Abstract
Introduction:
Type 2 Alcoholism is characterized by low serotonin system functioning and has a high degree of heritability, with offspring of alcoholics often showing a reduced response to the intoxicating effects of ethanol, which is thought to be marker for future alcohol use disorders (AUDs). As such, an important aim of studies investigating the origins of AUDs is understanding the relationship between serotonin system functioning and level of intoxication. A nonhuman primate model was used to evaluate observational ratings of sensitivity to ethanol and to further investigate the relationship between central serotonin activity and behavioral response to ethanol.
Materials and Methods:
Cerebrospinal fluid (CSF) concentrations of 5-hydroxyindoleacetic acid (5-HIAA) were obtained from four cohorts of alcohol-naïve, adolescent rhesus macaques (N=82, 45 females, 37 males). One to three months after the CSF sample, subjects were administered a standardized intravenous (IV) ethanol bolus (males: 2.1 g/kg body weight, females: 2.0 g/kg body weight), placed into an open top, clear Plexiglas™ chamber suspended from the ceiling, and their latency to escape was recorded as a measure of the degree of intoxication. Thereafter, subjects were rated using a Likert scale for the degree of intoxication during a 30-minute observation period.
Results:
Our results indicate that latency to escape from the chamber was associated with intoxication ratings (p = 0.0001) following the standardized IV administration of ethanol. Low CSF 5-HIAA concentrations predicted short escape latency (p = 0.006) and were associated with low intoxication ratings (p = 0.02), indicating that low CNS serotonin functioning is related to relative insensitivity to the intoxicating effects of alcohol.
Discussion:
Our study shows that, in monkeys exposed to alcohol for the first time, objective measures of intoxication are associated with subjective ratings for intoxication and both were associated with CSF 5-HIAA concentrations. Our data confirm and extend the finding that low CNS serotonin functioning is predictive of intrinsic low sensitivity to the intoxicating effects of ethanol.
Keywords: Alcohol Sensitivity, Alcohol Use Disorders, Serotonin, Rhesus Macaques, Tolerance
Introduction
Type 2 alcoholism, sometimes referred to as early-onset alcoholism, is characterized by antisocial and impulsive behavior (Bordukalo-Niksic et al., 2012), is more common among men, and is associated with the severity of the father’s alcoholism and the father’s criminality (Cloninger et al., 1996). Though outnumbered by men with Type 2 alcoholism substantially, Type 2 alcoholism is also present among women (Traber et al., 2009); however, in monkeys, Type 2-like alcohol use and its related behaviors are, on average, equally distributed between the sexes. There is evidence that etiologically, low CNS serotonin functioning mediates, at least in part, Type 2 alcoholism and alcohol abuse. Low concentrations of the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the cerebrospinal fluid (CSF), is characteristic of Type 2 alcoholics and is found in their first-degree alcoholic relatives, even during periods of sobriety (Ballenger et al., 1979; Fils-Aime et al., 1996; Hallikainen et al., 1999; Linnoila et al., 1994; Virkkunen & Linnoila, 1990; Virkkunen & Linnoila, 1993). Males at risk for early onset alcoholism exhibit decreased intoxication to identical doses of alcohol than do those with a non-alcoholic family history (Schuckit, 1985a). Furthermore, the intensity of response to an oral alcohol challenge in non-alcoholic individuals is predictive of later alcohol problems (Schuckit & Smith, 1997, Schuckit & Smith, 2006), with an effect size similar to that seen between family history and alcohol use disorders (AUDs) (Schuckit et al., 2006).
Human studies suggest that inherent sensitivity to alcohol or as Schuckit terms it, a “low level of response” to ethanol is heritable (Cloninger et al., 1981; Hinckers et al., 2006; Schuckit, 1984; Schuckit, 1985a; Schuckit, 1985b; Schuckit, 1985c). However, when tolerance is measured in the laboratory as a predictor of future alcohol-related problems, it is likely confounded by previous drinking history. Self-reported drinking history, for example, predicts the degree of intoxication among non-alcoholics when given identical doses of alcohol (Fillmore & Vogel-Sprott, 1996; Hiltunen, 1997). While Schuckit and colleagues have shown that this association remains even after statistically controlling for family history, research conducted with humans on intrinsic sensitivity to the psychomotor effects of alcohol and its contribution to future alcohol problems is compromised to some degree by an individual’s prior alcohol use history. Furthermore, due to the difficulties of working with self-reports, it is impossible to know whether the predictions are accurate.
To address the association between central serotonin functioning and alcohol sensitivity and rates of intoxication without the potential confounds in self-reports of alcohol-use history, we employ a nonhuman primate model. Rhesus macaques (Macaca mulatta) provide an excellent paradigm for human alcohol use and abuse because of their genetic similarities (Gibbs et al., 2007), parallels in social and sex differences, as well as parallels in temperament-related alcohol abuse (Higley et al., 1991). Like humans, rhesus monkeys show similar rates of high alcohol intake with about 10–20% drinking to intoxication on a daily basis (Higley et al., 1996; Baker et al., 2014), and rates of intake are higher among subjects raised in parentally-absent, peer-reared groups, although the effect of rearing is attenuated by social setting (Higley et al., 1991).
Other animal research has also demonstrated a relationship between heritable central serotonin deficits and low sensitivity to the intoxicating effects of alcohol. The selectively-bred, alcohol-preferring P-rat is relatively insensitive to the intoxicating effects of alcohol and exhibits low levels of CNS serotonin (Li et al.,1993; Li et al., 1986; Murphy et al., 1982). Barr and colleagues (2003a) showed that alcohol-naïve rhesus monkeys with low cisternal CSF 5-HIAA concentrations are rated as less intoxicated than monkeys with high CSF 5-HIAA concentrations, showing, for the first time, that individuals with chronic low CNS serotonin activity exhibit inherently low sensitivity to the intoxicating effects of alcohol. Low or impaired CNS serotonin functioning shows a high degree of inter-individual stability, beginning early in infancy, and is positively correlated with adult individual differences in macaques (Westergaard et al., 1999), suggesting that stable inter-individual differences in alcohol sensitivity may, in part, have roots in stable serotonin functioning. However, with the exception of our earlier studies using nonhuman primates, which measured the degree of intoxication using a Likert scale and a combination of behaviors thought to reflect the degree of intoxication (Barr et al., 2007; Schwandt et al., 2008), few studies using nonhuman primates focus on standardized, objective measurements of intoxication, and seldom do they assess intoxication in the context of potential etiological objective measurements of CNS functioning, such as measuring concentrations of 5-HIAA in the CSF. Moreover, monkeys that are active, (i.e., move a lot) are more likely to fall and stumble, confounding the measurement of intoxicated behaviors. Thus, one purpose of the present study is to assess the validity of the subjective ratings of intoxication through the use of objective measurements of intoxication, filling in the gap between subjective observational ratings and behavioral measurements, which can be biased by differences in general overall activity. Because nonhuman primates are a lot like humans, validating subjective ratings of intoxication in relation to CNS serotonin system functioning could provide insight to the underlying etiology, and lead to a standardized laboratory test that could be used as a diagnostic tool to assess potential risk for AUDs in humans.
This study seeks to assess the validity of subjective ratings of intoxication and to assess the relationship between CNS serotonin functioning, as measured by cisternal CSF 5-HIAA concentrations, and its relationship to sensitivity to the intoxicating effects of alcohol. We propose assessing subjects’ degree of intoxication by measuring their relative latency to escape from a tall, suspended Plexiglas™ chamber and their CSF 5-HIAA concentrations, and to test the relationship of both to subjective ratings for intoxication. Based on our earlier findings, we hypothesize that alcohol-naive subjects with low CSF 5-HIAA concentrations will show relative insensitivity to the intoxicating effects of alcohol, allowing them to more rapidly escape from the test chamber, such that a short latency to escape from chamber will be positively correlated with cisternal CSF 5-HIAA concentrations.
Materials and Methods
Subjects and Background
Subjects were N=82 (45 females, 54.9% of total sample), alcohol-naïve, adolescent rhesus macaques (mean age females: 39.35 months, mean age males: 38.81months), born over a four-year period (1991–1994), and housed at the National Institutes of Health Primate Laboratory in Poolesville, Maryland. As part of a larger research paradigm, all subjects were randomly assigned to one of three rearing conditions for the first six months of life, described in greater detail elsewhere (Higley et al., 1991; Higley et al., 1996a). Briefly, 1. Mother-reared subjects (MR, N=36) were reared in groups approximating natural conditions, living in indoor-outdoor runs, 2. Peer-reared (PR, N=30) subjects were removed from their mothers at birth, and raised in a neonatal nursery for their first month of life, after which they were placed with three to four other age-mates with whom they had constant companionship, 3. Surrogate-peer-reared (SPR, N=16) subjects were raised identically to the PR subjects for the first 30 days. Thereafter, SPR subjects were allowed visual, but no social contact with other agemates, except for a one-hour socialization period with 2–3 same-aged, similarly-reared peers, five days a week. Preliminary analyses showed no differences between the three rearing groups for the planned comparisons (p > 0.23), thus, they were statistically combined for the analyses.
For each birth cohort year, when subjects from the three rearing groups were 7 months of age, the MR subjects were separated from their mothers and placed in a new, larger cage with the same-aged PR and SPR subjects to form a permanent larger social group (Cohort size: N=20 in the first year cohort, N=27 in the second year cohort, N=14 in the third year cohort, and N=21 in the fourth year cohort), where they remained until the time of the study. With the exception of the early rearing experiences, all subjects received identical treatment. Preliminary analyses showed no effect of cohort for degree of intoxication ratings or any of the other variables (p > 0.44), thus they were statistically combined for analyses. All data were collected between 1994–1997.
Prior to beginning this research, protocols for the use of experimental animals were approved by the Institutional Animal Care and Use Committee of the National Institute of Child Health and Human Development indicating that the research was carried out in a manner that was humane and within the guidelines established by the National Institutes of Health.
CSF 5-HIAA Sample Collection and Assaying
One to three months prior to the intravenous ethanol infusion, subjects were captured in their home cage, immediately anesthetized with an intramuscular injection of ketamine hydrochloride (15 mg/kg), and a cisternal CSF sample was obtained from each subject using a 5mL syringe and 22-gauge needle. All samples were obtained within 30 minutes of ketamine administration and quick frozen in liquid nitrogen. The samples were stored at −70°C until assaying. CSF samples were assayed for concentrations of 5-HIAA using high-performance liquid chromatography (Scheinin et al., 1983). Inter- and intra-assay variabilities were less than 10% for all assays. Due to a freezer failure, the baseline CSF sample was lost for N = 22 subjects in the second cohort of animals. As inter-individual differences in CSF 5-HIAA concentrations are stable across time (Higley et al., 1998), for those animals, a CSF sample obtained three months earlier was used to assay for CSF 5-HIAA concentrations.
Administration of Intravenous Ethanol
To assess ethanol sensitivity, each subject was removed from its home cage and restrained on a flat surface. Subjects were intravenously infused in the saphenous vein at a constant rate over a 15-minute period with a 16.8 % (vol/vol) saline/United States Pharmacopeia ethanol solution. Our dosing levels were based on pilot testing that indicated that a lower dose of alcohol (1.0 g/kg) produced no visible signs of intoxication in some of the alcohol-naïve monkeys limiting the variability, while a higher dose of 3.0 g/kg led some monkeys to become unconscious. This dose led to 250–300 mg/100 dL blood alcohol concentrations. As male rhesus macaques have less body fat than females, and require a higher g/kg dose of alcohol to produce identical BACs (Baraona et al., 2001), we compared the BAC of males and females during pilot testing and found that males had lower BAC when compared to females infused with identical amounts of alcohol. Hence, in the study, males were infused with a higher dose of ethanol than females in order to produce identical BACs between the sexes—2.1 g/kg (males) or 2.0 g/kg (females). To assure that animals were not water-satiated at testing and that water had not been consumed immediately before the infusion, subjects’ water source was turned off the night before the infusion. To assure subjects’ safety, breathing, and heart rate were monitored during the infusion. A blood sample was taken from the femoral vein to quantify BAC at two, five, ten, and sixty minutes following the infusion. BACs were quantified enzymatically using a commercial kit (Sigma-Aldrich Corp™, St Louis, MO). Preliminary analyses showed that BAC at two, five, ten, and sixty minutes did not contribute statistically to any of the models and were thus excluded from the analyses. Given that all subjects received the same doses, the range of BAC was narrow and not related to either of the intoxication measures—average mean BAC = 267±4, 270±3, 269±3, 244±3 mg/100 dL alcohol at two, five, ten, and sixty minutes, respectively. The lower BAC at sixty minutes following alcohol administration indicates that the subjects’ BAC was on the descending limb, consistent with other studies (Hiltunen et al., 2000).
Assessment of Intoxication
Post-infusion, immediately after the ten-minute BAC blood draw, each subject was carried to and released inside the testing room. To prevent differential practicing of intoxicated motor movements after each subject was infused, their arms and legs were continually restrained from the time the alcohol infusion ended until they were released into the testing room. Investigators then immediately left the room, and observations were made through a one-way glass window by three observers experienced in behaviorally coding rhesus macaque intoxication. Observers used a portable computer program to record the frequency and duration of a variety of behaviors indicative of intoxication, as described in Barr et al., 2008. Briefly, they recorded falls, sways, collisions with the wall, missed jumps to perches, as well as time immobile, and time moving.
For the purposes of this investigation, after measuring the behavioral indices of intoxication for 30 minutes, investigators blind to the subject’s monoamine status rated the subject’s degree of intoxication using a previously established rating system (Barr et al., 2003a) on a 5-point Likert scale, with a score of 1 indicating minimal intoxication and a score of 5 indicating an extreme degree of intoxication. In those few cases of disagreement as to the rating of intoxication, investigators discussed the rationale of their rating and reached a consensus. The mean of the three ratings was used in the analyses. Inter-investigator reliability in rating intoxication was greater than r = 0.85.
After testing the first two cohorts of subjects, a Plexiglas™ chamber (height: 184cm; width: 92cm; depth: 60cm; see Figure 1) was added to the testing room. For ease of removal and placement of subjects in the chamber, it was suspended about 16cm off the surface from two chains attached to the ceiling of the room. The weight of the chamber and the size of the chains minimized chamber movement. To prevent injuries from falls, the floor of the testing room was covered with 30–45cm of wood shavings, except for the immediate area under the chamber. During pilot testing, it was evident that a portion of the intoxicated subjects failed to escape from the Plexiglas™ chamber over the course of an hour. Moreover, if a monkey did not escape within five minutes of being placed in the chamber, there was a low likelihood that it would escape at al. In order to determine whether subjects were able to escape in the non-intoxicated state, a week before alcohol testing, test subjects were placed twice in the Plexiglas™ chamber and allowed to escape by running into the Plexiglas™ walls. To prevent this, the experimenters used permanent ink markers to mark hatches on the walls. To further address this, and to facilitate escape, a 160cm long, bright, plastic chain was suspended from the ceiling extending down the middle of the chamber, terminating about 24cm from the bottom of the chamber—See Figure 1. This chain served two functions: first, the subjects could see the open top more readily, and second, to escape, the subjects could climb up the chain and out of the chamber. If the subject was unable to escape from the chamber after five minutes, an investigator opened the door of the chamber, allowing the subject to exit the chamber and the behavioral scoring session began.
Figure 1.

Plexiglas™ chamber (height: 184cm; width: 92cm; depth: 60cm) hanging by two chains from the ceiling of the room. To facilitate escape, a plastic chain was suspended from the ceiling extending to the middle of the bottom third of the chamber.
Identical to the first two cohorts, post-infusion, immediately after the ten-minute BAC blood draw, subjects tested in the Plexiglas™ chamber were carried to the testing room and placed in a supine position inside the chamber. Consistent with the first two cohorts, to prevent differential practicing of intoxicated motor movements, after each subject was infused, their arms and legs were continually restrained from the end of the alcohol infusion until they were placed in the Plexiglas™ chamber. Preliminary analyses showed no difference in intoxication ratings between the subset of animals tested in the Plexiglas™ chamber and a sample matched for sex, age, and distribution of rearing condition—See Table 1.
Table 1.
Comparison of intoxication ratings in subjects tested in the escape chamber and subjects not tested in the escape chamber. Subjects were matched for age, sex, and rearing condition. Three ANOVAs showed that there are no differences in essential variables between the two groups of subjects.
| Tested in the Escape Chamber? | Yes | No | |||||
|---|---|---|---|---|---|---|---|
| Mean | S.E. | Range | Mean | S.E. | Range | P-value | |
| Age (months) | 37.2 | 0.70 | (30.3–44.9) | 39.6 | 0.69 | (30.9–44.7) | 0.94 |
| Sex | Females: 15 Males: 8 |
Females: 15 Males: 8 |
0.99 | ||||
| Rearing Condition | MR: 13; PR: 3; SPR: 7 | MR: 13; PR: 3; SPR: 7 | 0.23 | ||||
| N | 23 | 23 | |||||
Data Analyses
All data were analyzed in SPSS, version 24 using between-groups, one-way ANOVAs or bivariate linear regressions. As there was only one subject with an intoxication rating of a 1 and only three subjects with ratings of 5, subjects that received a rating of 1 were combined with those rated as 2 and those with a rating of 5 were combined with those rated as 4. A secondary set of analyses using one-way between groups ANOVA were performed to assess subjects, with the groups split into levels of intoxication: high (intoxication ratings of 4–5), medium (intoxication ratings of 3), and low (intoxication ratings of 1–2). Preliminary analyses confirmed that there were no sex differences in intoxication ratings, baseline CSF 5-HIAA, or latency to escape from the Plexiglas™ chamber (p > 0.19) (see Table 1), thus males and females were statistically combined for analyses.
Results
Escape Latency and Intoxication Ratings
One-way ANOVA with intoxication rating as the independent variable and escape latency as the dependent variable showed that escape latency varied significantly by intoxication rating (F(2,25) = 12.93, p = .0009—See Figure 2). A posteriori planned comparisons showed that subjects rated as highly intoxicated (rating of 4–5) took significantly longer to escape from the chamber when compared to subjects with a low intoxication rating (rating of 1–2; p < 0.0009) and subjects that were, on average, in the middle of the ratings (rating of 3; p < 0.001).
Figure 2.

A between-groups one-way ANOVA comparing intoxication rating across the three intoxication groups showed that subjects rated as highly intoxicated exhibited a significantly longer escape time (M = 177.25±30.12) when compared to subjects rated as medium (M = 57.09±33.55) and subjects rated as low (M = 17.29±31.34); p = 0.0001. The asterisks represent a significant effect at the p < .001 level. The error bars represent standard errors of the mean.
Escape Latency and CSF 5-HIAA
A bivariate linear regression with time (in seconds) to escape from the chamber as the dependent variable and subjects’ baseline CSF 5-HIAA concentration as the independent variable showed that baseline CSF 5-HIAA concentrations predicted escape latency from the suspended chamber, with low CSF 5-HIAA concentrations predicting rapid escape (R = .61, F(1,18) = 10.06, p = .007—See Figure 3).
Figure 3.
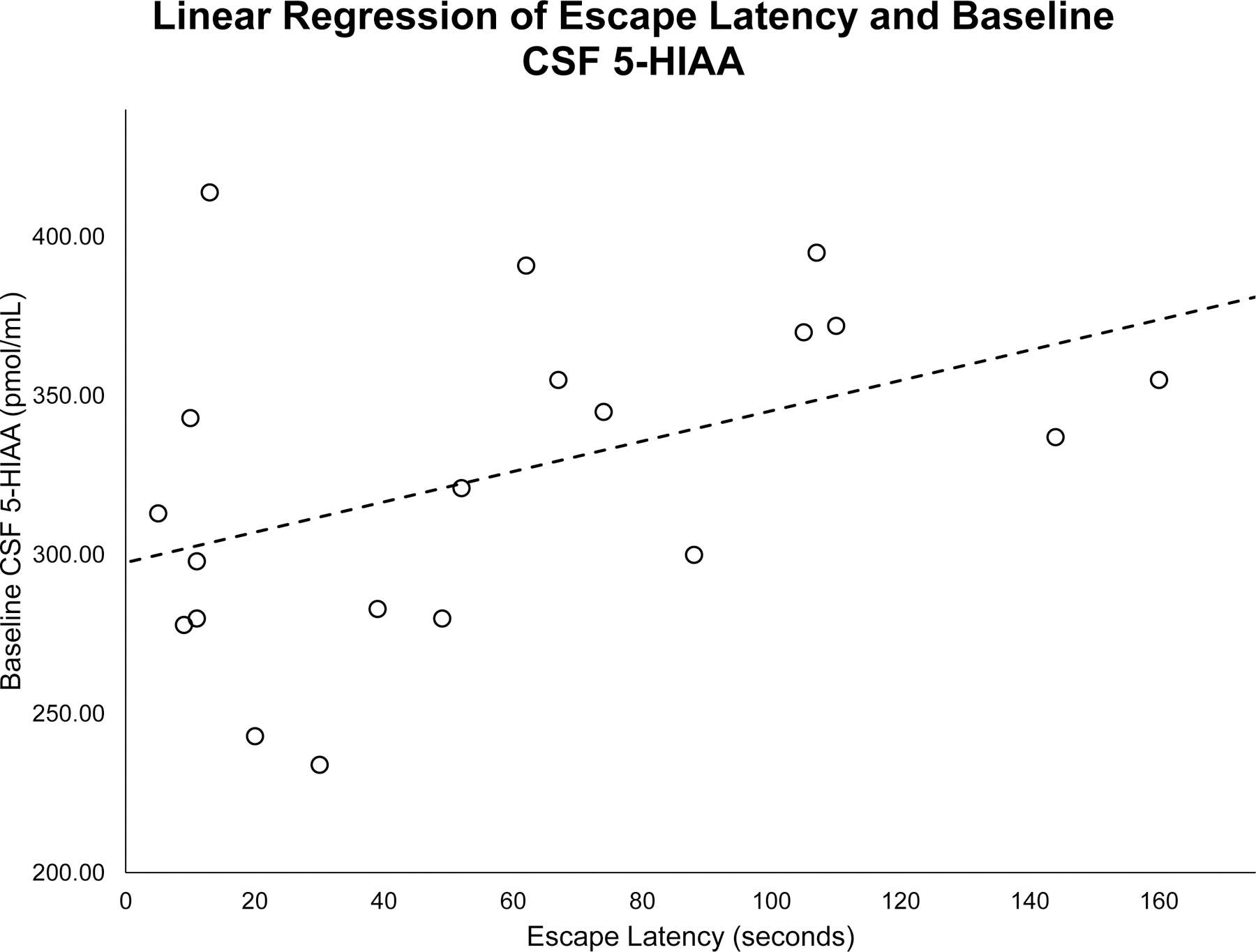
A linear regression found baseline 5-hydroxyindoleacetic acid (5-HIAA) concentrations in the cerebrospinal fluid (CSF) are significantly predictive of escape latency from the Plexiglas™ chamber (R = .61, F(1,18) = 10.06, p = .007).
Intoxication Ratings and CSF 5-HIAA
A between-groups one-way ANOVA comparing CSF 5-HIAA concentrations across the three intoxication groups (high (intoxication rating of 4–5), medium (intoxication rating of 3), or low (intoxication of 1–2)) showed that the medium and high intoxication groups had essentially the same mean baseline CSF 5-HIAA concentrations (Medium: M = 280.98±13.00; High: M = 278.98±10.51), whereas the subjects rated as low in intoxication exhibited lower CSF 5-HIAA concentrations when compared to the other groups (M = 241.46±11.47), although overall probability only approached statistical significance (F(2,76) = 2.86, p = 0.06—See Figure 4). A posteriori planned comparisons, however, showed a significant difference in CSF 5-HIAA concentrations when subjects rated as low in intoxication were compared to the other two higher-rated groups (p < 0.04), potentially an indication of a threshold effect. Moreover, to assure that the a posteriori effect was statistically sound, when the two groups rated as medium and high in intoxication ratings were combined and a one-way ANOVA compared the combined group with subjects with low intoxication ratings, statistical significance was achieved (F(1,76) = 5.79, p = 0.02), with subjects given a low intoxication rating exhibiting significantly lower CSF 5-HIAA concentrations (M = 241.46±11.47) than did the subjects given a high intoxication rating (M = 280.01±8.35).
Figure 4.

A between-groups one-way ANOVA comparing CSF 5-HIAA concentrations across the intoxication groups when they were collapsed into high (3–5) and low (1–2) groups showed that the subjects given a low intoxication rating exhibiting significantly lower CSF 5-HIAA concentrations (M = 241.46±11.47) than did the subjects given a high intoxication rating (M = 280.01±8.35); p = .02. The asterisk represents a significant effect at the p < .05 level. The error bars represent standard errors of the mean.
Discussion
The results of the present study lend support to our hypotheses and are consistent with other studies indicating that CNS serotonin is associated with inherent sensitivity to the intoxicating effects of alcohol. Monkeys with low concentrations of CSF 5-HIAA escaped from the suspended chamber more rapidly than those with higher concentrations (see Figure 3), and they exhibited lower ratings for intoxicated state (i.e., were less sensitive to the intoxicating effects of alcohol), as shown in the a posteriori analyses and confirmed with the combining of the two groups (see Figure 4), indicating a relationship between impaired or low CNS serotonin functioning and an inherent sensitivity to the intoxicating effects of alcohol. These data confirm other findings, showing that alcohol-naive monkeys with low CSF 5-HIAA concentrations are intrinsically less sensitive to the intoxicating effects of ethanol (Heinz et al., 1998; Heinz et al., 2003; Schwandt et al., 2008). Studies show that inherent low sensitivity to ethanol is predictive of high ethanol intake (Hinckers et al., 2006) and is linked to alcohol abuse and the development of alcohol dependency in humans (Schuckit & Smith, 2006), further supporting work by Schuckit and others that show subjective and objective measures of intoxication are biomarkers for the risk of excessive alcohol intake.
One of the more intriguing findings of the report is the finding that the relationship of CNS serotonin functioning and sensitivity to alcohol was not simply a linear relationship, but instead only the lowest tertile of subjects exhibiting relative insensitivity to the intoxicating effects of alcohol had low CSF 5-HIAA concentrations. Subjects that were mid and high in alcohol sensitivity were essentially identical in their CSF 5-HIAA concentrations. While somewhat speculative, our results suggest that the association between CNS serotonin and alcohol sensitivity is based on a threshold effect, suggesting that below a certain level the association between CNS serotonin functioning and alcohol sensitivity is robust and above that level, CNS serotonin has a reduced effect on alcohol sensitivity. Intriguingly, above a certain threshold, high CNS serotonin may show on average a protective effect with levels of intoxication resulting from low doses, leading to lower overall alcohol intake. Should this replicate in humans, Type 2 alcoholism, widely held to have genesis in CNS serotonin, may have a threshold biomarker of low or impaired serotonin, although this is not likely the whole story as two of the subjects with the lowest CSF 5-HIAA concentrations received an intoxication rating of 3, rather than 2.
Male rhesus monkeys with low CSF 5-HIAA concentrations exhibit hypoactivity in their frontal lobes (Doudet et al., 1995; Ichise et al., 2006). Serotonin axons from the raphe nucleus project to the frontal cortex and are known to synapse predominantly on inhibitory GABAergic interneurons (Smiley & Goldman-Rakic, 1996). Animals exhibiting tolerance to alcohol typically demonstrate cross-tolerance to other GABA-A agonists such as isoflurane and barbiturates (Ho & Yu, 1991). This may be a mechanism by which low CNS serotonin functioning is associated with hypofrontality (Doudet et al., 1995), aggression (Coccaro, 1989) and impulsivity (Higley et al., 1996), as well as intrinsic tolerance to ethanol.
Earlier work implicates low central serotonin in rhesus macaques as a risk factor for impulsivity (Mehlman et. al, 1994; Higley et. al, 1997), aggression (Higley et al., 1996), elevated alcohol consumption (Barr et. al, 2003b; Barr et. al, 2008) and impaired social competence (Higley et al., 1996b). Earlier studies from our laboratory indicated that subjects who were rated as highly intoxicated were more likely to stumble, fall, miss leaping to their perches, or lean against the wall (Barr et al., 2007; Schwandt et al., 2008). Furthermore, research with other animal models suggests that serotonin may mediate the constitutional sensitivity of an animal to the intoxicating effects of alcohol (Li et al., 1993; Li et al., 1986; Murphy et al., 1982), although our results suggest that this may be limited to subjects with the lowest CSF 5-HIAA concentrations. This result confirms that of a previous paper linking macaque CSF 5-HIAA concentrations to intrinsic sensitivity to ethanol (Heinz et al., 1998). Our results are consistent with an earlier study from out laboratory (Higley et al., 1996a, 1996b), showing that low or impaired central serotonin functioning is a high-risk trait for excessive long-term alcohol intake: high impulsivity, and relative insensitivity to the intoxicating effects of alcohol may lead to a high risk for overconsumption because subjects require more alcohol to experience its euphorigenic effects. The current study shows that sensitivity to the intoxicating effects of alcohol is based, at least in part, on the functioning of the serotonin system. Moreover, because subjects with low CSF 5-HIAA concentrations are impulse-impaired, once an individual with low central serotonin functioning begins drinking, they are likely to have difficulties terminating the drinking bout. This pair of traits, or the “terrible two” as we term them, potentially result in a forward-feed, where subjects with low CNS serotonin functioning drink more to experience the euphoriogenic effects of alcohol and then, because it is reinforcing, impulsive individuals continue to exhibit excess alcohol intake across drinking sessions. Our results also suggest that the heritable, early-life trait-like quality of low CNS serotonin activity may be a marker for AUDs and suggest that measurements of serotonin activity in very young subjects may be used to identify individuals that are at risk for the development of alcoholism.
Acknowledgements
The authors would like to acknowledge the research and animal care staff as well as the graduate students and post docs at the National Institutes of Health Animal Center for their assistance in data collection. We would also like to thank Kari Kruger for the artistry in developing Figures 1 and 2. This work was supported by Brigham Young University mentoring grants, the National Institute of Child Health and Human Development, and National Institute on Alcohol Abuse and Alcoholism Intramural Research Programs.
This research was supported by NIAAA Intramural Funds and by mentoring grants from Brigham Young University
Footnotes
The authors declare no conflicts of interest.
References
- Baker EJ, Farro J, Gonzales S, Helms C, & Grant KA (2014). Chronic alcohol self-administration in monkeys shows long-term quantity/frequency categorical stability. Alcoholism: Clinical and Experimental Research, 38: 2835–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballenger JC, Goodwin FK, Major LF, & Brown GL (1979). Alcohol and central serotonin metabolism in man. Archives of General Psychiatry, 36: 224–227. [DOI] [PubMed] [Google Scholar]
- Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, Scaefer C, & Lieber CS (2001). Gender differences in pharmacokinetics of alcohol. Alcoholism: Clinical and Experimental Research 25: 502–507. [PubMed] [Google Scholar]
- Barr CS, Becker ML, Suomi SJ, Higley JD (2003a). Relationships among CSF monoamine metabolite levels, alcohol sensitivity, and alcohol-related aggression in rhesus macaques. Aggressive Behavior 29: 288–301. [Google Scholar]
- Barr CS, Dvoskin RL, Yuan Q, Lipsky RH, Gupte M, Hu X, Zhou Z, Schwandt ML, Lindell SG, McKee M, & Becker ML (2008). CRH haplotype predicts CSF CRH, HPA axis activity, temperament, and alcohol consumption in rhesus macaques. Archives of General Psychiatry, 65: 934–944. 10.1001/archpsyc.65.8.934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD (2003b). Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macaques exposed to early-life stress. Alcoholism: Clinical and Experimental Research, 27: 812–817. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt M, Lindell SG, Chen SA, Goldman D, Suomi SJ, Higley JD, & Heilig M (2007). Association of a functional polymorphism in the μ-opioid receptor gene with alcohol response and consumption in male rhesus macaques. Archives of General Psychiatry, 64: 369–376. [DOI] [PubMed] [Google Scholar]
- Bordukalo-Niksic T, Stefulj J, Matosic A, Mokrovic G, & Cicin-Sain L (2012). Combination of polymorphic variants in serotonin transporter and monoamine oxidase-A genes may influence the risk for early-onset alcoholism. Psychiatry Research, 200: 1041–1043. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, & Sigvardsson S (1981). Inheritance of alcohol abuse: cross-fostering analysis of adopted men. Archives of General Psychiatry, 38: 861–868. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, & Bohman M (1996). Type I and type II alcoholism: an update. Alcohol Research and Health, 20: 18. [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF (1989). Central serotonin and impulsive aggression. British Journal of Psychiatry, Supplement 8: 52–62. [PubMed] [Google Scholar]
- Doudet D, Hommer D, Higley JD, Andreason PJ, Moneman R, Suomi SJ, & Linnoila M (1995). Cerebral glucose metabolism, CSF 5-HIAA levels, and aggressive behavior in rhesus monkeys. American Journal of Psychiatry, 152: 1782–1787. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, & Vogel-Sprott M (1996). Social drinking history, behavioral tolerance and the expectation of alcohol. Psychopharmacology, 127: 359–364. [DOI] [PubMed] [Google Scholar]
- Fils-Aime ML, Eckardt MJ, George DT, Brown GL, Mefford I, & Linnoila M (1996). Early-onset alcoholics have lower cerebrospinal fluid 5-hydroxyindoleacetic acid levels than late-onset alcoholics. Archives of General Psychiatry, 53: 211–216. [DOI] [PubMed] [Google Scholar]
- Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, … Batzer MA (2007). Evolutionary and biomedical insights from rhesus macaque genome. Science, 316: 222–234. [DOI] [PubMed] [Google Scholar]
- Hallikainen T, Saito T, Lachman HM, Volavka J, Pohjalainen T, Ryynanen OP, Kauhanen J, Syvalahti E, Hietala J, & Tiihonen J (1999). Association between low activity serotonin transporter promoter genotype and early onset alcoholism with habitual impulsive violent behavior. Molecular Psychiatry, 4: 385–388. [DOI] [PubMed] [Google Scholar]
- Heinz A, Higley JD, Gorey JG, Saunders RC, Jones DW, Hommer D, Zajicek K, Suomi SJ, Lesch KP, Weinberger DR & Linnoila M (1998). In vivo association between alcohol intoxication, aggression, and serotonin transporter availability in nonhuman primates. American Journal of Psychiatry, 155: 1023–1028. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Gorey JG, Bennet A, Suomi SJ, Weinberger DR, & Higley JD (2003). Serotonin transporter availability correlates with alcohol intake in non-human primates. Molecular Psychiatry, 8: 231–234. [DOI] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, & Linnoila M (1991). Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proceedings of the National Academy of Sciences, 88: 7261–7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, King ST, Hasert MF, Champoux M, Suomi SJ, Linnoila M (1996). Stability of interindividual differences in serotonin function and its relationship to severe aggression and competent social behavior in rhesus macaque females. Neuropsychopharmacology 14: 67–76. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Poland RE, Taub DT, Vickers J, Suomi SJ, & Linnoila M (1996). CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biological Psychiatry, 40: 1067–1082. [DOI] [PubMed] [Google Scholar]
- Higley JD & Linnoila M (1997). Low central nervous system serotonergic activity is traitlike and correlates with impulsive behavior: a nonhuman primate model investigating genetic and environmental influences on neurotransmission. Annals of the New York Academy of Sciences, 836: 39–56. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, & Linnoila M (1996a). A nonhuman primate model of type II excessive alcohol consumption? Part 1. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcoholism: Clinical and Experimental Research, 20: 629–642. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, & Linnoila M (1996b). A nonhuman primate model of type II alcoholism? Part 2. Diminished social competence and excessive aggression correlates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Alcoholism: Clinical and Experimental Research, 20: 643–650. [DOI] [PubMed] [Google Scholar]
- Hiltunen AJ (1997). Acute alcohol tolerance in cognitive and psychomotor performance: influence of the alcohol dose and prior alcohol experience. Alcohol, 14: 125–130. [DOI] [PubMed] [Google Scholar]
- Hiltunen AJ, Saxon L, Skagerberg S, & Borg S (2000). Acute tolerance during intravenous infusion of alcohol: comparison of performance during ascending and steady state concentrations—a pilot study. Alcohol, 22: 69–74. [DOI] [PubMed] [Google Scholar]
- Hinckers AS, Laucht M, Schmidt MH, Mann KF, Schumann G, Schuckit MA, & Heinz A (2006). Low level of response to alcohol as associated with serotonin transporter genotype and high alcohol intake in adolescents. Biological Psychiatry, 60: 282–287. [DOI] [PubMed] [Google Scholar]
- Li T, Lumeng L, McBride WJ, Waller MB, & Murphy JM (1986). Studies on an animal model of alcoholism. NIDA Research Monograph, 66: 41–49. [PubMed] [Google Scholar]
- Li T, Lumeng L, & Doolittle DP (1993). Selective breeding for alcohol preference and associated responses. Behavioral Genetics, 23: 163–170. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, George T, Eckardt M, Higley JD, Nielsen D, & Goldman D (1994). Serotonin, violent behavior and alcohol. Experientia Supplement, 71: 155–163. [DOI] [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M (1994). Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. The American Journal of Psychiatry, 151: 1485–1491.http://search.proquest.com/docview/220467715?accountid=4488 [DOI] [PubMed] [Google Scholar]
- Murphy JM, McBride WJ, Lumeng L, & Li TK (1982). Regional brain levels of monoamines in alcohol-preferring and -nonpreferring lines of rats. Pharmacology, Biochemistry, and Behavior, 16: 145–149. [DOI] [PubMed] [Google Scholar]
- Scheinin M, Chang WH, Kirk KL, & Linnoila M (1983). Simultaneous determination of 3-methoxy-4-hydroxyphenylglycol, 5-hydroxyindoleacetic acid, and homovanillic acid in cerebrospinal fluid with high-performance liquid chromatography using electrochemical detection. Analytical Biochemistry, 131: 246–253. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Barr CS, Suomi SJ, & Higley JD (2010). Age-dependent variation in behavior following acute ethanol administration in male and female adolescent rhesus macaques (Macaca mulatta). Alcoholism: Clinical and Experimental Research, 31, 228–237. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Higley JD, Suomi SJ, Heilig M, & Barr CS (2008). Rapid tolerance and locomotor sensitization in ethanol-naïve adolescent rhesus macaques. Alcoholism: Clinical and Experimental Research, 32: 1217–1228. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Lindell SG, Chen S, Higley JD, Suomi SJ, Heilig M, & Barr CS (2010). Alcohol response and consumption in adolescent rhesus macaques: life history and genetic influences. Alcohol, 44: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA (1984). Subjective responses to alcohol in sons of alcoholics and control subjects. Archives of General Psychiatry, 41: 879–884. [DOI] [PubMed] [Google Scholar]
- Schuckit MA (1985a). Behavioral effects of alcohol in sons of alcoholics. Recent Developments in Alcoholism, 3: 11–19. [DOI] [PubMed] [Google Scholar]
- Schuckit MA (1985b). Ethanol-induced changes in body sway in men at high alcoholism risk. Archives of General Psychiatry, 42: 375–379. [DOI] [PubMed] [Google Scholar]
- Schuckit MA (1985c). Studies of populations at high risk for alcoholism. Psychiatric Developments, 3: 31–63. [PubMed] [Google Scholar]
- Schuckit MA, & Smith TL (1997). Assessing the risk for alcoholism among sons of alcoholics. Journal of Studies on Alcohol, 58: 141–145. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, & Smith TL (2006). An evaluation of the level of response to alcohol, externalizing symptoms, and depressive symptoms as predictors of alcoholism. Journal of Studies on Alcohol, 67: 215–227. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Pierson J, Danko GP, & Beltran IA (2006). Relationships among the level of response to alcohol and the number of alcoholic relatives in predicting alcohol-related outcomes. Alcoholism: Clinical and Experimental Research, 30: 1308–1314. [DOI] [PubMed] [Google Scholar]
- Traber R, Würmle O, & Modestin J (2009). Two types of classification in female alcoholism. Archives of Women’s Mental Health, 12: 291–299. [DOI] [PubMed] [Google Scholar]
- Virkkunen M, & Linnoila M (1990). Serotonin in early onset, male alcoholics with violent behaviour. Annals of Medicine, 22: 327–331. [DOI] [PubMed] [Google Scholar]
- Virkkunen M, & Linnoila M (1993). Brain serotonin, type II alcoholism and impulsive violence. Journal of Studies on Alcohol Supplement, 11: 163–169. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Suomi SJ, Higley JD, & Mehlman PT (1999). CSF 5-HIAA and aggression in female macaque monkeys: species and interindividual differences. Psychopharmacology, 146: 440–446. [DOI] [PubMed] [Google Scholar]


