Abstract
Necrotizing enterocolitis (NEC) is one of the most common and devastating intestinal disorders in preterm infants. Therapies to meet the clinical needs for this special and highly vulnerable population are extremely limited. A specific human milk oligosaccharide (HMO), disialyllacto-N-tetraose (DSLNT), was shown to contribute to the beneficial effects of breastfeeding as it prevented NEC in a neonatal rat model and was associated with lower NEC risk in a human clinical cohort study. Herein, gram-scale synthesis of two DSLNT analogs previously shown to have NEC preventing effect is described. In addition, four novel disialyl glycans have been designed and synthesized by enzymatic or chemoenzymatic methods. Noticeably, two disialyl tetraoses have been produced by enzymatic sialylation of chemically synthesized thioethyl β-disaccharides followed by removal of the thioethyl aglycon. Dose-dependent and single-dose comparison studies showed varying NEC-preventing effects of the disialyl glycans in neonatal rats. This study helps to refine the structure requirement of the NEC-preventing effect of disialyl glycans and provides important dose-dependent information for using DSLNT analogs as potential therapeutics for NEC prevention in preterm infants.
Keywords: enzymatic synthesis, chemoenzymatic synthesis, oligosaccharide, necrotizing enterocolitis, disialyl glycan, sialic acid
TOC Graph

1. INTRODUCTION
Necrotizing enterocolitis (NEC) is one of the most common and devastating intestinal disorders in preterm infants.1,2 It affects nearly 10% of all very low-birth-weight infants and leads to a severe and often fatal destruction in the infant’s intestine.3 Therapies to meet the clinical needs for this special and highly vulnerable population are extremely limited. Current treatment of NEC includes cessation of enteral feeding, antibiotic therapy, and surgical removal of the necrotic intestine, but all of these treatments are accompanied by devastating long-term complications.4–6
It has been known that formula-fed infants have up to 10-fold higher risk of developing NEC than breast-fed infants.7,8 A major difference between infant formula and human milk is the lack of human milk oligosaccharides (HMOs) in common infant formula. This led to the hypothesis that HMOs contribute to the protective effects of breast-feeding against NEC.9 Indeed, recent studies have shown that a specific human milk oligosaccharide (HMO) called disialyllacto-N-tetraose (DSLNT, 1, in Fig. 1) contributes to the beneficial effects of breastfeeding in preventing NEC in preclinical neonatal rat model studies9 and in a human clinical cohort study.10 DSLNT has also been shown to be a potential marker for NEC-diagnosis.11 Due to challenges in obtaining DSLNT in large amounts for potential clinical therapeutic applications, five synthetic disialyl glycans have been produced by convenient enzymatic methods. Among these, two disialyl hexasaccharide analogs of DSLNT, disialyllacto-N-neotetraose (DSLNnT, 2) and DS′LNT (3), have shown promising effects in preventing NEC in a preclinical neonatal rat model.12
Fig. 1.
Structures of disialyllacto-N-tetraose (DSLNT, 1) found in human milk and disialyl glycans (2–7) synthesized and tested for NEC-preventing effects in this study.
Herein, gram-scale synthesis of the two promising compounds DSLNnT (2) and DS′LNT (3) using highly efficient one-pot multienzyme (OPME) systems is described. Both contain two α2–6-linked N-acetylneuraminic acid (Neu5Ac), the most common sialic acid form. These compounds have been used for dose-dependent studies in the established NEC neonatal rat model.12
To search for additional compounds with potentially enhanced NEC-preventing function, four additional novel disialyl glycans 4–7 (Fig. 1) have been designed and synthesized by chemoenzymatic or enzymatic methods. Their NEC-preventing effects have been compared to HMOs, DSLNnT (2), and DS′LNT (3). These include Neu5Gc-DS′LNT (4), a disialyl hexaose designed to replace the Neu5Ac residues in DS′LNT (3) by a non-human sialic acid form, N-glycolylneuraminic acid (Neu5Gc), which is presented in most animals and animal products including cow milk. The compound is designed to probe whether the sialic acid form affects the NEC-preventing effect of disialyl glycan. DS′LNnT (5) is also included. It is a disialyl glycan mimic of DSLNnT (2) in which the α2–6-sialyl linkage at the non-reducing end is replaced by an α2–3-sialyl linkage and the resulting terminal disaccharide structure resembles DSLNT (1) in human milk better. In addition, disialyl T-antigen tetraose (DSTa, 6) and disialyl galactobiose (DSGalB, 7) are designed to mimic the tetrasaccharide structure at the non-reducing end of DSLNT (1).
2. RESULTS AND DISCUSSION
Gram-Scale Enzymatic Synthesis of DSLNnT (2) and DS′LNT (3)
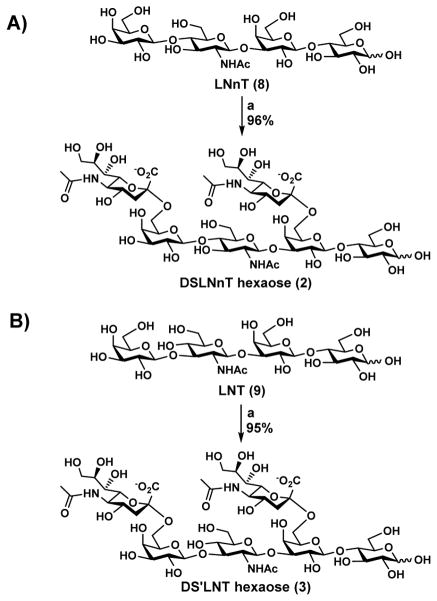
Gram-scale syntheses of DSLNnT and DS′LNT were carried out by sialylating lacto-N-neotetraose (LNnT, 8)12 and lacto-N-tetroase (LNT, 9), respectively, with two Neu5Ac residues in a one-pot two-enzyme (OP2E) system containing Neisseria meningitidis cytidine 5′-monophosphate (CMP)-sialic acid synthetase (NmCSS)13 and Photobacterium damselae α2–6-sialyltransferase (Pd2,6ST).14 As shown in Scheme 1A, Pd2,6ST-catalyzed OP2E sialylation of LNnT using N-acetylneuraminic acid (Neu5Ac) as the donor precursor (3 equivalents) produced 1.7 grams of the desired product Neu5Acα2–6Galβ1–4GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc (DSLNnT, 2) in an excellent 96% yield, comparable to the previous 99% yield for 236 mg-scale synthesis.12
Scheme 1. One-Pot Two-Enzyme (OP2E) Synthesis of A) DSLNnT (2) and B) DS′LNT (3).
Reagents and conditions: (a) Neu5Ac, CTP, MgCl2 (20 mM), Tris-HCl buffer (pH 8.5), NmCSS, Pd2,6ST, 37 °C. Enzymes used: NmCSS, Neisseria meningitidis CMP-sialic acid synthetase; Pd2,6ST, Photobacterium damselae α2–6-sialyltransferase.
Gram-scale synthesis of disialyl LNT (DS′LNT) hexaose (1.79 g) containing two Neu5Ac residues, Neu5Acα2–6Galβ1–3GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc (3), was completed similarly in an excellent yield (95%) using the OP2E sialylation system containing NmCSS and Pd2,6ST with a ratio of 3 to 1 for Neu5Ac to LNT (Scheme 1B). The yield for the gram-scale synthesis was also comparable to the previous 98% yield for 268 mg-scale synthesis.12
Enzymatic Synthesis of Neu5Gc-Containing DS′LNT (4)
In addition to the most abundant and the most common sialic acid form N-acetylneuraminic acid (Neu5Ac), non-human sialic acid N-glycolylneuraminic acid (Neu5Gc) is also broadly presented in tissues of animals other than human.15 Neu5Gc has also been shown to be expressed on glycoproteins and gangliosides in human melanoma, colon, retinoblastoma and breast cancers.16 As rats present both Neu5Ac and Neu5Gc on cell surfaces, it is interesting to test whether Neu5Ac and Neu5Gc-containing DS′LNTs have noticeable differences in protecting neonatal rats from NEC.
To obtain Neu5Gc-DS′LNT (4), the synthesis was carried out in the OP2E sialylation system containing NmCSS and Pd2,6ST using Neu5Gc as the donor precursor and LNT (9) as the acceptor substrate for Pd2,6ST. As shown in Scheme 2, hexasaccharide Neu5Gcα2–6Galβ1–3GlcNAcβ1–3(Neu5Gcα2–6)Galβ1–4Glc (Neu5Gc-DS′LNT, 4) (272 mg) was obtained in an excellent 94% yield.
Scheme 2. One-Pot Two-Enzyme (OP2E) Synthesis of Neu5Gc-DS′LNT (4).
Reagents and conditions: (a) Neu5Gc, CTP, MgCl2 (20 mM), Tris-HCl buffer (pH 8.5), NmCSS, Pd2,6ST, 37 °C. Enzymes used: NmCSS, Neisseria meningitidis CMP-sialic acid synthetase; Pd2,6ST, Photobacterium damselae α2–6-sialyltransferase.
Enzymatic Synthesis of DS′LNnT (5) Using a Sequential One-Pot Multienzyme (OPME) Glycosylation Approach
DS′LNnT (5) is a hexasaccharide containing the LNnT (8) core with a Neu5Ac α2–6-linked to the internal galactose (Gal) residue and a Neu5Ac α2–3-linked to the terminal Gal. This hexasaccharide is designed to better mimic the natural DSLNT (1) found in human milk. Enzymatic sialylation with different sialyl linkages in DS′LNnT (5) was achieved by using a glycosylation sequence different from the one used for the synthesis of DSLNnT (2). Instead of carrying out α2–6-sialylation to introduce two α2–6-linked Neu5Ac simultaneously after the formation of LNnT using a β1–4-galactosyltransferase-catalyzed one-pot multienzyme (OPME) reaction, the formation of α2–3- and α2–6-linked Neu5Ac in DS′LNnT (5) was accomplished by α2–6-sialylation of the trisaccharide core GlcNAcβ1–3Galβ1–4Glc (Lc3, 10) followed by sequential extension of the core with β1–4-galactosylation and α2–3-sialylation.17 As shown in Scheme 3, trisaccharide GlcNAcβ1–3Galβ1–4Glc (Lc3, 10) obtained from lactose in a one-pot four-enzyme GlcNAc-activation and transfer reaction12 containing recombinant enzymes Bifidobacterium longum strain ATCC55813 N-acetylhexosamine-1-kinase (BLNahK),18 Pasteurella multocida N-acetylglucosamine uridylyltransferase (PmGlmU),19 Pasteurella multocida inorganic pyrophosphatase (PmPpA),20 and Neisseria meningitidis β1–3-N-acetylglucosaminyltransferase (NmLgtA)21 was used for α2–6-sialylation by the OP2E system containing NmCSS and Pd2,6ST. Tetrasaccharide GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc (11) (0.3 g) was obtained in an excellent 96% yield. Next, galactosylation of this tetrasaccharide using a one-pot four-enzyme galactosylation system containing Escherichia coli galactokinase (EcGalK),22 Bifidobacterium longum UDP-sugar phosphorylase (BLUSP),23 Pasteurella multocida inorganic pyrophosphatase (PmPpA),20 and Neisseria meningitidis β1–4-galactosyltransferase (NmLgtB)20 produced pentasaccharide Galβ1–4GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc (12) (0.167 g) in 94% yield. This showed that oligosaccharides such as tetrasaccharide 11 is a better acceptor substrate than monosaccharide GlcNAc20 for NmLgtB. Finally, further sialylation of the pentasaccharide (12) using an OP2E α2–3-sialylation system containing NmCSS and Pasteurella multocida α2–3-sialyltransferase 1 (PmST1) M144D (PmST1_M144D) mutant24 produced the desired DS′LNnT (5) (121 mg) in 93% yield.
Scheme 3. Sequential One-Pot Multienzyme (OPME) Synthesis of DS′LNnT (5).
Reagents and conditions: (a) Neu5Ac, CTP, MgCl2 (20 mM), Tris-HCl buffer (pH 8.5), NmCSS, Pd2,6ST, 37 °C; (b) Gal, ATP, UTP, 20 mM MgCl2, Tris-HCl buffer (pH 8.0), EcGalK, BLUSP, NmLgtB, PmPpA, 30 °C; (c) Neu5Ac, CTP, MgCl2 (20 mM), Tris-HCl buffer (pH 8.5), NmCSS, PmST1_M144D, 37 °C. Enzymes used: NmCSS, Neisseria meningitidis CMP-sialic acid synthetase; Pd2,6ST, Photobacterium damselae α2–6-sialyltransferase; EcGalK, Escherichia coli galactokinase; BLUSP, Bifidobacterium longum UDP-sugar phosphorylase; PmPpA, Pasteurella multocida inorganic pyrophosphatase; NmLgtB, Neisseria meningitidis β1–4-galactosyltransferase; PmST1_M144D, Pasteurella multocida α2–3-sialyltransferase 1 M144D mutant.
Chemoenzymatic synthesis of tetrasaccharide DSTa (6)
The next disialyl glycan target is a tetrasaccharide containing a Galβ1–3GalNAc (T antigen or Ta) core. Ta is an essential component of glycans presented on glycoproteins (e.g. O-GalNAc glycan core-2 structure) and glycolipids, and also an important carbohydrate epitope involved in cell adhesion, signaling, fertilization, differentiation, development, and cancer metastasis.25 The disialyl Ta tetrasaccharide (DSTa, 6) has two α2–6-linked Neu5Ac residues on neighboring monosaccharide units in the disaccharide core, mimicking the situation of the terminal tetrasaccharide component in human milk DSLNT (1). It will be interesting to test whether it has NEC-preventing effects.
Galβ1–3GalNAc was synthesized in gram-scale from N-acetylgalactosamine (GalNAc), galactose (Gal), and adenosine 5′-triphosphate (ATP) using a one-pot two-enzyme (OP2E) galactosylation system containing EcGalK and Bifidobacterium infantis D-galactosyl-β1–3-N-acetyl-D-hexosamine phosphorylase (BiGalHexNAcP).26 Silica gel filtration and crystallization were used to obtain the pure product. Attempts for adding two Neu5Ac residues simultaneously by sialylating disaccharide Galβ1–3GalNAc using an excess amount (three equivalents) of Neu5Ac in the OP2E α2–6-sialylation system containing NmCSS and Pd2,6ST led to the formation of monosialylated glycan as the major product and the disialyl glycan was formed in a low yield. This was most likely due to the predominant presence of the α-anomer at the free reducing end of the Galβ1–3GalNAc (Ta) in the crystallized disaccharide. As Galβ1–3GalNAcβOR with a beta-linkage at the reducing end of the disaccharide is a suitable glycan for direct disialylation,27 disaccharide Galβ1–3GalNAcβSEt with a thioethyl group at the reducing end which can be selectively removed after the sialylation reaction was designed as the acceptor substrate for disialylation reactions. As shown in Scheme 4, GalNAcβSEt (14) was readily synthesized from galactosamine (GalNH2) using a reported method.28 The disaccharide Galβ1–3GalNAcβSEt (15) was then enzymatically synthesized from GalNAcβSEt via the OP2E galactosylation reaction containing EcGalK and BiGalHexNAcP.26 OP2E α2–6-sialylation of Galβ1–3GalNAcβSEt (15) using three equivalents of Neu5Ac formed the desired disialyl tetrasaccharide Neu5Acα2–6Galβ1–3(Neu5Acα2–6)GalNAcβSEt (16) in an excellent 93% yield. Removal of the thioethyl group in compound 16 using N-bromosuccinimide (NBS) in acetone-water led to the formation of DSTa (6) in 94% yield.
Scheme 4. Chemoenzymatic Synthesis of Disialyl T-Antigen DSTa (6).
Reagents and conditions: (a) i) Phthalic anhydride, NaOH (1 M); ii) Ac2O, pyridine; iii) EtSH, BF3·Et2O, CH2Cl2, 44% in three steps; (b) i) N2H4·H2O, MeOH; ii) Ac2O, MeOH, 93% in two steps; (c) Gal, ATP, MgCl2 (20 mM), Tris-HCl buffer (pH 6.8), EcGalK, BiGalHexNAcP, 37 °C; (d) Neu5Ac, CTP, MgCl2 (20 mM), Tris-HCl buffer (pH 8.5), NmCSS, Pd2,6ST, 37 °C; (e) NBS, acetone, H2O. Enzymes used: EcGalK, Escherichia coli galactokinase; BiGalHexNAcP, Bifidobacterium infantis D-galactosyl-β1–3-N-acetyl-D-hexosamine phosphorylase; NmCSS, Neisseria meningitidis CMP-sialic acid synthetase; Pd2,6ST, Photobacterium damselae α2–6-sialyltransferase.
Chemoenzymatic Synthesis of Disialyl Galactobiose DSGalB (7)
Galβ1–3Gal (galactobiose, GalB) exists in nature as a part of galactan structures in some bacteria and plants.29 It also constitutes galacto-oligosaccharide (GOS) structures that are currently used as prebiotics.30,31 Structurally, galactobiose differs from Galβ1–3GalNAc (Ta) only at the carbon-2 substitution of the monosaccharide at the reducing end (OH of the Gal in GalB versus NHAc of the GalNAc in Ta). It will be interesting to test whether a single substitution in the core disaccharide structure of DSTa tetrasaccharide (6) affects its NEC preventing efficiency.
A similar thioethyl β-glycoside acceptor strategy for the production of disialyl glycan DSTa (6) was used for the synthesis of DSGalB (7). GalβSEt (17)32 was synthesized chemically and used for producing Galβ1–3GalβSEt (18) using a one-pot four-enzyme (OP4E) galactosylation system containing EcGalK, BLUSP, PmPpA, and Campylobacter jejuni β1–3-galactosyltransferase (CjCgtB)33 (Scheme 5). To our delight, the monosaccharide galactoside GalβSEt (17) was a suitable acceptor for CjCgtB. A moderate 50% yield was obtained for the OP4E galactosylation reaction. The relatively low yield was due to GalβSEt (17) being a less optimal acceptor than GalNAcβOR in GM2 or GD2 ganglioside oligosaccharides.34
Scheme 5. Chemoenzymatic Synthesis of Disialyl Galactobiose (DSGalB) (7).
Reagents and conditions: (a) Gal, ATP, UTP, MgCl2 (20 mM), Tris-HCl buffer (pH 8.0), EcGalK, BLUSP, PmPpA, CjCgtB, 37 °C; (b) Neu5Ac, CTP, MgCl2 (20 mM), Tris-HCl buffer (pH 8.5), NmCSS, Pd2,6ST, 37 °C; (c) NBS, acetone, H2O. Enzymes used: EcGalK, Escherichia coli galactokinase; BLUSP, Bifidobacterium longum UDP-sugar phosphorylase; PmPpA, Pasteurella multocida inorganic pyrophosphatase; CjCgtB, Campylobacter jejuni β1–3-galactosyltransferase.
Disialylation of Galβ1–3GalβSEt (18) using the OP2E α2–6-sialylation system containing NmCSS and Pd2,6ST formed the desired tetrasaccharide Neu5Acα2–6Galβ1–3(Neu5Acα2–6)GalβSEt (19) in 84% yield. Removal of the thioethyl group using N-bromosuccinimide in acetone-water followed by purification produced DSGalB (7) in 87% yield.
In vivo Efficacy of Disialyl Glycans in Preventing Necrotizing Enterocolitis (NEC) in a Preclinical Neonatal Rat Model
The in vivo efficacy in preventing NEC in the neonatal rat model of the four new disialyl glycans Neu5Gc-DS′LNT (4), DS′LNnT (5), DSTa (6), and DSGalB (7) was tested and compared to two synthetic disialyl hexasaccharides DSLNnT (2), and DS′LNT (3), as well as HMOs. A single concentration of 300 μM was used for this purpose. The choice of this concentration was based on the concentration of DSLNT (1) determined by HPLC in the pooled HMOs supplemented to formula (10 mg/mL, similar to the level of HMO concentrations in mature milk) for in vivo neonatal rat feeding studies in a previous study.9 As shown in Fig. 2, similar to previously reported findings,12 DSLNnT (2) and DS′LNT (3) reduced the pathology scores significantly, although less effectively compared to HMOs. Replacing the Neu5Ac in DS′LNT (3) by Neu5Gc in Neu5Gc-DS′LNT (4) decreased the efficiency slightly. Replacing the terminal α2–6-linked Neu5Ac in DSLNnT (2) by the terminal α2–3-linked Neu5Ac in DS′LNnT (5) led to moderately decreased efficacy. On the other hand, disialyl tetraoses including DSTa (6) and DSGalB (7) did not have obvious NEC-preventing effect. These results indicate that Neu5Ac is a better sialic acid form than Neu5Gc for NEC-preventing disialyl compounds. In addition, disialyl hexasaccharides are more effective NEC-preventing compounds than disialyl tetrasaccharides. Furthermore, the distance between two sialic acid residues on the disialyl hexasaccharide is also important.
Figure 2.
In vivo efficacy testing in preclinical rat model of NEC. A. Comparing all synthesized glycans at a concentration of 300 μM. Number of animals per group: dam fed (DF): 22; formula fed (FF): 14; HMO: 20; DSLNnT: 18; DS′LNT: 17; Neu5GC-DS′LNT: 15; DS′LNnT: 13; DSTa: 11; DSGalB: 9.
Dose-dependent responses to DSLNnT (2) and DS′LNT (3) were tested at five different concentrations (30, 100, 300, 1,000, and 2,000 μM). These concentrations represent the range of DSLNT (1) in 10-fold diluted HMO sample to 7-fold concentrated HMO sample. As shown in Fig. 3, DSLNnT effects plateaued at high concentrations between 300 and 2,000 μM without reaching the maximum beneficial effects of HMOs. The effects of DS′LNT, however, continued to improve with higher concentrations, reaching similar protective effects as HMOs.
Figure 3.
Dose-dependent studies of DSLNnT and DS′LNT in the established neonatal rat NEC model. Each compound at each concentration was orally administered to 15 neonatal rats.
3. CONCLUSIONS
In summary, we have herein shown the efficient production of a group of disialyl glycans by chemoenzymatic or enzymatic synthetic approaches. Among them, DSLNnT (2) and DS′LNT (3) have been synthesized in gram-scales. Disialyl tetrasaccharides including disialyl Ta (DSTa, 6) and disialyl galactobiose (DSGalB, 7) have been synthesized using a novel chemoenzymatic method involving disialylation of β-thioethyl glycosides followed by removal of the thioethyl group. The effects of all six compounds synthesized have been studied in an established neonatal rat NEC model. The glycan size, sialyl linkages, the distance of two sialic acid residues, and sialic acid forms have been indicated as important factors for the NEC-preventing effects of disialyl glycans. The study helps to refine the structure requirement of the NEC-preventing effect of disialyl glycans and provides important dose-dependent information for using DSLNT analogs as potential therapeutics for NEC prevention in preterm infants. Longer disialyl glycans and glycans with more sialic acid residues can be explored further for potentially improved NEC-preventing effect.
4. EXPERIMENTAL SECTION
Materials and general methods
Chemicals and reagents were purchased from commercial sources and used without further purification unless stated otherwise. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance-800 NMR spectrometer and Bruker Avance-400 NMR spectrometer. High resolution electrospray ionization (ESI) mass spectra were obtained using Thermo Electron LTQ-Orbitrap Hybrid MS at the Mass Spectrometry Facility in the University of California, Davis. Silica gel 60 Å (230–400 mesh, Sorbent Technologies) was used for flash column chromatography. Thin-layer chromatography (TLC, Sorbent Technologies) was performed on silica gel plates using anisaldehyde sugar stain or 5% sulfuric acid in ethanol stain for detection. Gel filtration chromatography was performed using a column (100 cm × 2.5 cm) packed with Bio-Gel P-2 Fine resins (Bio-Rad). Lac, Gal, and GalNAc were purchased from Fisher Scientific, Neu5Ac was from Atomole Scientific, UTP and CTP were from Chemfun Medical Technology Co., and ATP was from Beta Pharm Inc. Lacto-N-tetraose (LNT) was from Elicityl (Crolles, France). Recombinant enzymes Bifidobacterium longum strain ATCC55813 N-acetylhexosamine-1-kinase (BLNahK),18 Pasteurella multocida N-acetylglucosamine uridyltransferase (PmGlmU),19 Pasteurella multocida inorganic pyrophosphatase (PmPpA),20 Neisseria meningitidis β1–3-N–acetylglucosaminyltransferase (NmLgtA),21 Escherichia coli galactokinase (EcGalK),22 Bifidobacterium longum UDP-sugar pyrophosphorylase (BLUSP),23 Neisseria meningitidis β1–4-galactosyltransferase (NmLgtB),20 Neisseria meningitidis CMP-sialic acid synthetase (NmCSS),13 Photobacterium damselae α2–6-sialyltransferase (Pd2,6ST),14 Pasteurella multocida α2–3-sialyltransferase 1 M144D mutant (PmST1 M144D),24 Bifidobacterium infantis D-galactosyl-β1,3-N-acetyl-D-hexosamine phosphorylase (BiGalHexNAcP),26 and Campylobacter jejuni β1–3-galactosyltransferase (CjCgtB)33 were expressed and purified as described previously. HMOs used were obtained previously.9 Major components are 2′-fucosyllactose (2′-FL), lacto-N-fucopentaose 1 (LNFP1), lacto-N-tetraose (LNT), lacto-N-fucopentaose 2 (LNFP2), 3′-sialyllactose (3′-SL), lacto-N-neotetraose (LNnT), 3-fucosyllactose (3FL), sialyllacto-N-tetraose c (LSTc), sialyllacto-N-tetraose b (LSTb), and disialyl lacto-N-tetraose (DSLNT).
One-pot two-enzyme gram-scale synthesis of Galβ1–3GalNAc (GNB)
A reaction mixture in Tris-HCl buffer (100 mM, pH 6.9) in a total volume of 270 mL containing GalNAc (3.00 g, 13.6 mmol), galactose (3.17 g, 17.6 mmol), ATP (9.73 g, 17.6 mmol), MgCl2 (20 mM), EcGalK (30 mg), and BiGalHexNAcP (50 mg) was incubated in a shaker at 37 °C by agitating at 140 rpm. The reaction was monitored by mass spectrometry. When an optimal yield was achieved (after 1 day), the enzyme reaction was terminated by incubating the reaction mixture in a boiling water bath for 15 min and the mixture was centrifuged to remove the precipitates. The supernatant was concentrated and purified by silica gel chromatography (EtOAc:MeOH:H2O = 2:1:0.1 by volume) to produce crude disaccharide Galβ1–3GalNAc as α- and β-anomers (4.6 g, over 90% purity). The crude product was further crystallized from water:EtOH = 1:1 (20 mL, 60 °C) to produce 1.63 g of GNB crystal as the α-anomer. The spectroscopic data coincided with a previous report.26
One-pot two-enzyme preparative-scale synthesis of Neu5Acα2–6Galβ1–4GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc (DSLNnT, 2)
A reaction mixture in a total volume of 50 mL containing Tris-HCl buffer (100 mM, pH 8.5), LNnT12 (1.05 g, 1.48 mmol), Neu5Ac (1.37 g, 4.43 mmol), CTP (2.35 g, 4.43 mmol), MgCl2 (20 mM), NmCSS (13 mg), and Pd2,6ST (16 mg) was incubated in a shaker at 37 °C for 2 d. The reaction was monitored by mass spectrometry. When an optimal yield was achieved, 50 mL of ethanol was added to the reaction mixture and the resulting mixture was incubated at 4 °C for 30 min. The precipitates were removed by centrifugation and the supernatant was concentrated and purified by a Bio Gel P-2 gel column (water was used as an eluent). Further purification was achieved by silica gel chromatography (EtOAc:MeOH:H2O = 4:3:2 by volume) and by Bio-Gel P-2 column (eluted with H2O) in the final step to produce Neu5Acα2–6Galβ1–4GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc hexasaccharide as white powder (2, 1.70 g, 96%). 1H NMR (800 MHz, D2O) δ 5.21 (d, J = 3.2 Hz, 0.4H), 4.71 (d, J = 8.0 Hz, 1H), 4.66 (d, J = 8.0 Hz, 0.6H), 4.43 (d, J = 8.0 Hz, 1H), 4.39 (d, J = 8.0 Hz, 1H), 4.17 (s, 1H), 4.00–3.28 (m, 37H), 2.70 (dd, J = 4.8 and 12.0 Hz, 1H), 2.66 (dd, J = 4.8 and 12.0 Hz, 1H), 2.04 (s, 3H), 2.02 (s, 6H), 1.72 (t, J = 12.0 Hz, 1H). 1.71 (t, J = 12.0 Hz, 1H). 13C NMR (151 MHz, D2O) δ 174.8, 173.4, 173.4, 103.4, 103.1, 103.1, 102.5, 100.1, 100.0, 95.5, 91.7, 82.0, 80.4, 79.6, 79.5, 74.5, 74.5, 74.1, 73.5, 73.1, 72.4, 72.4, 72.2, 72.2, 71.6, 71.6, 71.5, 70.9, 70.6, 69.8, 69.5, 69.5, 68.2, 68.2, 68.1, 68.0, 68.0, 63.3, 63.2, 62.5, 62.4, 60.1, 60.0, 59.9, 54.8, 51.7, 51.6, 40.0 39.9, 22.1, 21.9, 21.8. HRMS (ESI) m/z calculated for C48H78N3O37 [M−H]− 1288.4320, found 1288.4305.
One-pot two-enzyme preparative-scale synthesis of DS′LNT Neu5Acα2–6Galβ1–3GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc (DS′LNT, 3)
A reaction mixture in a total volume of 50 mL containing Tris-HCl buffer (100 mM, pH 8.5), LNT (1.0 g, 1.41 mmol), Neu5Ac (1.31 g, 4.24 mmol), CTP (2.23 g, 4.24 mmol), MgCl2 (20 mM), NmCSS (12 mg), and Pd2,6ST (15 mg) was incubated in a shaker at 37 °C for 2 d. The reaction was monitored by mass spectrometry. When an optimal yield was achieved, 50 mL of ethanol was added to the reaction mixture and the resulting mixture was incubated at 4 °C for 30 min. The precipitates were removed by centrifugation and the supernatant was concentrated and purified by a Bio Gel P-2 gel column (water was used as an eluent). Further purification was achieved by silica gel chromatography (EtOAc:MeOH:H2O = 4:3:2 by volume) and by Bio-Gel P-2 column (eluted with H2O) in the final step to produce Neu5Acα2–3Galβ1–3GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc hexasaccharide as white powder (3, 1.79 g, 95%). 1H NMR (800 MHz, D2O) δ 5.21 (d, J = 3.2 Hz, 0.4H), 4.72 (d, J = 8.0 Hz, 1H), 4.66 (d, J = 8.0 Hz, 0.6H), 4.43 (d, J = 8.0 Hz, 1H), 4.38 (d, J = 8.0 Hz, 1H), 4.18 (s, 1H), 3.99–3.28 (m, 37H), 2.72–2.69 (m, 2H), 2.03 (s, 6H), 2.02 (s, 3H), 1.73 (t, J = 12.0 Hz, 1H). 1.69 (t, J = 12.0 Hz, 1H). 13C NMR (151 MHz, D2O) δ 174.9, 174.8, 174.8, 173.4, 103.8, 103.1, 103.1, 102.5, 100.2, 100.1, 95.5, 91.7, 83.6, 82.0, 79.7, 79.6, 75.2, 74.6, 74.5, 74.2, 73.6, 73.5, 73.2, 72.4, 72.4, 72.3, 71.7, 71.7, 71.5, 70.9, 70.4, 69.9, 69.7, 69.7, 69.3, 68.6, 68.4, 68.3, 68.3, 68.2, 68.1, 68.1, 63.4, 63.4, 62.6, 60.6, 60.2, 60.0, 54.4, 51.7, 51.7, 40.1, 40.0, 22.2, 22.0. HRMS (ESI) m/z calculated for C48H78N3O37 [M−H]− 1288.4320, found 1288.4315.
One-pot two-enzyme preparative-scale synthesis of Neu5Gcα2–6Galβ1–3GlcNAcβ1–3(Neu5Gcα2–6)Galβ1–4Glc (Neu5Gc-DS′LNT, 4)
A reaction mixture in a total volume of 10 mL containing Tris-HCl buffer (100 mM, pH 8.5), LNT (150 mg, 0.21 mmol), Neu5Gc (206 mg, 0.63 mmol), CTP (335 mg, 0.63 mmol), MgCl2 (20 mM), NmCSS (3.0 mg), and Pd2,6ST (5.0 mg) was incubated in a shaker at 37 °C for 2 d. The reaction was monitored by mass spectrometry. When an optimal yield was achieved, 10 mL of ethanol was added to the reaction mixture and the resulting mixture was incubated at 4 °C for 30 min. The precipitates were removed by centrifugation and the supernatant was concentrated and purified by a Bio Gel P-2 gel column (water was used as an eluent). Further purification was achieved by silica gel chromatography (EtOAc:MeOH:H2O = 4:3:2 by volume) and by Bio-Gel P-2 column (eluted with H2O) in the final step to produce Neu5Gcα2–6Galβ1–3GlcNAcβ1–3(Neu5Gcα2–6)Galβ1–4Glc hexasaccharide as white powder (4, 272 mg, 94%). 1H NMR (800 MHz, D2O) δ 5.21 (d, J = 3.2 Hz, 0.4H), 4.72 (d, J = 8.0 Hz, 1H), 4.65 (d, J = 8.0 Hz, 0.6H), 4.41 (d, J = 8.0 Hz, 1H), 4.37 (d, J = 8.0 Hz, 1H), 4.17 (s, 1H), 4.10 (s, 4H), 3.99–3.28 (m, 37H), 2.73–2.70 (m, 2H), 2.01 (s, 3H), 1.74 (t, J = 12.0 Hz, 1H). 1.70 (t, J = 12.0 Hz, 1H). 13C NMR (201 MHz, D2O) δ 175.6, 175.5, 174.8, 173.4, 173.4, 103.8, 103.1, 103.1, 102.5, 100.2, 100.0, 95.5, 91.7, 83.5, 81.9, 79.7, 79.6, 75.2, 74.6, 74.5, 73.6, 73.5, 73.2, 72.3, 72.1, 72.1, 71.8, 71.7, 71.5, 70.9, 70.4, 69.8, 69.7, 69.6, 68.6, 68.3, 68.2, 68.1, 68.1, 68.0, 67.9, 63.4, 62.5, 60.9, 60.5, 60.1, 60.0, 54.4, 51.4, 51.4, 40.1, 40.0, 22.1. HRMS (ESI) m/z calculated for C48H78N3O39 [M−H]− 1320.4218, found 1320.4210.
One-pot two-enzyme preparative-scale synthesis of Neu5Acα2–3Galβ1–4GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc (DS′LNnT, 5)
(a) One-pot two-enzyme preparative-scale synthesis of GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc (11)
The reaction mixture in a total volume of 15 mL containing Tris-HCl buffer (100 mM, pH 8.5), Lc3 trisaccharide GlcNAcβ1–3Galβ1–4Glc12 (0.2 g, 0.367 mmol), Neu5Ac (0.17 g, 0.55 mmol), CTP (0.29 g, 0.55 mmol), MgCl2 (20 mM), NmCSS (4 mg), Pd2,6ST (5 mg) was incubated in a shaker with agitation (100 rpm) at 37 °C for 24 h. The product formation was monitored by TLC (i-PrOH:H2O:NH4OH = 5:2:1 by volume, and detected by p-anisaldehyde sugar stain) and mass spectrometry. When an optimal yield was achieved, ethanol (15 mL) was added and the mixture was incubated at 4 °C for 30 min. The precipitates were removed by centrifugation and the supernatant was concentrated and purified by a Bio-Gel P-2 gel column (water was used as an eluate). Further purification was achieved by silica gel chromatography (EtOAc:MeOH:H2O = 4:2:1 by volume) and finally Bio-Gel P-2 column (eluted with H2O) to produce GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc as white powder (11, 0.30 g, 96%). 1H NMR (800 MHz, D2O) δ 5.20 (d, J = 3.2 Hz, 0.4H), 4.66 (d, J = 8.0 Hz, 0.6H), 4.65 (d, J = 8.0 Hz, 1H), 4.41 (d, J = 8.0 Hz, 1H), 4.19–3.28 (m, 25H), 2.68 (dd, J = 4.8 and 12.0 Hz, 1H), 2.01 (s, 6H), 1.72 (t, J = 12.0 Hz, 1H). 13C NMR (201 MHz, D2O) δ 174.8, 173.4, 103.1, 102.8, 100.2, 95.5, 91.7, 82.0, 79.6, 79.5, 75.5, 74.6, 74.5, 73.6, 73.5, 73.1, 72.4, 71.7, 70.9, 69.9, 69.6, 68.2, 68.1, 63.3, 62.5, 60.4, 60.1, 55.5, 51.7, 40.0, 22.0, 21.9. HRMS (ESI) m/z calculated for C31H51N2O24 [M−H]− 835.2837, found 835.2833.
(b) One-pot four-enzyme preparative-scale synthesis of Galβ1–4GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc (12)
The reaction mixture in a total volume of 10 mL containing Tris-HCl buffer (100 mM, pH 8.0), tetrasaccharide 11 (0.15 g, 0.175 mmol), galactose (0.047 g, 0.261 mmol), ATP (0.144 g, 0.261 mmol), UTP (0.138 g, 0.261 mmol), MgCl2 (20 mM), EcGalK (3 mg), BLUSP (4 mg), NmLgtB (3 mg), and PmPpA (2 mg) was incubated in a shaker with agitation (100 rpm) at 30 °C for 2 days. The product formation was monitored by TLC (n-PrOH:H2O:NH4OH = 5:2:1 by volume, and detected by p-anisaldehyde sugar stain) and mass spectrometry. When an optimal yield was achieved, ethanol (10 mL) was added and the mixture was incubated at 4 °C for 30 min. The precipitates were removed by centrifugation and the supernatant was concentrated and purified by a Bio-Gel P-2 gel column (water was used as an eluate). Further purification was achieved by silica gel chromatography (EtOAc:MeOH:H2O = 4:2:1 by volume) and finally Bio-Gel P-2 column (eluted with H2O) to produce Galβ1–4GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc as white powder (12, 0.167 g, 94%). 1H NMR (800 MHz, D2O) δ 5.22 (d, J = 3.2 Hz, 0.4H), 4.68 (d, J = 8.0 Hz, 1H), 4.66 (d, J = 8.0 Hz, 0.6H), 4.47 (d, J = 8.0 Hz, 1H), 4.41 (d, J = 8.0 Hz, 1H), 4.19–3.28 (m, 31H), 2.70 (dd, J = 4.8 and 12.0 Hz, 1H), 2.02 (s, 6H), 1.72 (t, J = 12.0 Hz, 1H). 13C NMR (201 MHz, D2O) δ 174.8, 174.8, 173.4, 173.4, 103.1, 102.8, 102.7, 100.2, 95.5, 91.7, 82.1, 79.6, 79.5, 78.0, 75.2, 74.6, 74.5, 74.4, 73.6, 73.1, 72.4, 72.4, 72.1, 71.7, 71.5, 70.9, 70.9, 69.9, 69.6, 69.6, 68.5, 68.4, 68.2, 68.1, 63.3, 62.5, 60.9, 60.1, 60.0, 59.7, 55.1, 51.8, 51.7, 40.0, 22.1, 22.0. HRMS (ESI) m/z calculated for C37H61N2O29 [M−H]− 997.3365, found 997.3360.
(c) One-pot two-enzyme preparative-scale synthesis of DS′LNnT Neu5Acα2–3Galβ1–4GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc (5)
To prepare DS′LNnT, a reaction mixture in a total volume of 10 mL containing Tris-HCl buffer (100 mM, pH 8.5), pentasaccharide 12 (100 mg, 0.098 mmol), Neu5Ac (45 mg, 0.145 mmol), CTP (78 mg, 0.145 mmol), MgCl2 (20 mM), NmCSS (3.0 mg), and PmST1_M144D (3.0 mg) was incubated in a shaker at 37 °C for 24 h. The reaction was monitored by TLC (n-PrOH:H2O:NH4OH = 4:2:1 by volume and detected by p-anisaldehyde sugar stain) and mass spectrometry. When an optimal yield was achieved, 10 mL of ethanol was added to the reaction mixture and the resulting mixture was incubated at 4 °C for 30 min. The precipitates were removed by centrifugation and the supernatant was concentrated and purified by a Bio Gel P-2 gel column (water was used as an eluent). Further purification was achieved by silica gel chromatography (EtOAc:MeOH:H2O = 4:3:2 by volume) and by Bio-Gel P-2 column (eluted with H2O) in the final step to produce Neu5Acα2–3Galβ1–4GlcNAcβ1–3(Neu5Acα2–6)Galβ1–4Glc hexasaccharide as white powder (5, 121 mg, 93%). 1H NMR (800 MHz, D2O) δ 5.21 (d, J = 3.2 Hz, 0.4H), 4.68 (d, J = 8.0 Hz, 1H), 4.66 (d, J = 8.0 Hz, 0.6H), 4.54 (d, J = 8.0 Hz, 1H), 4.41 (d, J = 8.0 Hz, 1H), 4.18–3.26 (m, 38H), 2.74 (dd, J = 4.8 and 12.0 Hz, 1H), 2.70 (dd, J = 4.8 and 12.0 Hz, 1H), 2.01 (s, 9H), 1.80 (t, J = 12.0 Hz, 1H). 1.72 (t, J = 12.0 Hz, 1H). 13C NMR (201 MHz, D2O) δ 174.9, 174.8, 174.8, 173.8, 173.5, 173.4, 173.4, 103.1, 102.8, 102.7, 102.4, 100.2, 99.7, 95.5, 91.7, 82.0, 79.6, 79.5, 77.9, 75.3, 75.0, 74.6, 74.5, 74.4, 73.6, 73.1, 72.8, 72.4, 72.1, 71.7, 71.6, 70.9, 70.6, 69.6, 69.3, 68.1, 68.0, 67.4, 63.4, 63.4, 62.6, 62.5, 62.4, 60.9, 60.9, 60.1, 60.0, 59.7, 55.1, 51.7, 51.6, 40.0, 40.0, 39.5, 39.5, 22.1, 22.0, 22.0. HRMS (ESI) m/z calculated for C48H78N3O37 [M−H]− 1288.4320, found 1288.4318.
Synthesis of Neu5Acα2–6Galβ1–3(Neu5Acα2–6)GalNAc (DSTa, 6)
(a) Synthesis of ethyl-3,4,6-tri-O-acetyl-2-deoxy-2-phthalimido-1-thio-β-galactopyranoside (13)
D-Galactosamine hydrochloride (3.0 g, 13.9 mmol) was dissolved in a solution of 1 M NaOH (aq) (1.02 g, 24.7 mmol) (24 mL). Phthalic anhydride was added (3.1 g, 20.9 mmol) and the reaction mixture was stirred at room temperature for overnight to form a clear yellow aqueous solution which was washed with 3 × EtOAc (10 mL). The water was removed under reduced pressure to produce 2-(2-carboxybenzamido)-2-deoxy-D-galactopyranose as a yellow foam. The crude compound was dissolved in pyridine (20 mL) and acetic anhydride (14 mL, 0.15 mol) was added at 0 °C. The mixture was left to attain ambient temperature and was stirred for overnight. The reaction was quenched with MeOH at 0 °C. Solvents were evaporated and co-evaporated with toluene, and the brown suspension was dissolved in EtOAc and washed with brine, HCl solution (1 M), brine, satd. NaHCO3 (aq), and brine again. The solution was dried over MgSO4 and to remove residue solvent by treatment under reduced pressure to produce 1,3,4,6-tetra-O-acetyl-2-deoxy-2-phthalimido-D-galactopyranoside as a yellow foam. The crude compound (4.6 g, 9.6 mmol) was dissolved in dry CH2Cl2 (45 mL). At 0 °C, BF3·OEt2 (6.6 mL, 48 mmol) was added drop-wisely and the mixture was stirred for 10 min under argon. EtSH (2.2 mL, 29 mmol) was added and the reaction was stirred at room temperature for 20 h. TLC analysis indicated completion of the reaction and quenching was done with Et3N at 0 °C. After removal of the solvent, the crude residue was purified by flash chromatography (EtOAc:Hexane = 2:3 by volume) to produce pure compound ethyl-3,4,6-tri-O-acetyl-2-deoxy-2-phthalimido-1-thio-β-galactopyranoside 13 as white foam (2.9 g, 6.05 mmol). Yield was 43.5% over 3 steps. 1H NMR (400 MHz, CDCl3) δ 7.92–7.82 (m, 2H), 7.81–7.71 (m, 2H), 5.85 (dd, J = 11.0 and 3.3 Hz, 1H), 5.53 (d, J = 3.2 Hz, 1H), 5.48 (d, J = 10.5 Hz, 1H), 4.62 (t, J = 10.7 Hz, 1H), 4.27–4.08 (m, 3H), 2.80–2.60 (m, 2H), 2.21 (s, 3H), 2.07 (s, 3H), 1.86 (s, 3H), 1.23 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 170.4, 170.3, 169.8, 168.0, 167.4, 134.4, 134.3, 131.6, 131.3, 123.7, 123.6, 81.7, 74.5, 68.8, 67.0, 61.7, 50.2, 24.6, 20.8, 20.7, 20.5, 15.0.
(b) Synthesis of ethyl 2-deoxy-2-acetimido-1-thio-β-D-galactopyranoside (14)
Ethyl 3,4,6-tri-O-acetyl-2-deoxy-2-phthalimido-1-thio-β-D-galactopyranoside (13) (2.8 g, 5.17 mmol) was dissolved in dry MeOH (65 mL), and hydrazine monohydrate (15 mL, 0.31 mol, 5 eq.) was added. The mixture was refluxed under argon for 8 h. The reaction mixture was concentrated and co-evaporated 2 × with EtOH (10 mL). The residue was purified by silica gel flash chromatography (NH4OH:MeOH:EtOAc = 1:5:30 by volume) to yield ethyl 2-deoxy-2-amino-1-thio-β-D-galactopyranoside as a pale-yellow solid. The crude material was dissolved in MeOH, acetic anhydride (1.55 mL, 15.3 mmol) was added at 0 °C. The reaction mixture was stirred at room temperature for overnight. Upon solvent evaporation under reduced pressure, the crude product was purified by flash chromatography (EtOAc:MeOH:H2O = 60:10:1 by volume) to produce ethyl 2-deoxy-2-acetimido-1-thio-β-D-galactopyranoside as white foam (14, GalNAcβSEt) (1.44 g). Yield was 92.9% over two steps. 1H NMR (400 MHz, D2O) δ 4.49 (d, J = 10.4 Hz, 1H), 3.90 (d, J = 3.3 Hz, 1H), 3.87 (t, J = 10.4 Hz, 1H), 3.75–3.59 (m, 3H), 2.75–2.56 (m, 2H), 1.96 (s, 3H), 1.16 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, D2O) δ 174.6, 84.4, 78.9, 72.1, 67.9, 61.1, 51.3, 24.5, 22.2, 14.3.
(c) Synthesis of ethyl β-D-galactopyranosyl-(1–3)-2-acetamido-2-deoxy-β-D-glucopyranoside (15)
GalNAcβSEt 14 (200 mg, 0.75 mmol), Gal (203 mg, 1.13 mmol), ATP (574 mg, 1.13 mmol) were dissolved in water in a 50 mL centrifuge tube containing Tris-HCl buffer (100 mM, pH 6.8) and MgCl2 (20 mM). After the addition of appropriate amount of EcGalK (5.4 mg), BiGalHexNAcP (15 mg), water was added to bring the volume of the reaction mixture to 30 mL. The reaction was carried out by incubating the solution in an incubator shaker at 37 °C with agitating at 100 rpm. After 36 h, the reaction was quenched by adding methanol (30 mL) and incubating at 4 °C for 30 min. The mixture was then centrifuged to remove precipitates. The supernatant was concentrated and purified by silica gel chromatography (EtOAc:MeOH:H2O = 7:3:0.3 to 6:4:0.4 by volume) to produce a white solid. Then it was purified by Bio-Gel P-2 gel filtration column (2.5 × 80 cm) to produce ethyl β-D-galactopyranosyl-(1→3)-2-acetamido-2-deoxy-β-D-glucopyranoside as white powder (15, Galβ1–3GalNAcβSEt) (199 mg, 62%). 1H NMR (400 MHz, D2O) δ 4.53 (d, J = 10.5 Hz, 1H), 4.37 (d, J = 7.7 Hz, 1H), 4.13 (d, J = 3.1 Hz, 1H), 4.00 (t, J = 10.5 Hz, 1H), 3.82 (d, J = 3.4 Hz, 1H), 3.79 (dd, J = 10.4 and 3.1 Hz, 1H), 3.73–3.60 (m, 5H), 3.57 (dd, J = 7.8 and 4.5 Hz, 1H), 3.53 (dd, J = 10.0 and 3.6 Hz, 1H), 3.44 (dd, J = 9.8 and 7.8 Hz, 1H), 2.76–2.56 (m, 2H), 1.93 (s, 3H), 1.16 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, D2O) δ 174.5, 104.8, 84.2, 80.9, 78.6, 75.0, 72.5, 70.6, 68.6, 68.2, 61.0, 61.0, 50.0, 24.4, 22.3, 14.3. HRMS (ESI) m/z calculated for C16H29NNaO10S [M+Na]+ 450.1410, found 450.1422.
(d) Synthesis of tetrasaccharide thioglycoside 16
Galβ1–3GalNAcβSEt 15 (106 mg, 0.25 mmol), Neu5Ac (230 mg, 0.75 mmol), and CTP (392 mg, 0.75 mmol) were dissolved in water in a 15 mL centrifuge tube containing Tris-HCl buffer (100 mM, pH 8.5) and MgCl2 (20 mM). After the addition of appropriate amounts of NmCSS (3 mg) and Pd2,6ST (4 mg), water was added to bring the volume of the reaction mixture to 10 mL. The reaction was carried out by incubating the solution in an incubator shaker at 37 °C by agitating at 100 rpm. After 3 day, the reaction was quenched by adding 10 mL of methanol and the mixture was incubated at 4 °C for 30 min. The mixture was then centrifuged to remove precipitates. The supernatant was concentrated and purified by silica gel (EtOAc:MeOH:H2O = 4:2:0.8 by volume) and Bio-Gel P-2 gel filtration column (2.5 × 80 cm) to produce a white solid (16, 249 mg, 93%). 1H NMR (400 MHz, D2O) δ 4.49 (d, J = 10.5 Hz, 1H), 4.37 (d, J = 7.8 Hz, 1H), 4.10 (d, J = 3.1 Hz, 1H), 4.01 (t, J = 10.4 Hz, 1H), 3.89 (dd, J = 10.4 and 8.5 Hz, 1H), 3.85 (dd, J = 6.9 and 3.1 Hz, 1H), 3.83–3.69 (m, 8H), 3.68–3.47 (m, 13H), 3.42 (dd, J = 9.9, 7.7 Hz, 1H), 2.76–2.58 (m, 4H), 1.96 (s, 6H), 1.94 (s, 3H), 1.62 (t, J = 12.1 Hz, 1H), 1.61 (t, J = 12.1 Hz, 1H), 1.18 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, D2O) δ 175.1, 175.0, 174.5, 173.5, 173.4, 104.5, 100.6, 100.4, 84.6, 80.1, 80.1, 77.2, 74.3, 73.1, 72.7, 72.6, 72.3, 71.8, 71.6, 70.5, 69.5 68.4, 68.3, 68.2, 68.2, 68.1, 64.0, 63.2, 62.7, 59.3, 52.0, 51.9, 50.0, 40.2, 40.2, 24.7, 22.3, 22.0, 14.4. HRMS (ESI) m/z calculated for C38H62N3O26S [M−H]− 1008.3348, found 1008.3351.
(e) Synthesis of Neu5Acα2–6Galβ1–3(Neu5Acα2–6)GalNAc (6)
Tetrasaccharide thioglycoside 16 (100 mg, 0.099 mmol) was dissolved in acetone/water (5 mL, 50% v/v). The reaction mixture was cooled down to 0 °C and NBS (35 mg, 0.20 mmol) was added in one portion in the dark. After 30 min of vigorous stirring, acetone was evaporated under reduced pressure. The residue was purified by flash chromatography (EtOAc:MeOH:H2O = 4:2:1 by volume) to produce the desired disialyl glycan DSTa 6 as a white powder (90 mg, 94%). 1H NMR (800 MHz, D2O) δ 5.18 (d, J = 3.2 Hz, 0.6H), 4.64 (d, J = 8.0 Hz, 0.4H), 4.49 (d, J = 8.0 Hz, 0.6H), 4.42 (d, J = 8.0 Hz, 0.4H), 4.29–3.48 (m, 26H), 2.74–2.70 (m, 2H), 2.03 (s, 3H), 2.02 (s, 3H), 2.01 (s, 3H), 1.71–1.66 (m, 2H). 13C NMR (201 MHz, D2O) δ 174.9, 174.9, 174.9, 174.8, 174.5, 173.4, 173.4, 173.3, 104.4, 104.2, 100.5, 100.5, 100.3, 100.3, 95.1, 91.1, 79.2, 76.2, 73.4, 73.0, 72.6, 72.6, 72.5, 72.5, 72.3, 72.3, 71.7, 71.6, 71.4, 70.4, 70.4, 68.9, 68.6, 68.3, 68.2, 68.2, 68.1, 68.1, 68.1, 68.1, 68.1, 68.0, 64.2, 64.1, 63.1, 63.0, 62.6, 62.5, 62.5, 52.3, 51.9, 51.9, 51.7, 49.0, 40.2, 40.1, 40.1, 40.0, 22.2, 22.0, 21.9. HRMS (ESI) m/z calculated for C36H58N3O27 [M−H]− 964.3263, found 964.3241.
Synthesis of Neu5Acα2–6Galβ1–3(Neu5Acα2–6)Gal (DSGalB, 7)
(a) Synthesis of disaccharide thioglycoside 18
The reaction mixture in a total volume of 10 mL containing Tris-HCl buffer (100 mM, pH 8.0), GalβSEt32 (17, 60 mg, 0.27 mmol), galactose (0.072 g, 0.40 mmol), ATP (0.221 g, 0.40 mmol), UTP (0.212 g, 0.40 mmol), MgCl2 (20 mM), EcGalK (2 mg), BLUSP (3 mg), PmPpA (2 mg), and CjCgtB (3 mg) was incubated in a shaker with agitation (100 rpm) at 37 °C for 2 days. The reaction was quenched by adding 10 mL of ethanol and the mixture was incubated at 4 °C for 30 min. The mixture was then centrifuged to remove precipitates. The supernatant was concentrated and purified by flash chromatography (EtOAc:MeOH:H2O = 4:2:0.2 by volume) to produce disaccharide 18 as white solid (52 mg, 50%). 1H NMR (600 MHz, D2O) δ 4.48 (d, J = 7.7 Hz, 1H), 4.37 (d, J = 9.9 Hz, 1H), 4.07 (d, J = 3.1 Hz, 1H), 3.76 (d, J = 2.6 Hz, 1H), 3.67 (dd, J = 9.4 and 3.2 Hz, 1H), 3.64–3.36 (m, 9H), 2.68–2.53 (m, 2H), 1.12 (t, J = 7.4 Hz, 3H). 13C NMR (151 MHz, D2O) δ 104.2, 85.2, 83.2, 78.5, 75.0, 72.4, 71.0, 68.6, 68.6, 68.5, 60.9, 60.9, 24.0, 14.4.
(b) Synthesis of tetrasaccharide thioglycoside 19
Galβ1–3GalβSEt 18 (20 mg, 0.052 mmol), Neu5Ac (64 mg, 0.21 mmol), and CTP (109 mg, 0.21 mmol) were dissolved in water in a 15 mL centrifuge tube containing Tris-HCl buffer (100 mM, pH 8.5) and MgCl2 (20 mM). After the addition of appropriate amounts of NmCSS (2 mg) and Pd2,6ST (3 mg), water was added to bring the total volume of the reaction mixture to 5 mL. The reaction was carried out by incubating the solution in an incubator shaker at 37 °C by agitating at 100 rpm. After 2 day, the reaction was quenched by adding 5 mL of methanol and the mixture was incubated at 4 °C for 30 min. After being centrifuged for 30 min at 7,000 × g in a Sorvall legend T/RT bench-top centrifuge, the clear supernatant was transferred to a 100 mL round-bottom flask. The protein precipitates were washed with 4 mL of deionized water. The mixture was then centrifuged to remove precipitates. The supernatant was concentrated and purified by Bio-Gel P-2 gel filtration column (2.5 × 80 cm) and flash chromatography (EtOAc:MeOH:H2O = 4:2:0.8 by volume) to produce compound 19 as a white solid (38 mg, 84%). 1H NMR (800 MHz, D2O) δ 4.63 (d, J = 7.8 Hz, 1H), 4.50 (d, J = 9.9 Hz, 1H), 4.20 (d, J = 3.2 Hz, 1H), 3.95 (dd, J = 10.1 and 8.5 Hz, 1H), 3.92 (d, J = 3.4 Hz, 1H), 3.92–3.77 (m, 9H), 3.76–3.61 (m, 10H), 3.60–3.53 (m, 4H), 2.82–2.68 (m, 4H), 2.03 (s, 3H), 2.02 (s, 3H), 1.70 (t, J = 12.2 Hz, 1H), 1.69 (t, J = 12.2 Hz, 1H), 1.28 (t, J = 7.3 Hz, 3H). 13C NMR (201 MHz, D2O) δ 174.9, 174.9, 173.2, 173.1, 103.8, 100.3, 100.2, 95.1, 85.4, 82.7, 77.0, 73.2, 72.6, 72.5, 72.3, 71.5, 71.4, 70.9, 68.6, 68.4, 68.2, 68.1, 68.0, 68.0, 63.9, 63.1, 62.6, 62.5, 51.9, 51.7, 40.1, 40.0, 24.3, 21.9, 21.9, 14.5. HRMS (ESI) m/z calculated for C36H59N2O26S [M−H]− 967.3082, found 967.3078.
(c) Synthesis of Neu5Acα2–6Galβ1–3(Neu5Acα2–6)Gal (7)
Tetrasaccharide thioglycoside 19 (18 mg, 0.019 mmol) was dissolved in acetone/water (1 mL, 50% v/v). The reaction mixture was cooled down to 0 °C and NBS (8 mg, 0.046 mmol) was added in one portion in the dark. After 20 min of vigorous stirring, acetone was evaporated under reduced pressure. The residue was purified by flash chromatography (EtOAc:MeOH:H2O = 4:2:1 by volume) to produce DSGalB (7) as a white powder (15 mg, 87%). 1H NMR (800 MHz, D2O) δ 5.25 (d, J = 3.2 Hz, 0.4H), 4.61 (d, J = 8.0 Hz, 0.6H), 4.60 (d, J = 8.0 Hz, 1H), 4.25–3.51 (m, 26H), 2.74–2.69 (m, 2H), 2.03 (s, 6H), 1.73–1.66 (m, 2H). 13C NMR (201 MHz, D2O) δ 175.0, 174.9, 174.9, 173.4, 173.4, 173.4, 173.3, 103.8, 100.4, 100.3, 100.3, 100.3, 96.0, 92.0, 88.9, 81.9, 78.9, 74.4, 73.3, 73.1, 73.1, 72.5, 72.5, 72.5, 72.5, 72.3, 72.3, 71.6, 71.6, 71.4, 70.9, 70.9, 70.8, 68.9, 68.8, 68.4, 68.3, 68.2, 68.1, 68.0, 67.4, 64.2, 64.1, 63.1, 63.0, 62.6, 62.5, 59.4, 51.9, 51.9, 51.7, 40.2, 40.1, 40.0, 21.9, 21.9. HRMS (ESI) m/z calculated for C34H55N2O27 [M−H]− 923.2998, found 923.2991.
In vivo Efficacy of Disialyl Glycans in Preventing Necrotizing Enterocolitis (NEC) in a Preclinical Neonatal Rat Model
In vivo efficacy was tested in an established NEC model in neonatal rats as previously described.9 Single dose (300 μM) and dose-dependent (30, 100, 300, 1,000, and 2,000 μM) studies were carried out in the same manner. The concentration of HMOs used was 10 mg/mL, the same as that used previously.9,12 Briefly, pregnant time-dated Sprague-Dawley rats were induced at term using Pitocin (1–2 U per animal). Immediately after birth, neonatal rats were randomized into the different study groups. Animals in the dam-fed (DF) group remained with the dam. All other animals were separated from the dam, housed in a temperature- and humidity-controlled incubator and orally gavaged with a special rodent formula (0.2 mL; without and with different oligosaccharides) twice daily. The formula approximates the protein and caloric content of rat breast milk and consists of 15 g Similac 60/40 (Ross Pediatrics, Columbus, Ohio, USA) in 75 mL of Esbilac canine milk replacer (Pet-Ag, Hampshire, Illinois, USA). All animals, dam-fed and gavaged, were exposed to 10 min of hypoxia (5% O2, 95% N2) thrice daily in a modular chamber. All animals were sacrificed 96 h post-partum; their intestines were collected and inspected for the presence of gross necrotic changes or pneumatosis intestinalis. A 0.5 cm section of the terminal ileum was prepared for H&E staining per standard protocols and scored blindly by two investigators based on morphological changes that included epithelial sloughing, villus oedema, infiltration of neutrophils, apoptosis of villus enterocytes, crypt hyperplasia and misaligned nuclei in the epithelium. If at least one pathology sign was observed, a score of 0.5–1.5 was assigned depending on severity. Two or three signs together resulted in a score of 2–3. The maximum score of 4 was given in case of complete obliteration of the epithelium with or without intestinal perforation. The animal protocol was approved by The University of California San Diego Institutional Animal Care and Use Committee (IACUC), and complied with the Guide for the Care and Use of Laboratory Animals.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R43HD087077 (to H. Y.) and R01HD065122 (to X. C.) as well as a scholarship from the China Scholarship Council (to X. Y.). The support of the Family Larsson-Rosenquist Foundation (to L.B.) is gratefully acknowledged.
Footnotes
Notes
HY, YL, and XC are co-founders of Glycohub, Inc., a company focused on the development of carbohydrate-based reagents, diagnostics, and therapeutics.
Supporting Information. 1H and 13C NMR spectra of the glycans synthesized. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Neu J, Walker WA. N Engl J Med. 2011;364:255. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holman RC, Stoll BJ, Clarke MJ, Glass RI. Am J Public Health. 1997;87:2026. doi: 10.2105/ajph.87.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rees CM, Pierro A, Eaton S. Arch Dis Child Fetal Neonatal Ed. 2007;92:F193. doi: 10.1136/adc.2006.099929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dicken BJ, Sergi C, Rescorla FJ, Breckler F, Sigalet D. J Pediatr Surg. 2011;46:1618. doi: 10.1016/j.jpedsurg.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Clark RH, Gordon P, Walker WM, Laughon M, Smith PB, Spitzer AR. J Perinatol. 2012;32:199. doi: 10.1038/jp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blakely ML, Lally KP, McDonald S, Brown RL, Barnhart DC, Ricketts RR, Thompson WR, Scherer LR, Klein MD, Letton RW, Chwals WJ, Touloukian RJ, Kurkchubasche AG, Skinner MA, Moss RL, Hilfiker ML. Ann Surg. 2005;241:984. doi: 10.1097/01.sla.0000164181.67862.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schanler RJ, Lau C, Hurst NM, Smith EO. Pediatrics. 2005;116:400. doi: 10.1542/peds.2004-1974. [DOI] [PubMed] [Google Scholar]
- 8.Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O’Shea TM. J Perinatol. 2007;27:428. doi: 10.1038/sj.jp.7211758. [DOI] [PubMed] [Google Scholar]
- 9.Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, Choudhury B, Grishin AV, Ford HR, Bode L. Gut. 2012;61:1417. doi: 10.1136/gutjnl-2011-301404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Niekerk E, Autran CA, Nel DG, Kirsten GF, Blaauw R, Bode L. J Nutr. 2014;144:1227. doi: 10.3945/jn.113.187799. [DOI] [PubMed] [Google Scholar]
- 11.Autran CA, Kellman BP, Kim JH, Asztalos E, Blood AB, Spence EC, Patel AL, Hou J, Lewis NE, Bode L. Gut. 2017 doi: 10.1136/gutjnl-2016-312819. [DOI] [PubMed] [Google Scholar]
- 12.Yu H, Lau K, Thon V, Autran CA, Jantscher-Krenn E, Xue M, Li Y, Sugiarto G, Qu J, Mu S, Ding L, Bode L, Chen X. Angew Chem Int Ed. 2014;53:6687. doi: 10.1002/anie.201403588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu H, Yu H, Karpel R, Chen X. Bioorg Med Chem. 2004;12:6427. doi: 10.1016/j.bmc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 14.Yu H, Huang S, Chokhawala H, Sun M, Zheng H, Chen X. Angew Chem Int Ed. 2006;45:3938. doi: 10.1002/anie.200600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varki A. Am J Phys Anthropol. 2001;(Suppl 33):54. doi: 10.1002/ajpa.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cazet A, Julien S, Bobowski M, Burchell J, Delannoy P. Breast Cancer Res. 2010;12:204. doi: 10.1186/bcr2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C, Zhang Y, Xue M, Liu XW, Li Y, Chen X, Wang PG, Wang F, Cao H. Chem Commun. 2015;51:7689. doi: 10.1039/c5cc01330e. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Yu H, Chen Y, Lau K, Cai L, Cao H, Tiwari VK, Qu J, Thon V, Wang PG, Chen X. Molecules. 2011;16:6396. doi: 10.3390/molecules16086396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Thon V, Li Y, Yu H, Ding L, Lau K, Qu J, Hie L, Chen X. Chem Commun. 2011;47:10815. doi: 10.1039/c1cc14034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau K, Thon V, Yu H, Ding L, Chen Y, Muthana MM, Wong D, Huang R, Chen X. Chem Commun. 2010;46:6066. doi: 10.1039/c0cc01381a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Xue M, Sheng X, Yu H, Zeng J, Thon V, Chen Y, Muthana MM, Wang PG, Chen X. Bioorg Med Chem. 2016;24:1696. doi: 10.1016/j.bmc.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Liu Z, Zhang J, Zhang W, Kowal P, Wang PG. Chembiochem. 2002;3:47. doi: 10.1002/1439-7633(20020104)3:1<47::AID-CBIC47>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 23.Muthana MM, Qu J, Li Y, Zhang L, Yu H, Ding L, Malekan H, Chen X. Chem Commun. 2012;48:2728. doi: 10.1039/c2cc17577k. [DOI] [PubMed] [Google Scholar]
- 24.Sugiarto G, Lau K, Qu J, Li Y, Lim S, Mu S, Ames JB, Fisher AJ, Chen X. ACS Chem Biol. 2012;7:1232. doi: 10.1021/cb300125k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irazoqui FJ, Sendra VG, Lardone RD, Nores GA. Immunol Cell Biol. 2005;83:405. doi: 10.1111/j.1440-1711.2005.01348.x. [DOI] [PubMed] [Google Scholar]
- 26.Yu H, Thon V, Lau K, Cai L, Chen Y, Mu S, Li Y, Wang PG, Chen X. Chem Commun. 2010;46:7507. doi: 10.1039/c0cc02850a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng X, Yao W, Cheng J, Zhang X, Jin L, Yu H, Chen X, Wang F, Cao H. J Am Chem Soc. 2014;136:5205. doi: 10.1021/ja5000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellervik U, Magnusson G. Carbohydr Res. 1996;280:251. doi: 10.1016/0008-6215(95)00318-5. [DOI] [PubMed] [Google Scholar]
- 29.Ichinose H, Kuno A, Kotake T, Yoshida M, Sakka K, Hirabayashi J, Tsumuraya Y, Kaneko S. Appl Environ Microbiol. 2006;72:3515. doi: 10.1128/AEM.72.5.3515-3523.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben XM, Zhou XY, Zhao WH, Yu WL, Pan W, Zhang WL, Wu SM, Van Beusekom CM, Schaafsma A. Chin Med J. 2004;117:927. [PubMed] [Google Scholar]
- 31.Boehm G, Moro G. J Nutr. 2008;138:1818S. doi: 10.1093/jn/138.9.1818S. [DOI] [PubMed] [Google Scholar]
- 32.Das SK, Roy N. Carbohydr Res. 1996;296:275. [Google Scholar]
- 33.Malekan H, Fung G, Thon V, Khedri Z, Yu H, Qu J, Li Y, Ding L, Lam KS, Chen X. Bioorg Med Chem. 2013;21:4778. doi: 10.1016/j.bmc.2013.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu H, Li Y, Zeng J, Thon V, Nguyen DM, Ly T, Kuang HY, Ngo A, Chen X. J Org Chem. 2016;81:10809. doi: 10.1021/acs.joc.6b01905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.