Abstract
Aim
Continuous regional arterial infusion (CRAI) of protease inhibitors may be effective in the treatment of severe acute pancreatitis (SAP), but it is more invasive than i.v. infusion. The purpose of this study was to examine the effectiveness of continuous i.v. infusion (CIVI) for SAP compared with CRAI by unifying the dose and the administration period of nafamostat mesylate.
Methods
This study comprised 32 patients with SAP who were divided into two groups: the CRAI group and the CIVI group. The protease inhibitor, nafamostat mesylate, was continuously infused at a rate of 200 mg/day for 5 days in both groups. Clinical outcomes including in‐hospital mortality were examined.
Results
There were no significant between‐group differences in in‐hospital mortality and 90‐day mortality. The duration from admission to treatment was significantly shorter in the CIVI group (median, 7 h vs. 2 h, P = 0.0001; CRAI group vs. CIVI group). The rate of mechanical ventilation was significantly less in the CIVI group than in the CRAI group (93% vs. 47%, P = 0.007). The CIVI group showed a tendency toward decreased length of intensive care unit stay (median, 13 days vs. 4 days, P = 0.085) and hospital stay (median, 19 days vs. 11 days, P = 0.072). Total costs during hospitalization were significantly lower in the CIVI group (median, $18,320 vs. $11,641, P = 0.049).
Conclusion
The effectiveness of CIVI with early nafamostat mesylate treatment after the development of SAP could be equivalent to, or better than, that of CRAI.
Keywords: Continuous intravenous infusion, continuous regional arterial infusion, nafamostat mesylate, protease inhibitor, severe acute pancreatitis
Introduction
Protease inhibitors and prophylactic antibiotics are widely used to treat severe acute pancreatitis (SAP) in Japan, but the effectiveness of these treatments remains controversial. Protease inhibitors decrease the amount of activated pancreatic trypsin in pancreatitis in mice1, 2, 3 and prevent necrotic changes in the pancreas by suppression of pancreatic ischemia associated with vasospasm and the inhibition of an increase in the coagulability of the pancreatic microcirculation.4 Protease inhibitors also help to prevent ischemia‐reperfusion injury in organs such as the lungs and kidneys.5, 6, 7 Nafamostat mesylate, with a molecular weight of 540 Da and half‐life of 23.1 min, is expected to be an effective treatment for pancreatitis because it can easily penetrate into the pancreas.
Continuous regional arterial infusion (CRAI) of protease inhibitors has spread as a treatment for SAP in Japan since it was first proven to be effective for acute necrotizing pancreatitis.8 In the first randomized controlled trial for CRAI, in which patients were divided into two groups, the CRAI group (treated with CRAI of nafamostat mesylate and imipenem) and non‐CRAI group (treated i.v. with imipenem), it was revealed that CRAI was more effective in preventing septic complications and in reducing mortality rates in SAP than non‐CRAI.9
However, there are few reports showing that the continuous i.v. infusion (CIVI) of a protease inhibitor is effective for SAP. The reason might be related to the low dose of the administered protease inhibitor. It was reported that CRAI of nafamostat mesylate and imipenem decreased the need for surgical therapy and reduced mortality in patients with SAP compared with non‐CRAI, that is, i.v. infusion of nafamostat mesylate and imipenem.10 However, the comparison between CRAI and non‐CRAI was not appropriate because the dose of nafamostat mesylate was not equal in each patient, and the period of administration of nafamostat mesylate and imipenem was unclear.
It is necessary to reassess the effectiveness of CIVI of protease inhibitors because CRAI requires the skills of an interventional radiologist and is associated with complications of bleeding and catheter‐related infection. In our institution, we changed the treatment protocol of nafamostat mesylate for SAP on April 2012 from CRAI to CIVI, but other treatment practices were constant, including the dosage and infusion periods of the protease inhibitor.
The purpose of this study was to reassess the effectiveness of the i.v. infusion of the protease inhibitor and the prophylactic antibiotic for SAP in comparison with CRAI under conditions of identical dosages and periods of administration of nafamostat mesylate and imipenem in both CRAI and CIVI.
Methods
Patients and methods
Patients who were transported to our hospital between December 2009 and April 2014 and diagnosed as SAP were selected for study objects. Severe acute pancreatitis was diagnosed according to the scoring system of acute pancreatitis devised by the Research Committee for Intractable Diseases of the Pancreas of the Ministry of Health, Labor and Welfare, Japan. Those who were admitted between December 2009 and March 2012 received protease inhibitor and antibiotic by CRAI, and those who admitted between April 2012 and April 2014 received treatment by CIVI. Our hospital is a university hospital located in a major urban city in Japan. Our department has a closed intensive care unit (ICU; six beds) and accepts many critical patients. Patients were evaluated and stabilized following the general protocol of SAP, and managed at the ICU by emergency physicians. We obtained consent regarding the treatment protocols for SAP; the use of CRAI or CIVI depended on the period when the patients were admitted.
Continuous regional arterial infusion or continuous i.v. infusion of protease inhibitor and antibiotic
Continuous regional arterial infusion was carried out through a catheter inserted into either or both the celiac artery and the superior mesenteric artery. The catheter used for CRAI was the same as that used for angiography. Following computed tomography (CT) evaluation of the hypoenhanced area of the pancreas, angiography of the pancreas was carried out. The catheter tip was located in the artery perfusing the area containing the main lesion of hypoperfusion of the pancreas. Nafamostat mesylate was continuously infused at a rate of 200 mg/day for 5 days in both the CRAI and CIVI groups. Imipenem was also given to both groups at a dose of 0.5 g/h at 12‐h intervals for 5 days.
Japanese severity scoring system for acute pancreatitis
The severity of acute pancreatitis was determined on the basis of the Japanese severity score (prognostic factor score) determined by summing nine factors, along with the CT severity score.11, 12 Table 1 shows the details of this scoring system. Severe acute pancreatitis was diagnosed when the total prognostic factor score was 3 or higher, or the CT severity grade was 2 or higher.
Table 1.
Japanese severity scoring system for acute pancreatitis
| Scoring system | |
|---|---|
| Prognostic factor score (one point for each factor) | |
| Base excess ≤−3 mEq/L or shock (systolic blood pressure below 80 mmHg) | |
| PaO2 ≤ 60 mmHg (room air) or respiratory failure (respiratory assistance needed) | |
| BUN ≥40 mg/dL (or creatinine ≥2.0 mg/dL) or oliguria (daily urine output <400 mL even after i.v. fluid resuscitation) | |
| LDH at or above twice the upper limit of normal | |
| Platelet count ≤100,000/mm3 | |
| Serum calcium ≤7.5 mg/dL | |
| CRP ≥15 mg/dL | |
| Number of positive measures in SIRS criteria ≥3 | |
| Age ≥70 years | |
| CT grade based on contrast‐enhanced CT | |
| Scoring | A: Extrapancreatic progression of inflammation |
| 0 | Anterior pararenal space |
| 1 | Root of mesocolon |
| 2 | Beyond lower pole of kidney |
| B: Hypoenhanced lesion of the pancreas | |
| Pancreas divided into three segments (heads, body, and tail) | |
| 0 | Localized in each segment or surrounding only the pancreas |
| 1 | Extends to two segments |
| 2 | Occupies two whole segments or more |
| A + B = total score CT grade | |
| Total score = 0 or 1 Grade 1 | |
| Total score = 2 Grade 2 | |
| Total score = 3 or more Grade 3 | |
| Severe acute pancreatitis | Prognostic factor score ≥3 or CT grade ≥2 |
Measures within the systemic inflammatory response syndrome (SIRS) criteria include body temperature >38°C or <36°C, heart rate >90 b.p.m., respiratory rate >20 breaths/min or partial pressure of carbon dioxide in blood <32 torr, and white blood cell count >12,000 cells/mm3, <4,000 cells/mm3, or >10% immature (band) forms. BUN, blood urea nitrogen; CRP, C‐reactive protein; CT, computed tomography; LDH, lactate dehydrogenase; PaO2, partial pressure of oxygen in arterial blood.
Disseminated intravascular coagulation scoring system
The diagnosis of disseminated intravascular coagulation (DIC) was made on the basis of the Japanese Association for Acute Medicine's DIC diagnostic criteria.13
Outcomes
The primary outcomes were in‐hospital mortality and 90‐day mortality. Secondary and tertiary outcomes included length of ICU stay, length of hospital stay, number of ventilator‐free days, and receipt of mechanical ventilation, renal replacement therapy, or surgical intervention. Changes in the Sequential Organ Failure Assessment (SOFA) score, prognostic factor score, CT severity grade, and DIC score before and after the infusion were evaluated.
Statistical analyses
Continuous variables are given as the mean ± standard deviation or median and interquartile range, and were compared using Student's t‐test or the Mann–Whitney U‐test. Between‐group differences in categorical variables were compared using Fisher's exact test, a χ2‐test, or paired t‐test, as appropriate. Statistical analyses were carried out using JMP 10 (SAS Institute Inc., Cary, NC, USA). A two‐sided P‐value less than 0.05 was considered statistically significant.
Results
Patients
A total of 2,586 patients were admitted to the ICU in our department from December 2009 to April 2014. Thirty‐seven patients fulfilled the clinical diagnostic criteria for SAP. The CRAI group comprised 15 patients, and the CIVI group comprised 17 patients. Five patients were excluded from this study because three patients in the CRAI group and two patients in the CIVI group, respectively, were discharged within 5 days.
Patient characteristics
The clinical characteristics of the CIVI group and CRAI group are shown in Table 2. There were no significant differences in age, sex, APACHE II score, SOFA score, Charlson comorbidity index, prognostic factor score, CT grade, CT severity index, DIC score, or lactate level on admission. Time‐courses from onset of acute pancreatitis to treatment are shown in Figure 1. The duration from onset to admission tended to be shorter in the CIVI group, but the difference was not statistically significant (median, 48 [24–72] h vs. 24 [8.5–54] h, P = 0.07). The time from admission to treatment was significantly shorter in the CIVI group (median, 7 [4.5–22.5] h vs. 2 [1.3–3] h, P = 0.0001). The duration from onset to treatment was significantly shorter in the CIVI group (median, 53 [46.5–99] h vs. 40 [12–55.3] h, P = 0.013; CRAI group vs. CIVI group).
Table 2.
Characteristics of patients with severe acute pancreatitis treated with continuous regional arterial infusion (CRAI) or continuous i.v. infusion (CIVI) of protease inhibitors
| CRAI | CIVI | ||
|---|---|---|---|
| (n = 15) | (n = 17) | P‐value | |
| Age, years, mean | 60.1 ± 17.8 | 51.7 ± 18.1 | 0.25 |
| Sex, n | |||
| Female | 3 | 6 | |
| Male | 12 | 11 | 0.44 |
| Cause of pancreatitis, n (%) | |||
| Alcoholic | 7 (46.7) | 9 (52.9) | |
| Biliary | 4 (26.3) | 4 (23.5) | |
| Post‐ERCP | 1 (6.7) | 1 (5.9) | |
| Others | 3 (20.3) | 3 (17.7) | |
| APACHE II score, median (IQR) | 17 (14–21) | 18 (10–22) | 0.90 |
| SOFA score, median (IQR) | 4 (2–5) | 3 (2–6) | 0.70 |
| Charlson comorbidity index, median (IQR) | 4 (1–4) | 2 (0.5–5) | 0.74 |
| Prognostic factor score, median (IQR) | 5 (2–5) | 2 (2–5) | 0.34 |
| CT grade, median (IQR) | 2 (2–2) | 2 (2–3) | 0.11 |
| CT severity index, median (IQR) | 6 (4–6) | 6 (5.5–6) | 0.45 |
| DIC score, median (IQR) | 3 (3–5) | 3 (0–5.5) | 0.41 |
| Lactate on admission, mmol/L, median (IQR) | 1.3 (0.9–3.9) | 1.2 (0.9–3.0) | 0.80 |
APACHE, Acute Physiology and Chronic Health Evaluation; CT, computed tomography; DIC, disseminated intravascular coagulation; ERCP, endoscopic retrograde cholangiopancreatography; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
Figure 1.
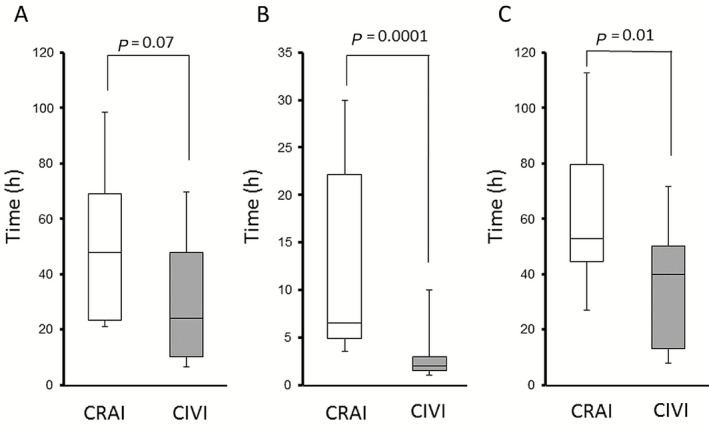
Time‐course from onset of acute pancreatitis to treatment. Time from onset to admission (A), time from admission to treatment (B), and time from onset to treatment (C) were investigated. White columns represent data for continuous regional arterial infusion (CRAI) and gray columns for continuous i.v. infusion (CIVI) of nafamostat mesylate. Lines denote median values, boxes represent 25th to 75th percentiles, and whiskers indicate the range.
Outcomes in the CIVI and CRAI groups
Outcomes in the CIVI and CRAI groups are summarized in Table 3. There were no significant differences in in‐hospital mortality or 90‐day mortality between the two groups. Two patients died in the CRAI group, one from acute myocardial infarction and the other from multiple organ dysfunction syndrome due to Pseudomonas aeruginosa pneumonia. Both patients died on hospital day 41. One patient died from multiple organ dysfunction syndrome in the CIVI group on hospital day 24. The percentage of patients undergoing mechanical ventilation was significantly lower in the CIVI group (93.3% vs. 47.1%, P = 0.007; CRAI group vs. CIVI group). The CIVI group showed a tendency toward decreased length of ICU stay (median, 13 [8–31] days vs. 4 [2–17] days, P = 0.085) and hospital stay (median, 19 [14–99] days vs. 11 [8–29]) days, P = 0.072). In addition, total costs during hospitalization were significantly lower in the CIVI group (median, $18,320 [$13,209–$80,847] vs. $11,641 [$8,645–$27,733], P = 0.049). There were no significant differences in factors relating to surgical intervention (Table 4).
Table 3.
Outcomes in continuous i.v. infusion (CIVI) and continuous regional arterial infusion (CRAI) patient groups for severe acute pancreatitis
| CRAI | CIVI | ||
|---|---|---|---|
| (n = 15) | (n = 17) | P‐value | |
| In‐hospital mortality, n (%) | 2 (13.3) | 1 (5.6) | 0.60 |
| 90‐day mortality, n (%) | 2 (13.3) | 1 (5.6) | 0.60 |
| Mechanical ventilation, n (%) | 14 (93.3) | 8 (47.1) | 0.01 |
| Ventilator‐free days, days, median (IQR) | 18 (0–21) | 28 (7.5–28) | 0.15 |
| Length of ICU stay, days, median (IQR) | 13 (8–31) | 4 (2–17) | 0.09 |
| Length of stay, (days, median (IQR) | 19 (14–99) | 11 (8–29) | 0.07 |
| Cost, $US, median (IQR) | 18,320 (13,209–80,847) | 11,641 (8,645–27,733) | 0.05 |
| Volume of infusion for 24 h, mL, median (IQR) | 6,400 (4,000–9,300) | 5,200 (3,800–10,000) | 0.48 |
| Ventilator‐associated pneumonia, n | 4 | 1 | 0.16 |
| Renal replacement therapy, n | 3 | 2 | 0.65 |
| Surgical intervention, n | 6 | 3 | 0.24 |
ICU, intensive care unit; IQR, interquartile range.
Table 4.
Surgical interventions in patients with severe acute pancreatitis treated with continuous i.v. infusion (CIVI) or continuous regional arterial infusion (CRAI)
| CRAI (n = 15) | CIVI (n = 17) | P‐value | |
|---|---|---|---|
| Surgery, n (%) | 6 (40.0) | 3 (17.6) | 0.24 |
| Procedures | |||
| Open surgery | 3 | 3 | |
| Open surgery after percutaneous drainage | 2 | 0 | |
| Endoscopic drainage | 1 | 0 | |
| Cause of surgery | |||
| Infected pancreatic necrosis | 5 | 1 | |
| Abdominal compartment syndrome | 2 | 1 | |
| Intestinal perforation | 0 | 2 | |
| Time from admission to surgery, days, median (IQR) | 20 (16–34) | 9 (4–27) | 0.52 |
IQR, interquartile range.
Changes in clinical parameters such as prognostic factor, CT grade, SOFA score, and DIC score before and after CIVI or CRAI are shown in Figure 2. The SOFA score in the CRAI group significantly increased after the treatment (median, 4 [2–5] vs. 5 [4–8], P = 0.016; before vs. after), but no significant differences were observed in the extent of change in the prognostic factor or DIC score in the two groups before and after the treatment.
Figure 2.
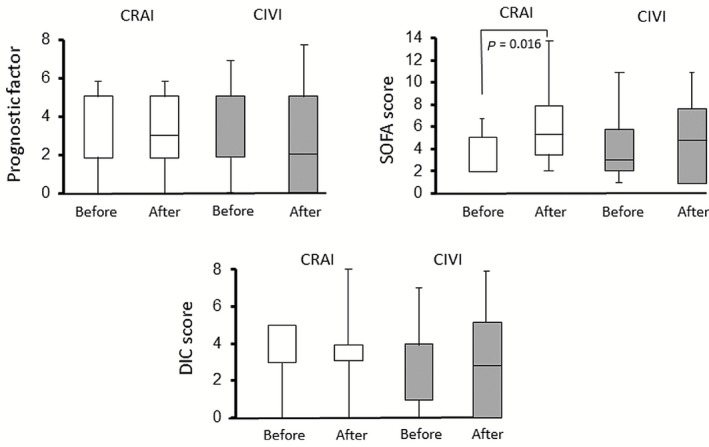
Changes in clinical parameters of acute pancreatitis. Prognostic factor, Sequential Organ Failure Assessment (SOFA) score, and disseminated intravascular coagulation (DIC) score before and after continuous regional arterial infusion (CRAI; white columns) or continuous i.v. infusion (CIVI; gray columns). Lines denote median values, boxes represent 25th to 75th percentiles, and whiskers indicate the range.
Discussion
Protease inhibitors are widely used to treat acute pancreatitis in Japan, but there are no international guidelines that describe their effectiveness. One of the major reasons is that protease inhibitors are not approved in many countries and unavailable for treatment of acute pancreatitis.
Protease inhibitors used for the treatment of acute necrotizing pancreatitis are thought not to easily reach the pancreas when given i.v. Because of ischemia or impaired microcirculation, they hardly penetrate into pancreatic tissue.14, 15 Continuous regional arterial infusion was contrived to improve this weak point in the administration of protease inhibitors.
Takeda et al. were the first group to report the usefulness of CRAI of a protease inhibitor and antibiotic as a treatment for SAP.8 They found that CRAI of a protease inhibitor and antibiotic was associated with significantly lower rates of mortality and pancreatic infection compared to i.v. infusion of these drugs. One mechanism of this can be explained by the high concentration of protease inhibitor when it was administered by CRAI. However, the effectiveness of i.v. infusion of protease inhibitors is unclear because the dosage and duration of administration of nafamostat were not comparable, and it is thought that the time from onset to admission and initial treatment differed widely in each patient.
One randomized controlled trial showed that CIVI of high‐dose gabexate mesylate, a low‐molecular‐weight antiprotease, reduced the necessity for surgical intervention and peritoneal lavage, and improved survival in patients of acute pancreatitis with organ dysfunctions compared with patients treated without protease inhibitors.16 In in vitro experiments, nafamostat inhibited the pancreatic protease activities 10–100 times more potently than gabexate,17 but there are few studies on CIVI of high‐dose nafamostat in SAP. There is some question as to the concentration of nafamostat mesylate we require to treat SAP. The local concentration of nafamostat mesylate in CIVI is not as high as in CRAI, but it is probable that CIVI of nafamostat mesylate is effective under conditions of high dosages of administration as well as CRAI.
Early CIVI of high‐dose nafamostat before the progression of pancreatic necrosis may improve SAP, comparable to that of CRAI. Therefore, we attempted to evaluate the effects of CIVI compared with CRAI by unifying the dosing period and the high dose of nafamostat. There were no significant differences in primary outcome between CRAI and CIVI, but our study showed that CIVI had some advantages compared with CRAI. The duration from admission to treatment was significantly shorter in the CIVI group. This was related to the time from admission to treatment, which was significantly shorter in the CIVI group. Continuous i.v. infusion could be started quickly because angiography was not required. The administration of a high dose protease inhibitor as soon as possible after the development of SAP may prevent pancreatitis from deteriorating before ischemia or impaired microcirculation occurs in the pancreatic tissue.
There were no significant differences in the number of ventilator‐free days, but the number of patients who underwent mechanical ventilation was significantly smaller in the CIVI group than in the CRAI group. Intubation or sedation was required for patients with CRAI to maintain the position of the intra‐arterial catheter. In our study, 5 of 15 patients actually received intubation to keep the patients at rest to maintain catheter position. Although the in‐hospital and 90‐day mortality were not different between the groups, the length of ICU and hospital stay tended to be shorter in the CIVI group. This may be due to the smaller number of patients with mechanical ventilation or smaller number requiring surgical intervention in CIVI as compared to CRAI groups.
The total costs during hospitalization were significantly lower in the CIVI group than in the CRAI group, which also may be related to the number of patients with mechanical ventilation or the length of ICU stay and hospital stay.
The SOFA score was exaggerated unexpectedly after treatment. This was associated with the deterioration of Glasgow Coma Scale scores. Almost all the patient with CRAI required mechanical ventilation and deep sedation to maintain the position of the intra‐arterial catheter.
Moving patients from the supine to sitting position during CRAI was difficult because of the necessity to maintain the position of the intra‐arterial catheter. With CIVI, however, postural change was easy and, therefore, it was possible to prevent ventilator‐associated pneumonia. Continuous regional arterial infusion is highly invasive for patients, compared with CIVI, because it requires intubation and angiography. Our results suggest that CIVI with early nafamostat treatment after the development of SAP could be more effective than CRAI.
Our study was a non‐randomized design and analyzed a limited number of patients from a single center. Furthermore, the starting periods for CRAI and CIVI were different. We changed from CRAI to CIVI in April 2012 to reassess the effectiveness of CIVI because the former required more invasive procedures. The treatment strategies for SAP, except for the administration methods, were the same. Therefore, the influence of the difference in starting periods was considered to be small.
We conclude that the effectiveness of CIVI with early nafamostat mesylate administration after the development of SAP could be equivalent to, or better than, that of CRAI.
Conflict of Interest
None.
References
- 1. Niederau C, Liddle RA, Ferrell LD, Grendell JH. Beneficial effects of cholecystokinin‐receptor blockade and inhibition of proteolytic enzyme activity in experimental acute hemorrhagic pancreatitis in mice. Evidence for cholecystokinin as a major factor in the development of acute pancreatitis. J. Clin. Invest. 1986; 78: 1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lankisch PG, Pohl U, Göke B et al Effect of FOY‐305 (camostat) on severe acute pancreatitis in two experimental animal models. Gastroenterology 1989; 96: 193–199. [DOI] [PubMed] [Google Scholar]
- 3. Suzuki M, Isaji S, Stanten R, Frey CF, Ruebner B. Effect of protease inhibitor FUT‐175 on acute hemorrhagic pancreatitis in mice. Int. J. Pancreatol. 1992; 11: 578–582. [PubMed] [Google Scholar]
- 4. Takeda K. Antiproteases in the treatment of acute necrotizing pancreatitis: Continuous regional arterial infusion. JOP 2007; 8 (Suppl. 4): 526–532. [PubMed] [Google Scholar]
- 5. Luh SP, Tsai CC, Shau WY et al Effects of gabexate mesilate (FOY) on ischemia‐reperfusion‐induced acute lung injury in dogs. J. Surg. Res. 1999; 87: 152–163. [DOI] [PubMed] [Google Scholar]
- 6. Xu L, Ren B, Li M, Jiang F, Zhanng Z, Hu J. Ulinastatin suppresses systemic inflammatory response following lung ischemia‐reperfusion injury in rats. Transplant. Proc. 2008; 40: 1310–1311. [DOI] [PubMed] [Google Scholar]
- 7. Inoue S, Sugitani A, Yamamoto H et al Effect of the synthetic protease inhibitor gabexate mesilate on the attenuation of ischemia/reperfusion injury in canine kidney autotransplantation. Surgery 2005; 137: 216–224. [DOI] [PubMed] [Google Scholar]
- 8. Takeda K, Matsuno S, Sunamura M, Kakugawa Y. Continuous regional arterial infusion of protease inhibitor and antibiotics in acute necrotizing pancreatitis. Am. J. Surg. 1996; 171: 394–398. [DOI] [PubMed] [Google Scholar]
- 9. Piaścik M, Rydzewska G, Milewski J et al The results of severe acute pancreatitis treatment with continuous arterial infusion of protease inhibitor and antibiotic: A randomized controlled study. Pancreas 2010; 39: 863–867. [DOI] [PubMed] [Google Scholar]
- 10. Imaizumi H, Kida M, Nishimaki H et al Efficacy of continuous regional arterial infusion of a protease inhibitor and antibiotic for severe acute pancreatitis in patients admitted to an intensive care unit. Pancreas 2004; 28: 369–373. [DOI] [PubMed] [Google Scholar]
- 11. Takeda K, Yokoe M, Takada T et al Assessment of severity of acute pancreatitis according to new prognostic factors and CT grading. J. Hepatobiliary Pancreat. Sci. 2010; 17: 37–44. [DOI] [PubMed] [Google Scholar]
- 12. Hamada T, Yasunaga H, Nakai Y et al Japanese severity score for acute pancreatitis well predicts in‐hospital mortality: A nationwide survey of 17,901 cases. J. Gastroenterol. 2013; 48: 1384–1391. [DOI] [PubMed] [Google Scholar]
- 13. Gando S, Iba T, Eguchi Y et al A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: Comparing current criteria. Crit. Care Med. 2006; 34: 625–631. [DOI] [PubMed] [Google Scholar]
- 14. Inoue K, Hirota M, Kimura Y et al Further evidence for endothelin as an important mediator of pancreatic and intestinal ischemia in severe acute pancreatitis. Pancreas 2003; 26: 218–223. [DOI] [PubMed] [Google Scholar]
- 15. Takeda K, Mikami Y, Fukuyama S et al Pancreatic ischemia associated with vasospasm in the early phase of human acute necrotizing pancreatitis. Pancreas 2005; 30: 40–49. [PubMed] [Google Scholar]
- 16. Chen HM, Chen JC, Hwang TL, Jan YY, Chen MF. Prospective and randomized study of gabexate mesilate for the treatment of severe acute pancreatitis with organ dysfunction. Hepatogastroenterology 2000; 47: 1147–1150. [PubMed] [Google Scholar]
- 17. Iwaki M, Ino Y, Motoyoshi A et al Pharmacological studies of FUT‐175, nafamostat mesilate. V. Effects on the pancreatic enzymes and experimental acute pancreatitis in rats. Jpn J. Pharmacol. 1986; 41: 155–162. [DOI] [PubMed] [Google Scholar]


