Abstract
Background
Diabetes results in pathophysiological changes, leading to tissue that is unable to withstand and adapt to the same loads, resulting in breakdown. Certain locations are more susceptible to breakdown, yet differences between locations are largely not well understood. We performed a histological and biochemical analysis of isolated plantar adipose tissue at six relevant locations.
Methods
Tissue from six plantar locations (thallux, first, third and fifth metatarsal heads, lateral midfoot and calcaneus) was taken from fresh cadaveric feet of older diabetic and older non-diabetic intact donors. Histomorpphological and biochemical analysis of isolated plantar tissue from both diabetic and non-diabetic feet at six relevant locations was performed.
Results
The main differences found between diabetic and non-diabetic tissue were in the thickness of the septal walls and the elastin content. Diabetic tissue had significantly thicker septal walls and an increased elastin concentration. When comparing the calcaneus to other locations, although there were no differences found in the thickness of the septal walls of diabetic tissue, elastin content was lower in the calcaneous tissue compared to the non-calcaneus sites.
Conclusions
Modifications in the structural and biochemical properties could translate to changes in the mechanical properties. This information could lead to an understanding of how the structural and biochemical changes result in an increase in susceptibility of tissue to breakdown with load at the different locations of the foot.
Keywords: Plantar soft tissue, histomorphological, biochemical, diabetic foot ulcer
Introduction
Diabetes has become one of the most prominent health threats of our time, with 21–33% of the U.S. population predicted to have diabetes by 2050 [1], if current trends in diabetes prevalence continue. One of the most common complications of diabetes is non-traumatic lower-extremity amputation (LEAs), which is preceded by foot ulcers approximately 85% of the time [2]. An estimated 15% of all diabetic patients will suffer from ulcerated feet in their lifetime and 5–24% of this population will require amputation within a period of 6–18 months after the first evaluation [3].
In healthy persons, the complex structure of the plantar tissue enables this tissue to endure the rigorous mechanical loading regimes of everyday life. However, tissue from people with systemic pathologies, such as diabetes, is often unable to withstand and adapt to the repeated loading regimes in the same manner, resulting in breakdown. All forms of diabetes are known to induce alterations in the metabolism of the macromolecules present in the body [4]. These changes lead to changes in tissue structure [5] and mechanics [6–8] resulting in diabetic plantar soft tissue having a lower threshold for injury. The biochemical changes in diabetes are complex [9], however, persistent hyperglycemia and the accelerated accumulation of advanced glycation end-products (AGEs) are major causes of the detrimental changes in the pathophysiology of diabetic tissue. These include alterations to the soft and hard tissue, nerve function, vascular structure, immunology, wound healing and plantar pressure [10–12]. Although the biochemistry of a number of tissues taken from persons with diabetes has been evaluated [9], the biochemistry of diabetic plantar soft tissue has been limited [13].
The plantar fat in the healthy foot comprises of elastic fibrous septa tissue separating closely packed fat cells [14]. This anatomic architecture acts to dissipate stresses to maintain normal plantar pressures [15]. Earlier histomorphometric studies of impaired plantar fat reported thicker, fragmented septal walls and a decrease in adipocyte area and diameter [16, 17]; however, all diabetic tissue from these studies came from limbs that were amputated due to vascular compromise. Waldecker and Lehr [18] examined biopsied metatarsal fat pad tissue but found no difference in adipocyte size between diabetic and non-diabetic tissue. Others have found that skin (on the dorsum of the foot and elsewhere) is thicker in people with diabetes [19, 20] and the expression of genes related to the remodeling of the extracellular matrix is impaired resulting in decreased mechanical properties [21]. Our previous stereological studies have supported the observation that diabetic tissue has thicker elastic septae measured at the heel and the first metatarsal [22]. These observations demonstrate that diabetes affects the histomorphology and the biochemistry of the plantar soft tissue that can be related to detrimental changes to the mechanical properties. However differences between location of the foot were not evaluated. Of the histomorphometric studies that have been performed, most have focused on the heel or a non-specified location, despite the variations in the mechanical properties [23] and incidence of ulceration [24, 25] in different locations of the foot. Evaluating both the histolomorphological and biomechanical changes that occur in diabetes over various plantar locations could give a more thorough understanding of why diseased tissue is so much more susceptible to breakdown and in the establishment of preventative measures.
The purpose of this study is to perform a histological and biochemical analysis of isolated plantar tissue from both diabetic and non-diabetic feet at six relevant locations. An understanding of both the histomorphological and biochemical changes in the soft tissue in relationship to the location on the foot can be used to understand the subsequent increase in susceptibility to breakdown in different locations.
Methods
Specimen Procurement
Plantar soft tissue samples were taken from fresh cadaveric feet of older diabetic and older non-diabetic donors. All specimens (Type 2) were obtained from the National Disease Research Interchange within 24 hours of death. Donors were of similar age and sex, but the diabetic patients had a significantly greater body mass index (BMI, Table 1). Specimens were not obtained from amputated limbs, but rather from intact donors. Only one of the diabetic donors died from a condition that has been closely linked with diabetes (congestive heart failure). All specimens were paired; one was used for biochemistry and histormorphological testing, while the other was used for mechanical testing [6–8].
Table 1.
Mean [range] donor information.
| Diabetic | Non-diabetic | p-value | |
|---|---|---|---|
| n | 4 | 9 | - |
| Age (years) | 70.5 [63–79] | 70.9 [61–79] | 0.7 |
| Gender (male:female) | 2:2 | 4:5 | - |
| Weight (kg) | 94.1 [68–126] | 64.7 [45–91] | 0.098 |
| Diabetes duration (years) | 20.3 [9–27] | - | - |
| BMI (kg/m2) | 33.0 [27.5–37.7] | 22.1 [16.8–29.1] | 0.013 |
Tissue was taken from six plantar locations, namely the hallux, first, third and fifth metatarsal heads, lateral midfoot and calcaneus. The specimens (2cm × 2cm), containing epidermis, dermis and plantar fat (hypodermis), were split for histomorphological or biochemical analysis. The histology samples were immediately placed in 10% neutral buffered formalin for fixation immediately following harvest. The biochemical samples were immediately flash frozen in liquid nitrogen and stored at −70° C until being processed. Histomorphological evaluation was performed using a combination of stereological methods and quantitative morphology as described previously [5]. Institutional Review Board approval was obtained for this study from the Human Subjects Division at the University of Washington. All samples were procured and dissected by National Disease Research Interchange (NDRI, Philadelphia, PA).
Histomorphological Evaluation
Vertical uniform random (VUR) sampling of the specimens [26] was used to obtain unbiased, isotropic sections when combined with stereological sampling probes as described previously [5]. Alternate unbiased, isotropic sections were stained with hematoxylin and eosin (H&E), picro sirius red for collagen and modified Hart’s for elastin, according to standard protocols. The histological sections were visualized using a Nikon microscope (Eclipse 80i, Melville, NY) and images were analyzed in a blind fashion using ImageJ 1.42 (National Institutes of Health, Bethesda, MD. Preliminary analysis was performed to determine the minimum number of images and measurements required so that only a negligible amount of the total variation was due to sampling error.
Elastic Septae Thickness
The thickness of the septal walls was measured in sections stained with modified Hart’s. A Stereological approach was used to make random measurements of the septal wall thickness as described previously [22]. An approximation of the true thickness was calculated using the harmonic and arithmetic mean, which overcomes the overestimation of the measured values [27, 28].
Adipocyte size
An optical-dissector probe (area of 25,000 µm2) was randomly placed over 10× images using systematic random sampling rules [29, 30]. The area, minimum Feret and Feret diameter were measured for adipocytes that followed the rules of the dissector. Adipocytes that were damaged or overly distorted due to processing were not included in the measurements.
Area Fraction
A point probe (area per point of 500,000 µm2) was randomly placed over 2× images of the adipose layer stained with modified Hart’s. Adipocytes and elastic septa were counted using systematic random sampling rules [31].
Biochemical Quantification of Collagen and Elastin
The adipose was carefully separated from the skin (epidermis and dermis), minced and subject to defatting using anhydrous acetone, acetone:ether (1:1) and methanol:chloroform (1:1) before being washed and lyophilized.
Collagen Content
The collagen content of samples was determined using the well-established hydroxyproline assay based on the protocol of Bergman and Loxley [32]. Briefly, defatted samples were lyophilized weighed and subjected to acid hydrolysis in 6M HCl. After neutralization with sodium hydroxide, chloromine T was added and incubated at room temperature for 20 minutes. Dimethylaminobenzaldehyde (Ehrlichs Reagent) was added and samples incubated at 60° C for 15 minutes before absorption was read at 550nm. Hydroxyproline concentrations were determined based on a generated standard curve for trans-4-hydroxy-L-proline (H1637, Sigma-Aldrich, St. Louis, MO). Total collagen per sample was calculated using a conversion factor of 6.94, based on the fact that hydroxyproline represents 14.4% of the amino acid composition of collagen in most mammalian tissues [33]. The collagen content is presented as mg per mg of tissue dry weight.
2.6.2 Elastin Content
The Fastin Elastin assay (Biocolor Ltd, Carrickfergus, U.K.) was used to quantify total soluble and insoluble elastin content. Defatted samples were lyophilized, weighed and subjected to sequential acid digestion with oxalic acid for 1 hour at 100° C and ethanolic potassium hydroxide for 1 hour at 37° C. The oxalic acid and subsequent ethanolic potassium hydroxide converts insoluble elastin to water soluble elastin which can be quantified. After each digestion, the samples were centrifuged and the supernatant was removed. The supernatant from all the oxalic acid digestions were pooled for analysis. The supernatants from the ethanolic potassium hydroxide digestions were dyalized against water over night before assaying. The extracted elastin was quantified as per the manufacturers instructions. Absorbance was read at 515 nm and elastin content was determined from a standard curve for α-elastin.
Collagen I:III Ratio
Collagen I:III ratio was determined by interrupted SDS gel electrophoresis based on methods of Sykes et al. [34] and the modifications made by Samuel [33]. Samples were ground in the presence of liquid nitrogen and then placed in 0.5M acetic acid for 48 hrs at 4° C with gentle agitation. Samples were digested exhaustively with a pepsin acetic acid solution (1mg/ml pepsin). The collagen chains were isolated by salt precipitation and the precipitate was re-suspended in 0.5M acetic acid and dyalized before lyophilizing. The lyophilized samples were reconstituted using loading buffer containing urea and heated before loading onto gels. 30% β-mercaptoethanol was added to each sample well for 60 minutes to separate the type I collagen chains from the type III collagen chains. Gels were stained with Coomassie blue and the collagen I and III band ratio was calculated using measurements made with a densitometer with quantitative software (GelDoc XR, Biorad, Hercules, CA).
Statistics
To determine differences between diabetic and non-diabetic tissue, linear mixed effects regression was used with the biochemical variable or histology variable as the outcome, diabetic status as the independent fixed effect, and foot as a random effect. Differences between calcaneus vs. other plantar locations were assessed using linear mixed effects regression with calcaneus vs. other as the independent fixed effect and random effects for foot and foot × calcaneus vs. other location interaction. To determine if differences by location differed by diabetic status, an interaction between diabetic status and calcaneus vs. other location was tested for significance. If significant, then pairwise comparisons were carried out for diabetic and non-diabetic tissue separately to test for differences between calcaneus vs. other locations. Significance was set at p<0.05. For the site comparisons, the calcaneus was selected based on differences in the mechanical properties found previously between the calcaneus and other locations [23, 35]. Hypothesis testing of ratio variables were carried using the numerator as the dependent variable, and the denominator as a model covariate, which can be interpreted as the significance of the association between the numerator and the independent fixed effect, adjusting for the denominator. Analyses were carried out using R 2.11.2 [36] using the lme4 package to carry out the linear mixed effects regressions [37]. Pairwise interactions were carried out using simultaneous inference from the multcomp package [38].
Results
All tissues were taken from visually healthy feet. None of feet displayed any indication of ulceration, breakdown or inflammation at the time of tissue harvest or during general histological evaluation.
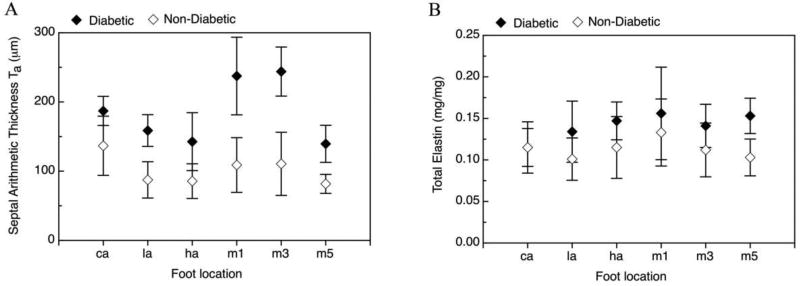
Stereological evaluation of the hypodermis reveals a statistically significant mean increase in the septal wall thickness by 100 µm (p<0.0001) in diabetic tissue vs. non-diabetic tissue (Table 2). An increase in septal wall thickness is observed in diabetic vs non-diabetic tissue when separated out by location (Figure 1). In the comparison of the calcaneus vs other locations, no difference is found between the calcaneus and non-calcaneus for the septal thickness of the diabetic patients. However, the septal thickness of the calcaneous in the non-diabetic tissue shows a significant mean difference compared to other locations (p=0.006) (Table 2).
Table 2.
Histological means [standard error] by diabetes status from linear mixed effects regression on diabetes status.
| Diabetic | Non-diabetic | Difference | p-value | |
|---|---|---|---|---|
| Septal Thickness (mm) | 234 [15] | 134 [10] | 100 [18] | <0.0001* |
| Septal Arithmetic Thickness (mm) | 184 [12] | 103 [8] | 81 [15] | <0.0001* |
| Calcaneus | 187 [21] | 137 [14] | ||
| Other location | 183 [11] | 95 [7] | ||
| p-value | 0.98† | 0.006† | 0.14‡ | |
| Adipose Area (mm2) | 2052 [290] | 1767 [193] | 285 [358] | 0.4* |
| Minimum Adipose Diameter (mm) | 42.9 [3.8] | 39.2 [2.5] | 3.7 [4.5] | 0.4* |
| Adipose area fraction | 0.336 [0.051] | 0.455 [0.034] | 0.119 [0.045] | 0.4* |
comparison between diabetic status from linear mixed effects regression of histological measure on diabetes status with foot ID as a random effect
comparison between calcaneus and other location from linear mixed effects regression of biochemical measure on location (heel vs. other)
significance of diabetes status by location (heel vs. other) interaction from linear mixed effects regression with foot ID and foot ID by location interaction as random effects
Figure 1.
Mean arithmetic thickness (left) and total elastin concentration (right) in diabetic and non-diabetic tissue at different foot locations: calcaneus (ca), lateral midfoot (la), hallus (ha), first, third and fifth metatarsal heads (m1, m3 and m5).
The mean total elastin concentration (Table 3) is also significantly increased in the diabetic tissue by 28 µg/mg compared to non-diabetic tissue (p=0.045). This increase is most prominent in non-calcaneus tissue (hallux, first, third and fifth metatarsal heads, lateral midfoot; Figure 1), but is not found in calcaneus tissue (location by diabetes status interaction, p=0.044, Table 3). Examining this result from the perspective of differences by location, mean total elastin concentration is lower at the calcaneus vs. other sites for diabetic tissue by 0.031 mg/mg (p=0.041) but not for non-diabetic tissue with only a 2 mg/mg difference between calcaneus and non-calcaneus sites, (p=0.98, Table 3). The increase in mean total elastin by diabetes status is more strongly reflected in soluble elastin (mean difference 0.023 mg/mg, p=0.077) than in insoluble elastin (mean difference 4 mg/mg, p=0.61, Table 3). The pattern of differences in soluble elastin by diabetes status and location is similar to that for total elastin (location by diabetes interaction p=0.020).
Table 3.
Biochemical means [standard error] by diabetes status. Total elastin and soluble elastin is further separated by calcaneus and other location.
| Diabetic | Non-diabetic | p-value | |
|---|---|---|---|
| Total elastin (mg/mg) | 0.141 [0.012] | 0.113 [0.008] | 0.045* |
| Calcaneus total elastin (mg/mg) | 0.115 [0.015] | 0.115 [0.010] | |
| Other location total elastin (mg/mg) | 0.146 [0.012] | 0.113 [0.008] | |
| p-value | 0.041† | 0.98† | 0.044‡ |
| Soluble elastin (mg/mg) | 0.115 [0.011] | 0.092 [0.007] | 0.077* |
| Calcaneus total elastin (mg/mg) | 0.088 [0.014] | 0.098 [0.009] | |
| Other location total elastin (mg/mg) | 0.120 [0.012] | 0.091 [0.008] | |
| p-value | 0.039† | 0.68† | 0.02‡ |
| Insoluble elastin (mg/mg) | 0.026 [0.008] | 0.022 [0.005] | 0.6* |
| Total collagen (mg/mg) | 0.488 [0.028] | 0.449 [0.019] | 0.3* |
| Collagen:Elastin ratio | 3.8 [0.5] | 4.2 [0.3] | 0.3* |
| Collagen I:III ratio | 1.11 [0.03] | 1.02 [0.02] | 0.061* |
comparison between diabetic status from linear mixed effects regression with foot ID as a random effect
comparison between calcaneus and other location from linear mixed effects regression of biochemical measure on location (heel vs. other)
significance of the diabetes status by location (heel vs. other) interaction from linear mixed effects regression with foot ID and foot ID by location interaction as random effects
Although the total mean collagen concentration tends to larger values in the diabetic tissue, this is not found to be statistically different (p=0.3). No differences are found in the collagen concentration when separating out by location (data not shown). Mean collagen adjusted for elastin and mean collagen I adjusted for collagen II did not significantly differ by diabetes status, (p=0.30, p=0.061 respectively, Table 3)
The mean adipocyte cell areas and minimum diameters were not significantly different in diabetic compared to non-diabetic tissue (Table 2). However, the mean values tend to larger values in the diabetic tissue (mean area difference, 285 µm2; mean min. diameter difference, 3.7 µm). The total area fraction of the adipose tissue tends to smaller values (0.336 ± 0.051 vs. 0.455 ± 0.034; p=0.4). However, no obvious qualitative differences in the adipose tissue compartments are observed in terms of orientation or morphology. Atrophy of the adipocytes is also not observed in any of the tissue specimens, although in some specimens the adipocytes in the diabetic tissue appeared to be qualitatively less uniform in shape.
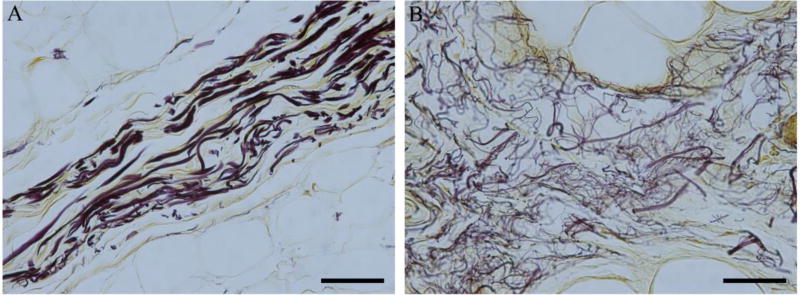
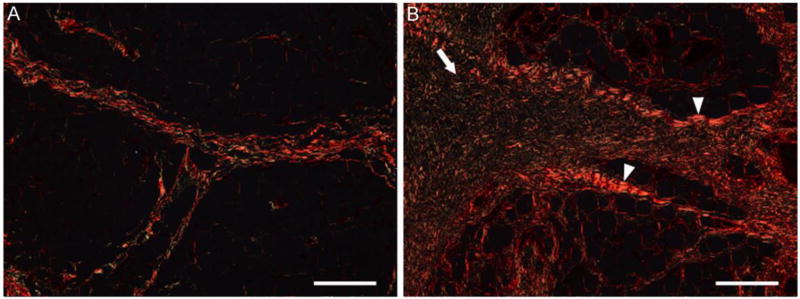
Histological evaluation reveals fragmentation/fraying of the elastin fibers within the septal walls of the diabetic tissue (Figure 2). In addition, the septal walls of the diabetic tissue contain collagen bundles that are thicker in sections, frayed in others, and without distinct band periodicity (Figure 3). There are no discernable qualitative differences observed between locations, for both conditions, beyond potential natural anatomical variation.
Figure 2.
Representative images of the elastic septa in a) non-diabetic and b) diabetic plantar tissue stained with modified Hart’s. Elastin fibres stain black. Elastic fibres in diabetic tissue are frayed and fragmented, often resulting in thicker septal walls. Scale bar represents 50 µm.
Figure 3.
Representative images of the elastic septa in a) non-diabetic and b) diabetic plantar tissue stained with Pico Sirus red. Under polarized light, collagen appears red-orange. Thicker collagen bundles (white arrow head) were observed in the diabetic tissue compared with the normal tissue. There were also regions of frayed and disordered collagen fibres (white arrow) observed in the diabetic septal walls compared with the normal tissue. Scale bar represents 200 µm.
Discussion
It is well known that diabetes causes detrimental changes in the pathophysiology of tissue, leading to a greater susceptibility to ulcerations. It is therefore important to evaluate the pathophysiological difference in the structural tissue and relating these changes to the biochemical and mechanical changes to provide insight into the complicated relationship between morphological, biochemical and mechanical properties. From these basic relationships new preventative measures may be developed.
In this study, a histomorpholicical and biochemical evaluation of diabetic and non-diabetic plantar, age-matched tissue at six locations (hallux, first, third and fifth metatarsal heads, lateral mid-foot and calcaneus) using a combination of stereological methods, quantitative morphological techniques, and biochemical analyses. A strength of this study was the adjustment of the thickness data to make random non-biased measurements in the histological sections [22] and inclusion of all pertinent regions of the plantar tissue [23]. The samples obtained display the characteristic structure of plantar soft tissue [22]. The muscle observed in the subcutaneous tissue of all foot locations do not show any obvious signs of damage nor are any differences observed between the diabetic and non-diabetic tissue. Cichowitz et al. suggested that pressure damage in the heel progressess with the initial injury involving the panniculus carnosus musle and subcutaneous fat [39]. It is therefore presumed that any changes in the tissue observed were not due to confounding effects of tissue breakdown and subsequent healing.
Although the increase in total elastin concentration in the diabetic tissue is only marginally significant (p=0.045), and there is no difference found in the total collagen concentration (p=0.2), the increase in the septal wall thickness is greatly significant (p<0.0001). This can be explained by the changes in the structure of the tissue components having a larger effect rather than increases in the amount of protein present. In non-diabetic tissue, elastin and collagen fibers are uniform and are mostly unidirectional. Both elastin and collagen are susceptible to nonenzymatic glycation via the Amadori rearrangement and advanced Maillard reactions. As a result, there will be both changes in the conformation of the protein networks resulting in an increase in the dimensions. In addition to possible changes due to the Amadori rearrangement, damage to the elastin fibres was observed as thinning and fraying. In the collagen fiber network, this was observed as a disorganization [40] and has been reported by others [41]. Both of these characteristics were observed in all of the diabetic tissue (Figure 2).
In the current study the mean adipocyte cell area and minimum diameter are numerically larger in the diabetic tissue, but not statistically greater. This agreed with previous studies evaluating non-damaged tissue [22, 42]. Hypertrophic adipocytes has also been associated with hyperinsulinemia, insulin resistance and dyslipidemia in other tissues [43, 44].
Aside from our previous study, which focused on two foot sites [22], most of the histological descriptions and evaluations of plantar tissue have focused on single sites [42, 45]. Only the mechanical properties have been evaluated for multiple sites of the plantar tissue [23, 46]. This study expands from our previous study to include all sites that have been evaluated mechanically. Results from the current study suggest that the tissue condition (diabetic status) rather than tissue site (calcaneus vs. other) has a greater effect on the biochemical and histological metrics evaluated in this study. For the site comparisons, the calcaneous was selected based on differences found previously between the calcaneous and other locations [23, 35]. However, it is possible that by selecting a different site/sites for the comparator more differences are discovered. Such a complicated comparison was not performed due to the small sample size and the need for a large number of pairwise comparisons.
The main structural difference found between diabetic and non-diabetic tissue was in the elastin content and thickness of the septal walls. When comparing the calcaneus to other locations, the elastin content and thickness was again found to be significantly different. The calcaneus was the only site that did not have an increase in elastin concentration despite the increase in septal wall thickness. This could translate to changes in the mechanical properties of the tissues and a greater susceptibility to breakdown. Future studies would involve correlating these biochemical and histomorphological metrics with the mechanical properties of the tissue [23, 47]. This could lead to an understanding of how the structural changes result in an increase in susceptibility of tissue to breakdown with load.
It is acknowledged that the current study contains a number of potential limitations. The small sample size for the diabetic group means that it was more difficult to identify differences smaller than the true effect size. The difference in BMI between the groups (the diabetic subjects were significantly larger) may have confounded the results. Due to the small number of specimens and the lack of much overlap between the groups, it was difficult to statistically account for differences in BMI. It is possible that tissue in the non-diabetic group was actually taken from subjects that had undiagnosed diabetes. However, given none of the non-diabetic group displayed histomorphological characteristics similar to the diabetic tissue, we do not think that this was the case. Finally, not all metrics that could be relevant to susceptibility to damage were evaluated, including collagen cross-linking, and vascularity.
Conclusion
Both morphological and biochemical differences were observed in diabetic plantar tissue compared to non-diabetic tissue, mainly related to the elastic septa. The differences measured could result in changes to the mechanical properties of the tissue, leading to greater susceptibility to breakdown in some locations over others.
Highlights/Brief Summary.
This paper presents basic research in the evaluation of the histomorphometric and biochemical changes that could occur in human plantar tissue with diabetes.
This is the first paper to evaluate the histomorphologic and biochemical properties separated by both disease status and foot location.
The main findings were differences in the elastin content and the septal wall thickness, with diabetes and location, which could translate to changes in the mechanical properties and subsequent sensitivities to ulcer formation
Acknowledgments
This study was supported by the National Institutes of Health grant 1R01 DK75633-03 and the Department of Veterans Affairs, Rehabilitation Research and Development Service grant A4843C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest to declare
References
- 1.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population health metrics. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputations in diabetes. In: Harris MI, editor. Diabetes in America. 2. Washington DC: U.S. Government Printing Office; 1995. pp. 409–28. [Google Scholar]
- 3.Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes therapy : research, treatment and education of diabetes and related disorders. 2012;3:4. doi: 10.1007/s13300-012-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22:1036–42. doi: 10.2337/diacare.22.7.1036. [DOI] [PubMed] [Google Scholar]
- 5.Wang YN, Lee K, Ledoux WR. Histomorphological evaluation of diabetic and non-diabetic plantar soft tissue. Foot Ankle Int. 2011;32:802–10. doi: 10.3113/FAI.2011.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pai S, Ledoux WR. The compressive mechanical properties of diabetic and non-diabetic plantar soft tissue. J Biomech. 2010;43:1754–60. doi: 10.1016/j.jbiomech.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pai S, Ledoux WR. The quasi-linear viscoelastic properties of diabetic and non-diabetic plantar soft tissue. Ann Biomed Eng. 2011;39:1517–27. doi: 10.1007/s10439-011-0263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pai S, Ledoux WR. The shear mechanical properties of diabetic and non-diabetic plantar soft tissue. J Biomech. 2012;45:364–70. doi: 10.1016/j.jbiomech.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sternberg M, Cohen-Forterre L, Peyroux J. Connective tissue in diabetes mellitus: biochemical alterations of the intercellular matrix with special reference to proteoglycans, collagens and basement membranes. Diabete & metabolisme. 1985;11:27–50. [PubMed] [Google Scholar]
- 10.Boucek P. Advanced Diabetic Neuropathy: A Point of no Return? Rev Diabet Stud. 2006;3:143–50. doi: 10.1900/RDS.2006.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiber GE, Lipsky BA, Gibbons GW. The burden of diabetic foot ulcers. American Journal of Surgery. 1998;176:5s–10s. doi: 10.1016/s0002-9610(98)00181-0. [DOI] [PubMed] [Google Scholar]
- 12.Cavanagh PR, Simoneau GG, Ulbrecht JS. Ulceration, unsteadiness, and uncertainty: the biomechanical consequences of diabetes mellitus. J Biomech. 1993;26:23–40. doi: 10.1016/0021-9290(93)90077-r. [DOI] [PubMed] [Google Scholar]
- 13.Tahrani AA, Zeng W, Shakher J, Piya MK, Hughes S, Dubb K, et al. Cutaneous structural and biochemical correlates of foot complications in high-risk diabetes. Diabetes Care. 2012;35:1913–8. doi: 10.2337/dc11-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahss MH, Michelson JD, Desai P, Kaye R, Kummer F, Buschman W, et al. Investigations into the fat pads of the sole of the foot: anatomy and histology. Foot Ankle. 1992;13:233–42. doi: 10.1177/107110079201300502. [DOI] [PubMed] [Google Scholar]
- 15.Jahss MH, Kummer F, Michelson JD. Investigations into the fat pads of the sole of the foot: heel pressure studies. Foot Ankle. 1992;13:227–32. doi: 10.1177/107110079201300501. [DOI] [PubMed] [Google Scholar]
- 16.Buschmann WR, Jahss MH, Kummer F, Desai P, Gee RO, Ricci JL. Histology and histomorphometric analysis of the normal and atrophic heel fat pad. Foot & Ankle International. 1995;16:254–8. doi: 10.1177/107110079501600502. [DOI] [PubMed] [Google Scholar]
- 17.Jahss MH, Michelson JD, Desai P, Kaye R, Kummer F, Buschman W, et al. Investigations into the fat pads of the sole of the foot: Anatomy and histology. Foot & Ankle. 1992;13:233–42. doi: 10.1177/107110079201300502. [DOI] [PubMed] [Google Scholar]
- 18.Waldecker U, Lehr HA. Is there histomorphological evidence of plantar metatarsal fat pad atrophy in patients with diabetes? J Foot Ankle Surg. 2009;48:648–52. doi: 10.1053/j.jfas.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Goodfield MJ, Millard LG. The skin in diabetes mellitus. Diabetologia. 1988;31:567–75. doi: 10.1007/BF00264762. [DOI] [PubMed] [Google Scholar]
- 20.Hanna W, Friesen D, Bombardier C, Gladman D, Hanna A. Pathologic features of diabetic thick skin. J Am Acad Dermatol. 1987;16:546–53. doi: 10.1016/s0190-9622(87)70072-3. [DOI] [PubMed] [Google Scholar]
- 21.Bermudez DM, Herdrich BJ, Xu J, Lind R, Beason DP, Mitchell ME, et al. Impaired biomechanical properties of diabetic skin implications in pathogenesis of diabetic wound complications. Am J Pathol. 2011;178:2215–23. doi: 10.1016/j.ajpath.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YN, Lee K, Ledoux WR. Histomorphological Evaluation of Diabetic and Non-Diabetic Plantar Soft Tissue. Foot & Ankle International. 2011;32:802–10. doi: 10.3113/FAI.2011.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pai S, Ledoux WR. The compressive mechanical properties of diabetic and non-diabetic plantar soft tissue. Journal of Biomechanics. 2010;43:1754–60. doi: 10.1016/j.jbiomech.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isakov E, Budoragin N, Shenhav S, Mendelevich I, Korzets A, Susak Z. Anatomic sites of foot lesions resulting in amputation among diabetics and non-diabetics. Am J Phys Med Rehabil. 1995;74:130–3. [PubMed] [Google Scholar]
- 25.Cowley MS, Boyko EJ, Shofer JB, Ahroni JH, Ledoux WR. Foot ulcer risk and location in relation to prospective clinical assessment of foot shape and mobility among persons with diabetes. Diabetes Research and Clinical Practice. 2008;82:226–32. doi: 10.1016/j.diabres.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–76. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 27.Ferrando RE, Nyengaard JR, Hays SR, Fahy JV, Woodruff PG. Applying stereology to measure thickness of the basement membrane zone in bronchial biopsy specimens. J Allergy Clin Immunol. 2003;112:1243–5. doi: 10.1016/j.jaci.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 28.Mayhew TM. The New Stereological Methods for Interpreting Functional-Morphology from Slices of Cells and Organs. Experimental Physiology. 1991;76:639–65. doi: 10.1113/expphysiol.1991.sp003533. [DOI] [PubMed] [Google Scholar]
- 29.Cruz-Orive LM, Weibel ER. Recent stereological methods for cell biology: a brief survey. Am J Physiol. 1990;258:L148–56. doi: 10.1152/ajplung.1990.258.4.L148. [DOI] [PubMed] [Google Scholar]
- 30.Nyengaard JR, Gundersen HJ. Sampling for Stereology in Lungs. Eur Respir Rev. 2006;15:107–14. [Google Scholar]
- 31.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 32.Bergman I, Loxley R. 2 Improved and Simplified Methods for Spectrophotometric Determination of Hydroxyproline. Anal Chem. 1963;35:1961-&. [Google Scholar]
- 33.Samuel CS. Determination of collagen content, concentration and sub-types in kidney tissue. Methods Mol Biol. 2009;466:223–35. doi: 10.1007/978-1-59745-352-3_16. [DOI] [PubMed] [Google Scholar]
- 34.Sykes B, Puddle B, Francis M, Smith R. Estimation of 2 Collagens from Human Dermis by Interrupted Gel-Electrophoresis. Biochem Bioph Res Co. 1976;72:1472–80. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- 35.Ledoux WR, Blevins JJ. The compressive material properties of the plantar soft tissue. Journal of Biomechanics. 2007;40:2975–81. doi: 10.1016/j.jbiomech.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Team RDC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 37.Bates D, Maechler M. lme4: Linear mixed-effects models using S4 classes. 2010 0.999375-35 Rpv, editor. [Google Scholar]
- 38.Torsten H, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical Journal. 2008;50:346–63. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 39.Cichowitz A, Pan WR, Ashton M. The heel: anatomy, blood supply, and the pathophysiology of pressure ulcers. Ann Plast Surg. 2009;62:423–9. doi: 10.1097/SAP.0b013e3181851b55. [DOI] [PubMed] [Google Scholar]
- 40.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:932–43. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 41.McDonald TO, Gerrity RG, Jen C, Chen HJ, Wark K, Wight TN, et al. Diabetes and arterial extracellular matrix changes in a porcine model of atherosclerosis. J Histochem Cytochem. 2007;55:1149–57. doi: 10.1369/jhc.7A7221.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waldecker U, Lehr HA. Is There Histomorphological Evidence of Plantar Metatarsal Fat Pad Atrophy in Patients with Diabetes? Journal of Foot & Ankle Surgery. 2009;48:648–52. doi: 10.1053/j.jfas.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Hoffstedt J, Arner E, Wahrenberg H, Andersson DP, Qvisth V, Lofgren P, et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia. 2010;53:2496–503. doi: 10.1007/s00125-010-1889-3. [DOI] [PubMed] [Google Scholar]
- 44.Veilleux A, Caron-Jobin M, Noel S, Laberge PY, Tchernof A. Visceral Adipocyte Hypertrophy is Associated With Dyslipidemia Independent of Body Composition and Fat Distribution in Women. Diabetes. 2011;60:1504–11. doi: 10.2337/db10-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jahss MH, Michelson JD, Desai P, Kaye R, Kummer F, Buschman W, et al. Investigations into the Fat Pads of the Sole of the Foot - Anatomy and Histology. Foot & Ankle. 1992;13:233–42. doi: 10.1177/107110079201300502. [DOI] [PubMed] [Google Scholar]
- 46.Pai S, Ledoux WR. The shear mechanical properties of diabetic and non-diabetic plantar soft tissue. Journal of Biomechanics. 2012;45:364–70. doi: 10.1016/j.jbiomech.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ledoux WR, Pai S, Shofer JB, Wang Y-N. The Association between Mechanical and Biochemical/Histological Characteristics in Diabetic and Non-Diabetic Plantar Soft Tissue. Journal of Biomechanics. 2016 doi: 10.1016/j.jbiomech.2016.08.021. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]