Abstract
Case
A 74‐year‐old woman underwent computed tomography‐guided transthoracic needle biopsy of a small lung mass. Immediately after the procedure, she lost consciousness. After resuscitation, her brain computed tomography scan confirmed a cerebral air embolism.
Outcome
As hyperbaric oxygenation was unavailable, she received controlled normothermia for neuroprotection. No cerebral symptoms were observed following treatment.
Conclusion
Air embolisms are rare, but fatal, complications of computed tomography‐guided transthoracic needle biopsy. Therefore, clinicians should be familiar with early diagnosis and prompt treatment. Preventing hyperthermia might be effective for treating hypoxic brain injury caused by cerebral air embolisms.
Keywords: Air embolism, complication, hypoxic brain damage, ischemia, targeted temperature management
Introduction
Computed tomography (CT)‐guided transthoracic needle biopsy is commonly used to diagnose pulmonary pathological conditions. Although this technique is generally safe, associated complications exist. Air embolisms are rare complications of CT‐guided transthoracic needle biopsy. Currently, there are no definite guidelines on managing accidental cerebral air embolisms (CAEs).
We report the case of a patient with CAE after CT‐guided transthoracic lung biopsy successfully treated with normothermia, resulting in favorable neurological outcomes.
Case
A 74‐year‐old female was admitted with a breast cancer history. She underwent repeat CT scans due to a solitary pulmonary nodule, suspicious for lung metastasis. Computed tomography‐guided transthoracic needle biopsy was requested to establish a histopathological diagnosis.
The procedure was carried out by an experienced radiologist using an 18‐gauge outer coaxial needle of a disposable core biopsy instrument under CT guidance. She was placed in the left lateral decubitus position and was cooperative, refraining from coughing or deep breathing. However, she coughed immediately after core needle removal. She then lost consciousness and developed shock with decreased oxygen saturation. Her electrocardiogram and echocardiogram were normal.
Although she became interactive after cardiopulmonary stabilization with 100% oxygen and a large hydration quantity, left‐side paralysis developed. Chest and head CT scans were immediately obtained. The former (Fig. 1A, B) showed regional pulmonary hemorrhage and presence of air in the right and left atria. The latter (Fig. 1C, D) with 5.0‐mm slice thickness viewed at 70‐HU window width and 33‐HU level showed air inflow in the right middle cerebral artery territory.
Figure 1.
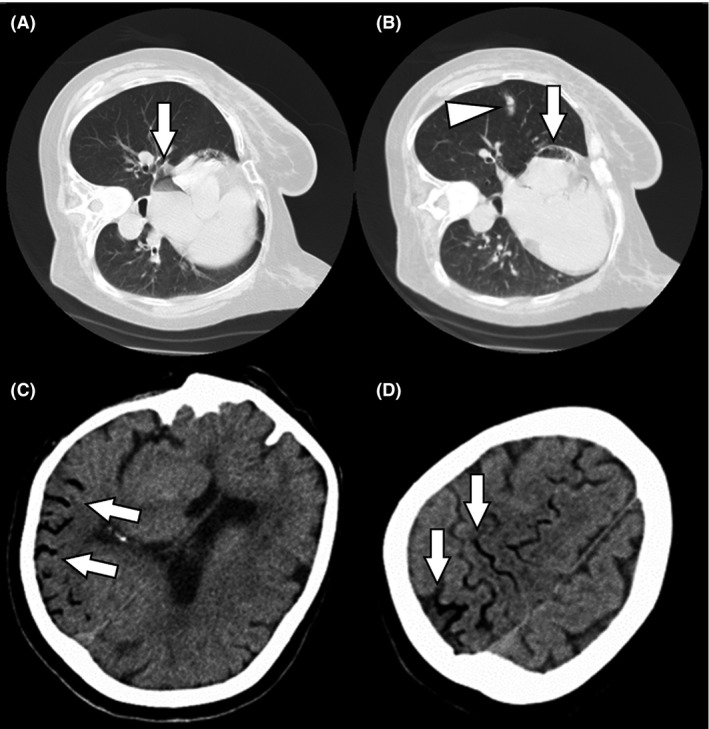
Computed tomography (CT) findings obtained at clinical onset of cerebral air embolism in a 74‐year‐old woman who underwent computed tomography‐guided transthoracic needle biopsy of a small lung mass. A, B, Chest CT showing small pulmonary hemorrhage (arrowhead) and air in the right and left atria (arrows). C, D, Brain CT showing air inflow in the right middle cerebral artery (arrows).
Hyperbaric oxygen (HBO) therapy was unavailable at our hospital, and non‐pressurized air ambulance flight over mountainous terrain was needed for transport to a nearby HBO facility. Because such transport may cause air expansion, she was transferred to our intensive care unit for further treatment and evaluation. Repeat CT and brain magnetic resonance imaging (MRI), including magnetic resonance (MR) angiography, were performed approximately 2 h after symptom onset. Brain CT demonstrated complete intracranial air resolution. Despite normal diffusion‐weighted (Fig. 2A) and T2‐weighted (Fig. 2B) imaging, MR angiography showed occlusion and segmental stenosis in the right middle cerebral artery (Fig. 2C). This helped establish the diagnosis of acute ischemic brain disease secondary to a cerebral arterial gas embolism. Immediately after MRI, she presented with generalized seizures developing from the left hand, not responding to i.v. diazepam and phenytoin. Her body temperature increased to 38.6 °C. Although fever can be caused by pulmonary aspiration due to being comatose, her temperature increased in a few hours. Thus, fever was likely due to acute ischemia. After tracheal intubation was carried out, she received controlled normothermia with a target bladder temperature of 36.0 °C for 24 h under sufficient sedation with propofol. Ventilator settings were adjusted according to the blood gas values to avoid hypocapnia, which may impair cerebral perfusion. Normothermia was immediately induced using a surface cooling system (Arctic Sun 2000; Medivance Inc., Louiseville, CO, USA).
Figure 2.
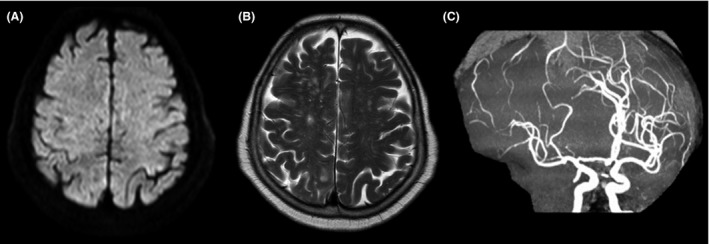
Magnetic resonance images of a 74‐year‐old woman who underwent computed tomography‐guided transthoracic needle biopsy of a small lung mass and developed cerebral air embolism. Images obtained 2 h after symptom onset. A, Diffusion‐weighted imaging showing no acute ischemic changes. B, T2‐weighted imaging showing no abnormality. C, Magnetic resonance angiography showing occlusion and segmental stenosis of the right middle cerebral artery.
Repeat post‐treatment brain MRI showed cortically localized areas of restricted diffusion along the gyri (Fig. 3A). T2‐weighted imaging (Fig. 3B) revealed diffuse high‐signal intensity mainly in the subcortical layer and deep white matter, indicating vasogenic edema in the brain swelling. The MR angiography (Fig. 3C) showed normal cerebral blood vessels. She increasingly regained consciousness without seizures and was transferred to a standard medical ward on the 6th day. Light left‐side paralysis completely improved with rehabilitation. She was discharged on the 183rd day with no CAE‐related symptoms.
Figure 3.
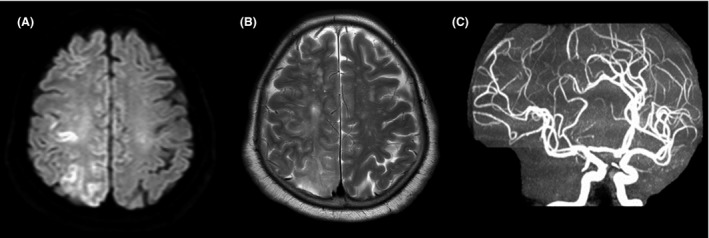
Magnetic resonance images obtained 1 day after symptom onset 74‐year‐old woman who underwent computed tomography‐guided transthoracic needle biopsy of a small lung mass and developed cerebral air embolism. A, Diffusion‐weighted imaging showing cortical areas with restricted diffusion. B, T2‐weighted imaging revealing diffuse high‐signal intensity. C, Magnetic resonance angiography showing complete occlusion and stenosis resolution.
Discussion
Detecting small peripheral pulmonary lesions has become routine since multi‐detector row CT was introduced. Computed tomography‐guided transthoracic needle biopsy may be the first diagnostic step in such cases, particularly for identifying very small, peripheral, and easily accessible lesions. However, it has risks. Systemic air embolisms have an incidence of 0.061%.1
The air embolism in our patient could derive from bronchial veins through a bronchovenous fistula created by the needle passing through the lung parenchyma. Furthermore, air can enter arterial circulation through the left atrium and ventricle through an intracardiac shunt or by directly crossing the pulmonary capillary bed, resulting in CAE.
Computed tomography is the imaging method of choice because it can be carried out quickly and provides the best way for visualizing cerebrovascular air. However, absence of intracerebral air on brain imaging does not exclude the diagnosis. A review of studies using CT, MRI, and single‐photon emission CT conclude that presently, no imaging technique has sufficient accuracy when used in isolation to warrant its use for diagnostic purposes; therefore, CAEs should be considered during lung needle biopsy.2
Arterial air emboli cause blood flow obstruction and inflammatory responses, resulting in endothelial damage, vasospasms, and vasogenic edema, which can induce seizures, secondary ischemia, or reperfusion injury.3, 4 Preventing ischemic/reperfusion injury is important in avoiding long‐term persistent sequelae of CAE. Acute ischemia triggers prostaglandin production, altering the thermoregulatory set point in the anterior hypothalamus and resulting in fever.5 A meta‐analysis on the relationship between hyperthermia and stroke mortality in acute stroke patients indicated a twofold increase in short‐term mortality in hyperthermia patients within the first 24 h of hospitalization.6 Targeted temperature management for 12–24 h is typically adopted to prevent hypoxic brain injury after cardiac arrest.7, 8 However, whether the reported treatment effect was due to hypothermia or the prevention of hyperthermia remains unclear. Numerous unknowns regarding indications, cooling method, optimal temperature, and cooling duration for targeted temperature management remain. A recent randomized trial was unable to detect any difference in mortality or adverse neurologic outcomes in patients treated at 33.0 °C compared with those treated at 36.0 °C.9 Therefore, controlled normothermia can be an efficient method for protecting against brain damage during ischemia/reperfusion injury caused by CAE.
Conclusion
Clinicians should be aware of symptomatic air embolisms and ready to provide emergent management for preventing long‐term brain injury. Although HBO therapy is standard for CAE, controlled normothermia application is effective for preventing secondary brain damage caused by CAE when HBO is unavailable. Further studies are required to definitively describe the effectiveness of controlled normothermia in managing hypoxic brain injury caused by CAE.
Conflict of Interest
None.
References
- 1. Tomiyama N, Yasuhara Y, Nakajima Y et al CT‐guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur. J. Radiol. 2006; 59: 60–4. [DOI] [PubMed] [Google Scholar]
- 2. Van Hulst RA, Klein J, Lachmann B. Gas embolism: pathophysiology and treatment. Clin. Physiol. Funct. Imaging 2003; 23: 237–46. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell S, Gorman D. The pathophysiology of cerebral arterial gas embolism. J. Extra Corpor. Technol. 2002; 34: 18–23. [PubMed] [Google Scholar]
- 4. Muth CM, Shank ES. Gas embolism. N. Engl. J. Med. 2000; 342: 476–82. [DOI] [PubMed] [Google Scholar]
- 5. Wrotek SE, Kozak WE, Hess DC, Fagan SC. Treatment of fever after stroke: conflicting evidence etiology of fever after stroke. Pharmacotherapy 2011; 31: 1085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prasad K, Krishnan PR. Fever is associated with doubling of odds of short‐term mortality in ischemic stroke: an updated meta‐analysis. Acta Neurol. Scand. 2010; 122: 404–8. [DOI] [PubMed] [Google Scholar]
- 7. Bernard SA, Gray TW, Buist MD et al Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002; 346: 557–63. [DOI] [PubMed] [Google Scholar]
- 8. Hypothermia after Cardiac Arrest Study Group . Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002; 346: 549–56. [DOI] [PubMed] [Google Scholar]
- 9. Nielsen N, Wetterslev J, Cronberg T et al Targeted temperature management at 33°C versus 36°C after cardiac arrest. N. Engl. J. Med. 2013; 369: 2197–206. [DOI] [PubMed] [Google Scholar]


