The science of dosage or posology (from Greek posos, how much, and logos, study) is a branch of pharmacology and therapeutics concerned with ‘treatment dosage’ and ‘dosage regimen’. Establishing optimum dosage underpins every clinical development plan for novel therapeutic candidates. Failure to select the adequate drug dose is a leading culprit for regulatory delays or denial of initial applications for new drugs and, more generally, inadequate dose selection contributes to the high attrition rate of pivotal clinical trials.1
Regulatory agencies are committed to facilitate the development and ultimate licensure of safe and effective regenerative therapies.2 To this end, dedicated programs applicable to stem cell therapies have been designed to expedite the advancement and approval of new products (e.g., breakthrough therapy designation, accelerated approval).3 However, with limited grasp on the disposition (pharmacokinetics) and action (pharmacodynamics) of stem cells, posology applied to the development of cardiovascular regenerative therapies is still a nascent area of investigation.
Without the establishment of standardized dose regimens, clinical trials continue to evaluate wide dose ranges.4 A case in point are clinical studies that have shown rather paradoxical results regarding the relationship between the stem cell dose and clinical benefit in the setting of heart disease.5 Accordingly, scientific, regulatory and medical communities remain challenged with critical gaps in knowledge required for successful clinical translation of a regenerative biotherapeutics.6 Principles that apply in conventional drug development may not be readily transferable to the evolving regenerative pharmacy reflecting the dichotomy of product classes (e.g., chemicals versus biologics). Traditional medicinal products (small chemical molecules) feature defined mechanisms of action and delineated processes of absorption, distribution, metabolism, and excretion. In contrast, emergent biotherapeutics (including live cell-based therapies) display complex and still poorly understood pharmacodynamics and pharmacokinetics.7 A closer understanding of determinants that define the dose-exposure-response triad is needed to inform dose and schedule selection (Figure), notwithstanding that to date only a limited number of studies have formally examined the posology of regenerative therapy.
Figure. Linking elements that determine the posology paradigm for regenerative therapy.
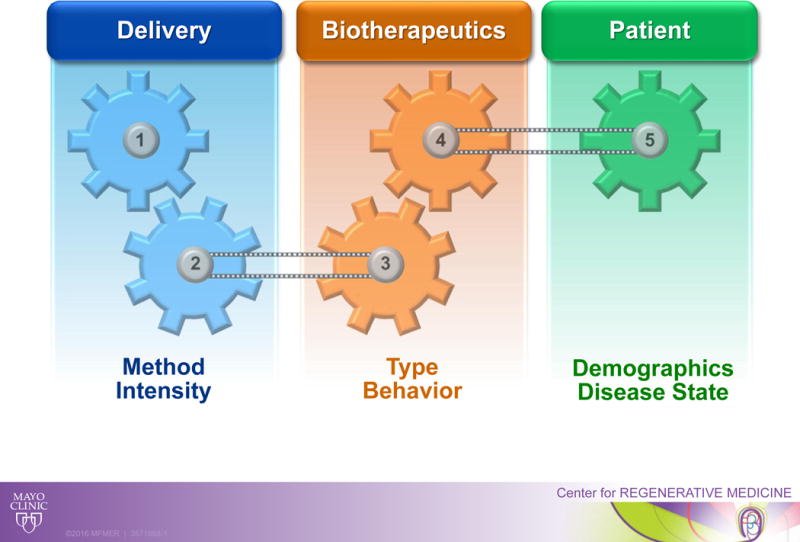
Regenerative biotherapies display complex pharmacodynamics and pharmacokinetics encompassing multiple factors including the delivery method and intensity, the biotherapeutics type and behavior, and the patient demographics and disease substrate.
The paucity of cardiovascular clinical trials designed to assess cell dosage is further accentuated by the limited information available on cell fate post-delivery, including the kinetics of engraftment or the dynamics of autocrine/paracrine signaling. Toward addressing this knowledge gap, the TRIDENT Study – presented in this issue of Circulation Research – compared outcomes following randomized treatment with two doses of allogeneic bone marrow-derived human mesenchymal stem cells (hMSC) in patients with chronic ischemic cardiomyopathy.8 Thirty patients received either 20 or 100 million cells identically delivered, in a blinded manner, via transendocardial injection (ten 0.5 cc injections/patient). At one year follow-up, both cell doses were safe and well tolerated with favorable impact on reducing post-infarction scar size, but only the larger dose was associated with improved ejection fraction. In the context of the TRIDENT trial, the higher dose may provide greater benefit than the lower dose suggesting a direct relationship between cell dose and clinical efficacy at least within the dose range tested, a conclusion supported by pro-BNP levels which remained stable only in the 100 million hMSC-treated group.8 The TRIDENT study therefore adds to an increasing compendium of clinical experience for use of cell-based technology in patients with heart disease. As recognized by the TRIDENT investigators, the study was limited by lack of a placebo group and small sample size testing two distinct doses. The TRIDENT study thus underscores the ongoing need for clinical trials designed to evaluate dosage regimens while incorporating cell dose ranges and well-defined patient populations with appropriate controls.
A monophasic dose-effect relationship has been previously documented with cells of mesenchymal origin delivered transendocardially in cardiomyopathic ventricles. This includes a dose-escalation study where the highest dose (150 million cells) produced greatest benefit.9 However, inverse or ‘U-shape’ relationships have also been reported. For example, the POSEIDON trial demonstrated an inverse relationship between the hMSCs dose delivered and clinical outcomes, with maximal efficacy achieved with a lower dose (20 million versus the larger 200 million cells).10 More recently, the CHART-1 trial addressed the effect of cardiopoiesis-based cell therapy in advanced heart failure.11 In this, to date, largest regenerative cardiovascular trial, cardiopoietic stem cells – also obtained from a mesenchymal source and delivered endomyocardially – showed significant reverse remodeling with improvement in left ventricular volumes especially in subgroups of patients who received an intermediate number of cell injections, indicating a ’ceiling effect’ as excessive therapeutic intensity may offset benefit.12
The act of cell delivery may cause myocardial damage, through multiple mechanisms that are both mechanical and biological in nature. Beyond cell quantity per se, a number of confounding factors may influence outcome including the delivery method intensity and/or disease substrate (Figure). It has been suggested that intracoronary injections, typically used in treating acute conditions, require cells to extravasate and migrate to the areas of injury which may result in lower engraftment rates than intramyocardial injections, thus requiring higher initial doses.8 Moreover, in the context of severe hypoxia and inflammation germane to acute myocardial infarction, the recently injured tissue is unlikely to mimic a chronic disease state and as such may dictate distinct doses and treatment schedules.
Going forward, establishing an evidence-based posology paradigm is required to ensure accurate titration of regenerative therapies and advance the science of regenerative medicine. Studies evaluating treatment schedules (e.g., singular versus repeat stem cell interventions),13 in tandem with the intricacy of the regenerative product behavior post-delivery within the host milieu, including the dynamics and kinetics of cross-talk with endogenous healing processes are needed. Furthermore, the formulation of regenerative therapeutics beyond first generation stem cell products into next generation acellular or engineered counterparts can profoundly impact the attributes of the pharmacokinetic and pharmacodynamic equations.14 Cell-free products mimicking the paracrine impact of cell-based therapies can potentially achieve standardized dosing reflecting more closely the pharmacology of small chemical molecules. Another avenue for posological standardization includes use of cyto-engineering and/or allogeneic strategies to overcome cell-to-cell variability in regenerative potency inherent to autologous cell therapies. Such approaches provide the consistency required to streamline the understanding of dosage parameters for regenerative products. The path to adoption in cardiology care15 will thus mandate a transdisciplinary effort bringing together multiple specialties to establish validated posology for regenerative therapy.
Acknowledgments
SOURCE OF FUNDING
Supported by Mayo Clinic Center for Regenerative Medicine, Marriott Foundation, Michael S. and Mary Sue Shannon Family, Russ and Kathy VanCleve Foundation, Leducq Fondation, Florida Heart Research Institute, and National Institutes of Health.
Footnotes
Sola dosis facit venenum
(The dosage makes it either a poison or a remedy, Paracelsus 1493–1541)
DISCLOSURES
None.
References
- 1.Musuamba FT, Manolis E, Holford N, Cheung S, Friberg LE, Ogungbenro K, Posch M, Yates J, Berry S, Thomas N, Corriol-Rohou S, Bornkamp B, Bretz F, Hooker AC, Van der Graaf PH, Standing JF, Hay J, Cole S, Gigante V, Karlsson K, Dumortier T, Benda N, Serone F, Das S, Brochot A, Ehmann F, Hemmings R, Rusten IS. Advanced methods for dose and regimen finding during drug development. CPT Pharmacometrics Syst Pharmacol. 2017;6:418–429. doi: 10.1002/psp4.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marks PW, Witten CM, Califf RM. Clarifying stem-cell therapy’s benefits and risks. N Engl J Med. 2017;376:1007–1009. doi: 10.1056/NEJMp1613723. [DOI] [PubMed] [Google Scholar]
- 3.Fujita Y, Kawamoto A. Regenerative medicine legislation in Japan for fast provision of cell therapy products. Clin Pharmacol Ther. 2016;99:26–29. doi: 10.1002/cpt.279. [DOI] [PubMed] [Google Scholar]
- 4.Fisher SA, Doree C, Mathur A, Martin-Rendon E. Meta-analysis of cell therapy trials for patients with heart failure. Circ Res. 2015;116:1361–1377. doi: 10.1161/CIRCRESAHA.116.304386. [DOI] [PubMed] [Google Scholar]
- 5.Golpanian S, Schulman IH, Ebert RF, Heldman AW, DiFede DL, Yang PC, Wu JC, Bolli R, Perin EC, Moyé L, Simari RD, Wolf A, Hare JM, Cardiovascular Cell Therapy Research Network Review and perspective of cell dosage and routes of administration from preclinical and clinical studies of stem cell therapy for heart disease. Stem Cells Transl Med. 2016;5:186–191. doi: 10.5966/sctm.2015-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terzic A, Behfar A, Filippatos G. Clinical development plan for regenerative therapy in heart failure. Eur J Heart Fail. 2016;18:142–144. doi: 10.1002/ejhf.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson TJ, Behfar A, Terzic A. Stem cells: biologics for regeneration. Clin Pharmacol Ther. 2008;84:620–623. doi: 10.1038/clpt.2008.146. [DOI] [PubMed] [Google Scholar]
- 8.Florea V, Rieger AC, DiFede DL, El-Khorazaty J, Natsumeda M, Banerjee MN, Tompkins BA, Khan A, Schulman IH, Landin AM, Mushtaq M, Lowery MH, Byrnes J, Hendel RC, Cohen MG, Valasaki K, Pujol MV, Ghersin E, Miki R, Delgado C, Abuzeid FA, Vidro-Casiano M, Saltzman R, DaFonseca D, Caceres LV, Ramdas KN, Mendizabal A, Heldman AW, Mitrani RD, Hare JM. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (The TRIDENT Study) Circ Res. doi: 10.1161/CIRCRESAHA.117.311827. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, Traverse JH, Krum H, Skerrett D, Zheng Y, Willerson JT, Itescu S, Henry TD. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res. 2015;117:576–584. doi: 10.1161/CIRCRESAHA.115.306332. [DOI] [PubMed] [Google Scholar]
- 10.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, Merkely B, Musialek P, Wojakowski W, Andreka P, Horvath IG, Katz A, Dolatabadi D, El Nakadi B, Arandjelovic A, Edes I, Seferovic PM, Obradovic S, Vanderheyden M, Jagic N, Petrov I, Atar S, Halabi M, Gelev VL, Shochat MK, Kasprzak JD, Sanz-Ruiz R, Heyndrickx GR, Nyolczas N, Legrand V, Guédès A, Heyse A, Moccetti T, Fernandez-Aviles F, Jimenez-Quevedo P, Bayes-Genis A, Hernandez-Garcia JM, Ribichini F, Gruchala M, Waldman SA, Teerlink JR, Gersh BJ, Povsic TJ, Henry TD, Metra M, Hajjar RJ, Tendera M, Behfar A, Alexandre B, Seron A, Stough WG, Sherman W, Cotter G, Wijns W, CHART Program Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J. 2017;38:648–660. doi: 10.1093/eurheartj/ehw543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teerlink JR, Metra M, Filippatos GS, Davison BA, Bartunek J, Terzic A, Gersh BJ, Povsic TJ, Henry TD, Alexandre B, Homsy C, Edwards C, Seron A, Wijns W, Cotter G, CHART Investigators Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: results from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) study. Eur J Heart Fail. 2017 doi: 10.1002/ejhf.898. [DOI] [PubMed] [Google Scholar]
- 13.Behfar A, Gersh B, Terzic A. Repetition rescues regenerative reserve. Eur Heart J. 2016;37:1667–1670. doi: 10.1093/eurheartj/ehv596. [DOI] [PubMed] [Google Scholar]
- 14.Behfar A, Terzic A. Make regeneration great again; stronger together. Eur Heart J. 2017;38:1094–1095. doi: 10.1093/eurheartj/ehx151. [DOI] [PubMed] [Google Scholar]
- 15.Terzic A, Behfar A. Regenerative medicine in the practice of cardiology. Eur Heart J. 2016;37:1089–1090. [PubMed] [Google Scholar]


