Abstract
Background:
Frontal plane gait mechanics are known risk factors for knee osteoarthritis (OA) but their role in early cartilage degeneration following ACL reconstruction (ACL-R) is not well understood.
Hypothesis/Purpose:
The objective was to evaluate the association of frontal plane gait mechanics with medial knee cartilage magnetic resonance (MR) relaxation times over 1-year in ACL-R subjects and controls. We hypothesized that (1) there will be an increase in frontal plane medial knee loading and medial knee MR relaxation times over time in the ACL-R subjects, and (2) increases in frontal plane medial knee loading will be associated with an increase in medial knee MR relaxation times.
Study Design:
Cohort study
Methods:
Subjects with ACL-R (n=37) underwent walking gait analyses and bilateral quantitative MRI before surgery (BL) and at 6-month (6M) and 12-month (12M) after ACL-R. Healthy control subjects (n=13) were evaluated at BL and 12M. Gait variables included, peak knee adduction moment (KAM), knee KAM impulse, and peak knee adduction angle. MRI variables included medial femur (MF) and medial tibia (MT) whole-compartment and sub-regional T1ρ and T2 relaxation times. Statistical analyses included a comparison of changes over-time for gait and MRI variables, correlations between changes in gait and MRI variables over-time, and differences in change in MRI variables in subjects who showed an increase vs. decrease in KAM impulse.
Results:
There were significant increases in medial T1ρ (Δ 4–11%) and T2 (Δ 2–10%) from BL to 6M for both knees in ACL-R group, and in KAM (Δ 13%) for the injured knee. From BL to 6M, subjects who had an increase in KAM impulse in the injured knee had a greater increase in medial T1ρ and T2 compared to those who did not have an increase in KAM impulse. Longitudinal changes for the control group were not significant.
Conclusion:
There is an increase in medial knee relaxation times over first 6-months after ACL-R. People with increase in medial knee loading show an increase in medial knee relaxation times compared to those who do not have an increase in medial knee loading over the first 6 months.
Clinical Relevance:
Strategies to reduce frontal plane loading over the first 6-months following ACL-R may be investigated to slow early cartilage degeneration.
Keywords: ACL, cartilage, MRI, Osteoarthritis, gait
INTRODUCTION
Individuals who undergo anterior cruciate ligament reconstruction (ACL-R) have an approximately 4 fold greater risk of developing post-traumatic osteoarthritis (PTOA) of the knee compared to those without ACL injury.1 Mechanisms underlying the development of early PTOA are not well understood. It is critical to identify potentially modifiable risk factors related to early PTOA following ACL-R so that potential interventions to prevent the onset of PTOA can be developed.8.
Altered gait patterns have been thought to contribute to PTOA following an ACL-R.11 A number of studies have identified abnormal walking mechanics in individuals with ACL-R compared to controls and the non-operated knee. A few studies report higher knee adduction moment (KAM) and peak knee adduction angle 4, 11, 16, 26, 42 whereas others report no difference in KAM11 or lower KAM in the ACL-R knees.42 High knee adduction angle and moment suggest greater loading over the medial compartment and are related to greater risk of progression of medial tibiofemoral cartilage damage in people with knee osteoarthritis. 6, 7, 17, 24 Quantitative T1ρ and T2 MRI relaxation times can be used to evaluate cartilage integrity and early osteoarthritis.2, 19, 25, 30, 41 Specifically, increases in T1ρ and T2 MR relaxation times are indicative of a reduction in proteoglycan content, disruption of collagen matrix, increase in water content, and a deterioration of the mechanical properties of the cartilage.13, 14 Loss of proteoglycans and collagen disruption occur early in the PTOA disease process.22 Hence, these MR parameters allow detection of PTOA (as an increase in the cartilage MR relaxation time parameters) in the early post-operative phase following ACL-R. However, it is not known if an increase in loading over the medial tibiofemoral compartment over time in people with ACL-R is related to progression of medial compartment degeneration. A recent pilot cross-sectional study showed that, in 9 individuals who were approximately 1.5 years post-surgery, those with high knee adduction moment in the ACL-R knee had significantly higher medial knee articular cartilage T1ρ and T2 MR relaxation times.16 However, this study was cross-sectional and included a very small sample. Hence, longitudinal studies with larger sample sizes are needed to ascertain if changes in walking mechanics are related to changes in cartilage composition in the early post-operative phase following ACL-R. If walking mechanics are related to early PTOA, then strategies to minimize gait abnormalities following ACL-R could be designed including improved surgical techniques and rehabilitation protocols.
The objective of this study was to quantify the longitudinal changes in frontal plane gait mechanics and medial knee cartilage MR relaxation times in subjects with ACL-R and control subjects over 12-months, and to evaluate the association of frontal plane gait mechanics with medial knee cartilage MR relaxation times from before surgery to 12-months after surgery in subjects with an ACL-R. We hypothesized that (1) there will be an increase in frontal plane medial knee loading and medial knee MR relaxation times over time in the ACL-R subjects, and (2) increases in frontal plane medial knee loading will be associated with an increase in medial knee MR relaxation times.
SUBJECTS AND METHODS
Subjects:
The subjects were recruited for a prospective, longitudinal, single-site, observational study from the Sports Medicine clinic at our Institution. Prospective patients with full-thickness ACL ruptures were referred by experienced fellowship trained sports orthopaedic surgeons (> 15 years of experience). The inclusion criteria were clinically diagnosed complete ACL rupture confirmed by increased anterior laxity on Lachman testing and diagnostic MRI, willingness to have an ACL-R, and capability to undergo the standard pre- and post-operative rehabilitation. Exclusion criteria included prior history of osteoarthritis, inflammatory arthritis, prior knee injury or surgery, or contraindications to MRI. Patients that required surgical intervention for associated knee ligaments or meniscus repair were excluded from the study. ACL-R was performed using standard surgical technique with independent drilling of femoral and tibial tunnels and soft tissue grafts by one of three board-certified and fellowship-trained orthopaedic surgeons. Physically active control subjects without knee injuries or clinical symptoms of OA were recruited from the community using frequency matching for age, BMI and sex.
All patients who underwent ACL-R and met the criteria between July 2011 and April 2014 were invited to participate in the study. Of the 87 patients who were eligible and invited to participate, 33 declined to participate. The remaining 54 patients were recruited into the study. In these analyses, we included data from subjects who were available at all time points (n=37). The ACL subjects underwent all testing at baseline (pre-operative), and 6 and 12 months post ACL-R. The control subjects (n=13) underwent all testing at the same time as baseline and 12 months post ACL-R. The mean duration between injury and the MRI was 47± 22 days. All ACL-R subjects underwent the same rehabilitation protocol that consisted of - bracing during walking, and range of motion and isometric exercises for 2 weeks, gradual weight bearing without brace and with crutches, and progressive strengthening, proprioception and balance training exercises from 2–6 weeks, return to daily activities, progressive strengthening, proprioception and balance training from 6–12 weeks, initiation of running and further strengthening exercises from 12–20 weeks, and return to sports at 6–8 months. This study was approved by the Committee for Human Research at our institution, and informed consent was obtained from all subjects. The study was Health Insurance Portability and Accountability Act (HIPAA) compliant.
Motion Analysis:
Three-dimensional kinematic data were collected at 250 Hz using a passive 10-camera system (VICON, Oxford Metrics, UK), and kinetic data were collected at 1000 Hz from two embedded force platforms (AMTI, Watertown, MA). Spherical retro-reflective markers with 14 mm diameter were placed on bony landmarks of bilateral lower extremities for identification of joint centers and rigid clusters placed on the lateral surface of the subject’s thighs, legs and heel counters were used to track segment motions 33. Data were collected while the subjects walked over-ground at a pace of 1.35 m/s. We selected a fixed walking speed to minimize the effects of walking speed on the biomechanical outcomes between subjects and over-time. A trial was considered acceptable when there was clean foot-strike on any of the force platforms and the speed was within ± 5% of the defined speed.Four successful trials were collected from both lower extremities. Kinematic and kinetics were calculated using Visual3D (C-motion, Georgetown, MD). All net joint moments were expressed as external moments. Variables were calculated for the stance phase when the foot was in contact with ground and included – knee adduction moment impulse (KAM impulse) over the stance phase (Nm*s and normalized to body weight and height as %BW*Ht*s), peak KAM (Nm and normalized to body weight and height as %BW*Ht) and peak knee adduction angle (degrees). KAM impulse was calculated as the integral of the positive portion of the KAM curve over stance. Peak KAM was the first peak of KAM in the first half of stance phase. Peak adduction angle was the maximum adduction angle during stance.
MR Imaging:
Imaging was performed using 3.0 Tesla MR scanners (General Electric, Milwaukee, WI, USA) and an 8-channel phased-array knee coil (Invivo, Orlando, FL, USA) with the patient in a supine position. The imaging protocol included – (1) high-resolution, 3D fast spin-echo (Cube) images for evaluation of cartilage, ligamentous, and meniscal morphology (repetition time (TR), 1500 ms; echo time (TE), 25 ms; echo train length, 32; matrix, 384 × 384; field of view (FOV), 16 cm; slice thickness, 1 mm (interpolated into 0.5mm)), and (2) 3D T1ρ/T2 quantification sequence21 (TR/TE=9/3ms, TSL: 0/10/40/80 msec, spin-lock frequency: 500Hz, FOV= 14cm, matrix=256×128, 4 mm slice thickness; for T2: preparation TE = 0/13.7/27.3/54.7 msec; total acquisition time ~ 9–10 mins).
Baseline injured medial femoral condyle (MF) and medial tibia (MT) cartilage compartments were segmented on multiple slices semi-automatically in high resolution Cube images using the in-house software developed with Matlab (Mathworks, Natick, MA, USA) based on edge detection and Bezier splines 5. The Cube images and the first echo of T1ρ were rigidly registered using Visualization Toolkit Computation Imaging Science Group (VTK CISG) registration toolkit.31 Piecewise rigid registration was applied along T1ρ and T2 echoes to take into account non-rigid movement of the articulation during the scan. Non-rigid registration was developed using Elastix registration library.15,28 All T1ρ and T2 echoes of the contralateral and longitudinal scans were registered to the first T1ρ-weighted image of the baseline injured knee to assure that the same anatomical regions of cartilage were being compared in the analysis.27 This allows for applying the ROIs identified on the baseline injured images on all contralateral and follow up scans. Intensity based multi-resolution pyramidal approach15 was applied to accomplish the non-rigid registration on the first echo and the transformation obtained was applied on the later echoes. The longitudinal registration strategy adopted in this study dropped considerably the human intervention, reduced to a simple quality check and local adjusting in case of poor results of the automatic procedure. T1ρ and T2 maps were reconstructed by fitting the T1ρ-and T2- weighted images pixel-by-pixel to the equations below19,20 using in-house developed software:
| (1) |
| (2) |
Where S is the image signal at a given time point – time of spin-lock (TSL) for T1ρ maps or echo time (TE) for T2 maps.
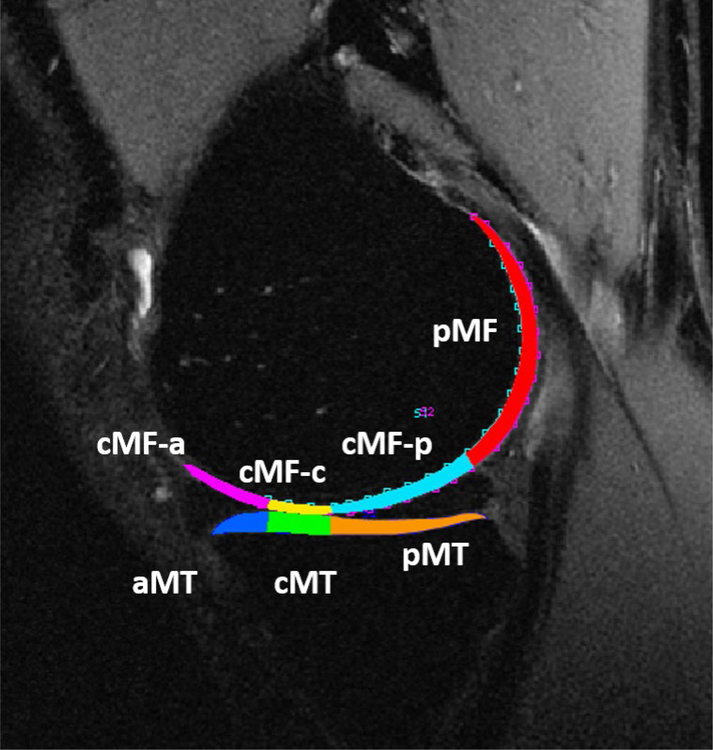
The cartilage contours generated from Cube images after segmentation were overlaid to the registered T1ρ and T2 maps. To reduce artifacts caused by partial volume effects with synovial fluid, pixels with relaxation time greater than 130 ms in T1ρ or 100 ms for T2 maps were removed from the data used forquantification.36 T1ρ and T2 relaxation times were calculated for the global MF and MT compartments. Additionally, the relaxation times were also calculated for weight-bearing subregions as shown in Figure 1.
Figure 1:
Medial knee compartments for MR relaxation time quantification. Global MF and MT consisted of the entire cartilage of medial femur and medial tibia including all shown subregions. The weight-bearing subregions were defined using the boundaries of the anterior and posterior meniscus horns. Hence, cMF-a and aMT were the cartilage regions of the MF and MT respectively adjacent to the anterior horn of the medial meniscus. The cMF-c and cMT subregions were the cartilage on cartilage regions of the MF and MT respectively. The cMF-p and pMT were the cartilage subregions of the MF and MT respectively adjacent to the posterior horn of medial meniscus. The pMF subregion was the posterior region of MF that is not considered weight-bearing.
During the course of this longitudinal study, the 3.0 Tesla HDx Long Bore MRI scanner was replaced with a 3.0 Tesla MR750 Wide Bore system. Potential variations in T1ρ and T2 values related to different MR systems were assessed by scanning phantoms and study subjects.20, 35 The T1ρ and T2 values were adjusted for scanner based on phantom and human subject calibration data, as detailed previously.35
Statistics:
Primary analyses were designed to test the stated hypotheses. These analyses included (a) Repeated-measures ANOVA with repeated contrasts (comparing BL to 6M and 6M to 12M) for with-in group changes in gait and MRI variables for ACL-R injured and uninjured knees, and for with-in group changes in the control group from BL to 12M, and (b) Correlations between the change in gait and MRI variables from BL to 6M and from 6M to 12M for ACL-R injured knee, and (c) Multivariate ANOVA comparing the change in MRI variables over time for ACL-R injured knee with the subjects stratified as those who showed an increase in KAM impulse compared to those who showed a decrease. The Benjamini-Hochberg procedure was used for controlling the false discovery rates for repeated contrast comparisons, correlations, and multivariate ANOVA.3 The FDR was set at 5% and adjusted P-values (i.e. q-values) < 0.05 were considered significant. All analyses were performed using IBM SPSS 23.
RESULTS
Subjects:
There were 54 subjects with ACL-R who consented to participate in this study. Out of these 17 subjects were excluded from this analysis due to data not being available at all time-points and 37 were included. Age (P = 0.766) and sex distribution (P = 0.965) were not different between the 17 subjects who were excluded compared to the 37 subjects who were included. However, the excluded subjects had higher BMI (mean difference = 2.8 kg/m2, P = 0.002) compared to the included group. Of the 37 ACL-R subjects, 27 received the semitendinosus autograft, 8 received the tibialis posterior allograft, 1 received the patellar tendon allograft, 1 received the semitendinosus allograft. The differences in age, BMI, and distribution of men and women between the ACL-R and control groups were not statistically significant (Table 1).
Table 1.
Age, BMI, sex distribution for the ACL-R and the Control groups.
| ACL-R | Control | P value | |
|---|---|---|---|
| Age (y) | 30.8 (5.3) | 29.4 (8.3) | 0.568 |
| BMI (kg/m2) | 23.3 (2.2) | 23.4 (2.6) | 0.921 |
| Men: Women | 22:15 | 9:4 | 0.532 |
ACL-R = Anterior cruciate ligament Reconstruction
Changes in frontal plane knee mechanics over time:
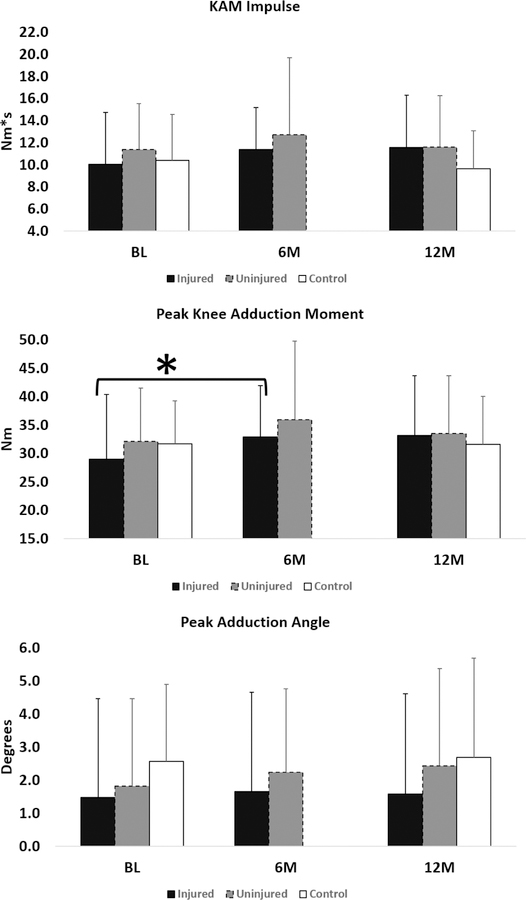
Longitudinal changes in frontal plane gait variables are shown in Figure 2. For the injured knee, the overall model was significant for longitudinal changes in peak KAM (P = 0.001) and KAM impulse (P = 0.026) but not for peak adduction angle (P=0.845). Repeated contrasts showed that peak KAM increased significantly from BL to 6M (Δ= 3.9±7.9 Nm; % change = 13.3%; Padj = 0.03) but not from 6M to 12M (Δ= 0.3±6.9 Nm; % change = 0.8%; Padj = 0.829). However, repeated contrasts showed that the changes in KAM impulse were not significant. For the uninjured knee, overall model was not significant for longitudinal changes in KAM impulse (P=0.206), peak adduction angle (P=0.231), and peak KAM (P=0.059). The changes in knee mechanics from BL to 12M were not significant in the control group (Figure 2).
Figure 2:
Frontal plane gait variables over-time for the injured (black column), uninjured (grey column), and control (white column) knees. *indicates a significant difference.
Changes in MR relaxation times over time:
Table 2 has the change (Δ) for T1ρ and T2 in ms and % from BL to 6M and from 6M to 12M for both knees of ACL-R subjects. Figure 3 shows the T1ρ and T2 parameters for baseline and 12-months for the control group. For the injured knee, there were significant longitudinal changes in T1ρ for global MF (P<0.001), cMFa (P< 0.001), cMFc (P<0.001), and cMFp (P<0.001) femoral compartments. Similarly, there were significant changes in T2 for global MF (P<0.001), cMFa (P< 0.001), cMFc (P<0.001), and cMFp (P<0.001) compartments. Contrasts showed that all of these parameters increased from BL to 6M (Table 2). For the tibia, the overall F-test was significant for longitudinal changes in T1ρ for global MT (P=0.019) and cMT (P=0.037). However, repeated contrasts showed that these differences were not significant. Similarly, although the overall F-test was significant for longitudinal changes in global MT T2 (P=0.006), aMT T2 (P=0.043), cMT T2 (0.009), and pMT T2 (0.028), repeated contrasts showed that none of these differences were significant.
Table 2.
Change in medial cartilage MRI T1ρ and T2 in the injured and uninjured knees for the ACL-R group.
| BL to 6M | 6M to 12M | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Injured | Uninjured | Injured | Uninjured | ||||||||||
| Variable | Δ (ms) | Δ (%) | Padj** | Δ (ms) | Δ (%) | Padj** | Δ (ms) | Δ (%) | Padj** | Δ (ms) | Δ (%) | Padj** | |
| T1ρ | Global MF | 2.9 (2.7) | 7.4 | <0.001* | 1.5 (2.2) | 3.8 | 0.002* | −0.2 (2.4) | −0.5 | 0.724 | −0.3 (1.3) | −0.8 | 0.207 |
| cMFa | 3.3 (4.5) | 9.4 | 0.001* | 2.0 (3.9) | 5.6 | 0.010* | −0.8 (4.2) | −2.0 | 0.447 | 0.1 (3.4) | 0.3 | 0.867 | |
| cMFc | 4.0 (5.8) | 10.7 | 0.001* | 1.3 (4.0) | 3.5 | 0.089 | −0.3 (5.2) | −0.7 | 0.855 | −0.6 (3.7) | −1.5 | 0.399 | |
| cMFp | 2.6 (3.6) | 7.2 | 0.001* | 1.8 (3.2) | 4.9 | 0.005* | −0.1 (4.0) | −0.1 | 0.936 | −0.5 (2.7) | −1.2 | 0.362 | |
| global MT | 0.5 (3.6) | 1.4 | 0.404 | 2.0 (2.5) | 5.7 | <0.001* | 1.1 (2.8) | 3.1 | 0.078 | −1.7 (3.4) | −4.5 | 0.012* | |
| aMT | 0.9 (3.4) | 2.7 | 0.211 | 0.6 (4.2) | 1.9 | 0.405 | 0.4 (4.1) | 1.3 | 0.673 | −0.5 (3.2) | −1.4 | 0.405 | |
| cMT | 0.6 (4.1) | 1.6 | 0.612 | 2.1 (3.6) | 6.1 | 0.004* | 1.2 (3.9) | 3.3 | 0.163 | −1.7 (4.2) | −4.7 | 0.034* | |
| pMT | 0.2 (4.5) | 0.6 | 0.869 | 2.4 (3.3) | 6.6 | 0.001* | 1.4 (2.9) | 3.7 | 0.027 | −2.1 (3.5) | −5.5 | 0.004* | |
| T2 | global MF | 2.2 (2.4) | 7.2 | <0.001* | 1.6 (1.6) | 5.2 | <0.001* | 0.1 (2.3) | 0.2 | 0.901 | −0.7 (1.9) | −2.1 | 0.071 |
| cMFa | 2.8 (3.7) | 10.3 | 0.001* | 1.8 (3.0) | 6.8 | 0.004* | −0.5 (3.0) | −1.6 | 0.579 | −0.3 (2.5) | −1.1 | 0.502 | |
| cMFc | 2.4 (4.1) | 8.6 | 0.004* | 1.7 (3.0) | 5.8 | 0.006* | 0.4 (3.7) | 1.3 | 0.673 | −1.4 (3.2) | −4.7 | 0.021* | |
| cMFp | 2.0 (3.2) | 7.2 | 0.003* | 1.8 (2.2) | 6.6 | <0.001* | 0.1 (3.2) | 0.4 | 0.901 | −0.7 (2.5) | −2.4 | 0.236 | |
| global MT | 0.8 (2.5) | 2.9 | 0.163 | 1.4 (2.2) | 5.1 | 0.004* | 0.7 (2.6) | 2.6 | 0.210 | −0.7 (2.3) | −2.7 | 0.096 | |
| aMT | 1.1 (3.1) | 4.3 | 0.107 | 0.7 (2.9) | 2.9 | 0.207 | 0.1 (2.9) | 0.4 | 0.901 | 0.1 (2.8) | 0.4 | 0.844 | |
| cMT | 1.1 (3.1) | 4.3 | 0.094 | 1.4 (3.1) | 5.3 | 0.011* | 0.7 (3.4) | 2.5 | 0.432 | −0.5 (3.0) | −1.9 | 0.362 | |
| pMT | 0.3 (2.9) | 1.1 | 0.673 | 1.5 (2.5) | 5.7 | 0.004* | 1.1 (3.0) | 3.9 | 0.094 | −1.2 (2.4) | −4.2 | 0.011* | |
indicates significant p value.
P values are from analyses adjusted using Benjamini-Hochberg procedure (q-value)
MRI = magnetic resonance imaging, ACL-R= anterior cruciate ligament, BL = baseline, 6M = 6 month, 12M = 12 month, MF = medial femur, cMFa = anterior portion of central medial femur, cMFc = central portion of central medial femur, cMPp = posterior portion of central medial femur, MT = medial tibia, aMT = anterior medial tibia, cMT = central medial tibia, pMT = posterior medial tibia
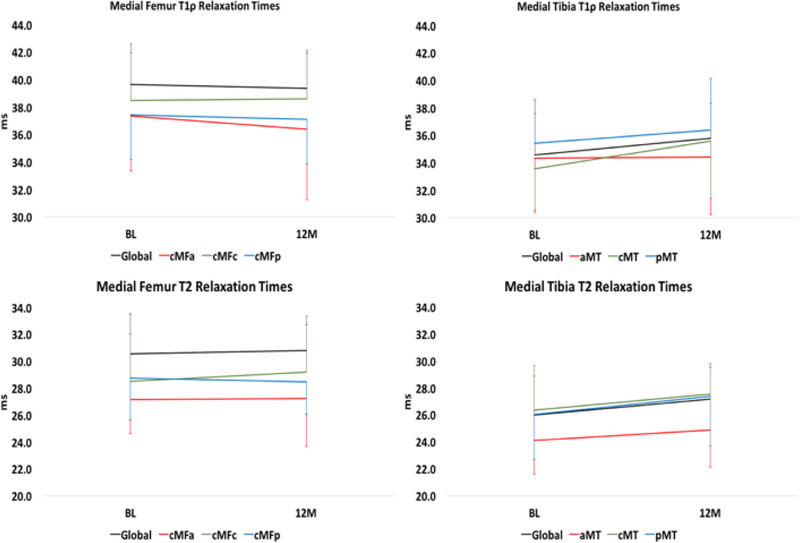
Figure 3.
Medial Femur T1ρ (top) and T2 (bottom) relaxation times over-time in the Control group. None of the changes between BL and 12M were significant.
For the uninjured knee, there were significant longitudinal changes in T1ρ for global MF (P<0.001), cMFa (P<0.001), and cMFp (P=0.002) femoral compartments. Contrasts showed that all of these parameters increased from BL to 6M but not from 6M to 12M (Table 2). Similarly, there were significant longitudinal changes in T2 for global MF (P<0.001), cMFa (P<0.001), cMFc (P=0.005), and cMFp (P<0.001) femoral compartments. Contrasts showed that all of these parameters increased from BL to 6M (Table 2). Additionally, T2 for cMFc decreased from 6M to 12M. For the tibia, there were significant longitudinal changes in T1ρ for global MT (P<0.001), cMT (P=0.004), and pMT (P<0.001). Contrasts showed that the global MT, cMT, and pMT T1ρ increased from 6M to 12M and decreased from 6M to 12M (Table 2). There were significant longitudinal changes in T2 for global MT (P=0.003), cMT (P=0.029), and pMT (0.002) compartments in the uninjured knee. Contrasts showed that the global MT, cMT, and pMT T2 increased from BL to 6M and pMT T2 decreased from 6M to 12M (Table 2).
Longitudinal changes in T1ρ and T2 from BL to 12M were not significant in the Control group (Figure 2).
Associations of frontal plane knee mechanics with cartilage MR relaxation times for the injured knee in ACL-R subjects:
Table 3 shows the results from the correlation analyses. None of the associations were significant. From BL to 6M, when the subjects were stratified into those with an increase (Δ KAM impulse = 3.4±2.6 Nm*s) vs. decrease (Δ KAM impulse = −2.1±1.8 Nm*s) in KAM impulse (Table 4), the subjects who showed an increase in the KAM impulse had a significant increase in global MF T1ρ (Padj=0.037), cMFc T1ρ (Padj=0.032), cMFp T1ρ (Padj=0.037), global MF T2 (Padj=0.037), cMFc T2 (Padj=0.032), and cMFp T2 (Padj=0.037). From 6M to 12M, when the subjects were stratified into those with an increase (Δ KAM impulse = 3.3±2.2 Nm*s) vs. decrease (Δ KAM impulse = −2.7±2.2 Nm*s) in KAM impulse from 6M to 12M (Table 4), the changes in MRI relaxation time parameters were not significant (Table 4).
Table 3:
Pearson’s correlations between change (Δ) in gait and MRI variables for the injured knee in ACL-R subjects. None of the correlations were significant.
| BL to 6M | 6M to 12M | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global | cMFa | cMFc | cMFp | Global | aMT | cMT | pMT | Global | cMFa | cMFc | cMFp | Global | aMT | cMT | pMT | ||
| Δ T1ρ | Δ T1ρ | ||||||||||||||||
| Δ KAM Impulse | r | 0.265 | 0.143 | 0.276 | 0.226 | −0.104 | 0.200 | −0.057 | −0.171 | −0.056 | −0.226 | −0.293 | 0.087 | −0.144 | −0.316 | 0.021 | −0.139 |
| Δ Peak KAM | r | 0.179 | −0.048 | 0.246 | 0.241 | −0.043 | 0.050 | 0.006 | −0.100 | −0.074 | −0.253 | −0.291 | 0.091 | 0.001 | −0.262 | 0.142 | −0.018 |
| Δ T2 | Δ T2 | ||||||||||||||||
| Δ KAM Impulse | r | 0.389 | 0.154 | 0.354 | 0.356 | −0.014 | 0.076 | 0.053 | −0.128 | −0.169 | −0.183 | −0.338 | −0.009 | −0.005 | −0.258 | 0.150 | −0.090 |
| Δ Peak KAM | r | 0.258 | −0.012 | 0.228 | 0.320 | −0.053 | −0.119 | 0.068 | −0.164 | −0.216 | −0.127 | −0.431 | −0.070 | 0.105 | −0.204 | 0.244 | 0.010 |
MRI = magnetic resonance imaging, ACL-R= anterior cruciate ligament, BL = baseline, 6M = 6 month, 12M = 12 month, MF = medial femur, cMFa = anterior portion of central medial femur, cMFc = central portion of central medial femur, cMPp = posterior portion of central medial femur, MT = medial tibia, aMT = anterior medial tibia, cMT = central medial tibia, pMT = posterior medial tibia, KAM = knee adduction moment
Table 4:
Change (Δ) in MRI variables for the injured knee between ACL-R subjects with increase vs. decrease in KAM impulse of the injured knee.
| BL to 6M | 6M to 12M | ||||||
|---|---|---|---|---|---|---|---|
| Decrease ( n= 14) | Increase (n=23) | Padj** | Decrease ( n= 19) | Increase (n=18) | Padj** | ||
| Δ T1ρ | Global Femur | 1.3 (2.7) | 3.8 (2.3) | 0.037* | 0.1 (2.5) | −0.3 (2.1) | 0.674 |
| cMFa | 2.1 (3.6) | 4.1 (5.0) | 0.446 | 0.5 (5.1) | −1.8 (2.8) | 0.291 | |
| cMFc | 0.3 (5.1) | 5.6 (4.1) | 0.032* | 1.6 (4.2) | −1.5 (4.8) | 0.170 | |
| cMFp | 0.5 (4.0) | 3.7 (2.6) | 0.037* | 0.2 (3.1) | 0.4 (3.8) | 0.883 | |
| Global Femur | 0.3 (3.2) | 0.5 (3.9) | 0.883 | 1.9 (1.7) | 0.6 (3.4) | 0.394 | |
| aMT | 0.0 (3.0) | 1.3 (3.6) | 0.499 | 1.1 (4.1) | −0.1 (4.1) | 0.618 | |
| cMT | 0.0 (3.1) | 0.7 (4.6) | 0.674 | 1.8 (2.3) | 1.1 (4.5) | 0.674 | |
| pMT | 0.7 (4.7) | 0.0 (4.5) | 0.802 | 2.1 (2.2) | 0.8 (3.4) | 0.407 | |
| Δ T2 | Global Tibia | 0.8 (2.7) | 3.0 (1.7) | 0.037* | 0.8 (2.1) | −0.7 (2.2) | 0.152 |
| cMFa | 2.0 (2.7) | 3.3 (4.2) | 0.568 | 0.5 (3.2) | −1.4 (2.6) | 0.170 | |
| cMFc | −0.1 (3.2) | 4.1 (3.2) | 0.032* | 1.8 (2.6) | −1.0 (4.0) | 0.082 | |
| cMFp | 0.1 (3.8) | 3.2 (2.1) | 0.037* | 0.5 (2.8) | −0.2 (3.6) | 0.764 | |
| Global Tibia | 0.6 (2.8) | 0.9 (2.4) | 0.843 | 1.2 (2.6) | 0.2 (2.7) | 0.499 | |
| aMT | 1.2 (3.2) | 1.0 (3.1) | 0.883 | 0.4 (2.4) | −0.3 (3.4) | 0.718 | |
| cMT | 0.5 (3.3) | 1.5 (3.3) | 0.568 | 0.8 (3.1) | 0.6 (3.7) | 0.883 | |
| pMT | 0.6 (3.4) | 0.2 (2.6) | 0.830 | 1.9 (3.2) | 0.3 (2.7) | 0.291 | |
indicates significant p value.
P values are from analyses adjusted using Benjamini-Hochberg procedure (q-value).
MRI = magnetic resonance imaging, ACL-R= anterior cruciate ligament, BL = baseline, 6M = 6 month, 12M = 12 month, MF = medial femur, cMFa = anterior portion of central medial femur, cMFc = central portion of central medial femur, cMPp = posterior portion of central medial femur, MT = medial tibia, aMT = anterior medial tibia, cMT = central medial tibia, pMT = posterior medial tibia
DISCUSSION
The objective of this study was to evaluate the association of frontal plane gait mechanics and medial knee MR relaxation times from baseline to 6- to 12-months after ACL-R surgery. The results support our first hypotheses with significant increases in medial knee MR relaxation times in the injured knees of the ACL-R group over time, mostly from BL to 6M. An increase in the MR relaxation times is suggestive of worsening cartilage composition (decrease in proteoglycan or increase in collagen disruption). The results partially support the second hypothesis since we observed increases in medial femoral MR relaxation times in subjects with an increase in frontal plane medial loading (KAM impulse) in the injured knee from BL to 6M. From 6M to 12M, we observed trends for decreases in medial tibiofemoral MR relaxation times in subjects with increase in KAM impulse, but these observations were not significant. These findings highlight the complex relationship between knee loading in the frontal plane and medial knee cartilage composition in the early post-operative phase following an ACL-R.
We observed significant increases (approximately 13%) in the peak KAM in the injured knee of the ACL-R group from BL to 6M but not from 6M to 12M. This is also reflected in the fact that a larger proportion of the ACL-R subjects (62% vs. 51%) showed an increase in the frontal plane loading from BL to 6M vs. 6M to 12M. Very few longitudinal studies have evaluated changes in frontal plane walking mechanics over the early period following ACL-R. A recent study by Wellsandt et al. reported KAM, KAM impulse, and medial knee contact force data at baseline (pre-surgery), 6M, and 12M time points in 22 patients with ACL-R as they walked at their self-selected walking speeds.40 They reported the data with the subjects stratified into those who did and did not develop radiographic OA 5-years after surgery. However, on averaging the data from the nonOA and the OA groups, it is seen that they observed a 28% increase in normalized peak KAM from BL to 6M and a 35% increase in the normalized KAM impulse over the same period. From 6M to 12M they reported a 5% decrease in peak KAM and a 14% decrease in KAM impulse. They did not report whether the changes over time were statistically significant for their cohort. We also observed approximately 13% increase in the KAM impulse and peak KAM from BL to 6M and a 7–9% decrease from 6M to 12M in the uninjured knees of the ACL-R subjects. However, only the increase in peak KAM from BL to 6M was statistically significant.
From pre-surgery (BL) to 6-months post-surgery there was an increase in the medial femur T1ρ and T2 relaxation times and in the medial tibia T2 relaxation times for the injured knee in the ACL-R subjects. Relatively less frequent changes were observed from 6M to 12M and none of these were significant. Hence, cartilage degeneration process may be slower after 6M but may still continue in the ACL-R injured knee. Other studies have reported increases in medial knee T1ρ and T2 relaxation times from baseline to 1–3 years in individuals with ACL-R.18, 34, 37 A recent large study in 111 subjects with ACL-R compared to 20 control subjects reported a high prevalence of MRI detected OA features in the ACL-R group one year after surgery.9 Our results support the findings from these earlier studies suggesting that cartilage degeneration is evident in the early post-operative phase after ACL-R. These findings support the evaluation of cartilage status at least at 6-months post ACL-R,29 and a potential need for an early clinical intervention considering the significant worsening of cartilage composition within 6M after the surgery. We also observed significant increases in T1ρ and T2 relaxation times for the uninjured knee in the ACL-R subjects from BL to 6M. From 6M to 12M, the MR relaxation times for the uninjured knee decreased overall whereas the injured knee tended to increase or not change. This suggests that the initial increase in MR relaxation times for the uninjured knee may be transient with the cartilage composition recovering over the longer term.29 Although not assessed in this study, it is possible that these changes in MR relaxation times for the uninjured knee may be partially related to the changes in gait mechanics which also tended to increase from BL to 6M and decrease from 6M to 12M. However, these changes may also be related to the systemic and local inflammatory environment. A recent systematic review reported that 5% of uninjured contralateral knees in people with ACL injuries present with knee OA.1 Further work is needed to evaluate the clinical significance of and mechanisms underlying these early changes in the cartilage composition for the uninjured knee.
Our pilot work in a different cohort showed that individuals with high KAM had higher medial knee MR relaxation times compared to those with low KAM approximately 1.5 years after ACL-R.16 Based on those findings, we had speculated that longitudinal changes in KAM may have a negative impact on medial knee MR relaxation times. The results from the current study partially support our speculation. Although, the positive associations between the increase in KAM impulse and the increase in medial knee MR relaxation times from BL to 6M in the ACL-R group were not significant, we observed increases in medial femoral MR relaxation times in subjects with increases in frontal plane loading from BL to 6M compared to those who showed a decrease in loading. Hence, these results indicate that over the very early post-operative period strategies to target reduction in frontal plane loading could be investigated in an attempt to slow the immediate changes in medial knee compartment cartilage composition. However, from these analyses it is not clear if the period of reduced loading before ACL-R may also be related to the observed increases in MR relaxation times. Interestingly, we observed that the subjects who showed an increase in KAM impulse from 6M to 12M had a decrease in medial MR relaxation times over the same period. However, these differences were not significant. The study by Wellsandt et al. reported that people who developed radiographic knee OA 5-years after ACL-R walked with lower KAM impulse and lower medial knee contact force in the early post-operative period.40 Therefore, the role of reduced knee loading following the initial injury in early cartilage degeneration warrants further investigation.
As is evident from Figure 2, over-time the ACL-R subjects in our cohort achieve symmetrical loading patterns. Gardinier et al. reported no overall differences asymmetry in medial knee contact force during walking 6M after ACL-R.10 The difference of 0.6° in peak adduction angle at 12M (Figure 2) between the injured and uninjured knees is likely clinically not meaningful. Further work is needed to evaluate the longitudinal changes in symmetry and their relationships with early post-operative cartilage changes in people with ACL-R. In our study, the changes in frontal plane gait mechanics over time were not significant in the control group as expected. Compared to the control subjects, injured knees had lower frontal loading compared to the uninjured knee and control subjects at BL but the differences were not significant. The lack of significance may be related to the fact that we controlled the walking speed for all of our subjects. We also did not observe significant differences in frontal plane gait mechanics between the ACL-R injured knee compared to the uninjured knee or control subjects at 6M or 12M. These finding are in line with a recent meta-analysis12 that reviewed previous conflicting studies4,11,32,42
The limitations of this work include the fact that the ACL-R group was not homogenous in terms of the surgical technique. Our study was not powered to investigate the differences between different graft choices. Similarly, we did not excluded subjects if they needed a meniscectomy or had cartilage lesions at baseline. It is known that people with ACL-R who also undergo a meniscectromy are at an even greater risk of knee OA23 it is possible that our results may be affected by inclusion of these individuals. To assess this, we repeated our primary analyses excluding the subjects with medial meniscectomy (n=2) or medial cartilage repair (n=0.). Our primary findings were unchanged with an increase in KAM (p =0.007), medial T1ρ (p <0.0001), and T2 (p ≤0.020) from BL to 6M. Subjects with an increase in KAM impulse showed a greater increase in medial MR relaxation times (P ≤0.007) compared to those who did not show an increase in KAM impulse from BL to 6M. Also, we only included a follow-up of 12-months after ACL-R. Future work should include longer follow-up to see if the trends observed in our study continue over longer periods. However, considering that interventions are most likely to be effective in the early period after ACL-R, our findings provide valuable information regarding previous speculations about the role of gait mechanics in early PTOA following ACL-R. Despite the extensive cross-calibration and validation between the two scanners used in this study, the change in scanners could still be a source of variability.
CONCLUSIONS
PTOA following ACL-R is likely multifactorial with mechanics playing a role but other factors including bone shape,29 concomitant cartilage and meniscus injuries, graft choice, trauma during injury/surgery, and inflammation may also be involved.38,39 From the results of the current study, we conclude that medial tibiofemoral T1ρ and T2 relaxation times increase from before surgery to 6-months after surgery in both knees of ACL-R subjects. From 6 to 12-months after surgery, the medial tibiofemoral MR relaxation times remain unchanged for the injured knee but decrease for the uninjured knee. Over the first 6-months the increase in medial MR relaxation times may be associated with a concomitant increase in medial knee loading for the injured knee. The associations of changes in frontal plane loading 6-months after ACL-R with medial tibiofemoral cartilage degeneration require further investigations.
What is known about the subject:
People with ACL-R are at an increased risk for knee OA and early cartilage degeneration can be detected with quantitative MRI techniques. Frontal plane loading is a known risk factor for medial knee OA.
What this study adds to the existing knowledge:
Increase in frontal plane loading over the first 6-months after ACL-R is associated with medial cartilage degeneration.
Acknowledgements:
The authors would like to thank Drew A. Lansdown, MD, Musa Zaid, MD, and Lauren Tufts for their help with data analyses. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number NIH NIAMS P50 AR065645. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Study performed at University of California, San Francisco, CA
REFERENCES
- 1.Ajuied A, Wong F, Smith C, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med 2014;42(9):2242–2252. [DOI] [PubMed] [Google Scholar]
- 2.Akella SV, Regatte RR, Gougoutas AJ, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med 2001;46(3):419–423. [DOI] [PubMed] [Google Scholar]
- 3.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological 1995;57(1):289–300. [Google Scholar]
- 4.Butler RJ, Minick KI, Ferber R, Underwood F. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med 2009;43(5):366–370. [DOI] [PubMed] [Google Scholar]
- 5.Carballido-Gamio J, Bauer JS, Stahl R, et al. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal 2008;12(2):120–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang A, Hochberg M, Song J, et al. Frequency of varus and valgus thrust and factors associated with thrust presence in persons with or at higher risk of developing knee osteoarthritis. Arthritis Rheum 2010;62(5):1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang AH, Chmiel JS, Moisio KC, et al. Varus thrust and knee frontal plane dynamic motion in persons with knee osteoarthritis. Osteoarthritis Cartilage 2013;21(11):1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu CR, Beynnon BD, Buckwalter JA, et al. Closing the gap between bench and bedside research for early arthritis therapies (EARTH): report from the AOSSM/NIH U-13 Post-Joint Injury Osteoarthritis Conference II. Am J Sports Med 2011;39(7):1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culvenor AG, Collins NJ, Guermazi A, et al. Early knee osteoarthritis is evident one year following anterior cruciate ligament reconstruction: a magnetic resonance imaging evaluation. Arthritis Rheumatol 2015;67(4):946–955. [DOI] [PubMed] [Google Scholar]
- 10.Gardinier ES, Di Stasi S, Manal K, Buchanan TS, Snyder-Mackler L. Knee contact force asymmetries in patients who failed return-to-sport readiness criteria 6 months after anterior cruciate ligament reconstruction. Am J Sports Med 2014;42(12):2917–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall M, Stevermer CA, Gillette JC. Gait analysis post anterior cruciate ligament reconstruction: knee osteoarthritis perspective. Gait Posture 2012;36(1):56–60. [DOI] [PubMed] [Google Scholar]
- 12.Hart HF, Culvenor AG, Collins NJ, et al. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med 2015. [DOI] [PubMed]
- 13.Hatcher CC, Collins AT, Kim SY, et al. Relationship between T1rho magnetic resonance imaging, synovial fluid biomarkers, and the biochemical and biomechanical properties of cartilage. J Biomech 2017;55:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keenan KE, Besier TF, Pauly JM, et al. Prediction of glycosaminoglycan content in human cartilage by age, T1rho and T2 MRI. Osteoarthritis Cartilage 2011;19(2):171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein S, Staring M, Murphy K, Viergever MA, Pluim JP. elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 2010;29(1):196–205. [DOI] [PubMed] [Google Scholar]
- 16.Kumar D, Kothari A, Souza RB, Wu S, Benjamin Ma C, Li X. Frontal plane knee mechanics and medial cartilage MR relaxation times in individuals with ACL reconstruction: A pilot study. Knee 2014;21(5):881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar D, Manal KT, Rudolph KS. Knee joint loading during gait in healthy controls and individuals with knee osteoarthritis. Osteoarthritis Cartilage 2013;21(2):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Tao H, Hua Y, Chen J, Li Y, Chen S. Quantitative magnetic resonance imaging assessment of cartilage status: a comparison between young men with and without anterior cruciate ligament reconstruction. Arthroscopy 2013;29(12):2012–2019. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Benjamin Ma C, Link TM, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage 2007;15(7):789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Pedoia V, Kumar D, et al. Cartilage T1rho and T2 relaxation times: longitudinal reproducibility and variations using different coils, MR systems and sites. Osteoarthritis Cartilage 2015;23(12):2214–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Wyatt C, Rivoire J, et al. Simultaneous acquisition of T and T quantification in knee cartilage: Repeatability and diurnal variation. J Magn Reson Imaging 2013. [DOI] [PMC free article] [PubMed]
- 22.Lohmander LS. Articular cartilage and osteoarthrosis. The role of molecular markers to monitor breakdown, repair and disease. J Anat 1994;184 ( Pt 3):477–492. [PMC free article] [PubMed] [Google Scholar]
- 23.Magnussen RA, Duthon V, Servien E, Neyret P. Anterior Cruciate Ligament Reconstruction and Osteoarthritis: Evidence from Long-Term Follow-Up and Potential Solutions. Cartilage 2013;4(3 Suppl):22S–26S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis 2002;61(7):617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol 2004;8(4):355–368. [DOI] [PubMed] [Google Scholar]
- 26.Noehren B, Wilson H, Miller C, Lattermann C. Long-term gait deviations in anterior cruciate ligament-reconstructed females. Med Sci Sports Exerc 2013;45(7):1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedoia V, Li X, Su F, Calixto N, Majumdar S. Fully automatic analysis of the knee articular cartilage T1rho relaxation time using voxel-based relaxometry. J Magn Reson Imaging 2016;43(4):970–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedoia V, Russell C, Randolph A, Li X, Majumdar S, Consortium A-A. Principal component analysis-T1rho voxel based relaxometry of the articular cartilage: a comparison of biochemical patterns in osteoarthritis and anterior cruciate ligament subjects. Quant Imaging Med Surg 2016;6(6):623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedoia V, Su F, Keiko A, et al. Analysis of the Articular Cartilage T1ρ And T2 Relaxation Times Changes After ACL Reconstruction in Injured and Contralateral Knees and Relationships with Bone Shape. J Orthop Res 2016;[Conditionally Accepted]. [DOI] [PMC free article] [PubMed]
- 30.Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging 2006;23(4):547–553. [DOI] [PubMed] [Google Scholar]
- 31.Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging 1999;18(8):712–721. [DOI] [PubMed] [Google Scholar]
- 32.Sanford BA, Zucker-Levin AR, Williams JL, Mihalko WM, Jacobs EL. Principal component analysis of knee kinematics and kinetics after anterior cruciate ligament reconstruction. Gait Posture 2012;36(3):609–613. [DOI] [PubMed] [Google Scholar]
- 33.Souza RB, Fang C, Luke A, Wu S, Li X, Majumdar S. Relationship between knee kinetics during jumping tasks and knee articular cartilage MRI T1rho and T2 relaxation times. Clin Biomech (Bristol, Avon) 2012;27(4):403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su F, Hilton JF, Nardo L, et al. Cartilage morphology and T1rho and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthritis Cartilage 2013;21(8):1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su F, Pedoia V, Teng HL, et al. The association between MR T1rho and T2 of cartilage and patient-reported outcomes after ACL injury and reconstruction. Osteoarthritis Cartilage 2016. [DOI] [PMC free article] [PubMed]
- 36.Subburaj K, Kumar D, Souza RB, et al. The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. Am J Sports Med 2012;40(9):2134–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theologis AA, Haughom B, Liang F, et al. Comparison of T1rho relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee Surg Sports Traumatol Arthrosc 2014;22(2):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Ginckel A, Verdonk P, Witvrouw E. Cartilage adaptation after anterior cruciate ligament injury and reconstruction: implications for clinical management and research? A systematic review of longitudinal MRI studies. Osteoarthritis Cartilage 2013;21(8):1009–1024. [DOI] [PubMed] [Google Scholar]
- 39.van Meer BL, Meuffels DE, van Eijsden WA, Verhaar JA, Bierma-Zeinstra SM, Reijman M. Which determinants predict tibiofemoral and patellofemoral osteoarthritis after anterior cruciate ligament injury? A systematic review. Br J Sports Med 2015;49(15):975–983. [DOI] [PubMed] [Google Scholar]
- 40.Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS, Snyder-Mackler L. Decreased Knee Joint Loading Associated With Early Knee Osteoarthritis After Anterior Cruciate Ligament Injury. Am J Sports Med 2015. [DOI] [PMC free article] [PubMed]
- 41.Xia Y Relaxation anisotropy in cartilage by NMR microscopy (muMRI) at 14-microm resolution. Magn Reson Med 1998;39(6):941–949. [DOI] [PubMed] [Google Scholar]
- 42.Zabala ME, Favre J, Scanlan SF, Donahue J, Andriacchi TP. Three-dimensional knee moments of ACL reconstructed and control subjects during gait, stair ascent, and stair descent. J Biomech 2013;46(3):515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]