Abstract
Aim
It is well known that continuous renal replacement therapy (CRRT) produces some amount of nitrogen loss, but there are few tools that are easily applied to measure it. This study aimed to evaluate nitrogen loss using blood urea nitrogen (BUN) measurement in patients receiving CRRT.
Methods
The subjects were 28 patients who received CRRT (except for liver failure) between 2010 and 2012. Nutrition data and nitrogen excretion in dialysate and urine were measured.
Results
The median age of the patients was 61 years, with an Acute Physiology and Chronic Health Evaluation score of 27 points and a Sequential Organ Failure Assessment score of 12 points. All‐cause hospital mortality was 50%. Median protein intake was 40 g/day. The daily urinary volume was 245 mL and volume of dialysate was 26,000 mL/day. The median amount of nitrogen loss was 10.58 g/day, with BUN showing a strong correlation (r = 0.804, P < 0.0001). There was a poor relation between protein intake (g/kg body weight) and nitrogen balance (r = 0.322, P = 0.002).
Conclusions
In patients receiving CRRT, the nitrogen loss showed a positive correlation with BUN but not with protein intake. According to the guidelines, recommended protein intake was 1.5–2.0 g/kg/day, but we should be careful to avoid elevating BUN at the same time. The results showed that BUN might be a useful marker to check nitrogen balance in the nutritional management of patients receiving CRRT.
Keywords: Continuous renal replacement therapy, nitrogen balance, nitrogen loss
Background
Appropriate nutritional management using multiple protocols, with positive nitrogen balance, in patients requiring intensive care has been reported to influence patient outcomes.1, 2
Protein catabolism is enhanced in critically ill patients, especially in patients with acute kidney injury (AKI),3 which is induced by the activation of the neuroendocrine system, free radicals, cytokines, and the prostaglandin system.4 Moreover, patients with AKI requiring renal replacement therapy lose nitrogen into dialysate and require greater protein intake compared with patients not receiving renal replacement therapy,5 with recommended energy intakes of 30–35 kcal/kg per day and protein intakes of 1.5–2.0 g/kg per day.6 Although previous studies have reported improved nitrogen balance with the administration of up to 2.5 g/kg per day amino acids, the efficacy of this supplementation for improving outcomes in critically ill patients with AKI remains unclear.7, 8 In addition, the dynamics of nitrogen loss through a dialysis membrane also remains to be elucidated.
Although the maintenance of appropriate nitrogen balance is considered to be important in patients requiring continuous renal replacement therapy (CRRT), an effective evaluation method easily applied with daily clinical activities has yet to be established. Nitrogen may be excreted into dialysate in a blood urea nitrogen (BUN)‐concentration dependent manner in patients receiving CRRT under fixed specific treatment conditions; therefore, BUN levels could be used as a marker of nitrogen excretion. Blood urea nitrogen can be measured in daily medical practice, and it could be considered a mandatory test in patients with AKI receiving intensive care.
This study aimed to examine whether protein intake could enhance nitrogen balance, and nitrogen loss could be clinically evaluated using BUN in patients receiving CRRT under uniform treatment conditions and whether this value could inform the appropriate administration of nitrogen in patients receiving CRRT.
Methods
The subjects were 28 adult patients (≥20 years old) who received CRRT between January 1, 2010 and December 31, 2012 in the Tohoku University Hospital Emergency Center (Sendai, Japan). This was a retrospective observational study of data collected as part of daily routine work and from existing documents, without any medical intervention. Data management and statistical analyses were processed anonymously. Based on these reasons, the subjects did not provide informed consent. Ethical approval was obtained from the Tohoku University Hospital ethical committee (2014‐1‐399).
The introduction of CRRT was based on the following four factors: (i) presence of pulmonary edema and cardiac failure; (ii) acidemia (arterial blood pH < 7.1, base excess <−10 mEq/L); (iii) hyperkalemia (K > 6.0 mmol/L); and (iv) anuria (<100 mL/day) or oliguria (<400 mL/day) lasting ≥ 48 h.
Subjects who suffered from liver failure were excluded because they would have different BUN production from normal subjects.
The Acute Physiology and Chronic Health Evaluation (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores were calculated on admission to evaluate the severity of each patient's condition.
Nitrogen loss was measured once a week for a total of 88 measurements in 28 subjects. The total amount of nitrogen excreted per day was calculated from the sum of the amounts of nitrogen in the urine and dialysate. Urine was pooled for 24 h for the calculation of urinary excretion, which was calculated from urinary excretion levels and the volume of urine pooled for 24 h. Nitrogen loss in the dialysate was measured according to Scheinkestel's method; 20 mL dialysate was collected every 8 h, and the nitrogen level in the 24‐h dialysate was measured and calculated using a mixed sample of 60 mL in total based on the total volume of dialysate.9 Urea nitrogen was measured using a urease–glutamate dehydrogenase, isocitrate dehydrogenase, UV method with LABOSPECT 008 (Hitachi High‐Technologies, Tokyo, Japan) and reagent UN‐SL (Serotec, Hokkaido, Japan). At the same time, total protein intake was measured.
Statistical analysis
The data are presented as median values (interquartile range), and Pearson's correlation coefficient was used to analyze the correlations between variables. Statistical analysis was carried out using JMP Pro version 11 (SAS Institute Japan Ltd., Tokyo, Japan), and P < 0.05 was considered statistically significant.
Results
All subjects were introduced to CRRT in at least one criterion of the four items listed above. A polymethyl‐methacrylate membrane hemofilter was used for CRRT with the following basic settings: pump flow rate, 80 mL/h; replacement fluid flow rate, 200 mL/h; and dialysate flow rate, 800 mL/h.
Table 1 presents the baseline characteristics of the patients. Continuous renal replacement therapy was used for renal support in all patients. The underlying diseases were acute renal failure related with dehydration, not with ureteral obstruction (n = 8), severe sepsis (n = 8), acute pancreatitis (n = 4), and malignant syndrome (n = 3); the APACHE II score was 27 points, and the SOFA score was 12 points. The period of continuous dialysis was 15.5 (8.75–33.0) days, and all‐cause hospital mortality was 50%.
Table 1.
Characteristics of patients receiving continuous renal replacement therapy (CRRT) (n = 28)
| Age, years | 61 (48.5 to 73.8) |
| Male, n (%) | 25 (89%) |
| Body weight, kg | 64.3 (56.8 to 73.0) |
| Body mass index | 24.4 (21.4 to 27.9) |
| APACHE II score | 27 (23 to 34.2) |
| SOFA score | 12 (9.8 to 13) |
| Underlying disease | |
| Acute renal failure | 8 |
| Severe sepsis | 8 |
| Severe acute pancreatitis | 4 |
| Malignant syndrome | 3 |
| Severe burn | 3 |
| Others | 2 |
| Hospital mortality, n (%) | 14 (50%) |
| Initial serum Cr, mg/dL | 3.0 (2.7 to 4.2) |
| Initial BUN, mg/dL | 44.0 (29.5 to 59.0) |
| Duration of CRRT, days | 15.5 (8.75 to 33.0) |
| Urinary volume, mL/day | 245 (66.5 to 659) |
| Dialysate volume, mL/day) | 26,000 (23,255 to 26,000) |
| Nitrogen loss in urine, g/day | 0.59 (0.05 to 1.48) |
| Nitrogen loss in dialysate, g/day | 9.1 (5.5 to 14.8) |
| Total protein intake, g/day | 40.0 (10.0 to 60.0) |
| EN protein intake, g/day | 0 (0 to 35) |
| PN protein intake, g/day | 12 (0 to 40) |
| Nitrogen balance, g/day | −8.5 (−4.0 to −14.7) |
Data are expressed as median (interquartile range) or n (%). APACHE, Acute Physiology and Chronic Health Evaluation; BUN, blood urea nitrogen; Cr, creatinine; EN, enteral nutrition; PN, Parenteral nutrition; SOFA, Sequential Organ Failure Assessment.
The daily urinary volume was 245 mL (range, 66–659 mL), and the volume of dialysate was 26,000 mL/day. The median amount of nitrogen excretion was 0.58 g/day in the urine and 9.1 g/day in the dialysate, showing a strong correlation between BUN and the amount of nitrogen excreted (r = 0.804, P < 0.0001; y = 0.275 × BUN − 0.31; Fig. 1), and there was poor relation between protein intake (g/kg body weight [BW]) and nitrogen balance (r = 0.322, P = 0.002; Fig. 2).
Figure 1.
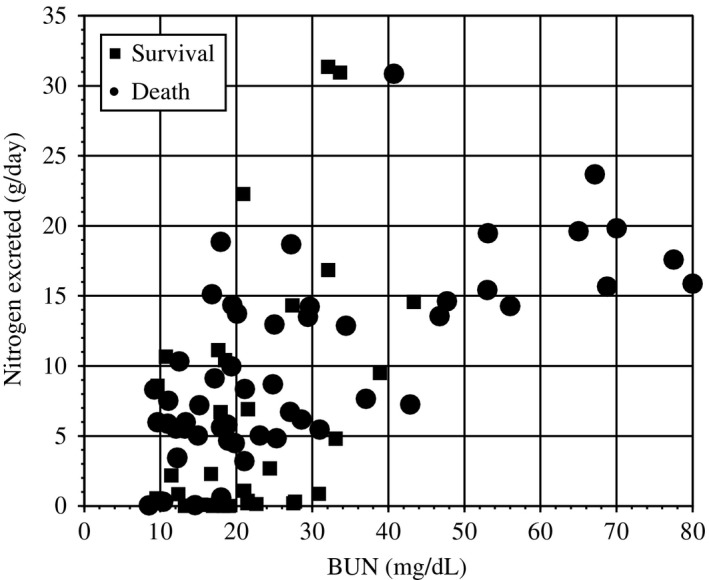
Correlation between nitrogen excreted and blood urea nitrogen (BUN) in patients receiving continuous renal replacement therapy. There was a strong relationship between nitrogen loss and BUN. Pearson's correlation, r = 0.794 (P < 0.001). An approximate line was plotted: nitrogen loss (g/day) = 0.275 × BUN (mg/dL)−0.31.
Figure 2.
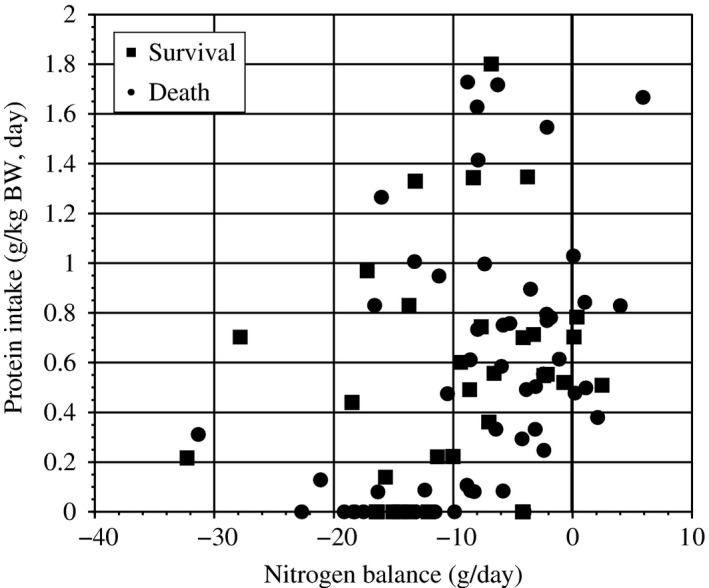
Protein intake and nitrogen balance in patients receiving continuous renal replacement therapy. There was poor relation between protein intake (g/kg body weight [BW]) and nitrogen balance (g/day).
Total protein intake was 40.0 g/day, 19.5 g/day from enteral nutrition and 20.5 g/day from parenteral nutrition, respectively. Calculated nitrogen balance was −8.5 (−4.0 to −14.7) g/day.
Discussion
The results of the present study showed a strong correlation between BUN and the amount of nitrogen loss in patients requiring CRRT. There was a poor relation between protein intake and positive nitrogen balance, indicating that nitrogen balance mainly results from mechanical excretion through a dialysis membrane that is primarily dependent on BUN.
Furthermore, there was loss of amino acids across the hemofilter, and the artificial kidney could not carry out active reabsorption. Thus, amino acid losses during hemodialysis were expected between 3% and 16%.10
Blood urea nitrogen was the final metabolite of protein and was elevated as a result of muscle catabolism and protein load with nutrition. The amount of anabolized protein was difficult to estimate to maintain appropriate nitrogen balance, protein intake, and BUN at a normal level.
The mortality rate was 50% in patients requiring CRRT, similar to that in other critically ill patients in Japan,11 and that patient information about nitrogen balance was rare before this study. According to this study, it may be possible to improve outcomes with nutritional therapy to correct nitrogen balance. To better achieve nitrogen balance, we should consider BUN as well as protein intake. The calculated ideal protein intake to ensure nitrogen balance was estimated to be 94.5 (70.8–156.6) g/day : 1.35 g/BW.
Patients receiving intensive care should be administered adequate protein while suppressing nitrogen loss to a minimum level. As nitrogen loss primarily occurs through a dialysis membrane in patients requiring CRRT, it can be estimated from the measurement of urea nitrogen in the dialysate, or BUN. Therefore, in these patients, an effort should be made to avoid an elevation in BUN as much as possible, while maintaining sufficient protein intake. Determination of appropriate nutrition for critically ill patients is still in progress. The BUN level is strongly influenced by disease condition. In some cases, a high BUN level and loss of protein and amino acid from dialysate might be unavoidable. If so, we should endeavor to withdraw CRRT as soon as possible.
Both BUN and urea nitrogen measurements are useful for nutritional management as a standard tool of nutritional assessment that can be easily carried out in the intensive care unit. Patients require not only appropriate protein administration but also detailed observation, with particular attention to BUN.
Although the present study does not provide information about the determination of an ideal protein dose from the BUN value, BUN provides important information for the appropriate management of nitrogen balance in patients with AKI requiring CRRT. To improve future therapy, nitrogen loss should be understood so that a method to measure nitrogen loss can be standardized. Further studies to examine the efficacy of maintaining positive nitrogen balance in patients receiving CRRT are warranted.
There are some limitations to our study. First, this is a retrospective observational study of data collected as part of daily routine work and from existing documents with a small sample size. Second, nitrogen loss in the dialysate was calculated in patients receiving CRRT within an almost uniform setting in this study. Therefore, the result may be varied under different or variable CRRT setting. Third, protein loss from stool was not calculated. The popularly used nitrogen balance equation does not consider the loss from diarrhea,12 but it might not be negligible, particularly in critically ill patients. Finally, amino acid loss through dialysate was uncountable in this study. The regular method of calculating nitrogen balance does not consider amino acid loss, but it might not be negligible in CRRT conditions.
Conclusion
According to this study, nitrogen loss shows a strong correlation with BUN in patients receiving CRRT under specific treatment conditions. There was poor relation between protein intake and nitrogen balance. Blood urea nitrogen may be used as a marker to determine the necessary amount of protein in nutritional management of patients receiving CRRT. Further research is required to determine the optimal methods of nitrogen administration.
Conflict of interest
None.
[The copyright line for this article was changed on 28 October 2016 after original online publication]
References
- 1. Cano NJ, Aparicio M, Brunori G et al ESPEN Guidelines on Parenteral Nutrition: adult renal failure. Clin. Nutr. 2009; 28: 401–14. [DOI] [PubMed] [Google Scholar]
- 2. Bellomo R. How to feed patients with renal dysfunction. Blood Purif. 2002; 20: 296–303. [DOI] [PubMed] [Google Scholar]
- 3. Schaefer RM, Shaefer L, Horl WH. Mechanisms for protein catabolism in acute renal failure. Nephrol. Dial. Transplant. 1994; 9: 44–7. [PubMed] [Google Scholar]
- 4. Wilmore DW. Catabolic illness: strategies for enhancing recovery. N. Engl. J. Med. 1991; 325: 695–702. [DOI] [PubMed] [Google Scholar]
- 5. Martinez JL, Sanchez‐Izquierdo RJA, Jimenez Jimenez FJ. Guidelines for specialized nutritional and metabolic support in the critically‐ill patient. Update. Consensus SEMICYUC‐SENPE: acute renal failure. Nutr. Hosp. 2011; 26: 21–6. [DOI] [PubMed] [Google Scholar]
- 6. McClave SA, Martindale RG, Vanek VW et al Guidelines for the provision and assessment of nutrition support therapy in adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enteral Nutr. 2009; 33: 277–316. [DOI] [PubMed] [Google Scholar]
- 7. Btaiche IF, Mohammad RA, Alaniz C et al Amino Acid requirements in critically ill patients with the acute kidney injury treated with continuous renal replacement therapy. Pharmacotherapy 2008; 28: 600–13. [DOI] [PubMed] [Google Scholar]
- 8. Bellomo R, Seacombe J, Daskalakis M et al A prospective comparative study of moderate versus high protein intake for critically ill patients with acute renal failure. Ren. Fail. 1997; 19: 111–20. [DOI] [PubMed] [Google Scholar]
- 9. Scheinkestel CD, Kar L, Marshall K et al Prospective randomized trial to assess caloric and protein needs of critically ill, anuric, ventilated patients requiring continuous renal replacement therapy. Nutrition 2003; 19: 909–16. [DOI] [PubMed] [Google Scholar]
- 10. Scheinketel CD, Adams F, Mahony L et al Impact of increasing parenteral protein loads on amino acid levels and balance in critically ill anuric patients on continuous renal replacement therapy. Nutrition 2003; 19: 733–40. [DOI] [PubMed] [Google Scholar]
- 11. Nagata I, Uchino S, Tokihira N et al Sepsis may not be a risk factor for mortality in patients with acute kidney injury treated with continuous renal replacement therapy. J. Crit. Care 2015; 30: 998–1002. [DOI] [PubMed] [Google Scholar]
- 12. Daley BJ, Bistrian BR. Nutritional assessment In: Zagola GP. (ed.). Nutrition in Critical Care. St. Louis: Mosby, 1994; 9–35. [Google Scholar]


