol. Methodological considerations regarding individual variability in response to dietary cholesterol and development of lesion type. Arterioscler Thromb Vasc Biol. 1996;16:1454–1464. doi: 10.1161/01.atv.16.12.1454. [DOI] [PubMed] [Google Scholar]
- 33.Jiang B, Qian K, Du L, Luttrell I, Chitaley K, Dichek DA. Helper-dependent adenovirus is superior to first-generation adenovirus for expressing transgenes in atherosclerosis-prone arteries. Arterioscler Thromb Vasc Biol. 2011;31:1317–1325. doi: 10.1161/ATVBAHA.111.225516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gruchala M, Bhardwaj S, Pajusola K, Roy H, Rissanen TT, Kokina I, Kholova I, Markkanen JE, Rutanen J, Heikura T, Alitalo K, Bueler H, Yla-Herttuala S. Gene transfer into rabbit arteries with adeno-associated virus and adenovirus vectors. J Gene Med. 2004;6:545–554. doi: 10.1002/jgm.535. [DOI] [PubMed] [Google Scholar]
- 35.Schulick AH, Dong G, Newman KD, Virmani R, Dichek DA. Endothelium-specific in vivo gene transfer. Circ Res. 1995;77:475–485. doi: 10.1161/01.res.77.3.475. [DOI] [PubMed] [Google Scholar]
- 36.Du L, Dronadula N, Tanaka S, Dichek DA. Helper-dependent adenoviral vector achieves prolonged, stable expression of interleukin-10 in rabbit carotid arteries but does not limit early atherogenesis. Hum Gene Ther. 2011;22:959–968. doi: 10.1089/hum.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hewing B, Parathath S, Barrett T, et al. Effects of native and myeloperoxidase-modified apolipoprotein a-i on reverse cholesterol transport and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2014;34:779–789. doi: 10.1161/ATVBAHA.113.303044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smythies LE, White CR, Maheshwari A, Palgunachari MN, Anantharamaiah GM, Chaddha M, Kurundkar AR, Datta G. Apolipoprotein a-i mimetic 4f alters the function of human monocyte-derived macrophages. Am J Physiol Cell Physiol. 2010;298:C1538–1548. doi: 10.1152/ajpcell.00467.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolfs IM, Stoger JL, Goossens P, Pottgens C, Gijbels MJ, Wijnands E, van der Vorst EP, van Gorp P, Beckers L, Engel D, Biessen EA, Kraal G, van Die I, Donners MM, de Winther MP. Reprogramming macrophages to an anti-inflammatory phenotype by helminth antigens reduces murine atherosclerosis. FASEB J. 2014;28:288–299. doi: 10.1096/fj.13-235911. [DOI] [PubMed] [Google Scholar]
- 40.Peled M, Fisher EA. Dynamic aspects of macrophage polarization during atherosclerosis progression and regression. Front Immunol. 2014;5:579. doi: 10.3389/fimmu.2014.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamane K, Leung KP. Rabbit m1 and m2 macrophages can be induced by human recombinant gm-csf and m-csf. FEBS Open Bio. 2016;6:945–953. doi: 10.1002/2211-5463.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tardif JC, Gregoire J, L'Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: A randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 43.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103:1084–1091. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 44.Sviridov D, Remaley AT. High-density lipoprotein mimetics: Promises and challenges. Biochem J. 2015;472:249–259. doi: 10.1042/BJ20150832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tangirala RK, Tsukamoto K, Chun SH, Usher D, Puré E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein a-i in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- 46.Belalcazar LM, Merched A, Carr B, Oka K, Chen KH, Pastore L, Beaudet A, Chan L. Long-term stable expression of human apolipoprotein a-i mediated by helper-dependent adenovirus gene transfer inhibits atherosclerosis progression and remodels atherosclerotic plaques in a mouse model of familial hypercholesterolemia. Circulation. 2003;107:2726–2732. doi: 10.1161/01.CIR.0000066913.69844.B2. [DOI] [PubMed] [Google Scholar]
- 47.Pastore L, Belalcazar LM, Oka K, Cela R, Lee B, Chan L, Beaudet AL. Helper-dependent adenoviral vector-mediated long-term expression of human apolipoprotein a-i reduces atherosclerosis in apo e-deficient mice. Gene. 2004;327:153–160. doi: 10.1016/j.gene.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 48.Ishiguro H, Yoshida H, Major AS, Zhu T, Babaev VR, Linton MF, Fazio S. Retrovirus-mediated expression of apolipoprotein a-i in the macrophage protects against atherosclerosis in vivo. J Biol Chem. 2001;276:36742–36748. doi: 10.1074/jbc.M106027200. [DOI] [PubMed] [Google Scholar]
- 49.Tavori H, Su YR, Yancey PG, Giunzioni I, Wilhelm AJ, Blakemore JL, Zabalawi M, Linton MF, Sorci-Thomas MG, Fazio S. Macrophage apoai protects against dyslipidemia-induced dermatitis and atherosclerosis without affecting hdl. J Lipid Res. 2015;56:635–643. doi: 10.1194/jlr.M056408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L, Tian F, Arias A, Yang M, Sharifi BG, Shah PK. Comparative effects of diet-induced lipid lowering versus lipid lowering along with apo a-i milano gene therapy on regression of atherosclerosis. J Cardiovasc Pharmacol Ther. 2016;21:320–328. doi: 10.1177/1074248415610216. [DOI] [PubMed] [Google Scholar]
- 51.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 52.Panzenboeck U, Balazs Z, Sovic A, Hrzenjak A, Levak-Frank S, Wintersperger A, Malle E, Sattler W. Abca1 and scavenger receptor class b, type i, are modulators of reverse sterol transport at an in vitro blood-brain barrier constituted of porcine brain capillary endothelial cells. J Biol Chem. 2002;277:42781–42789. doi: 10.1074/jbc.M207601200. [DOI] [PubMed] [Google Scholar]
- 53.Cavelier C, Rohrer L, von Eckardstein A. Atp-binding cassette transporter a1 modulates apolipoprotein a-i transcytosis through aortic endothelial cells. Circ Res. 2006;99:1060–1066. doi: 10.1161/01.RES.0000250567.17569.b3. [DOI] [PubMed] [Google Scholar]
- 54.Rubin EM, Ishida BY, Clift SM, Krauss RM. Expression of human apolipoprotein a-i in transgenic mice results in reduced plasma levels of murine apolipoprotein a-i and the appearance of two new high density lipoprotein size subclasses. Proc Natl Acad Sci U S A. 1991;88:434–438. doi: 10.1073/pnas.88.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dronadula N, Wacker BK, Van Der Kwast R, Zhang J, Dichek DA. Stable in vivo transgene expression in endothelial cells with helper-dependent adenovirus: Roles of promoter and interleukin-10. Hum Gene Ther. 2017;28:255–270. doi: 10.1089/hum.2016.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han CY, Tang C, Guevara ME, et al. Serum amyloid a impairs the antiinflammatory properties of hdl. J Clin Invest. 2016;126:266–281. doi: 10.1172/JCI83475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Getz GS, Krishack PA, Reardon CA. Serum amyloid a and atherosclerosis. Curr Opin Lipidol. 2016;27:531–535. doi: 10.1097/MOL.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 58.Wen S, Graf S, Massey PG, Dichek DA. Improved vascular gene transfer with a helper-dependent adenoviral vector. Circulation. 2004;110:1484–1491. doi: 10.1161/01.CIR.0000141574.78032.A9. [DOI] [PubMed] [Google Scholar]
- 59.Hara H, Yokoyama S. Interaction of free apolipoproteins with macrophages. Formation of high density lipoprotein-like lipoproteins and reduction of cellular cholesterol. J Biol Chem. 1991;266:3080–3086. [PubMed] [Google Scholar]
- 60.Choi HY, Rahmani M, Wong BW, Allahverdian S, McManus BM, Pickering JG, Chan T, Francis GA. Atp-binding cassette transporter a1 expression and apolipoprotein a-i binding are impaired in intima-type arterial smooth muscle cells. Circulation. 2009;119:3223–3231. doi: 10.1161/CIRCULATIONAHA.108.841130. [DOI] [PubMed] [Google Scholar]
- 61.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. Klf4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaul S, Rukshin V, Santos R, Azarbal B, Bisgaier CL, Johansson J, Tsang VT, Chyu KY, Cercek B, Mirocha J, Shah PK. Intramural delivery of recombinant apolipoprotein a-imilano/phospholipid complex (etc-216) inhibits in-stent stenosis in porcine coronary arteries. Circulation. 2003;107:2551–2554. doi: 10.1161/01.CIR.0000074042.19447.B1. [DOI] [PubMed] [Google Scholar]
- 63.Soma MR, Donetti E, Parolini C, Sirtori CR, Fumagalli R, Franceschini G. Recombinant apolipoprotein a-imilano dimer inhibits carotid intimal thickening induced by perivascular manipulation in rabbits. Circ Res. 1995;76:405–411. doi: 10.1161/01.res.76.3.405. [DOI] [PubMed] [Google Scholar]
- 64.Rye KA, Barter PJ. Antiinflammatory actions of hdl: A new insight. Arterioscler Thromb Vasc Biol. 2008;28:1890–1891. doi: 10.1161/ATVBAHA.108.173575. [DOI] [PubMed] [Google Scholar]
- 65.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. Hdl and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8:222–232. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 66.Sorci-Thomas MG, Thomas MJ. Why targeting hdl should work as a therapeutic tool, but has not. J Cardiovasc Pharmacol. 2013;62:239–246. doi: 10.1097/FJC.0b013e31829d48a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia P, Vadas MA, Rye KA, Barter PJ, Gamble JR. High density lipoproteins (hdl) interrupt the sphingosine kinase signaling pathway. A possible mechanism for protection against atherosclerosis by hdl. J Biol Chem. 1999;274:33143–33147. doi: 10.1074/jbc.274.46.33143. [DOI] [PubMed] [Google Scholar]
- 68.Lee MK, Moore XL, Fu Y, Al-Sharea A, Dragoljevic D, Fernandez-Rojo MA, Parton R, Sviridov D, Murphy AJ, Chin-Dusting JP. High-density lipoprotein inhibits human m1 macrophage polarization through redistribution of caveolin-1. Br J Pharmacol. 2016;173:741–751. doi: 10.1111/bph.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng AM, Handa P, Tateya S, Schwartz J, Tang C, Mitra P, Oram JF, Chait A, Kim F. Apolipoprotein a-i attenuates palmitate-mediated nf-kappab activation by reducing toll-like receptor-4 recruitment into lipid rafts. PLoS One. 2012;7:e33917. doi: 10.1371/journal.pone.0033917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaul S, Xu H, Zabalawi M, Maruko E, Fulp BE, Bluemn T, Brzoza-Lewis KL, Gerelus M, Weerasekera R, Kallinger R, James R, Zhang YS, Thomas MJ, Sorci-Thomas MG. Lipid-free apolipoprotein a-i reduces progression of atherosclerosis by mobilizing microdomain cholesterol and attenuating the number of cd131 expressing cells: Monitoring cholesterol homeostasis using the cellular ester to total cholesterol ratio. J Am Heart Assoc. 2016;5:e004401. doi: 10.1161/JAHA.116.004401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:1987–1994. doi: 10.1161/01.atv.15.11.1987. [DOI] [PubMed] [Google Scholar]
- 72.Wu BJ, Chen K, Shrestha S, Ong KL, Barter PJ, Rye KA. High-density lipoproteins inhibit vascular endothelial inflammation by increasing 3beta-hydroxysteroid-delta24 reductase expression and inducing heme oxygenase-1. Circ Res. 2013;112:278–288. doi: 10.1161/CIRCRESAHA.111.300104. [DOI] [PubMed] [Google Scholar]
- 73.Bortnick AE, Rothblat GH, Stoudt G, Hoppe KL, Royer LJ, McNeish J, Francone OL. The correlation of atp-binding cassette 1 mrna levels with cholesterol efflux from various cell lines. J Biol Chem. 2000;275:28634–28640. doi: 10.1074/jbc.M003407200. [DOI] [PubMed] [Google Scholar]
- 74.Lawn RM, Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, Seilhamer JJ, Vaughan AM, Oram JF. The tangier disease gene product abc1 controls the cellular apolipoprotein-mediated lipid removal pathway. J Clin Invest. 1999;104:R25–31. doi: 10.1172/JCI8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Langmann T, Klucken J, Reil M, Liebisch G, Luciani MF, Chimini G, Kaminski WE, Schmitz G. Molecular cloning of the human atp-binding cassette transporter 1 (habc1): Evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun. 1999;257:29–33. doi: 10.1006/bbrc.1999.0406. [DOI] [PubMed] [Google Scholar]
- 76.Tardy C, Goffinet M, Boubekeur N, Cholez G, Ackermann R, Sy G, Keyserling C, Lalwani N, Paolini JF, Dasseux JL, Barbaras R, Baron R. Hdl and cer-001 inverse-dose dependent inhibition of atherosclerotic plaque formation in apoe-/- mice: Evidence of abca1 down-regulation. PLoS One. 2015:e0137584. doi: 10.1371/journal.pone.0137584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arakawa R, Yokoyama S. Helical apolipoproteins stabilize atp-binding cassette transporter a1 by protecting it from thiol protease-mediated degradation. J Biol Chem. 2002;277:22426–22429. doi: 10.1074/jbc.M202996200. [DOI] [PubMed] [Google Scholar]
- 78.Arakawa LuR, Ito-Osumi R, Iwamoto C, Yokoyama NS. Apoa-i facilitates abca1 recycle/accumulation to cell surface by inhibiting its intracellular degradation and increases hdl generation. Arterioscler Thromb Vasc Biol. 2008:1820–1824. doi: 10.1161/ATVBAHA.108.169482. [DOI] [PubMed] [Google Scholar]
- 79.Duverger N, Kruth H, Emmanuel F, Caillaud JM, Viglietta C, Castro G, Tailleux A, Fievet C, Fruchart JC, Houdebine LM, Denefle P. Inhibition of atherosclerosis development in cholesterol-fed human apolipoprotein a-i–transgenic rabbits. Circulation. 1996;94:713–717. doi: 10.1161/01.cir.94.4.713. [DOI] [PubMed] [Google Scholar]
- 80.Pászty C, Maeda N, Verstuyft J, Rubin EM. Apolipoprotein ai transgene corrects apolipoprotein e deficiency-induced atherosclerosis in mice. J Clin Invest. 1994;94:899–903. doi: 10.1172/JCI117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang J, Srivastava RAK, Krul ES, Baumann D, Pfleger BA, Kitchens RT, Schonfeld G. In vivo regulation of apolipoprotein a-i gene expression by estradiol and testosterone occurs by different mechanisms in inbred strains of mice. J Lipid Res. 1991;32:1571–1585. [PubMed] [Google Scholar]
- 82.Vandenbrouck Y, Janvier B, Loriette C, Bereziat G, Mangeney-Andreani M. Thyroid hormone modulates apolipoprotein-ai gene expression at the post-transcriptional level in hep g2 cells. Eur J Biochem. 1995;231:126–132. doi: 10.1111/j.1432-1033.1995.tb20678.x. [DOI] [PubMed] [Google Scholar]
- 83.Van-Assche T, Huygelen V, Crabtree MJ, Antoniades C. Gene delivery strategies targeting stable atheromatous plaque. Curr Pharm Des. 2013;19:1626–1637. [PubMed] [Google Scholar]
- 84.Muck-Hausl M, Solanki M, Zhang W, Ruzsics Z, Ehrhardt A. Ad 2.0: A novel recombineering platform for high-throughput generation of tailored adenoviruses. Nucleic Acids Res. 2015;43:e50. doi: 10.1093/nar/gkv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.