Abstract
Case
A 51‐year‐old man presented with severe burns, with a burn index of 33.5. Relaxation incisions were made in the trunk and right arm. Ringer's solution (12,000 mL) was used as initial fluid therapy for the first 24 h. The patient's serum Na level gradually increased to 170 mEq/L; infusion was carried out to correct the hypernatremia. Continuous veno‐venous hemodialysis and filtration succeeded in maintaining the serum Na level at approximately 145 mEq/L.
Outcome
After the initiation of continuous veno‐venous hemodialysis and filtration, the skin graft survival rate improved markedly with the normalization of the Na level, and the patient recovered smoothly. He was discharged on foot.
Conclusion
Hypernatremia, frequently observed in patients with extensive burns, is considered to be markedly disadvantageous for the survival of skin grafts. Continuous veno‐venous hemodialysis and filtration may be one of the options for the treatment of refractory hypernatremia in severe burns.
Keywords: Burn, continuous veno‐venous hemodialysis and filtration, hypernatremia, renal replacement therapy, skin graft
Introduction
Hypernatremia is a frequent complication of severe extensive burns.1 It remains unclear whether refractory hypernatremia in burns should be managed by continuous veno‐venous hemodialysis and filtration (CVVH) or not. Although CVVH provides appropriate and consistent electrolyte management, it must be indicated carefully because the complications due to extracorporeal circulation are disadvantageous for the treatment of burns.
Herein, we report a patient with severe burns with refractory hypernatremia. The survival of skin grafts was very poor while hypernatremia persisted, but marked improvements were observed after CVVH, leading to a favorable outcome.
Case
A 51‐year‐old man sustained burns to his entire body while working as white gasoline for a stove caught fire. The patient was transported to an emergency unit. He had a history of Hartmann's operation for rectal cancer at the age of 42 years. An examination showed a body temperature of 35.8°C, blood pressure of 144/103 mmHg, heart rate of 78 b.p.m., respiratory rate of 20 breaths/min, and Glasgow Coma Scale of E3V4M6. Extensive grade II or severer burns were observed over 35% of the body surface, including the occipital region, left thoracoabdominal region, entire back, left upper extremity, and left buttock (Fig. 1). Particularly, areas other than the occipital region were painless, discolored to white, and later considered to have sustained grade III burns. There was no CO poisoning or burn of the respiratory tract. The total burn surface area according to the Lund and Browder chart was 6.0% for grade II and 30.5% for grade III, the burn index was 33.5, and the abbreviated burn severity index was 9 points, indicating a serious injury. Blood tests on admission showed: white blood cell count, 18,400/μL; red blood cell count, 523 × 104/μL; hemoglobin, 17.0 g/dL; aspartate aminotransferase, 62 IU/L; alanine aminotransferase, 51 IU/L; lactate dehydrogenase, 446 IU/L; creatine kinase, 359 IU/L; blood urea nitrogen, 14.0 mg/dL; creatinine, 0.7 mg/dL; Na, 139 mEq/L; K, 3.9 mEq/L; Cl, 97 mEq/L; and C‐reactive protein, 0.11 mg/dL. No other abnormality was noted.
Figure 1.
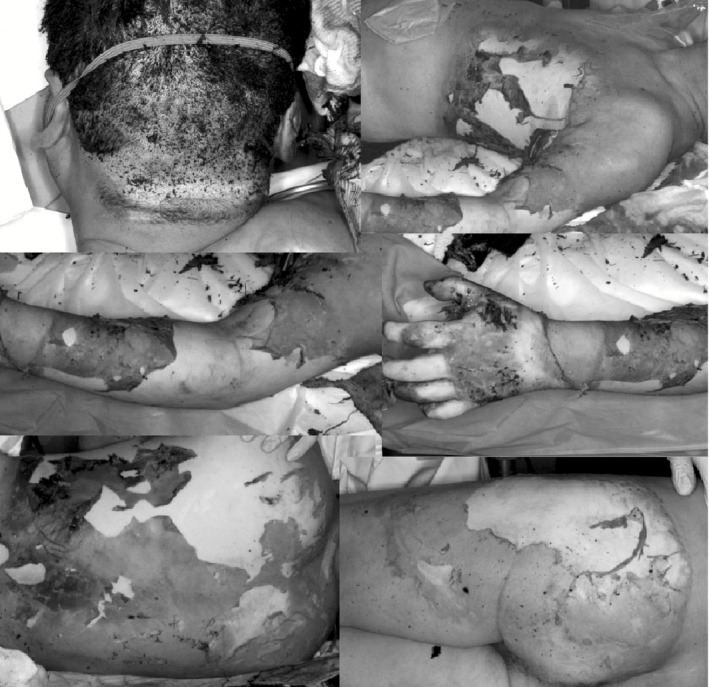
Photographs of a 51‐year‐old man who sustained severe burns, on admission. Grade II and III burns were observed over 35% of the body surface, including the occipital region, left thoracoabdominal region, entire back, left upper extremity, and left buttock. The total burn surface area according to the Lund and Browder chart was 6.0% for grade II and 30.5% for grade III, and the burn index was 33.5.
Relaxation incisions were made in the trunk and left arm, and systemic management including massive transfusion and mechanical ventilation was initiated. Figure 2 shows the clinical course. Lactate Ringer's solution and acetate Ringer's solution (12,000 mL) was given as initial fluid therapy for the first 24 h. Debridement and split‐thickness skin grafting were carried out five times. Operation time was 2–3 h in all surgeries. The serum Na level gradually elevated before and after the first skin grafting, carried out on day 4. Treatments including restriction of Na intake and high‐dose infusion of 5% glucose solution were used, but the Na level continued to increase despite monitoring the adequate urine output and all‐day overbalance (including gauze weight), and reached a maximum of 170 mEq/L. As the patient presented with not only hypernatremia but also in a septic condition, graft survival after the first skin grafting was limited to a 10% area (skin graft survival was evaluated from physical appearance by multiple dermatologists 7 days after skin graft operation). The patient's cardiac function, renal function, and hepatic function were maintained and refractory glucose tolerance, hypothermia, and coagulopathy did not occur throughout the course. Serum albumin was properly corrected over 1.5 g/dL with albumin administration in the five perioperative periods. Continuous veno‐venous hemodialysis and filtration was introduced for the management of refractory hypernatremia on day 10. For CVVH, a polysulfone membrane (EXCELFLO AEF‐10; Asahi Kasei Medical, Tokyo, Japan) was used and the dialysis conditions were initially set at: blood flow rate, 120 mL/min; dialysis fluid flow rate, 1,000 mL/h; and ultrafiltration rate, 200 mL/h. Continuous veno‐venous hemodialysis and filtration was continued until day 42, and the serum Na level could be maintained at approximately 145 mEq/L. Septic or, occasionally, septic shock conditions continued throughout the course and various antibiotics were given, however, the graft survival rate after the second skin grafting carried out on day 11 was approximately 60%, and those after all subsequent skin graftings were 50% or higher. Nafamostat mesilate was used as an anticoagulant for CVVH, and the activated coagulation time could be controlled under 150 s during treatment. Treatments for oozing from the wound surface were frequently carried out, but hemostasis could be achieved by appropriate procedures. The patient showed a smooth recovery, underwent rehabilitation, and was discharged after 4 months, being capable of independent ambulation. Figure 3 shows the good condition of the wound 1 year after injury.
Figure 2.
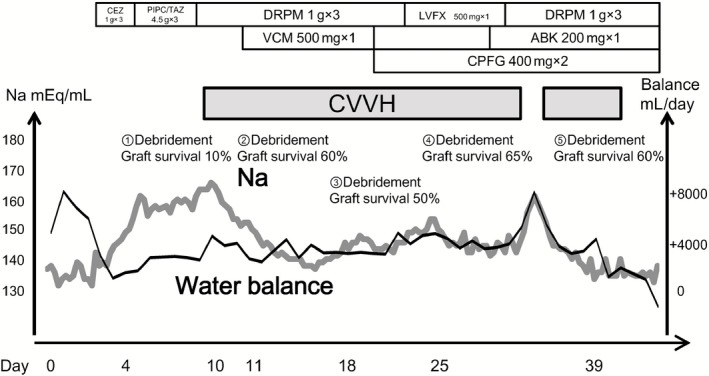
Clinical course of a 51‐year‐old man who sustained grade II and III burns to over 35% of his body. ABK, arbekacin; CEZ, cefazolin; CPFG, caspofungin; CVVH, continuous veno‐venous hemodialysis and filtration; DRPM, doripenem; LVFX, levofloxacin; PIPC/TAZ, piperacillin/tazobactam; VCM, vancomycin.
Figure 3.
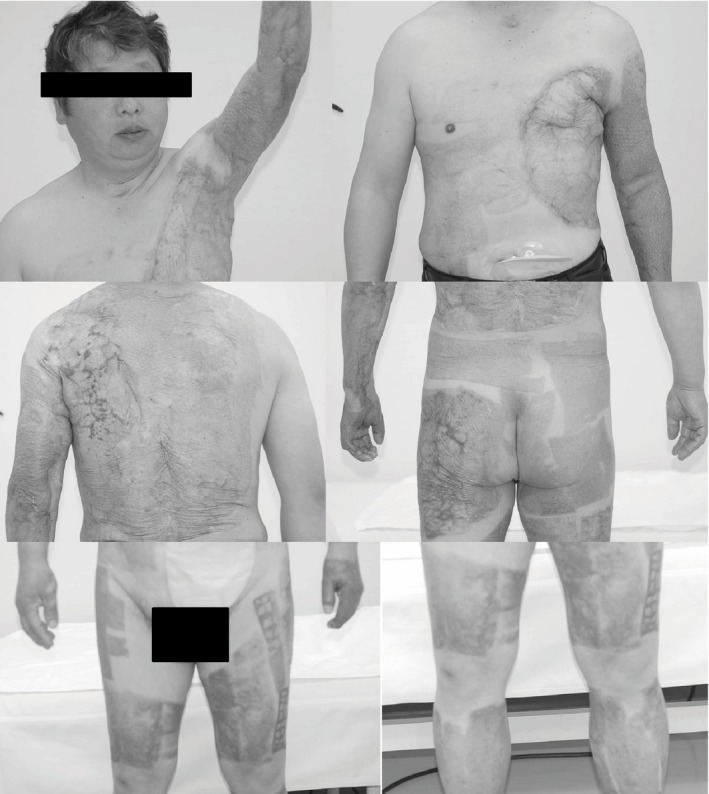
Photographs of injury sites 1 year after a 51‐year‐old man sustained grade II and III burns to over 35% of his body. The patient recovered with a little contracture in the left arm.
Discussion
We report the case of a severe burn patient with refractory hypernatremia in whom CVVH contributed to the management of the serum Na level with the improvement of skin graft survival.
Hypernatremia observed in severe burn patients is considered to be caused by dehydration in the ultra‐acute period2 and by the shift of Na in the infusion fluid into the blood vessels as well as dehydration in the subacute period.3 Actually, it is considered that hypernatremia in this case occurred by Na load from fluid therapy, consisting mainly of lactate Ringer's solution and acetate Ringer's solution in the first 3 days until the refilling state. Although the initial fluid load of 12,000 mL Ringer's solution was needed for the extensive burn and the wounds of relaxation incisions, it contained 1,560 mEq/L Na, equivalent to 92 g NaCl. In any event, hypernatremia in burns directly leads to hypertonic dehydration and is associated with intracellular dehydration. Indeed, the mortality rate is higher in patients with severe burns complicated with hypernatremia than in those without hypernatremia.4 Although hypernatremia in burn patients needs aggressive correction, it occasionally resists any treatment.4
Skin grafts for burns can survive only when the recipient site is free of infection, the blood supply is maintained, and nutrition and electrolytes are appropriately managed.5 However, it is rare that all these conditions are present in severe burn, and graft survival is often difficult. Although it may be possible that skin graft survival could have been higher in this case if we could have changed the timing of operations, the opportunity for surgery for severe burns is often subject to limitations, not only the patient's condition but also operating room or medical staff schedules. In particular, hypernatremia, which directly leads to intracellular dehydration, is considered markedly disadvantageous for skin graft survival. It was reported that hypernatremia induced more tissue damage and inflammation in the burned wound in experimental studies. Harada et al. reported that the apoptosis of hair follicle cells occured more severely under hypernatremia in the second‐degree burn wound in a rat model.6 Kuroda et al. reported that leukocytic infiltration was deepend in the second‐degree burn wound with hypernatremia in a rat model.7 Moreover, there is a report of a positive correlation between hypernatremia and the number of skin graft attempts in severe burn patients.8 Therefore, the correction of hypernatremia is important in the view of skin graft survival.
It remains unclear whether or not CVVH should be used for the management of refractory hypernatremia. Continuous veno‐venous hemodialysis and filtration facilitates the control of Na levels and corrects dehydration. Concerning the mechanism of the shift of Na from the given fluid therapy in the acute phase, the removal of Na by CVVH would be reasonable to a certain degree. Three cases of severe hypernatremia with burn were reported to have been treated with hypotonic hemodialysis and produced favorable outcomes.9 More recently, one report showed that renal replacement therapy had been carried out in 28.9% of hypernatremia (>150 mEq/L) with burns.10 Huang et al. reported nine cases of hypernatremia with severe burns in which mean blood Na concentrations were corrected and favorable outcomes were obtained following treatment with CVVH.11
Continuous veno‐venous hemodialysis and filtration requires extracorporeal circulation and is associated with the risk of infection and hemorrhage due to the anticoagulant. As hemorrhage associated with repeated debridement and skin grafting is an important problem, CVVH cannot be readily recommended. However, heparin‐free hemodialysis was reported as an effective and safe method in the treatment of hypernatremia in burns.12 Careful management of clotting function could greatly lower the risk of hemorrhage. In addition, infection is one of the greatest problems. The indication of CVVH, which is associated with the risk of bloodstream infection, must also be evaluated carefully. However, in burn patients, hypernatremia itself has been reported to increase the possibility of sepsis and the mortality rate.1 Neutrophil function was reported to be suppressed with increases of Na concentration.13 Therefore, CVVH may not do harm in infection through the management of hypernatremia.
In conclusion, initial fluid therapy for severe burn occasionally leads to severe hypernatremia. Continuous veno‐venous hemodialysis and filtration is an option worth considering in patients with severe burns showing refractory hypernatremia and it may exert favorable effects on skin grafting by correcting hypernatremia.
The content of this article was presented at the 41st Annual Meeting of the Japanese Association for Acute Medicine (Tokyo, 2013).
Conflict of Interest
None.
References
- 1. Ebrahim MK, George A, Bang RL. Only some septicaemic patients develop hypernatremia in the burn intensive care unit: Why? Burns 2002; 28: 543–547. [DOI] [PubMed] [Google Scholar]
- 2. Baxter CR. Fluid volume and electrolyte changes of the early postburn period. Clin. Plast. Surg. 1974; 1: 693–703. [PubMed] [Google Scholar]
- 3. Lin M, Liu SJ, Lim IT. Disorders of water imbalance. Emerg. Med. Clin. North Am. 2005; 23: 749–770, ix. [DOI] [PubMed] [Google Scholar]
- 4. Namdar T, Siemers F, Stollwerck PL, Stang FH, Mailänder P, Lange T. Increased mortality in hypernatremic burned patients. Ger. Med. Sci. 2010; 8: Doc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Archer SB, Henke A, Greenhalgh DG, Warden GD. The use of sheet autografts to cover extensive burns in patients. J. Burn Care Rehabil. 1998; 19 (1 Pt 1): 33–38. [DOI] [PubMed] [Google Scholar]
- 6. Harada T, Izaki S, Tsutsumi H, Kobayashi M, Kitamura K. Apoptosis of hair follicle cells in the second‐degree burn wound under hypernatremic conditions. Burns 1998; 24: 464–469. [DOI] [PubMed] [Google Scholar]
- 7. Kuroda T, Harada T, Tsutsumi H, Kobayashi M. Hypernatremia deepens the demarcating borderline of leukocytic infiltration in the burn wound. Burns 1997; 23: 432–437. [DOI] [PubMed] [Google Scholar]
- 8. Namdar T, Stollwerck PL, Stang FH et al Impact of hypernatremia on burn wound healing: Results of an exploratory, retrospective study. Ostomy Wound Manage. 2011; 57: 30–34. [PubMed] [Google Scholar]
- 9. Pazmiño PA, Pazmiño BP. Treatment of acute hypernatremia with hemodialysis. Am. J. Nephrol. 1993; 13: 260–265. [DOI] [PubMed] [Google Scholar]
- 10. Stewart IJ, Morrow BD, Tilley MA et al Dysnatremias and survival in adult burn patients: A retrospective analysis. Am. J. Nephrol. 2013; 37: 59–64. [DOI] [PubMed] [Google Scholar]
- 11. Huang C, Zhang P, Du R et al Treatment of acute hypernatremia in severely burned patients using continuous veno‐venous hemofiltration with gradient sodium replacement fluid: A report of nine cases. Intensive Care Med. 2013; 39: 1495–1496. [DOI] [PubMed] [Google Scholar]
- 12. Chai J, Diao L, Sheng Z, Guo Z, Gao W, Jia X. Heparin‐free hemodialysis in the treatment of hypernatremia in severely burned patients. Burns 2000; 26: 634–637. [DOI] [PubMed] [Google Scholar]
- 13. Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet 2004; 363: 1895–1902. [DOI] [PubMed] [Google Scholar]


