Abstract
Objectives
To assess bone density testing (BDT) use among prostate cancer survivors receiving ADT, and downstream implications for osteoporosis and fracture diagnoses as well as pharmacologic osteoporosis treatment in a national integrated delivery system.
Methods
We identified 17,017 men with prostate cancer who received any ADT between 2005 and 2014 using Veterans Health Administration cancer registry and administrative data. We identified claims for BDT within a 3-year period of ADT initiation. We then used multivariable regression to examine the association between BDT use and incident osteoporosis, fracture, and use of pharmacologic treatment.
Results
We found a minority of patients received BDT (n=2,502, 15%), however the rate of testing increased to over 20% by the end of the study period. Men receiving BDT were older at diagnosis and had higher-risk prostate cancer (both p<0.001). Osteoporosis and fracture diagnoses, use of vitamin D ± calcium, and bisphosphonates were all more common in men who received BDT. After adjustment, BDT, and to a lesser degree, 2 or more years of ADT, were both independently associated with incident osteoporosis, fracture, and osteoporosis treatment.
Conclusions
Bone density testing is rare among prostate cancer patients treated with ADT in this integrated delivery system. However, BDT was associated with substantially increased treatment of osteoporosis indicating an underappreciated burden of osteoporosis among prostate cancer survivors initiating ADT. Optimizing BDT use and osteoporosis management in this at-risk population appears warranted.
Keywords: prostatic neoplasms, bone density, osteoporosis, fractures, bone, anti-androgen effect
Introduction
Prostate cancer is a common malignancy in American men, many of whom eventually undergo androgen deprivation therapy (ADT) as part of their prostate cancer management.1,2 While ADT may be warranted to treat high-risk and advanced disease, it is associated with significant, often under-appreciated, adverse effects related to hypogonadism, including metabolic syndrome, cardiovascular disease, and decreased bone health.3
The effects of ADT on bone manifest as significantly decreased bone mineral density, and consequently increased fracture risk.4-8 Guidelines and existing literature recommend screening for osteoporosis at the time of ADT initiation to facilitate risk stratification and early pharmacological intervention where appropriate.9-13 The National Comprehensive Cancer Network's Task Force Report: Bone Health in Cancer Care states that “in patients who will be undergoing therapy that lowers sex steroids, the NCCN Guidelines for Breast and Prostate Cancers recommend evaluation with baseline and periodic follow-up DXA scans to evaluate bone health and risk of fracture.” 14 However, existing data demonstrate bone density testing (BDT) rates remain below optimal levels.15-20 Bone health assessment is especially warranted in patients with other risk factors for skeletal-related events such as smoking, alcohol use, and low vitamin D levels.21 These risk factors disproportionately afflict US veterans, who subsequently have higher rates of mortality following fractures, further magnifying the need for BDT.22 In spite of these increased risks, the national patterns of BDT use and subsequent osteoporosis management in this population have not been well-categorized.
In this context, we characterized BDT use and outcomes in a national integrated delivery system cohort of veteran prostate cancer patients treated with ADT. We evaluated BDT rates at the initiation of ADT, and assessed downstream skeletal-related outcomes including osteoporosis, fracture, and pharmacologic treatment for osteoporosis. Better understanding bone health practice patterns and outcomes through this study will define the burden of bone disease among high-risk prostate cancer patients and opportunities to improve the quality of their care.
Materials and Methods
Study population
We used the Veterans Administration (VA) Central Cancer Registry to identify patients with an incident diagnosis of pathologically-confirmed prostate cancer between 2005 and 2008 who were treated with ADT, defined as surgical orchiectomy or medical castration with an injectable gonadotropin releasing hormone agonist, using inpatient and outpatient pharmacy and utilization coding.16 More than 99% of the men in this cohort received medical castration, among whom 93% received goserelin, 4% leuprolide, and 3% another agent. We excluded patients with other cancer diagnoses, death within 30 days of diagnosis, or diagnosis at autopsy. We linked these data with VA administrative files containing inpatient, outpatient, laboratory, radiology, pharmacy, and facility data with follow up through the year 2014. This allowed us to examine ADT use as well as BDT and other skeletal-related outcomes. We identified a cohort of 17,017 patients.
Outcomes
Our primary outcome was receipt of BDT at the patient level, consisting of either dual x-ray absorptiometry or quantitative computed tomography. We assessed BDT by identifying claims submitted within 18 months prior to or following initiation of ADT, which should capture both recommended testing prior to ADT initiation as well as any delayed or follow up monitoring. We utilized a larger time window than previous studies in order to maximize our capture of BDT performed surrounding ADT. As such our BDT rates may be biased to be slightly higher than those of other studies on this topic. Our secondary outcomes were downstream bone health measures, including any administrative codes suggesting a new diagnosis of osteoporosis or any fracture following ADT initiation. We also queried pharmacy claims for any new prescriptions suggesting osteoporosis treatment after induction of ADT. Specifically, we assessed for dispensing of vitamin D ± calcium (calcium carbonate, calcium citrate, calcium acetate, calcium) as recommended for all patients initiating ADT, bisphosphonates (alendronate, pamidronate, risedronate, zoledronic acid, ibandronate), or denosumab, a bone health treatment for metastatic prostate cancer which has also been demonstrated to increase BMD and lower fracture rates in men receiving ADT.23
Statistical analysis
We used descriptive statistics to assess differences in demographics, disease, and treatment characteristics between prostate cancer patients treated with ADT who received BDT and those who did not. We examined covariates including age, race, ethnicity, marital status, employment status, Gleason score, D'Amico prostate cancer risk group, primary prostate cancer treatment, and Charlson comorbidity score (calculated using healthcare claims for the 12 months prior to prostate cancer diagnosis).24,25 We used student's t-tests and chi-square testing as appropriate.
To assess the independent association of BDT use with our secondary outcomes of incident osteoporosis, fracture, and osteoporosis treatment, we fit separate multiple logistic regression models for each outcome with the primary exposure of BDT. Given its particularly detrimental impact on bone health, we adjusted these models using an indicator variable for ≥2 years of ADT,4-8 as well as the following covariates: age, race, ethnicity, marital status, D'Amico risk score, prostate cancer treatment type, and Charlson comorbidity score.
All analyses were conducted using SAS software (SAS Institute, Cary, NC) and all testing was two-sided using an alpha of 0.05. This study was approved by the VA Ann Arbor Healthcare System Institutional Review Board.
Results
Among the 17,017 prostate cancer patients receiving ADT, a minority received BDT during the study period (2,502, 15%). As shown in Table 1, men receiving BDT were older and diagnosed with higher risk disease (p <0.001 for both). Among patients who received BDT there were slightly lower rates of initial treatment with combination radiation therapy and ADT (p <0.001). Testing rates increased consistently over the years of the study period, as illustrated in Figure 1.
Table 1.
Characteristics of patients on androgen deprivation therapy according to bone density testing.
| Demographics | No bone density testing (N=14,515) |
Bone density testing (N=2,502) |
p-value |
|---|---|---|---|
| Mean Age at Diagnosis, y (Std. Dev.) | 68.6 (9.1) | 70.1 (9.3) | <0.001 |
| Race, % | 0.05 | ||
| White | 67 | 66 | |
| Black | 29 | 31 | |
| Other/Unknown | 4 | 3 | |
| Ethnicity, % | <0.001 | ||
| Hispanic | 5 | 10 | |
| Non-Hispanic | 94 | 89 | |
| Unknown | 1 | 1 | |
| Marital Status, % | 0.30 | ||
| Married | 49 | 49 | |
| Divorced/Separated | 29 | 27 | |
| Single/Never Married | 8 | 9 | |
| Widowed | 13 | 15 | |
| Unknown | <1 | <1 | |
| Status, % | 0.07 | ||
| Alive | 74 | 72 | |
| Dead | 26 | 28 | |
| Employment Status, % | 0.50 | ||
| Full-Time | 7 | 6 | |
| Part Time | 3 | 3 | |
| Retired | 53 | 54 | |
| Self-Employed | 2 | 2 | |
| Unemployed | 34 | 34 | |
| Active Military | <1 | <1 | |
| Unknown | 1 | <1 | |
| Gleason Score, % | <0.001 | ||
| 6 | 25 | 21 | |
| 7 | 42 | 38 | |
| 8-10 | 33 | 41 | |
| Risk Group, % | <0.001 | ||
| Low | 14 | 11 | |
| Intermediate | 32 | 27 | |
| High | 54 | 63 | |
| Initial Treatment, % | <0.001 | ||
| Observation | 6 | 6 | |
| ADT Monotherapy | 36 | 38 | |
| Surgery | 8 | 10 | |
| Radiation | 5 | 6 | |
| Surgery + Radiation | 1 | <1 | |
| Radiation + ADT | 35 | 28 | |
| Other | <1 | 1 | |
| Unknown | 8 | 9 | |
| Comorbidity (Charlson Index), % | 0.22 | ||
| 0 | 42 | 40 | |
| 1 | 27 | 27 | |
| 2+ | 31 | 33 | |
| 2 or more years ADT, % | 32 | 38 | <0.001 |
Figure 1.
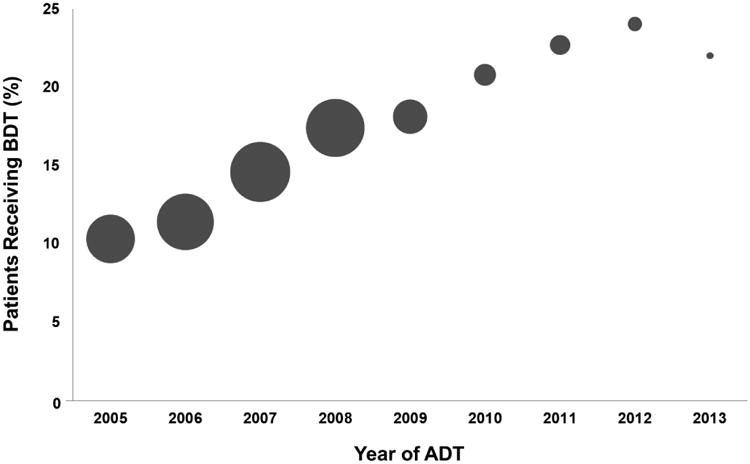
Rate of bone density testing across years of the study period, with the number of men initiating ADT each year represented by the size of the circle. Year 2014 is not shown as there were fewer than 50 patients initiating ADT.
Bone health outcomes among men with prostate cancer treated with ADT who did and did not receive BDT are shown in Table 2. Recipients of BDT were significantly more likely to be diagnosed with osteoporosis and fracture and more likely to receive treatment for osteoporosis (p <0.001 for all). As illustrated in Figure 2, differences in bone health outcomes among patients following the receipt of BDT were dramatic. For example, after BDT, diagnoses of osteoporosis and fracture increased nearly 10- and 3-fold respectively. In addition, rates of vitamin D use more than doubled after BDT, while bisphosphonate use also increased approximately 10-fold. Denosumab was rare but its use also increased.
Table 2.
Osteoporosis, fracture, and pharmacologic osteoporosis treatment among prostate cancer survivors on ADT according to bone density testing.
| Characteristic | No bone density testing (N=14,515) |
Bone density testing (N=2,502) |
p-value |
|---|---|---|---|
| Osteoporosis diagnosis | 752 (5%) | 669 (27%) | <0.001 |
| Fracture diagnosis | 1132 (8%) | 270 (11%) | <0.001 |
| Osteoporosis treatment | |||
| Vitamin D * ± Calcium ** | 5067 (35%) | 1848 (74%) | <0.001 |
| Bisphosphonate *** | 1983 (14%) | 1033 (41%) | <0.001 |
| Denosumab | 31 (0.2%) | 14 (0.6%) | <0.001 |
(vitamin D, ergocalciferol, cholecalciferol, 1,25 dihydroxycholecalciferol)
(calcium carbonate, calcium citrate, calcium acetate, calcium)
(alendronate, pamidronate, risedronate, zoledronic acid, ibandronate)
Figure 2.
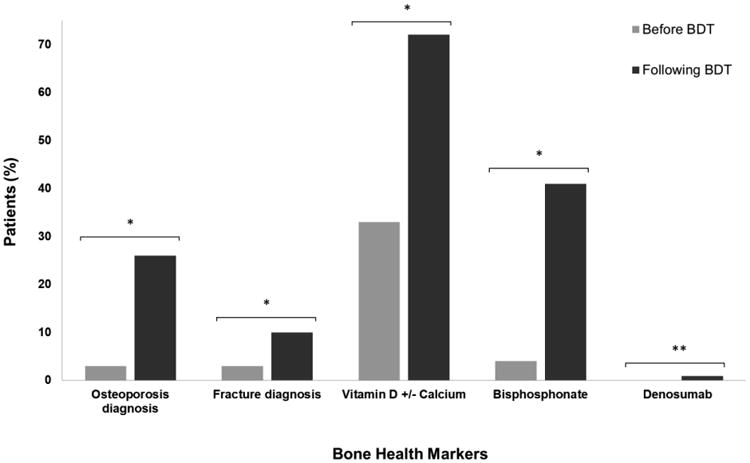
Comparison of bone health related diagnoses and treatments among men with prostate cancer receiving ADT before and after undergoing bone density testing. Among prostate cancer patients initiating ADT, BDT was associated with dramatic increases in the diagnosis of osteoporosis and fracture. In addition, bisphosphonate use increased approximately 10-fold, while vitamin D use more than doubled after BDT. * p<0.001. ** p value not obtainable as no patients were taking denosumab prior to receiving BDT
As shown in Table 3, after adjustment for patient and disease characteristics, BDT remained associated with the diagnosis (adjusted odds ratio (aOR) 6.21, 95% CI 5.40 – 7.15) and treatment of osteoporosis (aOR 6.47, 95% CI 5.66 – 7.39), as well as slightly increased odds of fracture diagnosis (aOR 1.29, 95% CI 1.08 – 1.53). Similarly, though to a lesser degree, the receipt of 2 or more years of ADT was significantly associated with diagnosis (aOR 1.47, 95% CI 1.28 – 1.69) and treatment of osteoporosis (aOR 1.86, 95% CI 1.71 – 2.03), in addition to incident fracture diagnosis (aOR 1.21, 95% CI 1.06 – 1.40).
Table 3.
Multivariable regression results for bone density testing and 2 or more years of ADT after adjustment for patient and disease characteristics.
| Outcome | Bone density testin Adjusted Odds Ratio (95% CI) * |
2+ Years ADT Adjusted Odds Ratio (95% CI) * |
|---|---|---|
| Osteoporosis Diagnosis | 6.21 (5.40 – 7.15) | 1.47 (1.28 – 1.69) |
| Osteoporosis Treatment | 6.47 (5.66 – 7.39) | 1.86 (1.71 – 2.03) |
| Fracture Diagnosis | 1.29 (1.08 – 1.53) | 1.21 (1.06 – 1.40) |
Adjusted for age, race, ethnicity, marital status, D'Amico risk score, prostate cancer treatment type, and Charlson comorbidity score.
Discussion
We found that roughly 1 in 7 patients with prostate cancer receiving ADT underwent BDT within the 3 years surrounding initiation of castration in this national integrated delivery system. However, by the end of the study period in 2014 this number had increased to more than one in five. Bone density testing was associated with dramatic increases in the diagnosis and treatment of osteoporosis, suggesting a non-trivial underlying burden of bone disease in untested men. While good clinical judgment regarding which patients would benefit most from testing may lead to selection bias, it is unlikely that the nearly 90% of untested men in our cohort were at uniformly low-risk of osteoporosis and fracture. Even after controlling for patient and disease characteristics, both BDT and, to a lesser degree, an extended duration of ADT were independent predictors of osteoporosis and fracture diagnoses. These findings confirm efforts are necessary to encourage bone health testing and osteoporosis treatment in this high-risk population of prostate cancer survivors to decrease avoidable harms of castration with ADT.
Our findings are consistent with prior data showing low rates of BDT in patients receiving ADT. Within veterans specifically, our finding of a 13% BDT rate is congruent with previously published rates of approximately 13% a decade ago, indicating a persistent quality gap.19,20 Though the uptrend in testing rates observed in this study is encouraging, 20% of patients undergoing BDT is still well below an ideal testing rate. While prior studies in veterans were limited to smaller geographical areas capturing several hundred patients, our national cohort is much larger, representing veterans across the United States, and reflects more contemporary practice patterns. Indeed, data from large studies in Medicare populations have also found persistently low rates varying from 6% to 14.5%, albeit with trends towards increasing utilization over time, signaling systematic poor compliance with recommended care.16-18 Recent results from other clinical contexts also underscore that the problem of low rates of BDT use among patients at high risk for bone-related complications extends beyond the realm of prostate cancer into breast cancer.26
The osteoporosis and fracture diagnoses identified in this study underscore the importance of appropriate bone health testing among high-risk prostate cancer survivors. The striking 5-fold difference in osteoporosis diagnosis between those who did and did not receive BDT suggests a significant amount of underlying disease in the 87% of men who were not tested. Moreover, the 10-fold increase in osteoporosis diagnosis following BDT, and 2- to 3-fold higher rates of osteoporosis treatment in the tested group compared to untested men demonstrate that testing yields actionable information for clinicians, who can intervene to potentially help avoid downstream bone complications especially in light of initiating ADT. Though the increases in fracture diagnoses were more modest, presumably discovered incidentally during evaluation for osteoporosis, this is a morbid complication highlighting that improvements in identification and treatment of skeletal fractures may be warranted.
In combination with the existing literature, our findings support efforts to increase rates of appropriate BDT in men undergoing ADT, and suggest that such testing could in turn yield improved diagnosis and treatment of ADT's adverse effects on the skeletal system. However, exploring the behaviors and norms of physicians and patients which contribute to persistently poor compliance with guideline-recommended bone health assessment among men on ADT is critical and should help inform subsequent intervention design. At least four addressable reasons may be driving our observations. First, providers may be unaware of the guideline recommendations to screen men receiving ADT for osteoporosis issued by groups such as the National Comprehensive Cancer Network, though the negative impacts of ADT on bone health have long been established.14,27 Second, many clinicians may not feel comfortable utilizing instruments such as the fracture risk assessment model (FRAX) tool, which combines BDT results with clinical risk factors to guide treatment (though it can be calculated without BMD).28 Third, there may be fragmentation among providers caring for these patients. While specialists order and manage ADT, in the absence of metastatic disease it is often primary care clinicians who are tasked with the diagnosis and treatment of osteoporosis in men with prostate cancer. Last, the evidence supporting the impact of vitamin D, calcium, and bisphosphonates on decreasing clinically-relevant fractures is mixed, generating confusion about the efficacy of these interventions and decreasing the likelihood of their utilization.29,30 However, current recommended care includes placing men older than 50 on vitamin D and calcium supplementation, a target not achieved in the majority of this cohort.31 It is possible some men in this study may have obtained these supplements over the counter, but this is unlikely given VA pharmacy coverage and cost differences. Future work must better clarify the impacts of BDT and pharmacologic intervention on subsequent fracture rates. Taken together, these findings suggest further study is needed to address this gap in high-risk prostate cancer care and men's health in general.
There are several limitations to this study. First, our results may not be generalizable to all prostate cancer patients treated with ADT, as additional risks and unmeasured differences may be present among veterans. However, our findings are consistent with results from other non-VA datasets, and the issues of low rates of BDT and low rates of subsequent treatment are not unique to this population. Further, our nationally-representative cohort and the lack of age exclusions as in Medicare studies increases generalizability. Second, although our study includes patients with follow up through 2014, the nine-year span of our cohort from incident cancer diagnosis to last follow up may suggest that our observed BDT rate could be an underestimate of current rates. However, the consistency of findings across studies and negligible increases indicate a persistent gap in care. Third, these retrospective data do not include the actual indications for BDT (other than initiating ADT) and therapeutic interventions, only whether or not they were received. Nonetheless, our use of incident diagnosis and pharmacy codes, coupled with our study design, support our conclusions of significantly underappreciated bone disease burden among these patients regardless of testing indication. It is possible that our analysis may be an underestimate of the rates at which physicians are assessing osteoporotic fracture risk in these men since we do not capture assessment methods that do not include BMD. However, given that BMD testing is the gold standard method and is the approach recommended by the NCCN we believe that these methods likely capture most of the bone health assessments being performed in this population. It is also important to note that BDT is not causally associated with osteoporosis or fracture. Rather, the use of BDT allows for the identification of potentially subclinical bone disease, which can subsequently lead to earlier intervention and long-term reduction of harm. Lastly, our analysis is subject to the inherent limitations of observational research and while we have attempted to control for confounding with multiple regression techniques, we were not able to fully account for unobserved confounders.
These limitations notwithstanding, our results have important implications for men receiving ADT and those involved their care. First, urologists must be vigilant to minimize the burdens related to the adverse effects of ADT on bone health. In spite of evidence recommending BDT in patients on ADT and trends towards increased use, rates of appropriate testing remain well below optimal levels. Second, from the standpoint of payers and policymakers, the costs of ADT-related adverse effects are significant, and interventions focused on mitigating the skeletal impacts of ADT have been found to be cost-effective.32 Increased attention should be directed towards encouraging the use of BDT in these patients. Last, the effects of ADT, osteoporosis, and fractures have significant negative implications for quality of life and survival. Increased use of appropriate BDT can potentially facilitate improved patient wellbeing and outcomes.
There appears to be significant under-diagnosis and treatment of osteoporosis among men with prostate cancer receiving ADT. Our findings suggest substantial opportunities exist to reduce bone-related complications by improving use of BDT at ADT initiation to allow for early intervention. Better understanding how providers care for these patients who are at high risk for bone-related complications, and how to most effectively target interventions to increase bone health assessment is justified. In addition, quantifying the degree to which improved detection and treatment of osteoporosis can help to lower clinically-relevant fracture rates in this high-risk population may help foster guideline concordant care. Efforts to optimize BDT among prostate cancer survivors initiating ADT may lead to increased quality of life and care.
Acknowledgments
Funding: Ruth L. Kirschstein National Research Service Award 4TL1TR000435-10 (PSK), National Cancer Institute T32-CA180984 (TB), National Institute on Aging (R01-AG-048071) (BKH), VA HSR&D Career Development Award − 2 (CDA 12−171) (TAS)
Footnotes
The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government
Conflicts of Interest: None
References
- 1.SEER Cancer Stat Facts: Prostate Cancer. [Accessed Jan 2017]; http://seer.cancer.gov/statfacts/prost.html.
- 2.Shahani S, Braga-Basaria M, Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab. 2008 Jun;93(6):2042–2049. doi: 10.1210/jc.2007-2595. [DOI] [PubMed] [Google Scholar]
- 3.Ostergren PB, Kistorp C, Bennedbaek FN, Faber J, Sonksen J, Fode M. The use of exercise interventions to overcome adverse effects of androgen deprivation therapy. Nat Rev Urol. 2016 Jun;13(6):353–364. doi: 10.1038/nrurol.2016.67. [DOI] [PubMed] [Google Scholar]
- 4.Wei JT, Gross M, Jaffe CA, et al. Androgen deprivation therapy for prostate cancer results in significant loss of bone density. Urology. 1999 Oct;54(4):607–611. doi: 10.1016/s0090-4295(99)00301-5. [DOI] [PubMed] [Google Scholar]
- 5.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005 Jan 13;352(2):154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 6.Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005 Dec;90(12):6410–6417. doi: 10.1210/jc.2005-0183. [DOI] [PubMed] [Google Scholar]
- 7.Morgans AK, Fan KH, Koyama T, et al. Bone complications among prostate cancer survivors: long-term follow-up from the prostate cancer outcomes study. Prostate Cancer Prostatic Dis. 2014 Dec;17(4):338–342. doi: 10.1038/pcan.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shao YH, Moore DF, Shih W, Lin Y, Jang TL, Lu-Yao GL. Fracture after androgen deprivation therapy among men with a high baseline risk of skeletal complications. BJU Int. 2013 May;111(5):745–752. doi: 10.1111/j.1464-410X.2012.11758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae DC, Stein BS. The diagnosis and treatment of osteoporosis in men on androgen deprivation therapy for advanced carcinoma of the prostate. J Urol. 2004 Dec;172(6 Pt 1):2137–2144. doi: 10.1097/01.ju.0000141515.67372.e5. [DOI] [PubMed] [Google Scholar]
- 10.Bienz M, Saad F. Androgen-deprivation therapy and bone loss in prostate cancer patients: a clinical review. Bonekey Rep. 2015;4:716. doi: 10.1038/bonekey.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saad F, Adachi JD, Brown JP, et al. Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol. 2008 Nov 20;26(33):5465–5476. doi: 10.1200/JCO.2008.18.4184. [DOI] [PubMed] [Google Scholar]
- 12.Skolarus TA, Caram MV, Shahinian VB. Androgen-deprivation-associated bone disease. Curr Opin Urol. 2014 Nov;24(6):601–607. doi: 10.1097/MOU.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 13.Zhumkhawala AA, Gleason JM, Cheetham TC, et al. Osteoporosis management program decreases incidence of hip fracture in patients with prostate cancer receiving androgen deprivation therapy. Urology. 2013 May;81(5):1010–1015. doi: 10.1016/j.urology.2012.11.066. [DOI] [PubMed] [Google Scholar]
- 14.Gralow JR, Biermann JS, Farooki A, et al. NCCN Task Force Report: Bone Health in Cancer Care. J Natl Compr Canc Netw. 2009 Jun;7(3):S1–32. doi: 10.6004/jnccn.2009.0076. quiz S33-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer. 2005 Jan 15;103(2):237–241. doi: 10.1002/cncr.20766. [DOI] [PubMed] [Google Scholar]
- 16.Shahinian VB, Kuo YF. Patterns of bone mineral density testing in men receiving androgen deprivation for prostate cancer. J Gen Intern Med. 2013 Nov;28(11):1440–1446. doi: 10.1007/s11606-013-2477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgans AK, Smith MR, O'Malley AJ, Keating NL. Bone density testing among prostate cancer survivors treated with androgen-deprivation therapy. Cancer. 2013 Feb 15;119(4):863–870. doi: 10.1002/cncr.27830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suarez-Almazor ME, Peddi P, Luo R, Nguyen HT, Elting LS. Low rates of bone mineral density measurement in Medicare beneficiaries with prostate cancer initiating androgen deprivation therapy. Supp Care Cancer. 2014 Feb;22(2):537–544. doi: 10.1007/s00520-013-2008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilcox A, Carnes ML, Moon TD, et al. Androgen deprivation in veterans with prostate cancer: implications for skeletal health. Ann Pharmacother. 2006 Dec;40(12):2107–2114. doi: 10.1345/aph.1H209. [DOI] [PubMed] [Google Scholar]
- 20.Yee EF, White RE, Murata GH, Handanos C, Hoffman RM. Osteoporosis management in prostate cancer patients treated with androgen deprivation therapy. J Gen Intern Med. 2007 Sep;22(9):1305–1310. doi: 10.1007/s11606-007-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang P, Regan MM, Ferrer M, et al. Relief of Urinary Symptom Burden after Primary Prostate Cancer Treatment. J Urol. 2016 Sep 1; doi: 10.1016/j.juro.2016.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bass E, Campbell RR, Werner DC, Nelson A, Bulat T. Inpatient mortality of hip fracture patients in the Veterans Health Administration. Rehabil Nurs. 2004 Nov-Dec;29(6):215–220. [PubMed] [Google Scholar]
- 23.Smith MR, Egerdie B, Hernandez Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009 Aug 20;361(8):745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005 Nov;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 26.Stratton J, Hu X, Soulos PR, et al. Bone Density Screening in Postmenopausal Women With Early-Stage Breast Cancer Treated With Aromatase Inhibitors. J Oncol Pract. 2017 Mar 07; doi: 10.1200/JOP.2016.018341. JOP2016018341. [DOI] [PubMed] [Google Scholar]
- 27.Theriault RL, Biermann JS, Brown E, et al. NCCN Task Force Report: Bone Health and Cancer Care. J Natl Compr Canc Netw. 2006 May;4(2):S1–20. quiz S21-22. [PubMed] [Google Scholar]
- 28.Saylor PJ, Smith MR. Bone health and prostate cancer. Prostate Cancer Prostatic Dis. 2010 Mar;13(1):20–27. doi: 10.1038/pcan.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moyer VA. U.S. Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013 May 07;158(9):691–696. doi: 10.7326/0003-4819-158-9-201305070-00603. [DOI] [PubMed] [Google Scholar]
- 30.Saylor PJ. Bone targeted therapies for the prevention of skeletal morbidity in men with prostate cancer. Asian J Androl. 2014 May-Jun;16(3):341–347. doi: 10.4103/1008-682X.122591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Network NCC. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer v2.2017. [Accessed Jun 2017]; https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 32.Ito K, Elkin EB, Girotra M, Morris MJ. Cost-effectiveness of fracture prevention in men who receive androgen deprivation therapy for localized prostate cancer. Ann Intern Med. 2010 May 18;152(10):621–629. doi: 10.7326/0003-4819-152-10-201005180-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]


