Abstract
Designing a nanomaterials platform with high target-to-background ratios has long been one of the major challenges in the field of nanomedicine. Here, we introduce a “target-or-clear” multifunctional nanoparticle platform that demonstrates high tumor-targeting efficiency and retention while minimizing off-target effects. Encouraged by the favorable preclinical and clinical pharmacokinetic profiles derived after fine-tuning surface chemical properties of radioiodinated (124I, t1/2 = 100.2 h) ultrasmall cRGDY-conjugated fluorescent silica nanoparticles (C dots), we sought to investigate how the biological properties of these radioconjugates could be influenced by the conjugation of radiometals such as zirconium-89 (89Zr, t1/2 = 78.4 h) using two different strategies: chelator-free and chelator-based radiolabeling. The attachment of 89Zr to newer, surface-aminated, integrin-targeting C′ dots using a two-pot synthesis approach led to favorable pharmacokinetics and clearance profiles as well as high tumor uptake and target-to-background ratios in human melanoma models relative to biological controls while maintaining particle sizes below the effective renal glomerular filtration size cutoff <10 nm. Nanoconjugates were also characterized in terms of their radiostability and plasma residence half-lives. Our 89Zr-labeled ultrasmall hybrid organic–inorganic particle is a clinically promising positron emission tomography tracer offering radiobiological properties suitable for enhanced molecularly targeted cancer imaging applications.
Graphical abstract

INTRODUCTION
Despite the promising preclinical research results of various types of solid (or inorganic-based) nanomaterials in small animals,1 very few of these platforms have progressed to first-inhuman clinical trials after more than 3 decades.2,3 In addition to manufacturing and regulatory challenges, rapidly rising clinical trial costs, and the increasing complexity of trial designs,4 many existing nanomaterials show limited in vivo specific targeting efficacy5 and high liver accumulation rates6 (i.e., 30–99% of administered particles from the bloodstream), which are major hurdles that need to be addressed. For nanomaterials with a hydrodynamic (HD) size larger than 10 nm, even with the protection of stealth polymers (e.g., polyethylene glycol [PEG]) and functionalized with tumor-homing ligands (e.g., peptides or antibodies), it is still extremely common to see predominant reticuloendothelial system (RES) (i.e., liver and spleen) uptake, tumor-to-liver activity concentration ratios less than 1, and relatively low tumor-to-background (i.e., blood or muscle) ratios.6 High RES uptake also raises long-term in vivo toxicity concerns due to extremely slow and generally unpredictable hepatobiliary clearance rates from liver, with resulting delays in obtaining Investigational New Drug (IND) approval from the United States Food and Drug Administration (FDA).7 Therefore, even though larger-sized (i.e., >10 nm) solid nanomaterials have the advantage of significantly enhanced drug-loading capacity relative to their sub-10 nm sized counterparts, clinical translation of such materials may be hindered by low tumor targeting efficacy and high off-target (i.e., liver) accumulations associated with dose-limiting toxicity.5,6
Fast renal clearance, relatively short blood circulation half-lives (ranging from several minutes to several hours), and low RES uptake (on the order of 5% ID/g or less) represent defining biological features for ultrasmall (sub-10 nm) renally clearable nanoparticles (Table S1).8–17 Although suitable PEGylation techniques have been developed to improve the blood circulation half-life (up to >10 h) of such platforms,12,18 the ability to precisely control physicochemical properties, including surface ligand number, in a manner that facilitates bulk renal clearance while preserving tumor specific targeting capabilities, has long posed a significant challenge to the field. At present, and to the best of our knowledge, ultrasmall dye-encapsulating, αvβ3 integrin-targeting cyclic(arginine-glycine-aspartic acid-D-tyrosine-cysteine) (cRGDY) peptide carrying and PEG-functionalized fluorescent core–shell silica nano-particles (also known as Cornell dots or cRGDY-PEG-C dots) are congruent with the foregoing description of highly desirable characteristics that are achievable for such nanoscale materials.16,17,19,20 Next-generation Cornell prime dots synthesized in water-based environments,21 cRGDY-PEG-C′ dots, are currently being utilized to study modulations in biological responses22 and as an iodine-124- (124I, t1/2 = 100.2 h) labeled, PET-optical platform for preclinical oncological indications23,24 and early phase clinical trials (NCT01266096 and NCT02106598).
Having a physical half-life comparable to that of 124I, zirconium-89 (89Zr, t1/2 = 78.4 h) is now a widely used positron-emitting radioisotope (Table S2) in preclinical studies25–27 and clinical trials.28 Moreover, 89Zr has a much lower mean β+ energy (396 vs 820 keV), which may improve positron emission tomography (PET) spatial resolution. In contrast to radioiodine, which is prone to dehalogenation after cellular uptake (the extent to which depends on the nature of the chelator), 89Zr has been reported to residualize stably within cells after internalization,29 underscoring its potential to enhance targeted particle accumulations and target-to-background ratios. Herein, we investigate and compare chelator-based and chelator-free radiolabeling strategies for attaching surface radiometals (i.e., 89Zr) to the water-based synthetic product, cRGDY-PEG-C′ dots.21 We sought to determine whether (1) chelator-free radiolabeling procedures, previously applied to larger size (porous and nonporous) silica particles,30,31 could be successfully extended to particle sizes below 10 nm and (2) resulting 89Zr-labeled peptide- and PEG-functionalized C′ dots (or cRGDY-PEG-C′ dots) yielded high targeted uptake and target-to-background ratios in well-established integrin-expressing melanoma models while maintaining sub-10 nm sizes to facilitate renal excretion. Results of these findings could also inform development of a targeted radiotherapeutic platform by substitution of the diagnostic for a therapeutic radiolabel such as lutetium-177.
The chelator-free strategy was achieved by 89Zr labeling of the intrinsic deprotonated silanol groups (i.e., −Si−O−) on the surface and within micropores of each particle at elevated temperature (75 °C, pH 8 Scheme 1a). A traditional chelator-based 89Zr labeling technique (37 °C, pH 7.5) was also developed by carefully controlling the surface density of the selected chelator (i.e., p-SCN-Bn-deferoxamine or DFO-NCS) to maximize specific activity and radiochemical yields while maintaining the renal clearance property (Scheme 1b). Radiolabeled nanoconjugates were extensively characterized in terms of their radiostability, pharmacokinetic, clearance and dosimetry profiles, as well as their tumor targeting and target-to-background ratios. To the best of our knowledge, this is the first-of-its-kind 89Zr-labeled and renally clearable targeted organic–inorganic hybrid particle for dual-modality PET-optical imaging. On the basis of its favorable biological properties, including extended blood circulation half-life (∼15 h), high tumor targeting uptake (>10% ID/g), renal clearance (>60% ID within 1–2 days), low liver accumulation (∼5% ID/g), and high tumor-to-background ratios (tumor:muscle >9; tumor:liver >2), this platform is a clinically promising diagnostic imaging tool for cancer-specific detection and localization in patients with melanoma while offering the potential to be further adapted as a targeted radiotherapeutic probe for treating disease.
Scheme 1. 89Zr-Radiolabeling Strategies of cRGDY-PEG-C′ Dotsa.

a(a) Chelator-free strategy: the surface and/or internal deprotonated silanol groups (−Si−O−) from the (1) cRGDY-PEG-C′ dots are functioning as the inherent oxygen donors (or hard Lewis bases) for the successful labeling of 89Zr (a hard Lewis acid) at 75 °C, pH 8, forming (2) cRGDY-PEG-[89Zr]C′ dots. (b) Chelator-based strategy: DFO chelators are conjugated to the surface of amine-functionalized NH2-cRGDY-PEG-C′ dots by reacting DFO-NCS with the amine groups on the silica surface of the C′ dots. As synthesized (4) DFO-cRGDY-PEG-C′ dots are then labeled with 89Zr at 37 °C, pH 7, forming (5) 89Zr-DFO-cRGDY-PEG-C′ dots. The molecular structures of the chelated radiometal for both strategies are rendered in 3D and 2D on the right. The atoms of silicon, oxygen, carbon, nitrogen, sulfur, hydrogen, and zirconium in the 3D renderings are colored in purple, red, gray, blue, yellow, white, and light green, respectively.
RESULTS AND DISCUSSION
Chelator-Free Zirconium-89 Radiolabeling of cRGDY-PEG-C′ Dots
Nanoparticle-based chelator-free radiolabeling has emerged as a novel intrinsic radiolabeling technique in the last several years,32 especially for radioisotopes (e.g., arsenic-72 [72As, t1/2 = 26 h],33,34 germanium-69 [69Ge, t1/2 = 39.1 h],35 and titanium-45 [45Ti, t1/2 = 3.8 h]36), for which suitable chelators are not currently available. Developing a chelator-free radiolabeling technique for ultrasmall renal clearable nano-particles is of particular interest because the introduction of additional surface modification steps may increase the particle’s hydrodynamic radius and, in turn, reduce or eliminate renal clearance while promoting high liver uptake. Due to the presence of the intrinsic silanol groups (−Si−OH) on the surface or in micropores of each nanoparticle,37 silica is known to be one of the most versatile nanoplatforms for successful chelator-free labeling using a variety of radiometals, including 89Zr.30,31 The mechanism of labeling is thought to be due to strong interactions between a hard Lewis acid (i.e., radiometal of 89Zr4+) and a hard Lewis base (i.e., deprotonated silanol groups, −Si−O−, on silica surfaces).30 Although a large part of the outer surface silanol groups have been quenched after the surface PEGylation step using PEG-silane,18 we hypothesized that internal silanol groups from each microporous C′ dot are still accessible for the chelator-free 89Zr labeling.
To that end, we radio-labeled cRGDY-PEG-C′ dots using 89Zr4+ via the chelator-free strategy. C′ dots were synthesized using a previously reported protocol.21 Near-infrared fluorescent Cy5 dyes were covalently encapsulated into the silica matrix of C′ dots, endowing C′ dots with fluorescent properties; cancer targeting cRGDY peptides were then covalently attached to the outer surface of the C′ dots during PEGylation, allowing for improved tumor targeting. The resulting cRGDY-PEG-C′ dots were purified and subjected to quality control analysis (Figure 1). The gel permeation chromatography (GPC) elugram of the purified cRGDY-PEG-C′ dot showed a single peak at around 9 min, corresponding to C′ dot nanoparticles (Figure 1a). The peak was well fit by a single Gaussian distribution, suggesting no detectable impurities and narrow particle size distributions (Figure 1a). The average hydrodynamic diameter of the purified cRGDY-PEG-C′ dots was 6.4 ± 0.2 nm (Figure 1b), as measured by fluorescence correlation spectroscopy (FCS) and consistent with transmission electron microscopy (TEM) observations (Figure 1a). In addition to particle size, FCS also provides the particle concentration, which we used to estimate the number of functional groups per particle, including dyes, targeting peptides, and 89Zr radioisotopes.38 The UV–vis spectra of the purified cRGDY-PEG-C′ dots exhibited strong absorption at wavelength around 650 nm, corresponding to the absorption maximum of Cy5 fluorescent dye (Figure 1c). As compared to C′ dots without cRGDY surface modification (PEG-C′ dots), an additional absorption peak was identified at a wavelength around 275 nm, attributed to the tyrosine residues on the cRGDY peptides (Figure 1c). By dividing the concentrations of Cy5 and cRGDY calculated from the UV–vis spectra by the concentration of C′ dots measured by FCS, the numbers of Cy5 and cRGDY per C′ dot were estimated to be around 1.6 and 20, respectively.
Figure 1.

Characterization of cRGDY-PEG-C′ dots and NH2-cRGDY-PEG-C′ dots. GPC elugram with fit (a), FCS Characterization of cRGDY-PEG-C′ dots and NH2-cRGDY-PEG-C′ dots. GPC elugram with fit (a), FCS correlation curve with fit (b), and UV–vis absorbance spectra (c) of cRGDY-PEG-C′ dots as compared to those of PEG-C′ dots. GPC elugram with fit (d), FCS correlation curve with fit (e), and UV–vis absorbance spectra (f) of amine-functionalized NH2-cRGDY-PEG-C′ dots as compared to those of PEG-C′ dots.
For radiolabeling procedures, 4 nmols of purified cRGDY-PEG-C′ dots were mixed with 1 mCi of 89Zr-oxalate in HEPES buffer (pH 8) at 75 °C. Radiochemical yields were monitored by radio-TLC. Results showed that, within the first hour, over 50% 89Zr labeling yield was achieved. A total of ∼75% 89Zr was successfully attached to the particle over a 4 h radiolabeling period (Figure 2a). As expected, the labeling process was dependent on the particle concentration: the higher the particle-to-89Zr (nmol-to-mCi) ratio, the higher the 89Zr labeling yield (Figure 2a). The specific activity of chelator-free 89Zr-labeled cRGDY-PEG-C′ dots (denoted as cRGDY-PEG-[89Zr]C′ dots) was found to be in the range of 100–500 Ci/mmol.
Figure 2.

Chelator-free and chelator-based 89Zr radiolabeling studies. (a) Concentration-dependent chelator-free 89Zr labeling of cRGDY-PEG-C′ dots. Labeling temperature was set to 75 °C; labeling pH was set to 8, and C′ dot (nmol) to 89Zr (mCi) ratio was in the range of 0–7.5 nmol/mCi. (b) pH-Dependent chelator-free 89Zr labeling. Labeling temperature: 75 °C; C′ dot to 89Zr ratio: 7.5 nmol/mCi; labeling pH range: 2–9. (c) Temperature-dependent chelator-free 89Zr labeling. Labeling pH: 8; C′ dot to 89Zr ratio: 7.5 nmol/mCi; labeling temperature range: 25–75 °C. (d) Chelator-free 89Zr labeling comparison between C′ dots with regular PEGylation procedures and PEGylated C′ dots further modified with additional small silane molecules (i.e., DEDMS: diethoxy dimethyl silane). Labeling temperature: 75 °C; labeling pH: 8; C′ dot to 89Zr ratio: 7.5 nmol/mCi. (e) Concentration-dependent chelator-based 89Zr labeling of DFO-cRGDY-PEG-C′ dots. Labeling temperature: 37 °C; labeling pH: 7.5; C′ dot to 89Zr ratio range: 0–0.75 nmol/mCi. (f) MP-AES testing of the number of natZr per DFO-cRGDY-PEG-C′ dot particles synthesized with varied particle to DFO-NCS ratios. The radiolabeling yield was evaluated once per time point (a–e). MP-AES measurements of natZr concentrations were repeated in triplicate (f).
Deprotonated silanol groups play a vital role in the chelator-free 89Zr labeling of silica nanoparticles.30 When the pH is below the isoelectric point of silica (pH ∼ 2–3), the surface silanol groups of C′ dots will become protonated, making them unsuitable for chelating with positively charged 89Zr. This was evidenced by the fact that less than 1% labeling yield was observed at pH 2 and 75 °C (Figure 2b). Chelator-free 89Zr labeling was also demonstrated to be temperature-dependent, with higher labeling temperatures leading to faster 89Zr labeling (Figure 2c). The optimized labeling pH and temperature ranges are pH 8–9 and 50–75 °C, respectively.
To further demonstrate specific 89Zr labeling of deprotonated silanol groups, remaining silanol groups on the C′ dot surface after PEGylation were quenched via the addition of diethoxy dimethyl silane (DEDMS). The resulting modified cRGDY-PEG-C′ dots were expected to exhibit a lower surface density of reactive silanol groups, thereby reducing the efficiency of chelator-free radiolabeling.39 Indeed, an approximate 25% reduction of 89Zr labeling yield was observed in this case (Figure 2d). Considering that the average specific activity of 89Zr-oxalate is about 833 Ci/mmol of zirconium with a >99.9% radiochemical purity,40 about 0.14–0.63 89Zr per cRGDY-PEG-C′ dot was estimated for cRGDY-PEG-[89Zr]C′ dots (Table S3). The number of Zr atoms per particle could be further increased by labeling with cold Zr (or natZr) at varied ratios. As shown in Figure S1, a natZr density of 2.27 ± 0.08 could be achieved by labeling cRGDY-PEG-C′ dots with natZr at a molar ratio of 1 to 10. To date, silica-based 89Zr chelator-free radiolabeling has focused exclusively on nanoparticles with a diameter larger than 100 nm to provide sufficient silanol groups (>105/particle).30,31,41 Herein, we show successful 89Zr chelator-free labeling of ultrasmall (6–7 nm) PEGylated silica nanoparticles with a significantly reduced surface and internal silanol group number.
Chelator-Based Zirconium-89 Radiolabeling of cRGDY-PEG-C′ Dots
To achieve traditional chelator-based 89Zr labeling, we used DFO-NCS providing six oxygen donors (Scheme 1b).42 In our initial studies, we attached DFO chelator to maleimide functionalized C′ dots (mal-cRGDY-PEG-C′ dots) by introducing glutathione (GSH) as a linker, thereby converting the maleimide groups on C′ dot surfaces to primary amine groups for DFO-NCS conjugation. The resulting GSH-modified C′ dots were first purified using a PD-10 column and then conjugated with DFO-NCS chelator via the GSH amine groups, resulting in DFO-cRGDY-PEG-C′ dots for 89Zr labeling. Although high chelator-based labeling yields (>80%) were achieved after PD-10 purification (to remove free, nonlabeled 89Zr), very high intestinal uptake of 89Zr-DFO-cRGDY-PEG-C′ dots was observed in a screening PET study (Figure S2a); this finding was hypothesized to be due to the detachment of 89Zr-DFO-GSH from the particles. No obvious bone uptake was observed at 24 h postinjection, indicating no detachment of free 89Zr from the radio-conjugates (Figure S2a).
To solve this problem, primary amine groups were attached directly to the C′ dot surface using a recently developed post-PEGylation surface modification by insertion (PPSMI) method.43 To that end, after C′ dot PEGylation, additional amino-silane molecules were added to the reaction and inserted into the PEG layer, attaching to the silica surface underneath. The resulting NH2-cRGDY-PEG-C′ dots contained reactive amine groups on the silica surface under the PEG layer, allowing for further conjugation with e.g., NCS functionalized DFO chelators. After purification, the NH2-cRGDY-PEG-C′ dots exhibited good product quality, similar to that of cRGDY-PEG-C′ dots without amine functionalization (Figures 1d–f). The average diameter of the purified NH2-cRGDY-PEG-C′ dots was around 6.5 nm. The numbers of Cy5 and cRGDY peptides per C′ dot were estimated to be around 1.5 and 18, respectively (Figures 1d–f). The purified NH2-cRGDY-PEG-C′ dots were then conjugated with DFO-NCS using a reaction molar ratio of 1:20 between the particles and DFO-NCS, followed by purification using a PD-10 column to remove unreacted DFO-NCS. Labeling of 89Zr-oxalate to the resulting DFO-cRGDY-PEG-C′ dots was performed at 37 °C for 60 min. A nearly 100% labeling yield was achieved by using a particle-to-89Zr ratio of 0.4 nmol/1 mCi (Figure 2e). The specific activity was estimated to be in the range of 1300–4300 Ci/ mmol, significantly higher than results from the chelator-free method. About 1.59–5.14 89Zr per C′ dot was estimated in the final 89Zr-DFO-cRGDY-PEG-C′ dot product (Table S4). To estimate the number of accessible DFO per particle, as-synthesized DFO-cRGDY-PEG-C′ dots were first labeled with natZr and then subjected to natZr quantification using microwave plasma-atomic emission spectroscopy (MP-AES). Our results revealed an average of 3.42 ± 0.13 natZr per C′ dot for natZr-DFO-cRGDY-PEG-C′ dots synthesized at a particle to DFO ratio of 1:10 and 4.76 ± 0.13 for a 1:30 ratio (Figure 2f). Because excess natZr was used during the labeling and unreacted natZr was removed by chelating with EDTA, the number of natZr per C′ dot (about 3–5) should be a good measure of the number of accessible DFO per DFO-cRGDY-PEG-C′ dot. A subsequent pilot PET study showed a significantly reduced intestinal uptake using amine-based 89Zr-DFO-cRGDY-PEG-C′ dots (Figure S2b).
Radiostability and Blood Circulation Half-Lives of 89Zr-Labeled cRGDY-PEG-C′ Dots
We next investigated the in vitro and in vivo radiostability and blood circulation half-lives of the chelator-free and chelator-based 89Zr-labeled cRGDY-PEG-C′ dot probes. High radiostability is vital because PET detects the radioisotope itself rather than the particles. Both 89Zr-labeled cRGDY-PEG-C′ dot probes were synthesized and purified using PD-10 columns. Figure S3 shows representative elution profiles of chelator-free and chelator-based 89Zr-labeled cRGDY-PEG-C′ dot probes in PD-10 columns. The fraction from 2.5 to 4.0 mL was collected for subsequent studies.
The radiostabilities of both 89Zr-labeled cRGDY-PEG-C′ dot probes were found to be comparable in phosphate-buffered saline (PBS) over a 120 h time interval at 37 °C under stirring at 650 rpm. Both 89Zr-DFO-cRGDY-PEG-C′ dots and cRGDY-PEG-[89Zr]C′ dots also demonstrated greater than 99% radiopurity in PBS (Figure 3a). Similar results were found when incubating both 89Zr-labeled cRGDY-PEG-C′ dot probes in human serum under the same conditions (Figure 3b). To evaluate in vivo radiostability of 89Zr-labeled probes, non tumor-bearing nude mice were intravenously (i.v.)-injected with ∼200 μCi (∼7.4 MBq) of 89Zr-labeled cRGDY-PEG-C′ dots. Whole blood specimens were collected at 2, 24, and 48 h postinjection, and the plasma fraction (which contained >98% of the 89Zr-labeled cRGDY-PEG-C′ dots) was separated from the whole blood at different postinjection time points by centrifugation at 8000 rpm for 10 min and used to test radiopurity. The nonspecific association of 89Zr-labeled cRGDY-PEG-C′ dots with red blood cells was estimated to be less than 2%. The percentage of intact 89Zr-labeled cRGDY-PEG-C′ dots was also measured by radio-TLC. As shown in Figure 3c, >98% of intact 89Zr-DFO-cRGDY-PEG-C′ dots was estimated at 48 h postinjection in mouse plasma, while <75% was found for mice injected with cRGDY-PEG-[89Zr]C′ dots. The latter finding suggests detachment of free 89Zr during its circulation in vivo and highlights the dramatic difference in product radiostability found under in vitro and in vivo conditions.
Figure 3.
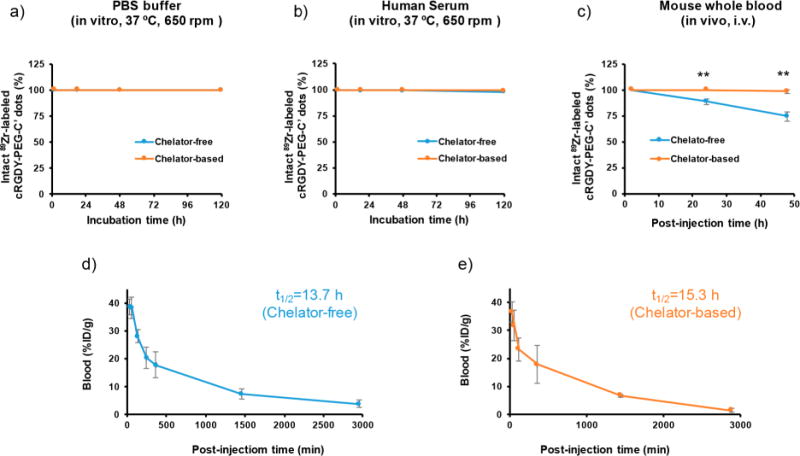
Comparison of chelator-free and chelator-based 89Zr-labeled C′ dot properties. Radiostability of 89Zr-labeled cRGDY-PEG-C′ dots in (a) PBS, 37 °C under stirring at 650 rpm, (b) human serum, 37 °C under stirring at 650 rpm, and (c) in vivo, i.v.-injected into healthy female athymic nu/nu mice (6–8 weeks old). Plasma (which contained >98% of the 89Zr-labeled cRGDY-PEG-C′ dots) was separated from whole blood at different postinjection time points and used to assess radiopurity. The nonspecific association of 89Zr-labeled cRGDY-PEG-C′ dots to red blood cells was estimated to be less than 2%. Blood circulation half-life fitting for (d) chelator-free 89Zr-labeled cRGDY-PEG-C′ dots (n = 3) and (e) chelator-based 89Zr-labeled cRGDY-PEG-C′ dots (n = 3). (**p < 0.005). For each time point, radiopurity of 89Zr-labeled cRGDY-PEG-C′ dots was evaluated in triplicate. Note: error bars in panels a and b are smaller than the size of the data points.
To evaluate the circulation half-life, blood from mice i.v.-injected with 89Zr-labeled cRGDY-PEG-C′ dots was sampled at various postinjection time points and assayed by gamma counting (n = 3). Blood uptake values were converted to a percentage of the injected dose per gram (% ID/g), and fit with a two-compartment model. As shown in Figures 3d and e, nearly equivalent blood circulation half-lives of about 15 h were measured, greater than those previously published for earlier generation radioiodinated particles (Table S1).16,17
Dynamic PET Imaging Using 89Zr-Labeled cRGDY-PEG-C′ Dots
PET is a widely used molecular imaging modality for noninvasively and quantitatively tracking the pharmacokinetics (PK) of various types of radiolabeled probes in vivo with high sensitivity.44 Limited by tissue penetration depths, it is well-known that optical imaging is generally not suitable for in vivo whole body screening and quantification of particle distributions within tissues. To track the distribution and fast renal clearance of systemically injected C′ dots, particularly in the early postinjection time period, a 60 min dynamic PET imaging study was performed in representative mice, each animal injected with one of the two 89Zr-labeled cRGDY-PEG-C′ dot probes. As shown in Figures 4a and b, maximum intensity projection (MIP) images show marked activity of 89Zr-labeled cRGDY-PEG-C′ dots in the mouse heart immediately after i.v. injection. Gradually reduced heart activity was observed in both cases with overall activity concentration estimated to be 20.5% ID/g at 60 min postinjection for mice injected with cRGDY-PEG-[89Zr]C′ dots (Figure 4c) and 19.3% ID/g for mice injected with 89Zr-DFO-cRGDY-PEG-C′ dots (Figure 4d). A similar trend was observed for hepatic uptake with 60 min postinjection uptake values of both probes estimated to be ∼6.5% ID/g. Significant kidney and bladder uptake was observed as early as 5 min postinjection, observed in both the MIP images and time-activity curves, clearly highlighting renal clearance capabilities of both 89Zr-labeled cRGDY-PEG-C′ dot probes. Short videos of the first 1 h dynamic biodistribution patterns of mice i.v.-injected with 89Zr-labeled cRGDY-PEG-C′ dot probes were also provided as Videos S1 and S2.
Figure 4.
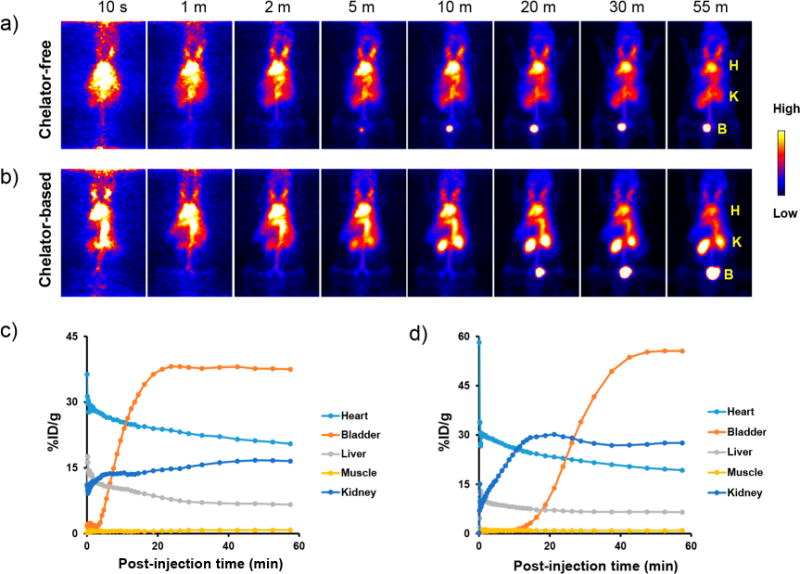
Comparison of dynamic PET imaging results in mice for chelator-free and chelator-based 89Zr-labeled C′ dots. (a) Chelator-free 89Zr-labeled cRGDY-PEG-C′ dots and (b) chelator-based 89Zr-labeled cRGDY-PEG-C′ dots. H: heart; K: kidney; B: bladder. The first 60 min time– activity curves for major organs (i.e., heart, bladder, liver, muscle, and kidney) in mice i.v.-injected with (c) chelator-free 89Zr-labeled cRGDY-PEG-[89Zr]C′ dots and (d) chelator-based 89Zr-labeled 89Zr-DFO-cRGDY-PEG-C′ dots. All images in panels a and b are coronal MIP PET images. For each group, a representative mouse was used to acquire dynamic PET data.
In Vivo Pharmacokinetics and Radiation Dosimetry Studies
Detailed biodistribution studies were performed to investigate the uptake of both 89Zr-labeled cRGDY-PEG-C′ dot probes at various postinjection time points after harvesting, weighing, and assaying major organs/tissues of interest from mice at the termination of the study (i.e., 5, 24, and 72 h, Tables S5 and S6, Figure 5). As evidenced in the dynamic PET imaging studies (Figure 4), the biodistribution studies confirmed significant activity of both 89Zr-labeled cRGDY-PEG-C′ dot probes in the blood compartment (Figures 5a and b). Plasma activity concentrations were twice as high as those for whole blood (Figure S4). Not surprisingly, urine activity at early postinjection time points varied from mouse to mouse, ranging from <10% ID/g to >20% ID/g. A total of 60–70% ID of 89Zr-labeled cRGDY-PEG-C′ dot probes was cleared within 72 h postinjection in the current study. As opposed to representative findings for >10 nm sized nanoparticles, usually revealing marked hepatic uptake (i.e., 30–99% ID),6 both 89Zr-labeled cRGDY-PEG-C′ dot probes exhibited significantly lower hepatic uptake (<5% ID/g). Interestingly, 89Zr-labeled cRGDY-PEG-C′ dots also showed only 2–4% ID/g in the kidney, which was significantly lower (about 5–10 fold less) than what has previously been described for other renally clearable ultrasmall particles such as ultrasmall quantum dots9 or gold nanoparticles.11,12 This suggests that in addition to size, optimized surface chemical particle properties are important for low off-target particle accumulation.
Figure 5.
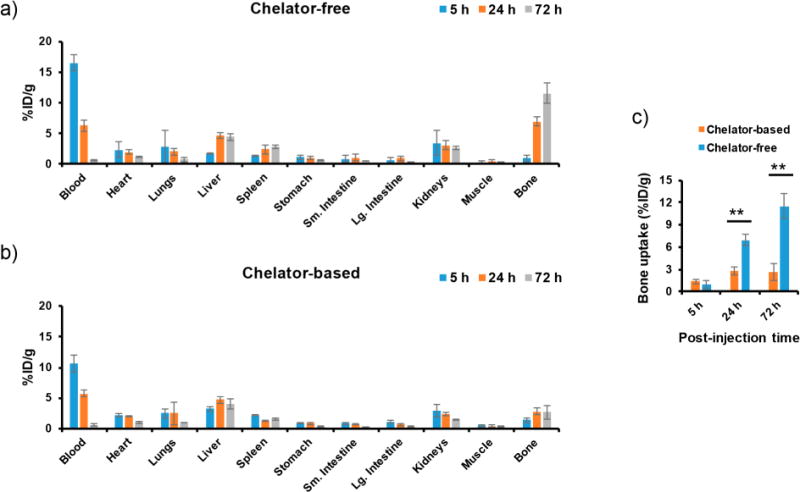
Biodistribution studies in mice for chelator-free and chelator-based 89Zr-labeled C′ dots. (a) Chelator-free 89Zr-labeled cRGDY-PEG-[89Zr]C′ dots and (b) chelator-based 89Zr-labeled 89Zr-DFO-cRGDY-PEG-C′ dots in healthy mice (n = 3). (c) Comparison of time-dependent bone uptake in mice injected with the 89Zr-labeled cRGDY-PEG-C′ dots (**p < 0.005).
A noticeable difference in overall bone uptake was found between the two 89Zr-labeled cRGDY-PEG-C′ dot probes. Values started to increase beyond 5 and 10% ID/g at the 24 and 72 h post i.v.-injection time points, respectively, for cRGDY-PEG-[89Zr]C′ dots (Figure 5c, p < 0.005). Such high bone uptake likely does not reflect marrow accumulation of cRGDY-PEG-[89Zr]C′ dot probes but rather indicates ongoing detachment of the free 89Zr4+ from the cRGDY-PEG-[89Zr]C′ dots due to relatively low radiostability in vivo (Figure 3b). Free 89Zr4+ is an osteophilic cation which could be readily accreted into bone mineral,45 as shown in Figure S5. Monitoring the change in bone uptake over time has also been demonstrated as one of the best ways to study the in vivo stability of 89Zr-labeled nanoprobes.30 Attempts to reduce the bone uptake of cRGDY-PEG-[89Zr]C′ dots by removing the less well-chelated surface 89Zr from cRGDY-PEG-[89Zr]C′ dots using EDTA challenge prior to injection was demonstrated to be only marginally effective in minimizing bone uptake (Figure S6). Only ∼20% bone uptake reduction was observed even after overnight EDTA challenge (conditions: 10 mM EDTA, 37 °C, kept under stirring at 650 rpm, Figure S6c). PET imaging in Figure S6b reveals obvious and persistent bone and joint uptake of cRGDY-PEG-[89Zr]C′ dots that were subjected to an additional EDTA challenge process. As reported previously,45 vthe clearance of 89Zr from the bones of mice was found to be slow with no significant reduction after 1 week (Figure S7). The excess and retained accumulation of radioactive 89Zr4+ in the bone marrow can increase the radiation dose to this compartment (an especially radiosensitive tissue), potentially hindering clinical translation.
To estimate mean organ absorbed doses and the effective dose in a 70-kg standard man, dosimetry calculations for both 89Zr-labeled cRGDY-PEG-C′ dot probes were performed based on the biodistribution data shown in Figure 5 and using the OLINDA computer program (yielding doses expressed in mSv/MBq 89Zr administered).46 Table S7 compares the estimated tissue-absorbed dose in humans for both 89Zr-labeled cRGDY-PEG-C′ dot probes. A slightly higher absorbed dose (0.084 mSv/MBq) in red marrow was found for the chelator-free 89Zr-labeled cRGDY-PEG-[89Zr]C′ dot probe when compared to the chelator-based 89Zr-labeled 89Zr-DFO-cRGDY-PEG-C′ dot probe (0.062 mSv/MBq). An absorbed dose ∼0.1 mSv/MBq was estimated for both 89Zr-labeled cRGDY-PEG-C′ dot probes in the human liver, only one-tenth of a previously reported value for 89Zr-DFO-trastuzumab (liver uptake was ∼12% ID, average estimated absorbed dose in liver was 1.54 mSv/MBq).47 Although significantly higher bone uptake was observed in the small-animal study, the estimated radiation dosimetry in a 70-kg standard man showed only a minor increase (<20%) in both the total-body and effective dose for the chelator-free 89Zr-labeled cRGDY-PEG-[89Zr]C′ dot product. Taken together, in vivo pharmacokinetic studies confirmed the renal clearance and extended blood circulation of 89Zr-labeled cRGDY-PEG-C′ dot probes within the first 24 h postinjection. All major organs, especially liver, spleen and kidney, showed minor (<5% ID/g) uptake throughout the study period. The only major difference between the chelator-free and the chelator-based 89Zr-labeled cRGDY-PEG-C′ dot probes is the lower in vivo radiostability and significantly (2–4 fold) higher bone uptake of the former at 24 h postinjection. However, the radiation dosimetry analysis showed favorable total-body and effective doses for both 89Zr-labeled cRGDY-PEG-C′ dot probes, which encouraged us to explore in vivo tumor-specific targeting of both radio-labeled nanoprobes in well-characterized integrin αvβ3-expressing human melanoma xenograft models.
In Vivo Tumor-Targeting by PET Imaging
Designing a “target-or-clear” multifunctional nanoparticle platform that can specifically localize in the target of interest after systemic administration while maintaining low nonspecific accumulations in the RES has long been one of the major challenges in the field of nanomedicine. Table S1 lists the current research status of ultrasmall nanoparticles exhibiting both renal clearance and in vivo tumor-targeting capabilities. Following systematic investigations and comparisons of 89Zr labeling strategies, we turned our attention to studying in vivo tumor-specific targeting and renal clearance profiles for both 89Zr-labeled cRGDY-PEG-C′ dot probes in αvβ3 integrin-expressing melanoma xenograft models.
As shown in Figure 6, significant bladder activity was observed in the 2 h MIP images for mice injected with cRGDY-PEG-[89Zr]C′ dots (Figure 6a) and 89Zr-DFO-cRGDY-PEG-C′ dots (Figures 6b and c). The high cardiac uptake observed (∼20% ID/g) clearly indicated the circulation of 89Zr-labeled cRGDY-PEG-C′ dots in the blood compartment. Time-activity curves shown in Figures 6d–f show the clearance of 89Zr-labeled cRGDY-PEG-C′ dots from the blood with uptake values estimated to be about 5–6 and 1–2% ID/g at 24 and 72 h postinjection, respectively. The clearance of 89Zr-labeled cRGDY-PEG-C′ dots by the RES (e.g., liver) was estimated to be only 5–6% ID/g at 2 h postinjection with slight reductions down to 4–5% ID/g after 3 days; these values are markedly lower than previously reported values for particles larger than 10 nm6 and close to that of early generation 124I-labeled C dots having a similar HD size (liver uptake: 2–4% ID/g).16 Splenic uptake was found to be only half of that found for liver uptake over the course of three days. Muscle uptake was found to be as low as ~1% ID/g. Given findings compatible with bulk renal clearance, significantly reduced RES uptake, and very low background activity levels in muscle, together with blood circulation half-life of ∼15 h, significantly enhanced tumor-to-background ratios may therefore be achievable.
Figure 6.

In vivo tumor-targeted coronal PET images of mice and their analysis. Mice injected with (a) cRGDY-PEG-[89Zr]C′ dots, chelator-free labeling, in M21 tumor-bearing mice (n = 3), (b) 89Zr-DFO-cRGDY-PEG-C′ dots, chelator-based labeling, in M21 tumor-bearing mice (n = 3), and (c) 89Zr-DFO-cRGDY-PEG-C′ dots, chelator-based labeling, in M21L tumor-bearing mice (n = 3). MIP images at 2 and 72 h are presented to reveal the extended blood half-lives of the particles, renal clearance of particles into the bladder at 2 h postinjection, and bone and joint uptake at 72 h postinjection. Time activity curves showing (d) chelator-free 89Zr-labeled cRGDY-PEG-[89Zr]C′ dot in M21 xenografts, (e) chelator-based 89Zr-labeled 89Zr-DFO-cRGDY-PEG-C′ dots in M21 xenografts, and (f) chelator-based 89Zr-labeled 89Zr-DFO-cRGDY-PEG-C′ dots in M21L xenografts. Comparisons of (g) tumor uptake, (h) tumor-to-blood ratios, (i) tumor-to-liver ratios, and (j) tumor-to-muscle ratios among three groups. N = 3 for each group.
As shown in Figures 6a and b, high M21 (αvβ3-positive) tumor uptake was observed in mice injected with both cRGDY-PEG-[89Zr]C′ dots (Figure 6a, 10.1 ± 2.1% ID/g) and 89Zr-DFO-cRGDY-PEG-C′ dots (Figure 6b, 10.5 ± 4.0% ID/g) at 2 h postinjection. The tumor uptake peaked at 24 h postinjection with an additional slight increase to about 10.7 ± 1.3 and 12.0 ± 1.4% ID/g, respectively (Figure 6g). Over 5-fold enhancement of tumor uptake was estimated when compared with our first-generation C dots (cRGDY ligand density: ∼ 6) labeled with 124I (maximal M21 tumor uptake: ∼ 2% ID/g at 4 h postinjection).16 Retention of particle activity over the 72 h time period was observed in M21 tumor-bearing mice injected with both types of 89Zr-labeled cRGDY-PEG-C′ dot probes (Figures 6d and e), noting only a very slow wash-out rate. As expected, mice injected with the chelator-free 89Zr-labeled cRGDY-PEG-C′ dots showed detachment of free 89Zr, with its accumulation in bone, joint, and spine (Figures 6a and S8), while significantly reduced bone and joint uptake was found in mice injected with 89Zr-DFO-cRGDY-PEG-C′ dots (Figures 6b and S8). Maximizing target tissue uptake and retention as well as target-to-background ratios in preclinical models are key considerations for the rational design of targeted nanoprobes for clinical cancer care.5 Recent debates in the field of nanomedicine have focused on nanoparticle delivery efficiency, the extent to which enhanced permeability and retention effects promote target accumulation, nanoparticle-tumor interactions, and nonspecific uptake at off-target sites, among others, highlighting the significant challenges encountered in the quest to translate nanoprobes to the clinic.48–53 Other areas of active investigation critical to the successful navigation of these challenges require the integration of high-resolution analytical approaches to improve particle characterization, facilitate particle clearance, and enhance particle penetration and diffusion within tumors.
A control study was performed in M21-L tumor-bearing mice (αvβ3-negative) following injection of 89Zr-DFO-cRGDY-PEG-C′ dots to further demonstrate target specificity of 89Zr-labeled cRGDY-PEG-C′ dots. Findings showed similar particle distributions in major organs/tissues such as bladder, heart, liver, and muscle with significantly lower uptake in the M21-L tumors (on average 2–3% ID/g), as shown in Figures 6c, 6f, and S9. For mice injected with either cRGDY-PEG-[89Zr]C′ dots or 89Zr-DFO-cRGDY-PEG-C′ dots, tumor accumulation rates and tumor-to-background ratios demonstrated no statistically significant differences (Figures 6g–j and S9). For mice injected with 89Zr-DFO-cRGDY-PEG-C′ dots, maximum tumor-to-muscle ratios were found to be 9.6 ± 2.5 at 72 h p.i., which is 3-fold higher than that measured in M21-L tumor-bearing mice (i.e., 2.8 ± 0.7, Figure 6j) and 2-fold higher than that found for 124I-labeled C dots (maximal tumor-to-muscle: ∼ 5).16 Finally, on the basis of high tumor uptake and low RES accumulation, we observed tumor-to-liver ratios of about 2 or higher in M21 tumor-bearing mice injected with cRGDY-PEG-[89Zr]C′ dots or 89Zr-DFO-cRGDY-PEG-C′ dots (Figure 6i), one of the unique features distinguishing 89Zr-labeled cRGDY-PEG-C′ dot probes from other tumor-targeting particles.6 Taken together, we successfully demonstrated in vivo targeting specificity and concomitant renal clearance for both 89Zr-labeled cRGDY-PEG-C′ dot preparations in αvβ3 integrin-expressing melanoma xenograft models.
CONCLUSIONS
In conclusion, to address challenges in the radiolabeling of ultrasmall renally clearable cRGDY-PEG-C′ dots, we developed and compared two 89Zr-radiolabeling strategies based on their biological and dosimetric properties. Although comparable in vitro radiostability was found for both radioconjugates, chelator-based radiolabeling showed a significantly higher in vivo radiostability than chelator-free preparations. Both PK studies and PET imaging evaluations confirmed renal clearance, low RES accumulation, enhanced tumor uptake, and high target-to-background ratios for both products in αvβ3 integrin-expressing human melanoma xenograft models. While there are limitations to rodent tumor models, they nonetheless provide useful information that contributes to the clinical translation of nanoparticles and other agents. In particular, the findings presented herein point to the favorable translatability of these novel target-or-clear 89Zr-labeled cRGDY-PEG-C′ dot tracers to human subjects for systemic targeted detection of cancer.
EXPERIMENTAL SECTION
Synthesis, Purification, and Characterization of cRGDY-PEG-C′ Dots and Amine-Functionalized NH2-cRGDY-PEG-C′ Dots
cRGDY-PEG-C′ dots and NH2-cRGDY-PEG-C′ dots were synthe-sized as described previously,21,43 the latter using a recently reported post-PEGylation surface modification by insertion approach. More specifically, a typical synthesis of cRGDY-PEG-C′ dots started with dissolving 5.7 μmol of NHS ester/maleimido functionalized heterofunctional polyethylene glycol (PEG) with molar mass around 866, referred to as mal-PEG-NHS, in 24.8 μL of dimethyl sulfoxide (DMSO). The solution was mixed with 5.2 μmol of (3-aminopropyl)-triethoxysilane (amine-silane) at room temperature under nitrogen. The reaction mixture was kept at room temperature under nitrogen for two days to complete conjugation of mal-PEG-NHS with amine-silane, forming mal-PEG-silane. Afterward, 6.3 μmol of cyclo(Arg-Gly-Asp-D-Tyr-Cys) peptide (cRGDY), dissolved in 300 μL DMSO, was further added into the mixture at room temperature under nitrogen. The reaction mixture was then kept at room temperature under nitrogen overnight to complete conjugation of mal-PEG-silane with cRGDY peptide, forming cRGDY-PEG-silane. At the same time, 0.5 μmol of maleimido functionalized Cy5 dye (Cy5-mal), dissolved in 30 μL DMSO, was mixed with 9.5 μmol of (3-mercaptopropyl)-trimethoxysilane (thiol-silane). The mixture was kept at room temperature under nitrogen overnight to complete conjugation of Cy5-mal with thiol-silane, forming Cy5-silane.
In the next step, 68 μL of tetramethyl orthosilicate (TMOS liquid) and conjugated Cy5-silane were added into 10 mL of aqueous solution of ammonium hydroxide at pH around 8.5. The addition was conducted at room temperature under vigorous stirring (600 rpm). The reaction solution was then kept at room temperature under vigorous stirring overnight. Afterward, cRGDY-PEG-silane prepared in the previous step was added into the reaction mixture at room temperature under vigorous stirring, followed by the addition of 100 μL of silane functionalized PEGs (PEG-silane liquid) with molar mass around 500. Afterward, the reaction solution was kept at room temperature overnight under vigorous stirring. The reaction solution was then kept at 80 °C without stirring overnight to further enhance the covalent attachment of PEG-silane and cRGDY-PEG-silane to silica nanoparticle surface. After cooling the reaction solution to room temperature, the resulting cRGDY-PEG-Cy5-C′ dots were purified by GPC, filtered by sterile syringe filters, and finally stored at 4 °C. Where applicable, remaining silanol groups on cRGDY-PEG-C′ dots could be terminated by adding diethoxydimethylsilane (DEDMS) to the synthesis mixture at a concentration of 7.3 mM after the reaction temperature was reduced from 80 °C to room temperature. The reaction solution was then kept at room temperature under vigorous stirring overnight before purification, allowing DEDMS to covalently attach to the remaining silanol groups on the silica particle surface. The rest of the synthesis remained the same as that of the regular cRGDY-PEG-C′ dots.21
The synthesis of NH2-cRGDY-PEG-C′ dots followed the same protocol as that for cRGDY-PEG-C′ dots, except that additional amine-silane was inserted into the PEG layer of cRGDY-PEG-C′ dots and attached to the silanol groups underneath, as described in detail in ref 43. More specifically, after the reaction temperature was reduced from 80 °C to room temperature in the synthesis of cRGDY-PEG-C′ dots, 8.6 μmol of amine-silane was added into the reaction solution at room temperature under vigorous stirring. The reaction was kept at room temperature overnight under vigorous stirring before particle purification to complete amine functionalization.43
Purification and characterization methods for different C′ dots, including GPC purification as well as TEM, FCS, and UV–vis measurements, are detailed in our previous publications.21,43 In short, GPC purification and characterization were performed using a BioLogic LP system equipped with a 275 nm UV detector. The column used was an Econo-Column chromatography column packed with Superdex 200 resin from GE healthcare. TEM images were taken on a FEI Tecnai T12 Spirit TEM operated at an acceleration voltage of 120 kV. FCS measurements were conducted using a home-built FCS setup with a 633 nm solid state laser as the excitation source. FCS error bars were obtained from a combination of the standard deviation from multiple measurements and a systemic error resulting from FCS alignment variations. UV–vis measurements were performed using a Varian Cary 5000 spectrophotometer.
89Zr-Oxalate Production
89Zr was produced at Memorial Sloan Kettering Cancer Center on a TR19/9 cyclotron (Ebco Industries Inc.) via the 89Y(p,n)89Zr reaction and purified to yield 89Zr with a specific activity of 5.28–13.43 mCi/μg (470–1195 Ci/mmol) of zirconium.40 Activity measurements were performed using a CRC-15R Dose Calibrator (Capintec). For the quantification of activities, experimental samples were counted on an Automatic Wizard2 γ-Counter (PerkinElmer). All in vivo experiments were performed according to protocols approved by the Memorial Sloan Kettering Institutional Animal Care and Use Committee. A purity of greater than 95% was confirmed using radio-TLC for all of the 89Zr-labeled cRGDY-PEG-C′ dots.
Chelator-Free 89Zr Radiolabeling of c(RDGyC)-PEG-C′ Dots
For a typical chelator-free 89Zr labeling of cRGDY-PEG-C′ dots, 4 nmol of cRGDY-PEG-C′ dots was mixed with 1 mCi of 89Zr-oxalate in HEPES buffer (pH 8) at 75 °C. The radiolabeling yield of cRGDY-PEG-C′ dots at 1, 30, 60, 120, and 240 min was monitored using salicylic acid impregnated instant thin-layer chromatography paper (ITLCSA) (Agilent Technologies), and analyzed either on a Bioscan AR-2000 radio-TLC plate reader using Winscan Radio-TLC software (Bioscan Inc., Washington, DC) or an Automatic Wizard2 γ-Counter (PerkinElmer). After incubation, 5 μL aliquots were withdrawn and mixed with 50 μL of EDTA (50 mM, pH 5–6) before analyzing by ITLC using EDTA (50 mM, pH 5–6) as a mobile phase solvent. Free 89Zr formed an instantaneous complex with EDTA and eluted with the solvent front, while 89Zr-labeled cRGDY-PEG-C′ dots remained at the origin. For more accurate quantification, strips were cut in half, and γ-ray emissions at 909 keV were counted on a calibrated γ-counter (PerkinElmer) using a dynamic energy window of 800–1000 keV. Similar procedures were introduced when studying the pH-, concentration-, and temperature-dependent chelator-free labeling of cRGDY-PEG-C′ dots. The specific activity of chelator-free 89Zr-labeled cRGDY-PEG-C′ dots was found to be in the range of 100–500 Ci/mmol.
Synthesis and Chelator-Based 89Zr Labeling of DFO-cRGDY-PEG-C′ Dots
A traditional chelator-based 89Zr labeling technique was introduced by reacting NH2-cRGDY-PEG-C′ dots with DFO-NCS (molar ratio was 1:20) for 1–2 h at 22 °C, pH 8–9, and kept under stirring at 640 rpm. As synthesized DFO-cRGDY-PEG-C′ dots were then purified by passing the particles through a PD-10 column using phosphate-buffered saline (PBS) as the mobile phase. For chelator-based 89Zr labeling, 0.2–0.75 nmol of DFO-cRGDY-PEG-C′ dots were then mixed with 1 mCi of 89Zr-oxalate in HEPES buffer (pH 8) at 37 °C for 1, 30, and 60 min; final labeling pH was kept as 7–7.5. The labeling yield was monitored as described above. An EDTA challenge process was introduced to remove any nonspecifically bound89Zr. As synthesized 89Zr-DFO-cRGDY-PEG-C′ dots were then purified by using a PD-10 column. The final radiochemical purity was measured using ITLC. The specific activity was found to be in the range of 1300–4300 Ci/mmol.
MP-AES Quantification of the Number of natZr per DFO-cRGDY-PEG-C′ Dot
To quantify the number of natZr per DFO-cRGDY-PEG-C′ dot, 0.75 nmol of DFO-cRGDY-PEG-C′ dots were mixed with excess natZrCl4 (15 nmol) at 37 °C for 60 min. The final labeling pH was kept at 7–7.5. After labeling, the mixture was combined with EDTA and incubated for more than 30 min to eliminate any nonspecific natZrCl4. The sample was then purified with a PD-10 column. The amount of total labeled natZr was then measured using MP-AES. Samples were prepared by a 10× dilution of natZr-labeled DFO-cRGDY-PEG-C′ dot in H2O into 0.1 M HCl for a final matrix concentration of 0.09 M HCl. The instrument was calibrated with increasing concentrations of ZrCl4 in 0.09 M HCl with a 3 s signal integration time and automatic background compensation. The 343.823 and 339.198 nm Zr atomic emission wavelengths were used for calibration and analysis. The number of natZr per DFO-cRGDY-PEG-C′ dots was calculated using the following equation:
Because excess natZrCl4 was used for labeling, the number of natZr per natZr-DFO-cRGDY-PEG-C′ dot should roughly be equal to the number of accessible DFO per DFO-cRGDY-PEG-C′ dot. Similar procedures were used for the quantification of the number of natZr-per-cRGDY-PEG-C′ dots (chelator-free radiolabeling method).
Blood Circulation Half-Life Evaluations
To estimate the blood circulation half-lives of both 89Zr-labeled cRGDY-PEG-C′ dot probes, healthy female athymic nu/nu mice (6–8 weeks old, Taconic Farms Inc.) were i.v.-injected with radioactive particles. Blood sampling was performed at various postinjection time points, and these radioactive samples were counted using an Automatic Wizard2 γ-Counter (PerkinElmer). Blood uptake values were presented as a percentage of the injected dose per gram (% ID/g) and fit with a two-compartment model by using Prism 7 software.
In Vitro and in Vivo Radio-Stability Studies
To study in vitro radio stability, both chelator-free and chelator-based 89Zr-labeled cRGDY-PEG-C′ dot probes (∼100 μCi in 100 μL PBS) were kept in PBS (1×, 900 μL) and human serum (1×, 900 μL) at 37 °C under stirring at 650 rpm. Radiochemical purity was measured over a 5-day period by ITLC at various time points from the end of synthesis. The measurement was repeated three times at each time point. For in vivo radio stability, healthy female athymic nu/nu mice (6–8 weeks old, Taconic Farms Inc.) were injected with ∼200 μCi (∼7.4 MBq) of chelator-free (or chelator-based) 89Zr-labeled cRGDY-PEG-C′ dots. Whole blood was collected at 2, 24, and 48 h postinjection, the plasma fraction (which contained >98% of the 89Zr-labeled cRGDY-PEG-C′ dots) was separated from the whole blood at different postinjection time points by centrifugation at 8000 rpm for 10 min and used to test the radiopurity. The nonspecific association of 89Zr-labeled cRGDY-PEG-C′ dots to the red blood cells was estimated to be less than 2%. The percentage of the intact 89Zr-labeled cRGDY-PEG-C′ dots was then measured by using ITLC with the plates analyzed on a Bioscan AR-2000 radio-TLC plate reader using Winscan Radio-TLC software (Bioscan Inc., Washington, DC). The measurement was repeated three times at each time point.
Animal Models and Tumor Inoculation
All animal experiments were done in accordance with protocols approved by the Institutional Animal Care and Use Committee of Memorial Sloan-Kettering Cancer Center and followed NIH guidelines for animal welfare. M21 and M21-L xenografts were generated by coinjecting equal volumes of cells (∼5 × 106 cells/100 μL) and Matrigel subcutaneously into the hind legs of female athymic nu/nu mice (6–8 weeks old, Taconic Farms Inc.). Average tumor volumes of 200 mm3 were used for all studies.
Dosimetry
Time–activity curves derived for each tissue were analytically integrated, accounting for radioactive decay, to yield the corresponding cumulative activity. Organ absorbed doses were then calculated by multiplying the cumulative activity by the 89Zr equilibrium dose constant for nonpenetrating radiations (positrons), assuming complete local absorption of such radiations and ignoring the contribution of penetrating radiations (i.e., γ-rays). Mouse normal organ cumulated activities were converted to human normal organ cumulated activities by taking into account differences in total-body and organ masses between mice and humans (assuming 70-kg standard human). Calculated human normal-organ cumulated activities were entered into the OLINDA dosimetry program to compute standard human organ absorbed doses using formalism of the Medical Internal Dosimetry Committee of the Society of Nuclear Medicine.46 This human dosimetry model is a normal (i.e., tumor-free) anatomic model.
In Vivo Static PET, Dynamic PET Imaging, and ex Vivo Biodistribution Studies
For static PET imaging, tumor-bearing mice (n = 3) were i.v.-injected with 200–300 μCi (7.4–11.1 MBq) cRGDY-PEG-[89Zr]C′ dots (labeling condition: 4 nmol/mCi, pH 8, 75 °C, 120 min) or 89Zr-DFO-cRGDY-PEG-C′ dots (labeling condition: 0.75 nmol/mCi, pH 7.4, 37 °C, 60 min). Approximately 5 min prior to the acquisition of PET images, mice were anesthetized by inhalation of 2% isoflurane (Baxter Healthcare, Deerfield, IL)/ oxygen gas mixture and placed on the scanner bed; anesthesia was maintained using 1% isoflurane/gas mixture. PET imaging was performed in a small-animal PET scanner (Focus 120 microPET; Concorde Microsystems) at 2, 24, 48, and 72 h postinjection. An energy window of 350–700 keV and a coincidence timing window of 6 ns were used. Data were sorted into 2D histograms by Fourier rebinning, and transverse images were reconstructed by filtered back-projection into a 128 × 128 × 63 (0.72 × 0.72 × 1.3 mm3) matrix. The PET imaging data were normalized to correct for nonuniformity of response, dead-time count losses, positron branching ratio, and physical decay to the time of injection; no attenuation, scatter, or partial-volume averaging corrections were applied. The counting rates in the reconstructed images were converted to activity concentrations (percentage injected dose per gram of tissue, % ID/g) by use of a system calibration factor derived from the imaging of a mouse-sized water-equivalent phantom containing 89Zr. Region-of-interest (ROI) analyses of the PET data were performed using IRW software.
For dynamic PET scanning, healthy mice were i.v. injected with ∼400 μCi (∼14.8 MBq) of cRGDY-PEG-[89Zr]C′ dots or 89Zr-DFO-cRGDY-PEG-C′ dots. A 60 min dynamic scan was performed in a small-animal PET scanner (Focus 120 microPET; Concorde Micro-systems) and framed into 46 frames: 12 × 5, 6 × 10, 6 × 30, 10 × 60, 6 × 150, and 5 × 300 s. Image reconstruction and ROI analysis were performed using IRW software and presented as % ID/g.
For biodistribution studies, tumor-bearing (n = 3) mice were injected with ∼100 μCi (∼3.7 MBq) cRGDY-PEG-[89Zr]C′ dots or 89Zr-DFO-cRGDY-PEG-C′ dots. Accumulated activity in major intraparenchymal organs was assayed at 24 h using an Automatic Wizard2 γ-Counter (PerkinElmer) and presented as % ID/g (mean ± SD).
Statistics
All comparisons were based on statistical significance from pairwise t tests from an analysis of variance model based on three replicates.
Supplementary Material
Acknowledgments
Funding
This study was funded by grants from the National Institutes of Health (1R01CA161280-01A1 to M.B. and U.W.; 1U54 CA199081-01 to M.B. and U.W.) and Sloan Kettering Institute (Core Grant P30 CA008748CCSG).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemmater.7b02567.
Tables of previously reported renally excreted nano-particles, decay properties of widely used PET isotopes, estimation of number of 89Zr per C′ dots, organ uptake details, radiation dosimetry, figures of natZr number estimation, PET imaging of 89Zr-DFO-cRGDY-PEG-C′ dots (using GSH and APTES as linkers), PD-10 elution profiles, mouse plasma uptake details, MIP images of free 89r-oxalate in mouse, effect of EDTA challenge, MIP PET images of tumor-bearing mice, and biodistribution studies (PDF)
First 1 hour Dynamic distribution video of mouse injected with chelator-free 89Zr labeled cRGDY-PEG-C′ dots (AVI)
First 1 hour Dynamic distribution video of mouse injected with chelator-based 89Zr labeled cRGDY-PEG-C′ dots (AVI)
ORCID
Feng Chen: 0000-0001-6495-1030
Kai Ma: 0000-0003-4415-6894
Ulrich Wiesner: 0000-0001-6934-3755
Michelle S. Bradbury: 0000-0003-3147-4391
Author Contributions
Product preparation was performed by F.C. and K.M.; experimental design was done by F.C., K.M., P.Z., U.W., and M.S.B., and data acquisition was done by F.C., K.M., L.Z.. Data analysis and interpretation was done by F.C., K.M., L.Z., P.Z., U.W., and M.S.B., and manuscript preparation was done by F.C., M.S.B., K.M., P.Z., and U.W. All authors approved the final version of the manuscript.
Notes
The authors declare no competing financial interest.
References
- 1.Smith BR, Gambhir SS. Nanomaterials for in Vivo Imaging. Chem Rev. 2017;117:901–986. doi: 10.1021/acs.chemrev.6b00073. [DOI] [PubMed] [Google Scholar]
- 2.Thakor AS, Gambhir SS. Nanooncology: the Future of Cancer Diagnosis and Therapy. Ca-Cancer J Clin. 2013;63:395–418. doi: 10.3322/caac.21199. [DOI] [PubMed] [Google Scholar]
- 3.Anselmo AC, Mitragotri S. A Review of Clinical Translation of Inorganic Nanoparticles. AAPS J. 2015;17:1041–54. doi: 10.1208/s12248-015-9780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anselmo AC, Mitragotri S. Nanoparticles in the Clinic. Bioeng Transl Med. 2016;1:10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of Nanoparticle Delivery to Tumours. Nat Rev Mater. 2016;1:16014. [Google Scholar]
- 6.Zhang YN, Poon W, Tavares AJ, McGilvray ID, Chan WC. Nanoparticle-Liver Interactions: Cellular Uptake and Hepatobiliary Elimination. J Controlled Release. 2016;240:332–348. doi: 10.1016/j.jconrel.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 7.D’Mello SR, Cruz CN, Chen ML, Kapoor M, Lee SL, Tyner KM. The Evolving Landscape of Drug Products Containing Nanomaterials in the United States. Nat Nanotechnol. 2017;12:523–529. doi: 10.1038/nnano.2017.67. [DOI] [PubMed] [Google Scholar]
- 8.Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal Clearance of Quantum Dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi HS, Liu W, Liu F, Nasr K, Misra P, Bawendi MG, Frangioni JV. Design Considerations for Tumour-Targeted Nano-particles. Nat Nanotechnol. 2010;5:42–7. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou C, Long M, Qin Y, Sun X, Zheng J. Luminescent Gold Nanoparticles with Efficient Renal Clearance. Angew Chem, Int Ed. 2011;50:3168–72. doi: 10.1002/anie.201007321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou C, Hao G, Thomas P, Liu J, Yu M, Sun S, Oz OK, Sun X, Zheng J. Near-infrared Emitting Radioactive Gold Nanoparticles with Molecular Pharmacokinetics. Angew Chem, Int Ed. 2012;51:10118–22. doi: 10.1002/anie.201203031. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Yu M, Ning X, Zhou C, Yang S, Zheng J. PEGylation and Zwitterionization: Pros and Cons in the Renal Clearance and Tumor Targeting of Near-IR-Emitting Gold Nano-particles. Angew Chem, Int Ed. 2013;52:12572–6. doi: 10.1002/anie.201304465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou M, Li J, Liang S, Sood AK, Liang D, Li C. CuS Nanodots with Ultrahigh Efficient Renal Clearance for Positron Emission Tomography Imaging and Image-Guided Photothermal Therapy. ACS Nano. 2015;9:7085–96. doi: 10.1021/acsnano.5b02635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen F, Goel S, Hernandez R, Graves SA, Shi S, Nickles RJ, Cai W. Dynamic Positron Emission Tomography Imaging of Renal Clearable Gold Nanoparticles. Small. 2016;12:2775–82. doi: 10.1002/smll.201600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns AA, Vider J, Ow H, Herz E, Penate-Medina O, Baumgart M, Larson SM, Wiesner U, Bradbury M. Fluorescent Silica Nanoparticles with Efficient Urinary Excretion for Nano-medicine. Nano Lett. 2009;9:442–448. doi: 10.1021/nl803405h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benezra M, Penate-Medina O, Zanzonico PB, Schaer D, Ow H, Burns A, DeStanchina E, Longo V, Herz E, Iyer S, Wolchok J, Larson SM, Wiesner U, Bradbury MS. Multimodal Silica Nanoparticles are Effective Cancer-Targeted Probes in a Model of Human Melanoma. J Clin Invest. 2011;121:2768–80. doi: 10.1172/JCI45600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y, Humm J, Gonen M, Kalaigian H, Schoder H, Strauss HW, Larson SM, Wiesner U, Bradbury MS. Clinical Translation of an Ultrasmall Inorganic Optical-PET Imaging Nano-particle Probe. Sci Transl Med. 2014;6:260ra149. doi: 10.1126/scitranslmed.3009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma K, Zhang DH, Cong Y, Wiesner U. Elucidating the Mechanism of Silica Nanoparticle PEGylation Processes Using Fluorescence Correlation Spectroscopies. Chem Mater. 2016;28:1537–1545. [Google Scholar]
- 19.Herz E, Ow H, Bonner D, Burns A, Wiesner U. Dye Structure-Optical Property Correlations in Near-Infrared Fluorescent Core-Shell Silica Nanoparticles. J Mater Chem. 2009;19:6341–6347. [Google Scholar]
- 20.Ow H, Larson DR, Srivastava M, Baird BA, Webb WW, Wiesner U. Bright and Stable Core-Shell Fluorescent Silica Nanoparticles. Nano Lett. 2005;5:113–7. doi: 10.1021/nl0482478. [DOI] [PubMed] [Google Scholar]
- 21.Ma K, Mendoza C, Hanson M, Werner-Zwanziger U, Zwanziger J, Wiesner U. Control of Ultrasmall Sub-10 nm Ligand-Functionalized Fluorescent Core-Shell Silica Nanoparticle Growth in Water. Chem Mater. 2015;27:4119–4133. [Google Scholar]
- 22.Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I, Conrad M, Turker MZ, Gao M, Jiang X, Monette S, Pauliah M, Gonen M, Zanzonico P, Quinn T, Wiesner U, Bradbury MS, Overholtzer M. Ultrasmall Nanoparticles Induce Ferroptosis in Nutrient-Deprived Cancer Cells and Suppress Tumour Growth. Nat Nanotechnol. 2016;11:977–985. doi: 10.1038/nnano.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradbury MS, Phillips E, Montero PH, Cheal SM, Stambuk H, Durack JC, Sofocleous CT, Meester RJ, Wiesner U, Patel S. Clinically-translated Silica Nanoparticles as Dual-Modality Cancer-Targeted Probes for Image-Guided Surgery and Interventions. Integr Biol (Camb) 2013;5:74–86. doi: 10.1039/c2ib20174g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradbury MS, Pauliah M, Zanzonico P, Wiesner U, Patel S. Intraoperative Mapping of Sentinel Lymph Node Metastases using a Clinically Translated Ultrasmall Silica Nanoparticle. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:535–53. doi: 10.1002/wnan.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. 89Zr-DFO-J591 for Immunopet of Prostate-Specific Membrane Antigen Expression In Vivo. J Nucl Med. 2010;51:1293–300. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland JP, Evans MJ, Rice SL, Wongvipat J, Sawyers CL, Lewis JS. Annotating MYC Status with 89Zr-transferrin Imaging. Nat Med. 2012;18:1586–91. doi: 10.1038/nm.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Medina C, Tang J, Abdel-Atti D, Hogstad B, Merad M, Fisher EA, Fayad ZA, Lewis JS, Mulder WJ, Reiner T. PET Imaging of Tumor-Associated Macrophages with 89Zr-Labeled High-Density Lipoprotein Nanoparticles. J Nucl Med. 2015;56:1272–7. doi: 10.2967/jnumed.115.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clinicaltrials.gov. https://clinicaltrials.gov/ct2/results?term=89Zr&cntry1=NA%3AUS (accessed September 6, 2017)
- 29.Lee FT, Scott AM. Immuno-PET for Tumor Targeting. J Nucl Med. 2003;44:1282. [PubMed] [Google Scholar]
- 30.Chen F, Goel S, Valdovinos HF, Luo H, Hernandez R, Barnhart TE, Cai W. In Vivo Integrity and Biological Fate of Chelator-Free Zirconium-89-Labeled Mesoporous Silica Nanopar-ticles. ACS Nano. 2015;9:7950–9. doi: 10.1021/acsnano.5b00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaffer TM, Wall MA, Harmsen S, Longo VA, Drain CM, Kircher MF, Grimm J. Silica Nanoparticles as Substrates for Chelator-Free Labeling of Oxophilic Radioisotopes. Nano Lett. 2015;15:864–8. doi: 10.1021/nl503522y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goel S, Chen F, Ehlerding EB, Cai W. Intrinsically Radiolabeled Nanoparticles: an Emerging Paradigm. Small. 2014;10:3825–30. doi: 10.1002/smll.201401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen F, Ellison PA, Lewis CM, Hong H, Zhang Y, Shi S, Hernandez R, Meyerand ME, Barnhart TE, Cai W. Chelator-free Synthesis of a Dual-Modality PET/MRI Agent. Angew Chem, Int Ed. 2013;52:13319–23. doi: 10.1002/anie.201306306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellison PA, Chen F, Goel S, Barnhart TE, Nickles RJ, DeJesus OT, Cai W. Intrinsic and Stable Conjugation of Thiolated Mesoporous Silica Nanoparticles with Radioarsenic. ACS Appl Mater Interfaces. 2017;9:6772–6781. doi: 10.1021/acsami.6b14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakravarty R, Valdovinos HF, Chen F, Lewis CM, Ellison PA, Luo H, Meyerand ME, Nickles RJ, Cai W. Intrinsically Germanium-69-Labeled Iron Oxide Nanoparticles: Synthesis and In-Vivo Dual-Modality PET/MR Imaging. Adv Mater. 2014;26:5119–23. doi: 10.1002/adma.201401372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen F, Valdovinos HF, Hernandez R, Goel S, Barnhart TE, Cai W. Intrinsic Radiolabeling of Titanium-45 using Mesoporous Silica Nanoparticles. Acta Pharmacol Sin. 2017;38:907–913. doi: 10.1038/aps.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhuravlev LT. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf, A. 2000;173:1–38. [Google Scholar]
- 38.Larson DR, Ow H, Vishwasrao HD, Heikal AA, Wiesner U, Webb WW. Silica Nanoparticle Architecture Determines Radiative Properties of Encapsulated Fluorophores. Chem Mater. 2008;20:2677–2684. [Google Scholar]
- 39.Lin YS, Abadeer N, Hurley KR, Haynes CL. Ultrastable, Redispersible, Small, and Highly Organomodified Mesoporous Silica Nanotherapeutics. J Am Chem Soc. 2011;133:20444–57. doi: 10.1021/ja208567v. [DOI] [PubMed] [Google Scholar]
- 40.Holland JP, Sheh Y, Lewis JS. Standardized Methods for the Production of High Specific-Activity Zirconium-89. Nucl Med Biol. 2009;36:729–39. doi: 10.1016/j.nucmedbio.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goel S, Chen F, Luan S, Valdovinos HF, Shi S, Graves SA, Ai F, Barnhart TE, Theuer CP, Cai W. Engineering Intrinsically Zirconium-89 Radiolabeled Self-Destructing Mesoporous Silica Nanostructures for In Vivo Biodistribution and Tumor Targeting Studies. Adv Sci (Weinh) 2016;3:1600122. doi: 10.1002/advs.201600122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meijs WE, Herscheid JD, Haisma HJ, Pinedo HM. Evaluation of Desferal as a Bifunctional Chelating Agent for Labeling Antibodies with Zr-89. Int J Rad Appl Instrum A. 1992;43:1443–7. doi: 10.1016/0883-2889(92)90170-j. [DOI] [PubMed] [Google Scholar]
- 43.Ma K, Wiesner U. Modular and Orthogonal Post-PEGylation Surface Modifications by Insertion Enabling Penta-functional Ultra-small Organic-Silica Hybrid Nanoparticles. Chem Mater. 2017;29:6840–55. [Google Scholar]
- 44.Gambhir SS. Molecular Imaging of Cancer with Positron Emission Tomography. Nat Rev Cancer. 2002;2:683–93. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 45.Abou DS, Ku T, Smith-Jones PM. In Vivo Biodistribution and Accumulation of 89Zr in Mice. Nucl Med Biol. 2011;38:675–81. doi: 10.1016/j.nucmedbio.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the Second-Generation Personal Computer Software for Internal Dose Assessment in Nuclear Medicine. J Nucl Med. 2005;46:1023. [PubMed] [Google Scholar]
- 47.Laforest R, Lapi SE, Oyama R, Bose R, Tabchy A, Marquez-Nostra BV, Burkemper J, Wright BD, Frye J, Frye S, Siegel BA, Dehdashti F. [89Zr]Trastuzumab: Evaluation of Radiation Dosimetry, Safety, and Optimal Imaging Parameters in Women with HER2-Positive Breast Cancer. Mol Imaging Biol. 2016;18:952–959. doi: 10.1007/s11307-016-0951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bjornmalm M, Faria M, Caruso F. Increasing the Impact of Materials in and beyond Bio-Nano Science. J Am Chem Soc. 2016;138:13449–13456. doi: 10.1021/jacs.6b08673. [DOI] [PubMed] [Google Scholar]
- 49.McNeil SE. Evaluation of Nanomedicines: Stick to the Basics. Nat Rev Mater. 2016;1:16073. [Google Scholar]
- 50.Torrice M. Does Nanomedicine Have a Delivery Problem? ACS Cent Sci. 2016;2:434–7. doi: 10.1021/acscentsci.6b00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilhelm S, Tavares AJ, Chan WCW. Reply to “Evaluation of Nanomedicines: Stick to the Basics”. Nat Rev Mater. 2016;1:16074. [Google Scholar]
- 52.Chan WCW. Nanomedicine 2.0. Acc Chem Res. 2017;50:627–632. doi: 10.1021/acs.accounts.6b00629. [DOI] [PubMed] [Google Scholar]
- 53.van der Meel R, Lammers T, Hennink WE. Cancer Nanomedicines: Oversold or Underappreciated? Expert Opin Drug Delivery. 2017;14:1–5. doi: 10.1080/17425247.2017.1262346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


