Abstract
The small G protein Ras is a central regulator of cellular signal transduction processes, functioning as a molecular switch. Switch mechanisms utilizing conformational changes in nucleotide-binding motifs have been well studied at the molecular level. Azobenzene is a photochromic molecule that undergoes rapid and reversible isomerization between the cis and trans forms upon exposure to ultraviolet and visible light irradiation, respectively. Here, we introduced the sulfhydryl-reactive azobenzene derivative 4-phenylazophenyl maleimide (PAM) into the nucleotide-binding motif of Ras to regulate the GTPase activity by photoirradiation. We prepared four Ras mutants (G12C, Y32C, I36C, and Y64C) that have a single reactive cysteine residue in the nucleotide-binding motif. PAM was stoichiometrically incorporated into the cysteine residue of the mutants. The PAM-modified mutants exhibited reversible alterations in GTPase activity, nucleotide exchange rate, and interaction between guanine nucleotide exchange factor and Ras, accompanied by photoisomerization upon exposure to ultraviolet and visible light irradiation. The results suggest that incorporation of photochromic molecules into its nucleotide-binding motif enables photoreversible control of the function of the small G protein Ras.
Abbreviations: DMF, N,N-dimethylformamide; DTT, dithiothreitol; GTP, guanosine 5ʹ-triphosphate; NBD-GTP, 2ʹ(3ʹ)-O-{6-(N-(7-nitrobenz-2-oxa-l,3-diazol-4-yl)amino)hexanoic}–GTP; GEF, guanine nucleotide exchange factor; HEPES, 4-(2-hydroxyethyl)-1-piperazineethane-sulfonic acid; PAM, 4-phenylazomaleinanil; UV, ultraviolet; Vis, visible
Keywords: Photocontrol, GTPase, Small G protein, Ras, Photochromic molecule, Azobenzene
Highlights
-
•
Photochromic molecule was incorporated into functional site of small GTPase Ras.
-
•
GTPase of Ras modified with photochromic molecule was altered photoreversibly.
-
•
Interaction of Ras with GEF was regulated with photochromic molecules photoreversibly.
-
•
The function of Ras was shown to be controlled using photochromic molecule.
1. Introduction
The small guanine nucleotide binding protein Ras, which is known as a molecular switch, is a central regulator of cellular signal transduction processes leading to transcription, cell cycle progression, growth, migration, cytoskeletal changes, apoptosis, cell survival, and senescence [1], [2]. Its switch mechanism involves its activation by the exchange of bound GDP with GTP and its inactivation by hydrolysis of GTP into GDP.
Interestingly, recent studies have shown that the ATP-driven motors, myosin and kinesin, and G proteins, which have a common nucleotide-binding motif, evolved from a common nucleotide-binding ancestral protein. It is believed that these biomolecular machines share a common molecular mechanism utilizing a nucleotide hydrolysis cycle [3]. Recently, structural and mechanistic studies have suggested that some biomolecular machines, for example, the motor protein myosin, have driving mechanisms that work similar to a camshaft [4].
It is expected that biomolecular machines that have incorporated an external stimulation-responsive molecular device into their driving mechanism might be regulated by extrinsic stimuli. Photochromic molecules are regarded as the striking external stimulation-responsive molecular devices. The structure and biophysical properties of a photochromic molecule reversibly change upon irradiation by light with two different wavelengths. Azobenzene is one of the most well-known photochromic molecules, which reversibly isomerizes between cis and trans forms upon exposure to UV and VIS light irradiation, respectively. Azobenzene has been applied to biological systems as a photoswitch [5], [6], [7]. We previously demonstrated the possible application of an azobenzene derivative in regulating a conformational change in skeletal muscle myosin. The azobenzene photochromic crosslinker, 4,4′-azobenzene-dimaleimide, was incorporated into the SH1 (C707)–SH2 (C697) region of skeletal muscle myosin subfragment-1 (S1), which plays a key role in the conformational changes of the myosin head during force generation coupled to ATP hydrolysis [8]. We also previously demonstrated the possible application of an azobenzene derivative in regulating the ATPase activities of the kinesin by photoirradiation. The azobenzene-derived photochromic molecule 4-phenylazophenyl maleimide (PAM) was incorporated into the L11 or L12 loop region of the microtubule-binding site of conventional kinesin [9] or L5 in the key region of ATPase activity of mitotic kinesin Eg5 [10]. Therefore, it is expected that the function of the small G protein Ras modified with photochromic molecules would be photocontrollable by photoirradiation.
In this paper, we demonstrated the possible application of an azobenzene derivative in regulating the GTPase activity of Ras and its interaction with guanine nucleotide exchange factor (GEF) by photoirradiation. The azobenzene photochromic molecule 4-phenylazophenyl maleimide (PAM) was incorporated into the nucleotide-binding motif. It is expected that Ras signaling would be photocontrollable by the introduction of a photochromic molecule into GTPase cycle regulators or effectors. It is also suggested that introduction of a photochromic molecule into Ras might enable the reversible photocontrol of signal transduction processes.
2. Materials and methods
Ligation enzymes were purchased from Toyobo (Osaka, Japan), unless stated otherwise. Oligonucleotides were obtained from Eurofins Genomics (Tokyo, Japan). The apparatus for affinity chromatography on Co2+-NTA agarose was procured from Clontech (Mountain View, CA, USA). Chemicals reagents were purchased from Wako Pure Chemicals (Osaka, Japan) unless stated otherwise. 4-Phenylazomaleinanil (PAM) was procured from Sigma-Aldrich, St. Louis, MO, USA).
2.1. Photoisomerization of PAM
The structure of the free PAM in N,N-dimethylformamide (DMF) was changed by irradiation with UV or Vis light. UV light irradiation (Black-ray lamp, 366 nm, 16 W, UVP Inc., San Gabriel, CA, USA) was used to convert PAM in trans form to cis form, while Vis light irradiation (Fluorescent lamp 27 W, FML27EX-N, Mitsubishi Inc., JP) was used to convert cis form to trans form. The isomerizations of PAM incorporated into Ras were performed at 0 °C at a distance of 5 cm above the surface of the solution (15 µM Ras modified with PAM, 120 mM NaCl, 30 mM Tris–HCl at pH 7.5, 2 mM MgCl2). Under these UV irradiation conditions, significant damage to Ras was not observed and the irradiated Ras retained most of its GTPase activity.
2.2. Expression and purification of Ras
The cDNA of Mouse K-Ras (residues 1–176), which eliminates 13 amino acids of the C terminal plasmid kindly provided by Dr. Ando (SOKA Univ.), was amplified by polymerase chain reaction and ligated into the pET15b vector. Ras plasmid has three cysteine residues at position of 51, 80, and 118. We first constructed Cys-light Ras plasmids by mutating cysteine 118 to serine. We generated four mutants (G12C, Y32C, I36C, and Y64C) having single reactive cysteine residues based on the Cys-light Ras plasmid, for further modification with PAM. Mouse K-Ras expression plasmids were used to transform Escherichia coli BL21 (DE3). Mouse K-Ras was purified by using a Co2+-NTA column. The Co2+-NTA column was washed with lysis buffer containing 10 mM Imidazole; bound Mouse K-Ras was eluted with lysis buffer containing 100 mM Imidazole. The obtained fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Purified Mouse K-Ras was dialyzed with buffer (30 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1 mM MgCl2, and 0.5 mM DTT) and stored at −80 °C until further use.
2.3. Expression and purification of SOS-505
cDNA for the catalytic domain, CDC25 domain, and a part of the Rem domain, of mouse SOS1 (SOS-505, residues 584–1088) [11] was amplified by polymerase chain reaction and ligated into the pColdI vector. SOS-505 expression plasmids were used to transform E. coli BL21 (DE3). SOS-505 was purified by using a Co-NTA column and stored at −80 °C until further use.
2.4. Pull-down assay of Ras with effectors: RalGDS and c-Raf
Solutions of 15 =M K-Ras and 1.4 μM GST-GFP-RalGDS (RBD) or c-Raf (RBD) in 100 μl assay buffer (25 mM HEPES-NaOH, pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.05% TritonX-100, 1 mg/ml BSA) were mixed with 30 μL glutathione-Sepharose 4B beads (GE Healthcare) suspended in assay buffer. The mixture was incubated at 30 °C for 5 min with 100 μM GTP, GDP, NBD-GTP, or NBD-GDP for the nucleotide exchange reaction. The reaction was terminated by adding 10 mM MgCl2. Then, the mixture was incubated at 4 °C for 1.5 h under continuous stirring. After incubation, the resin was washed with 400 μl wash buffer (25 mM HEPES-NaOH, pH 7.4, 150 mM NaCl, 2 mM MgCl2, 0.05% TritonX-100, 1 mg/ml BSA) three times. The bound proteins were eluted from the resin by boiling in SDS sample buffer (6.3 mM Tris–HCl, 10% Glycerol, 5% β-mercaptoethanol, 0.25 mg/mL Bromophenol Blue) and were analyzed by western blot using anti-His antibodies and anti-GST antibodies (Santa Cruz Biotechnology). The bound antibodies were detected using chemiluminescence (ATTO, EzWestLumi plus).
2.5. GTPase activity of Ras
GTPase activity of 50 µM Ras was measured at 37 °C in assay buffer (45 mM Tris–HCl pH 7.5, 180 mM NaCl, 2 mM MgCl2). The GTPase reaction was initiated by adding 1 mM GTP and terminated by adding 10% trichloroacetic acid. The released inorganic phosphate (Pi) in the supernatant was measured using the method described by Youngburg and Youngburg [12].
2.6. Modification of the cysteine residue in the core nucleotide-binding motif by using PAM
The concentration of the photochromic molecule was varied to suit the type of cysteine. Ras WT was incubated with 15-folds as much as PAM dissolved in DMF in modification buffer (45 mM Tris–HCl pH 7.5, 180 mM NaCl, 2 mM MgCl2, and 3% DMF), whereas G12C, Y32C, I36C, and Y64C were incubated with 25-folds as much as PAM in the modification buffer, all for 1 h at 25 °C. The reaction was terminated by the addition of DTT to a final concentration of 10 mM. The modified Ras was isolated from the unreacted excess PAM by using a 10DG column (Bio-Rad, Hercules, CA, USA) equilibrated with modification buffer. The stoichiometry of the incorporated PAM against that of Ras was determined based on the absorption spectra obtained using an extinction coefficient of 10,620 M−1 cm−1 at 350 nm for the PAM in 45 mM Tris–HCl pH 7.5, 180 mM NaCl, 2 mM MgCl2, and 3% DMF.
2.7. Measurement of GTPase activity of Ras following modification with PAM and photoirradiation
The isomerization of PAM-Ras modified with PAM as stated above “Modification of the cysteine residue in the core nucleotide-binding motif by using PAM” was performed at 0 °C in modification buffer using UV light irradiation (Black-ray lamp, 366 nm, 16 W, UVP Inc., San Gabriel, CA, USA) for induction of the cis state for 3 min and using Vis light irradiation (Fluorescent lamp 27 W, FML27EX-N, Mitsubishi Inc., JP) of the trans state for 10 min and then repeated. The GTPase activity of PAM-Ras was measured at 25 °C in modification buffer as stated above “GTPase activity of Ras”.
2.8. Measurement of nucleotide exchange of Ras induced by GEF
The GDP dissociation rate was estimated using the fluorescently labeled GDP analog NBD-GDP. To the complex of 1 μM PAM-Ras/NBD-GDP or PAM-Ras/NBD-GDP/GEF (SOS-505) in 120 mM Tris–HCl (pH 7.5) with 2 mM MgCl2, 100 μM GTP was added, and the decrease in fluorescence was monitored at 25 °C with an F-2500 spectrofluorometer (Hitachi, Tokyo, Japan). The data were fitted to a single exponential curve by using GraphPad Prism (GraphPad Software, La Jolla, CA, USA), which was used to comprehensive curve fitting the scientific data, from which the rate constants were calculated.
2.9. CD spectrum measurements
Near-UV CD spectra of 0.4 mg/ml sample and Far-UV CD spectra of 0.1 mg/ml sample were measured in CD buffer (150 mM NaCl, 30 mM Tris–HCl, pH 7.5, 1 mM MgCl2) at 25 °C using a J-720 CD spectropolarimeter (JASCO, MD, USA). Samples were Ras, Irradiated Ras by UV irradiation (366 nm) for 10 min on ice and Denatured Ras by final 8 M Urea. Cuvettes with 1 and 10 mm path lengths were used for the measurements in Near- and Far-UV regions, respectively. Each spectrum represents the average of 4 scans.
3. Results
3.1. Preparation of Ras mutants with a single cysteine residue in the core nucleotide-binding motif
In order to incorporate a photochromic molecule into the functional region of Ras, we prepared four mutants that have a single reactive cysteine residue in the functional site of Ras. In the Ras WT construct used in this study, the C-terminal 13 amino acids were removed, and it had three cysteine residues at positions 51, 80, and 118. To give priority to the reactive cysteine residue introduced into the functional region of Ras, the intrinsic reactive cysteine C118 was replaced with serine (C118S) to produce cys-light Ras. The other intrinsic internal cysteine residues C51 and C80 were not reactive with SH-modifying reagents and were therefore preserved. Subsequently, a single cysteine residue was introduced into the nucleotide-binding motif of the cys-light Ras. We selected four hydrophobic amino acid residues, G12, Y32, I36, and Y64 (Fig. 1A), for cysteine replacement. These four residues are well conserved among Ras family members and are key residues for Ras function [13], [14]. Crystallographic studies have revealed that the residues are located on the surface of the Ras core domain and are exposed to solvent [15]. G12 is located in the P-loop, and Y32 and I36 are located in the switch I region, which is the primary effector-binding region and is involved in downstream effector binding as well as interactions with GTPase-activating proteins [16]. The residue Y64 in the switch region is known to be important for binding to downstream effectors for Ras [17].
Fig. 1.
(A) Locations of the amino acids replaced with cysteine, in order to introduce PAM in the crystal structure of Ras. The α-carbons of the amino acids G12C, Y32C, I36C, Y64C, and C118 mutated to cysteine are indicated as white bowls. The 3D structure was prepared using the molecular graphics program Mol Feat 4.0 by using the coordinate data (3GFT) of Ras from the Protein Data Bank database. The GTP analog and Mg2+ are shown in bowl representation. (B) The GTPase activity of Ras. GTPase assays were performed at 37 °C for 1 h in 45 mM Tris–HCl pH 7.5, 180 mM NaCl, 2 mM MgCl2. The GTPase reactions were started by adding 1 mM GTP at 37 °C to 50 μM Ras. GTPase activity was measured in three independent proteins. Error bars represent SD.
The four Ras mutants G12C, Y32C, I36C, and Y64C were prepared using established molecular biological methods and bacterial expression systems. The GTPase activities of the Ras mutants are shown in Fig. 1B. Cys-light Ras exhibited an approximately two-fold higher GTPase activity than that of WT. GTPase activities of the Ras mutants G12C and Y32C were almost identical to that of cys-light. On the other hand, the I36C and Y64C mutants exhibited GTPase activities that were about one-half that of cys-light. To prepare the Cys-light Ras, Cys 118 on the surface of Ras was substituted with serine. The amino acid located between Asn 116 and Asp 119 interacts directly with guanine ring of GTP. Therefore, the mutation induced distortion in the conformation at the nucleotide binding region particularly the base moiety recognition site, resulting in enhanced GTPase activity. For the mutants I36C and Y64C, I36 affects switch I and Y64 affects switch II located at near the Mg2+ binding region. Therefore, the mutation in these residues may alter the stability of Mg2+, reducing the GTPase activities. We have added these points to the revised manuscript.
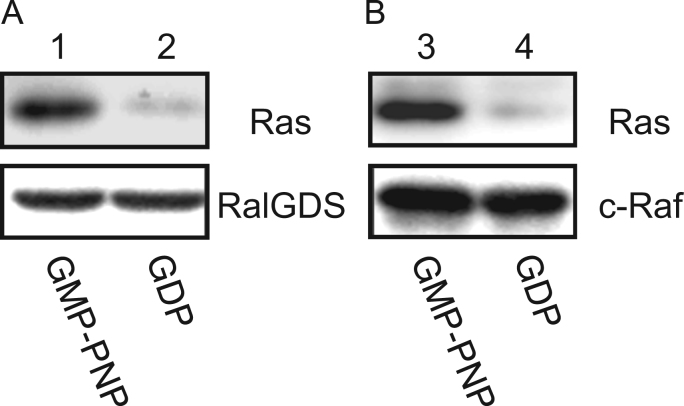
We have examined the functional assay for the interaction of K-RasΔ13 (lacking 13 C-terminal amino acids) with its target factors, Ral-GDS or c-Raf, the primary factors in the downstream signal transduction of Ras, using a pull-down assay. As shown in Fig. 2 (lane 1, 3), GMP-PNP bound Ras interacted with RalGDS and c-Raf. In contrast, GDP bound Ras did not bind to RalGDS and c-Raf (lane 2, 4). These results suggest that K-RasΔ13 retained its biological function. This is also supported by a previous report suggesting that Ras lacking the C-terminal 18 amino acid residues showed same biological activity compared with the full-length Ras [18].
Fig. 2.
In vitro binding assay of K-Ras to (A) RalGDS or (B) c-Raf. 15 μM Ras in the presence of (A) 1.4 μM RalGDS or (B) 1.4 =M c-Raf was loaded with GMP-PNP (lanes 1,3) or GDP (lanes 2,4). Proteins absorbed on the resin were dissolved in SDS-PAGE (15%) solution. Western blotting analysis was carried out using an anti-His-tag antibody or anti-GST-tag antibody. The experimental condition in detail is as described in Section 2 “Pull-down assay of Ras with effectors: RalGDS and c-Raf”.
3.2. Modification of Ras mutants with PAM and photoregulation of the GTPase activities
We employed the sulfhydryl reactive azobenzene derivative PAM (Fig. 3A) to incorporate a photochromic moiety into the functional region of Ras. It has been well known that the configurational state (cis or trans) of azobenzene and its derivatives can be monitored by UV/Vis light absorption spectroscopy [6]. The UV/Vis light absorption spectrum of PAM was similar to that of azobenzene (Fig. 3B). The trans form of azobenzene exhibited maximum absorption at 330 nm in a solution of DMF. The irradiation of the trans form of PAM solution by UV light (366 nm) resulted in a significant reduction of the peak at 330 nm in the absorption spectrum. Following irradiation for 14 min, the alteration of the spectrum was saturated. In comparison with the spectral data previously reported [19], the solution was estimated to contain approximately 75% cis and 25% trans forms of free PAM.
Fig. 3.
(A) Schematic representation of the photoisomerization of PAM. The molecular structures of cis and trans PAM. UV light irradiation converts the trans form of PAM into the hydrophilic cis form; Vis light irradiation reverses this conversion. Change in the absorption spectrum of free PAM induced by (B) UV and (C) Vis light irradiation. (C) Free PAM (75 μM) was irradiated using a UV lamp (Blak-Ray lamp, 16 W) at 366 nm for 0–14 min at room temperature in 100% DMF. (D) Free PAM irradiated using a UV lamp (Blak-Ray lamp, 16 W) at 366 nm for 14 min was subsequently irradiated using a fluorescent light (Fluorescent lamp 27 W, FML27EX-N, Mitsubishi Inc., JP) for 0–12 min at room temperature.
The Ras mutants with a single reactive cysteine were stoichiometrically modified with PAM. The time course and PAM concentration dependence of the modification of WT, which has a single reactive cysteine (C118), are shown in Fig. 4 A and B. The modification reaction was completed within 60 min and the incorporation of PAM into Ras was saturated at the addition of approximately 15-fold molar excess of PAM over Ras. The mutants were also modified by PAM in a manner similar to that of C118. The incorporated ratios of PAM for each Ras mutant are summarized in Table 1. Incorporated amount of PAM into Ras was determined using an extinction coefficient of PAM as described in Section 2. Quantitative analysis of the amount of PAM incorporated into WT or Cys-light Ras showed that C80 and C51 were not reactive to PAM and that non-specific binding was negligible even at higher concentrations of PAM, as shown in Fig. 4B of the revised manuscript. The incorporated ratios of PAM for Cys-light under an identical reaction condition used for other mutants are also shown in Table 1 of the revised manuscript. The amount of PAM incorporation for WT was 0.87. In contrast, PAM for Cys-light Ras, in which C118 was substituted with serine was 0.09. Therefore, PAM was incorporated into C118 nearly stoichiometrically. The incorporated ratios of PAM for each mutant, including G12C, Y32C, I36C, and Y64C, were 0.85, 0.61, 065, and 0.83, respectively. Therefore, C51 and C80 may be non-reactive for PAM under same experimental conditions and PAM was specifically modified at G12C, Y32C, I36C, and Y64C.
Fig. 4.
(A) Time-course and (B) concentration-dependence of PAM incorporation into Ras WT. (A) Ras WT (15 μM) was incubated with 235 μM PAM for (0–1 h) at 25 °C in modification buffer (45 mM Tris–HCl pH 7.5, 180 mM NaCl, 2 mM MgCl2 and 3% DMF). (B) 15 μM Ras WT (■) or Cys-light Ras (□) was incubated with 0–750 μM PAM for 1 h at 25 °C in modification buffer. All reactions were terminated by the addition of final 10 mM DTT. Change in the absorption spectrum of PAM-Ras WT induced by (C) UV and (D) Vis light irradiation. (C) PAM-Ras WT (60 μM) was irradiated using a UV lamp (Blak-Ray lamp, 16 W) at 366 nm for 0, 0.5, 1, 2, and 3 min at room temperature in a solution of 45 mM Tris–HCl pH 7.5, 180 mM NaCl, 2 mM MgCl2. (D) PAM-Ras WT irradiated using a UV lamp (Blak-Ray lamp, 16 W) at 366 nm for 3 min was subsequently irradiated using a fluorescent light (Fluorescent lamp 27 W, FML27EX-N, Mitsubishi Inc., JP) for 0, 1, 2, 6, and 8 min at room temperature.
Table 1.
Incorporation efficiency of photochromic molecule into Ras.
| PAM/Ras (mol/mol) | |
|---|---|
| WT | 0.87±0.02 |
| Cys-light | 0.09±0.03 |
| G12C | 0.85±0.03 |
| Y32C | 0.61±0.03 |
| I36C | 0.65±0.03 |
| Y64C | 0.83±0.03 |
Incorporation efficiency was measured in three independent experiments.
The configurational state (cis or trans) of PAM incorporated into Ras was monitored by UV/Vis light absorption spectroscopy (Fig. 4 C and D). The UV/VIS absorption spectrum for PAM was similar to that of free PAM. Irradiation of the trans-PAM-Ras solution by UV light (366 nm) led to a marked reduction in the peak at 330 nm, reflecting trans to cis isomerization, that approached a minimum at around 3 min, as shown in Fig. 4C. By comparing the absorbance at 330 nm of the saturated spectra on time course of UV and visible light irradiations, and also spectral data previously reported [19] and free PAM (Fig. 3B), the ratio of cis/trans was estimated to be 63% cis and 37% trans. The subsequent irradiation of the cis-PAM-Ras solution with a fluorescent room light resulted in a significant increase in the peak at 330 nm in the absorption spectrum and reached the same level as that of the initial trans-PAM-Ras solution at 8 min, as shown in Fig. 4D. The other Ras mutants modified with PAM also showed reversible spectral changes upon UV/Vis light irradiation.
We measured the near and far UV CD spectra of Ras irradiated by ultraviolet light. As shown in Fig. 5A and B, even after UV light irradiation at 366 nm for 10 min, the CD spectra were nearly identical to those before UV irradiation. This strongly suggests that UV irradiation under the conditions used in this study do not induce significant damage. We have also confirmed that Ras modified with PAM showed no oligomerization and aggregation based on size-exclusion chromatography coupled with high-performance liquid chromatography (data not shown).
Fig. 5.
Near and far UV CD spectrum of K-Ras. (A) Far-UV CD spectra of 0.1 mg/ml K-Ras. (B) Near-UV CD spectra of 0.4 mg/ml K-Ras. Each spectrum represents the average of 4 scans. The bold line indicates K-Ras before UV irradiation (366 nm). The dotted line indicates K-Ras after UV irradiation (366 nm) for 10 min. The narrow line indicates K-Ras denatured by 8 M Urea.
The modification of the mutants with PAM induced alterations in GTPase activity, as shown in Table 2. The activities of G12C and Y32C were particularly susceptible; modification with PAM significantly reduced their GTPase activity by 20–30% of those of the unmodified proteins.
Table 2.
Photocontrol of the GTPase activity of PAM-Ras WT.
| GTPase activity (GTP μM/Ras μM/h) | |||||
|---|---|---|---|---|---|
| Unmodified | UV 1st | Vis 1st | UV 2nd | Vis 2nd | |
| WT | 0.79±0.05 | 1.25±0.01 | 1.23±0.03 | 1.24±0.01 | 1.21±0.02 |
| G12C | 1.78±0.08 | 0.51±0.02 | 0.43±0.02 | 0.52±0.02 | 0.43±0.02 |
| Y32C | 2.35±0.11 | 0.53±0.02 | 0.67±0.03 | 0.52±0.01 | 0.70±0.03 |
| I36C | 0.88±0.06 | 0.98±0.01 | 1.11±0.03 | 1.02±0.02 | 1.13±0.02 |
| Y64C | 1.05±0.02 | 0.72±0.01 | 0.87±0.02 | 0.73±0.02 | 0.90±0.02 |
The GTPase activities of Ras modified with PAM were measured in the assay solution including 50 µM PAM-Ras WT, according to the method described in Section 2. GTPase activities were measured in three independent proteins. Data are presented as means±SD.
We examined the effect of the isomerization of PAM incorporated into the functional site of Ras on the GTPase activity. Transition of the PAM on Ras mutants between cis- and trans-isomers was achieved by UV/Vis light irradiation, as described in Materials and Methods. Ras mutants I36C, Y64, Y32C, and G12C exhibited apparent changes in GTPase activity upon exposure to UV/Vis light irradiation, as shown in Fig. 6. The GTPase activity of the I36C, Y64C, and Y32C mutants increased by 13%, 21%, and 26%, respectively, after the cis to trans isomerization of mutant-bound PAM induced by the first VIS light irradiation. Even after alternating UV/Vis light irradiation two times, the reversible changes in GTPase activity were retained. The GTPase activity of un-photoirradiated PAM-Ras was identical to that of visible-light irradiated PAM-Ras. The azobenzene moiety of the un-photoirradiated PAM-Ras exists as almost trans isomer. This is supported by the absorption spectra of un-photo-irradiated PAM-Ras. Trans to cis isomerization by UV irradiation induces a change in GTPase activity compared with un-photoirradiated PAM-bound Ras. These results suggest that the changes in GTPase activity of the Ras mutants modified with PAM are indeed due to the cis–trans photoisomerization of PAM upon UV/Vis light irradiation. Interestingly, contrary to the other mutants, PAM modification of G12C resulted in a decrease in GTPase activity accompanied by cis–trans isomerization of PAM. As shown in Fig. 6, the second UV light irradiation induced an approximately 21% increase in GTPase activity and the second VIS light irradiation reduced GTPase activity to the level of the first Vis light irradiation. On the other hand, WT modified with PAM at the reactive intrinsic cysteine residue (C118), which is located outside the GTPase region, did not show any change in GTPase activity upon UV/Vis light irradiation.
Fig. 6.
The photocontrol of GTPase activity of PAM-Ras. PAM-Ras was irradiated with UV light at 366 nm for 3 min or room light for 10 min on ice alternately. The GTPase assays were performed at 37 °C for 1 h in 45 mM Tris–HCl pH 7.5, 180 mM NaCl, 2 mM MgCl2 and 3% DMF. The GTPase reactions were started by adding 1 mM GTP to 50 μM PAM-Ras at 37 °C. GTPase activity was measured in three independent proteins. Error bars represent SD.
3.3. Photoregulation of the interaction between GEF and Ras modified with PAM
We also examined the effect of the isomerization of PAM incorporated into the functional site of Ras on the interaction with GEF (SOS-505). The exchange of GDP for GTP induced by SOS-505 was monitored using the fluorescently labeled GDP analog NBD-GDP. The addition of excess GTP to the Ras/NBD-GDP in the presence of SOS-505 resulted in an immediate decrease in NBD fluorescence, accompanied by the release of NBD-GDP from the GTP-binding site. From the decrease in fluorescence, the exchange rate was estimated according to the methods described in Materials and Methods. The nucleotide exchange rates of PAM-WT, PAM-G12C, and PAM-Y32C in the presence of SOS-505 were not altered by cis–trans isomerization of PAM. Conversely, the nucleotide exchange rates of PAM-I36C and PAM-Y64C were apparently altered upon UV/VIS light irradiation as shown in Fig. 7 and Table 3. PAM-I36C irradiated by VIS light displayed 13% higher nucleotide exchange rate than that irradiated by UV light. On the other hand, the nucleotide exchange rate of PAM-Y64C irradiated by VIS light was 23% lower than that of PAM-Y64C irradiated by UV. For both of the mutants modified with PAM, the reversible and repeated changes in the nucleotide exchange rates were observed with two alternating UV–vis light irradiations.
Fig. 7.
Photocontrol of the nucleotide exchange rate of (A) PAM-I36C and (B) PAM-Y64C with GEF. PAM-Ras (1 μM), premixed with 0.5 μM NBD-GDP and (A) 1 μM or (B) 0.5 μM SOS-505, was mixed with 100 μM GTP in 30 mM Tris–HCl pH 7.5, 120 mM NaCl, 2 mM MgCl2, and 3% DMF at 25 °C. The nucleotide exchange rates were measured in three independent proteins. The zero-point errors were corrected. k: exchange rate constant for GDP to GTP.
Table 3.
The nucleotide exchange rate of PAM-Ras with GEF.
| I36C |
Y64C |
|
|---|---|---|
|
ak |
ak |
|
| (×10−3 S−1) | (×10−3 S−1) | |
| Unmodified | 3.36±0.06 | 35.6±3.9 |
| UV 1st | 4.16±0.02 | 38.8±1.0 |
| Vis 1st | 4.81±0.06 | 30.0±2.9 |
| UV 2nd | 4.22±0.06 | 36.8±0.6 |
| Vis 2nd | 4.89±0.07 | 28.6±2.6 |
The nucleotide exchange rates were measured in three independent proteins. Data are presented as means±SD.
k: exchange rate constant from GDP to GTP.
4. Discussion
The aim of this study was to artificially control the function of the G-protein Ras, which is considered a bionanomachine that performs cell signaling, by using a switchable photochromic molecule. Previous crystallographic analysis demonstrated that the GTPase core region of G-proteins is strikingly very similar in structure to those of the ATP-driven motor proteins [3]. For myosin and kinesin, some possible mechanisms for energy transduction including Feynman-ratchet models have been suggested. From a crystallographic comparison of the transition intermediates, another possible model; the chemical energy of ATP is mechanically transduced to the motor activities utilizing cam-shaft motion via subdomain steric interactions, which was also deduced. We previously showed that we can photoreversibly control the function of motor proteins by incorporating an azobenzene derivative into their energy-transducing regions [8], [9], [10]. Therefore, we expected that the introduction of a switching device such as a photochromic molecule into the signal-transducing region might enable the functional control of G-proteins.
In this study, we employed the azobenzene derivative PAM as a photochromic nanodevice to control function of Ras. It is well known that azobenzene changes its structure, physico-chemical properties such as polarity drastically by photoisomerization induced by UV and VIS light irradiation [5], [6], [7]. The dipole moment of azobenzene changes from 0.5 to 3.1 Debye, accompanied by photoisomerization of the trans to the cis form [20]. Therefore, cis–trans isomerization of azobenzene moiety of PAM bound to Ras changes hydrophobic or hydrophilic circumstances at the cysteine residues modified with PAM and induces structural distortion at the catalytic core of Ras. We believe that the photoconversions between the cis and trans isomers of azobenzene act in a manner similar to that of amino acid substitution by point mutation to influence the activity of Ras. Therefore, it is strongly expected that the photoisomerization of PAM incorporated into the functional region would affect Ras functions, such as GTPase activity and interaction with regulatory factors and effectors in a photoreversible manner.
In this study, we selected four hydrophobic amino acid residues, Y32, I36, Y64, and G12, in the region consisting of the active site components switch I, switch II, and the P-loop, to mutate to cysteine and modify with PAM for the following reasons. The conserved residue T35 is necessary for stabilization of switch I in the GTP-bound form, and maybe a candidate site for incorporating a photochromic molecule. However, it has been shown that the mutation of T35 to other amino acids results in a disordered switch I [21]. Therefore, we chose the hydrophobic amino acid residue I36 that is adjacent to T35. The residue Y64 in switch II is an important binding site for downstream effectors [17]. G12 is in the well-known P-loop, and the mutation of G12 is commonly seen in oncogenic Ras [22]. Adjacent G13 binds to the γ-phosphate of GTP via hydrogen-bonding. Therefore, modifying G12C with PAM may induce distortion of the P-loop and reduction of GTPase activity. Y32, which is located in the switch I, interacts with the γ-phosphate oxygen of GTP via a water molecule [16]. It is thought that modifying Y32C with PAM abolishes the interaction resulting in reduced GTPase activity. Indeed, photoisomerization of PAM covalently bound to these cysteine-mutated residues induced photoreversible changes in GTPase activity and interaction with GEF, as shown in Fig. 6. In contrast, I36C and Y64 are located in the functional switches I and II, respectively, and do not interact directly with amino acids involved in catalytic core and GTP nucleotide. Therefore, modification of these amino acids residues with PAM may not induce significant changes in GTPase activities.
Interestingly, I36C and Y64C were affected differently by the cis and trans isomers of PAM in terms of their interactions with GEF. As shown in Fig. 7, the exchange rates were different significantly for I36 and Y64C. The nucleotide exchange rates of I36C induced by SOS-505 were nearly the same as those of observed WT, while those of Y64C induced by SOS-505 were approximately 10-fold higher than those for WT. The nucleotide dissociation rate of the mutant Y64A was also higher than that of WT. Crystallographic studies revealed that Y64 in switch II is located at the interface between SOS and Ras [23]. Therefore the mutation at the Y64 may influence the interaction of Ras with SOS, accelerating the nucleotide exchange rate.
The nucleotide exchange rate of PAM-Y64C after 2nd UV irradiation was considerably lower than that after the 1st UV irradiation. In this experiment, light irradiations were performed on a single small dish for each sample solution. An aliquot was taken from the solution for the assay after each UV or visible light irradiation. The volume of the sample solutions decreased at each irradiation step. After the final irradiation step, the sample volume had decreased by 60% of the original volume. Therefore, the effect of light irradiation on the sample solution may be slightly altered at each irradiation step. We measured the CD spectra of Ras to examine the protein degradation induced by UV irradiation. Even after 10 min UV irradiation, the CD spectra were nearly identical to those before UV irradiation (Fig. 5). Thus, the possibility of the protein degradation was excluded. One possible explanation may be related to the photo-bleaching of fluorescent nucleotide bound to the PAM-Ras mutants.
In the present study, the extent of the photoreversible change in GTPase activity and nucleotide exchange rate induced by GEF was not significantly different. One of the possible causes may be the thermal stability of the cis-PAM-Ras. We have conducted the experiments to measure the half-life of the cis-PAM-Ras. The half-life of the cis-PAM-Ras was approximately 2 h at 25 °C. The half-life of the cis-PAM-Ras was approximately 1.5 h at 37 °C. For the nucleotide exchange assay, the effect of the stability of the cis-PAM-Ras was not significant as the assay was completed within 10 min at 25 °C. However, 1 h is required to complete the GTPase activity assay. Therefore, as suggested by the reviewer, the stability of the cis-PAM-Ras may influence the photoregulation of the GTPase activity of PAM-Ras. Approximately 35% of cis-PAM-Ras is converted to trans-PAM-Ras after 1 h of the GTPase assay at 37 °C.
Basically the changes in the conformation and characteristics of azobenzene accompanied by its photo-isomerization may not be sufficient to induce significant changes in GTPase activity and exchange rate. If other photochromic molecules such as spiropyran derivatives that drastically change their structures and properties [10] are designed to fit functional region of Ras, highly efficient switching of Ras function may be possible. We also expected that the bifunctional photochromic molecule bound to two cysteines would induce significant conformational changes to function as a photoswitching nanodevice. Indeed, we previously succeeded in inducing significant conformational changes of the skeletal muscle myosin motor domain by using the bifunctional azobenzene derivative 4,4'-azobenzene-dimaleimide (ABDM), which cross-linked the two reactive cysteine residues SH1 and SH2 in the functional region [8]. In the case of skeletal muscle myosin, it is well established that the two intrinsic reactive cysteine residues can be cross-linked by using bifunctional cross-linkers. In contrast, for G-protein, two cysteine residues should be introduced by genetic engineering for cross-linking through bifunctional photochromic molecules. The location on the surface of Ras in the tertiary structure must be well designed. We previously attempted to cross-link the two cysteines of the engineered kinesin by using ABDM. However, the two cysteine residues were not cross-linked intramolecularly and stoichiometrically. Thus, it is difficult to cross-link the cysteine residues on the surface of proteins intramolecularly. Therefore, we are not currently using bifunctional photochromic molecules. Additionally the photochromic molecules that perform photoisomerization with VIS light instead of UV light may be applicable to photoswitching devices in living cell, since they do not cause damage to cell function.
In conclusion, we demonstrated the ability to photocontrol the GTPase activity of Ras and its interaction with regulatory factors by using a photochromic azobenzene derivative. This strategy may also be applicable to the photocontrol of the interaction of Ras with its effectors. It is expected that introduction of a photochromic molecule into Ras might enable the reversible photocontrol of cell signaling.
Acknowledgments
This work was supported by grant from Japan Society for the Promotion of Science: Grant-in-Aid for Scientific Research (C) 15K07060.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2015.10.002.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Fernández-Medarde A., Santos E. Ras in cancer and developmental diseases. Genes Cancer. 2011;2:344–358. doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell S.L., Khosravi-Far R., Rossman K.L., Clark G.J., Der C.J. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 3.Kull F.J., Vale R.D., Fletterick R.J. The case for a common ancestor: kinesin and myosin motor proteins and G proteins. J. Muscle Res. Cell Motil. 1998;19:877–886. doi: 10.1023/a:1005489907021. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y., Nejad M., Patterson B. Dictyostelium myosin II G680V suppressors exhibit overlapping spectra of biochemical phenotypes including facilitated phosphate release. Genetics. 1999;153:107–116. doi: 10.1093/genetics/153.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) H.-J. Timpe, Photochromism: Molecules and Systems, Herausgeber: Dürr, H., Bouas-Laurent, H. 1. Auflage, 1068 S. Elsevier, Amsterdam, Oxford, New York, Tokyo, 1990. Schriftenreihe: Studies in organic chemistry, 40, ISBN:0-444-87432-1, (b) J. Prakt. Chem., 333, 1991, pp. 811–812, 10.1002/prac.19913330522.
- 6.Beharry A.A., Woolley G.A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 2011;40:4422–4437. doi: 10.1039/c1cs15023e. [DOI] [PubMed] [Google Scholar]
- 7.Beharry A.A., Sadovski O., Woolley G.A. Azobenzene photoswitching without ultraviolet light. J. Am. Chem. Soc. 2011;133:19684–19687. doi: 10.1021/ja209239m. [DOI] [PubMed] [Google Scholar]
- 8.Umeki N., Yoshizawa T., Sugimoto Y., Mitsui T., Wakabayashi K., Maruta S. Incorporation of an azobenzene derivative into the energy transducing site of skeletal muscle myosin results in photo-induced conformational changes. J. Biochem. 2004;136:839–846. doi: 10.1093/jb/mvh194. [DOI] [PubMed] [Google Scholar]
- 9.Yamada M.D., Nakajima Y., Maeda H., Maruta S. Photocontrol of kinesin ATPase activity using an azobenzene derivative. J. Biochem. 2007;142:691–698. doi: 10.1093/jb/mvm183. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa K., Tamura Y., Maruta S. Photocontrol of mitotic kinesin Eg5 facilitated by thiol-reactive photochromic molecules incorporated into the loop L5 functional loop. J. Biochem. 2014;155:195–206. doi: 10.1093/jb/mvt111. [DOI] [PubMed] [Google Scholar]
- 11.Coccetti P., Mauri I., Alberghina L., Martegani E., Parmeggiani A. The minimal active domain of the mouse ras exchange factor CDC25Mm. Biochem. Biophys. Res. Commun. 1995;206:253–259. doi: 10.1006/bbrc.1995.1035. [DOI] [PubMed] [Google Scholar]
- 12.Youngburg G.E., Youngburg M.V. A system of blood phosphorus analysis. J. Lab. Clin. Med. 1930;16:158–166. [Google Scholar]
- 13.Paduch M., Jeleń F., Otlewski J. Structure of small G proteins and their regulators. Acta Biochim. Pol. 2001;48:829–850. [PubMed] [Google Scholar]
- 14.Cox A.D., Der C.J. Ras history: the saga continues. Small GTPases. 2010;1:2–27. doi: 10.4161/sgtp.1.1.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lander Harry M., Milbank Aaron J. Redox regulation of cell signalling. Nature. 1996;381:380–381. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 16.Scheffzek K., Ahmadian M.R., Kabsch W. The Ras–RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277 doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- 17.Stieglitz B., Bee C., Schwarz D. Novel type of Ras effector interaction established between tumour suppressor NORE1A and Ras switch II. EMBO J. 2008;27:1995–2005. doi: 10.1038/emboj.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita-Yoshigaki Junko, Ito Yutaka. Guanine-nucleotide binding activity, interaction with GTPase-activating protein and solution conformation of the human c-Ha-Ras protein catalytic domain are retained upon deletion of C-terminal 18 amino acid residues. J. Protein Chem. 1992;11:731–739. doi: 10.1007/BF01024974. [DOI] [PubMed] [Google Scholar]
- 19.Behrendt R., Renner C., Schenk M. Photomodulation of the conformation of cyclic peptides with azobenzene moieties in the peptide backbone. Angew. Chem. Int. Ed. Engl. 1999;38:2771–2774. doi: 10.1002/(sici)1521-3773(19990917)38:18<2771::aid-anie2771>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 20.Seki T., Sekizawa H. Inherent and cooperative photomechanical motions in monolayers of an azobenzene containing polymer at the air–water interface. J. Phys. Chem. 1998;102:5313–5321. [Google Scholar]
- 21.Spoerner M., Herrmann C., Vetter I.R., Kalbitzer H.R., Wittinghofer A. Dynamic properties of the Ras switch I region and its importance for binding to effectors. PNAS. 2001;98:4944–4949. doi: 10.1073/pnas.081441398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prior I.A., Lewis P.D., Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72:2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margarit S.M., Sondermann H. Structural evidence for feedback activation by Ras. GTP of the Ras-specific nucleotide exchange factor SOS. Cell. 2003;112:685–695. doi: 10.1016/s0092-8674(03)00149-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material