INTRODUCTION
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease.1 It usually starts in early infancy, and often remains a life-long therapeutic challenge, especially in its moderate-to-severe form.2 Its etiology remains only partly understood but is believed to be multifactorial, most likely rooted in a complex combination of immune deviation, impaired barrier function, and environmental risk factors.3–5 Skin sensitization to allergens seems to be a phenomenon that happens secondarily to a barrier defect, but it is an ongoing debate whether this is a result of genetic mutations affecting the epidermal barrier (outside-in-model) or due to an inflammatory process inhibiting epidermal differentiation (inside-out-model).6 As AD has been identified as a heterogeneous disease with activation of more than one inflammatory pathway rather than a uniform condition with a single pathogenic immune axis such as psoriasis, both models are likely to be relevant, with varying nuances depending on the AD population being evaluated.1,7 AD heterogeneity has not only been shown for intrinsic vs. extrinsic,8 pediatric vs. adult,9 and Caucasian vs. Asian AD.10 Also, the age of onset can be quite different, with a minority of patients showing the advent of late-onset AD during adulthood, a subset that can also have some diagnostic challenges due to atypical clinical presentation.11 Elucidating this complex disease is an ongoing endeavor, but previous work has already led to its increased understanding. Importantly, these data have led to the development of new therapeutics which have already been either approved, or which show promise in clinical trials.12 An important recent concept is that the immune, epithelial and microbial abnormalities in AD extend beyond the inflamed skin to also involve the “clinically normal” AD skin, possibly reflecting the systemic aspects of this disease (Figure 1).
Figure 1. “The roof is on fire” –
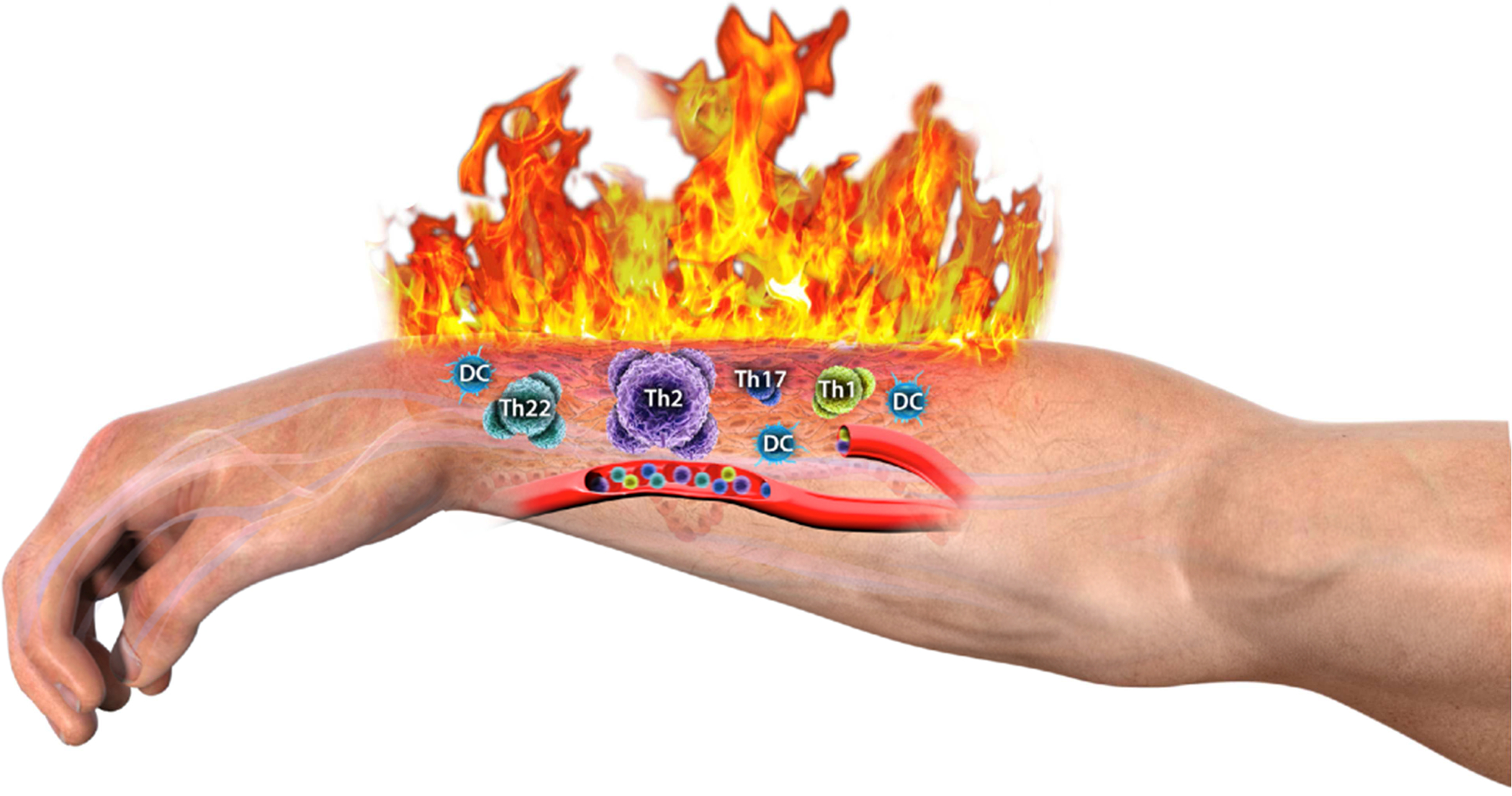
Immune cell subsets mediating local as well as potentially systemic inflammation.
AD is more than skin deep
AD is often associated with a variety of atopic/allergic comorbidities, including food allergy, allergic rhinoconjunctivitis, and asthma.13 Circulating skin-homing T-cells in severe patients show activation markers at levels higher than in healthy controls and even psoriasis,14 as well as alterations in polarizing cytokines, with a primary Th2/Th22 skewing.15,16 Abnormalities such as immune activation and barrier abnormalities are not only found in lesional, but also in non-lesional AD skin, both in longstanding as well as early onset pediatric disease.10,17–19 There are also increases in serum biomarkers, with CCL17 (a Th2-associated chemokine) being a robust marker of disease severity.20–22
Recently, AD has now also been linked to other, non-allergic conditions.23,24 Similar to what has been shown in psoriasis, adult AD patients showed increases in cardiovascular risk factors.25 Those included increases in body mass index (BMI), higher odds of heavy smoking, sedentary lifestyle, arterial hypertension, and lifetime pre-diabetes, increased alcohol intake, and decreased rates of vigorous physical activity compared to non-AD individuals.26–30 Recently, severe AD patients without any history of cardiovascular events were reported to show an increased prevalence of coronary artery disease, with coronary plaques in 48.1% of AD patients, compared to only 21.2% in healthy control subjects, as assessed by coronary computed tomography angiography.31 Whether AD is itself an independent risk factor for cardiovascular disease is an ongoing debate, and there might be heterogeneity among AD populations.32
AD is also associated with higher rates of neuropsychiatric disorders such as depression, anxiety, suicidal ideation, attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD).32–41 The exact mechanisms are still obscure but may be due to the harmful effects of AD on quality of life,42,43 such as debilitating chronic itch accompanied by sleeplessness.44 However, psychiatric disorders including depression, anxiety and autism have been linked to increased levels of pro-inflammatory cytokines,45,46 that are capable of penetrating the blood-brain barrier47 and thus could potentially influence and modulate behavior and emotions.32,39,48–50 Future studies will hopefully show whether AD skin disease itself is capable of shaping systemic disease, and influencing systemic immune abnormalities, or vice versa.
Elucidating the AD immune map via clinical investigation
The fact that AD is primarily a T-cell driven disease51,52 has initially been proven by the therapeutic efficacy of T-cell targeting agents such as cyclosporine, efalizumab, and alefacept.53,54,55 As no single animal model reflects the complex phenotype of AD, clinical trials using targeted therapeutics in humans are mandatory to fully understand this disease.56–58
Broadly acting therapies
Broadly immunosuppressive therapies such as cyclosporine A (CsA), systemic corticosteroids, and NB-UVB have been used for decades to treat moderate-to-severe AD, but their advent is more based on serendipity than on a detailed understanding of their mode of action.59 It has lateron been demonstrated that CsA and NB-UVB improve measures of epidermal hyperplasia and immune infiltrates, including the decrease of dendritic cell and T-cell infiltrates, but with less effects on terminal differentiation genes such as loricrin and filaggrin.59–61 Overall, CsA resulted in more rapid and stronger improvement in genomic and cellular dysregulation, and had stronger effects on non-lesional skin.60 Also, “inert” moisturizers such as petrolatum can robustly modulate antimicrobials and epidermal differentiation, in line with its beneficial response in AD patients.62 While such studies can help to identify disease biomarkers, the disease mediating cytokines need to be assessed using targeted therapies.
Th2 cytokines
It has taken a considerable amount of time since the first identification of IL-4 and IL-13 up-regulation in AD in 1994, until the approval of dupilumab, a humanized monoclonal antibody that inhibits signaling of these two key Th2 cytokines via IL-4 receptor (IL-4R) blockade. Dupilumab was recently FDA-approved for treatment of moderate-to-severe AD patients not well controlled with topical treatments.63–66 It has been evaluated in three phase III trials, with a total of 2119 adult patients, not adequately controlled by topical medications.67,68 Patients with an IGA score (investigator global assessment; ranging from 0 to 4, with higher numbers showing more severe disease) above 3 were included. The primary endpoint was the proportion of patients reaching an IGA of 0 (clear) or 1 (almost clear) and at least a 2-point improvement. Additional endpoints included EASI-75 (improvement of at least 75% in Eczema Area and Severity Index/EASI from baseline). Endpoints were assessed at week 16 of treatment. All studies showed significantly higher clinical improvements with dupilumab compared with placebo. The percentage of drug-treated patients reaching the primary endpoint with the now approved dosing (300mg s.c. q2w) were 38%, 36% and 39% in the three trials, as compared to placebo with 10%, 8% and 12%, respectively. EASI-75 responses were 51%, 44% and 69% with dupilumab, but only 15%, 12% and 23% with placebo, respectively. Dupilumab was generally well tolerated. Of note, patients on dupilumab had overall higher rates of conjunctivitis than placebo.67,68 However, the mechanism of this adverse event remains to be elucidated.
The dupilumab trial data prove that Th2 cytokines are drivers of AD in adult patients, and clinical trials in pediatric populations are ongoing (NCT02407756, NCT02612454, NCT03054428).
Whether the blockade of single Th2 cytokines (such as IL-4 or IL-13) alone is able to control the disease similarly to IL-4R antagonism remains to be determined. While there are no clinical data on IL-4 blockade, studies with anti-IL-13 antibodies tralokinumab and lebrikizumab showed clinical efficacy in phase II studies.69 However, the high placebo responses in these trials, possibly due to topical corticosteroid use, does not allow comparison with dupilumab monotherapy data.70 The concurrent application of topical corticosteroids might have masked drug effects, as demonstrated by previous publications of trials in AD.71,72 IL-5, another Th2-associated cytokine, has been implicated in eosinophil recruitment, and elevated blood eosinophil levels are known to occur in AD patients. Mepolizumab, a humanized antibody directed against free IL-5, was assessed in moderate-to-severe AD, but only led to modest improvements in clinical severity, albeit in a very short (2 week study),73 and a phase II study is currently being conducted (NCT03055195). Another Th2 centered treatment approach is blockade of IL-31 responses, a cytokine that has been implicated as an itch mediator.74 Nemolizumab, a humanized antibody blocking the IL-31 receptor A component, showed significant reductions in pruritus compared to placebo in a phase II trial, with concomitant reductions in clinical scores.75
Therefore, IL-31 blockade seems to be a promising strategy for AD, and possibly also for other pruritic skin diseases. Another Th2-centered treatment approach is blockade of the TSLP-OX40 (OX40L) pathway, which has been suggested to be involved in Th2 immune activation and tolerance induction.76–78 Blockers of OX40 (NCT02683928) and related mechanisms, including TSLP or its receptor TSLPR (NCT00757042, NCT01732510) are currently being investigated in early phase clinical trials.
Cytokine targeting beyond Th2 – IL-17 and IL-22
Despite the fact that blockade of Th2-centered inflammation shows promising results, the immune map in AD is more complex and heterogeneous than mere Type 2 cytokine-centric inflammation. While EASI-75 responses were achieved in approximately half of patients receiving dupilumab in phase III studies, the other half did not attain adequate responses,67 but the cause remains to be elucidated. While Th2 pathway activation is common to all studied AD subsets, including adult European American and Asian, as well as infant and adolescent patients,9,10,51 other T helper cell axes are detected variably in different AD populations, and their relative roles need to be still clarified. The Th1 axis seems to increase in chronic, adult AD, with low activation levels found in early-onset disease in adults and children.9,79 Asian AD populations showed additional, strong Th17 activation, with some overlap to psoriasis.10 Early-onset pediatric AD also showed increased Th17-skewing compared with adults.10 Ongoing and future trials (NCT02594098) should demonstrate whether Th17 blockade can control AD skin disease, and whether these results differ depending on ethnicity or age. Another distinct T helper cell subset that is strongly upregulated in AD is the Th22 pathway.80,81 IL-22 receptors are present primarily in epithelial cells, including the epidermis.82 In vitro studies suggested that IL-22 inhibits epidermal differentiation and promotes barrier defects,83,84 a concept that is currently being tested in a phase II clinical trial with the anti-IL-22 antibody fezakinumab (NCT01941537).
IL-12/IL-23p40
Ustekinumab, a fully human monoclonal antibody blocking the subunit of IL-12 and IL-23, thereby inhibiting Th1, Th17 as well as Th22 responses, is effective for psoriasis.85 A phase II placebo-controlled trial conducted in the United States using the approved psoriasis dosing showed clear trends of efficacy in AD but effects were likely diminished by concomitant topical glucocorticosteroid use.72 Also, treatment intervals seemed too long, with disease recurrence between the dosing periods.72 Similarly, there was no difference in ustekinumab and placebo effects in a Japanese cohort, but again, topical steroid use might have masked possible treatment effects.86 Consequently, trials with higher dosing and shorter treatment intervals will be needed to fully assess the potential of this therapeutic agent.
IgE
A considerable proportion of AD patients show increases in total serum IgE, and are often sensitized to multiple environmental allergens. Dupilumab treatment indeed suppressed blood levels of specific IgEs for a wide range of allergens in serum from AD patients, after 16 weeks of treatment.87 IgE has long been a logical candidate for AD treatment development. Randomized trials, however, failed to show clinical effects of IgE blockade using omalizumab,88,89 a humanized antibody that is highly efficacious for treating chronic spontaneous urticaria.90 These results, together with higher immune activation in patients with intrinsic than extrinsic AD,8 suggest that IgE is likely an epiphenomenon rather than a driver of AD, mediating primarily atopic/allergic comorbidities, but not directly impacting the atopic skin inflammation.
IL-6
IL-6 is increased in skin and blood of AD patients, but its role in disease pathogenesis is unclear.91,92 Tocilizumab, a humanized monoclonal antibody blocking the IL-6 receptor, is currently approved by the FDA for rheumatoid arthritis treatment. In a case series of three AD patients suffering from severe disease that were treated with tocilizumab, all patients had substantial decreases in pruritus, and all obtained an EASI-50 response. However, the study was not placebo-controlled, and two patients developed signs of bacterial infection,92 consistent with increased infectious complications of this agent in RA patients.93 This makes IL-6 blockade less attractive for AD treatment, especially in light of other potentially safer therapeutic approaches.
TNF-α
TNF-α, which has been linked to Th1/Th17 responses, is central to several inflammatory diseases, including psoriasis.94 While TNF-α can be increased in skin and blood of AD patients,95,96 there is no convincing evidence that TNF-blockade alleviates skin inflammation in these patients.97,98 Conversely, there are several reports showing that TNF-blockade can lead to AD exacerbation and blood eosinophilia,99–102 indicating that TNF-α might even have a protective effect in AD.
Small molecules
Several small molecules have also been assessed for treatment in AD. Crisaborole is a topical phosphodiesterase 4 (PDE4) inhibitor recently approved for the treatment of mild-to-moderate AD in 2 years of age or older patients.103 PDE4 activity is increased in AD skin, resulting in decreased intracellular levels of cyclic adenosine monophosphate, which in turn leads to increased production of proinflammatory cytokines.104 Crisaborole decreases levels of TNF-alpha, IL-12 and IL-23 and other inflammatory mediators105 that are increased in AD. Other small molecules with potentially promising results include the oral PDE4 inhibitor apremilast,106,107 the janus kinase blockers including tofacitinib, baricitinib and PF-04965842 (NCT02001181, NCT02576938, NCT02780167),108–110 and the histamine H4 receptor (H4R) antagonist ZPL389,51,111 but their efficacy needs to be demonstrated in larger clinical trials.
In sum, it has been demonstrated that T-cells are essential drivers of AD, but a considerable contribution by a specific T-cell immune axis has so far only been consistently proven in humans for the Th2 axis (IL-4, IL-13, IL-31). Other immune T-cell axes (Th1, Th17/IL-23, Th22) might also contribute to the complex and heterogeneous phenotype of AD (Figure 2), and their blockade (Table 1) could possibly close the therapeutic gap of patients not sufficiently responding to various Th2-centered treatment approaches.
Figure 2. Potential “driver” cytokines in AD.
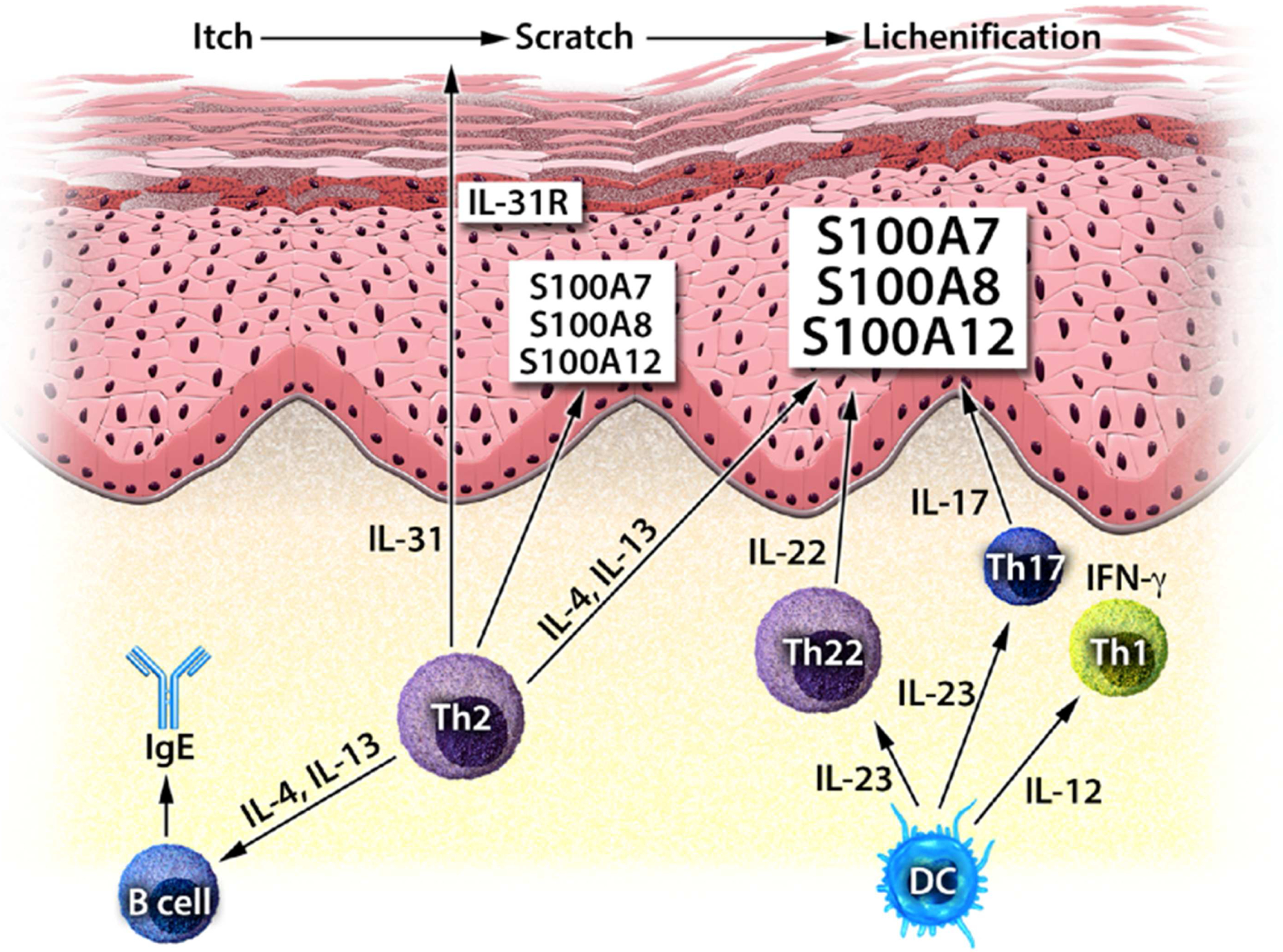
Immune mediators that have been shown to contribute to AD skin inflammation (IL-4, IL-13, IL-31, IL-12/IL-23), and that are potentially involved in shaping the disease phenotype, that are currently being investigated in clinical trials (IL-22, IL-17).
Table 1:
Biologics approved or currently being investigated in clinical trials for the treatment of AD.
| Agent | Target | Phase | Manufacturer | ClinicalTrials.gov |
|---|---|---|---|---|
| Dupilumab | IL-4Rα | Phase III published | Regeneron | NCT01949311 |
| Nemolizumab | IL-31R | Phase II published | Chugai | NCT01986933 |
| Ustekinumab | IL-12/23p40 | Phase II published | Janssen | NCT01806662 |
| Tralokinumab | IL-13 | Phase II completed | MedImmune | NCT02347176 |
| Lebrikizumab | IL-13 | Phase II completed | Hoffmann-La Roche | NCT02340234 |
| QGE031 | IgE | Phase II completed | Novartis | NCT01552629 |
| Fezakinumab | IL-22 | Phase II completed | Pfizer | NCT01941537 |
| GBR830 | OX40 | In Phase II | Glenmark | NCT02683928 |
| Secukinumab | IL-17 | In Phase II | Novartis | NCT02594098 |
| BMS-981164 | IL-31 | Phase I completed | BMS | NCT01614756 |
| Tezepelumab | TSLP | Phase I completed | Amgen | NCT00757042 |
| MK-8226 | TSLPR | In Phase I | Merck | NCT01732510 |
TSLP thymic stromal lymphopoietin; TSLPR thymic stromal lymphopoietin receptor.
Epidermis – victim and perpetrator
While a breach in the epidermal barrier is a characteristic component of AD, its pathogenic role for disease development is less clear. Loss-of-function mutations of the FLG gene results in a defective expression of the barrier protein filaggrin, which is currently the strongest risk factor for AD.112–114 Filaggrin is a key component of terminal differentiation and skin barrier function that also involves pH regulation and epidermal hydration.115 FLG loss-of-function mutations have been linked to more severe and persistent AD, leading to a higher degree of immune dysregulation with type 1 interferon-mediated stress responses and increased IL-1 cytokine levels, as well as elevated rates of allergic sensitization and skin infections.116–124 Nevertheless, this mutation is found only in a fraction of patients, and it is rarely present in African-American patients.124 Conversely, patients with FLG mutations can outgrow their disease.122 Also, dupilumab has been demonstrated to work equally well both in filaggrin mutated and wild type patients.64 Importantly, filaggrin is downregulated in all adult AD patients irrespective of FLG mutation status, most likely due to Th2/Th22-skewed inflammation. IL-4 and IL-13 significantly reduce filaggrin gene expression of keratinocytes in vitro,125 an effect that was also observed with IL-22 treatment of full thickness skin equivalents that have been designed to reflect cytokine effects in vivo.83 These data suggest that Th2/Th22 responses in adult patients with AD directly compromise the skin barrier. Surprisingly, though, early-onset pediatric AD did not show such a decrease in filaggrin expression despite the presence of Th2 and Th22-associated inflammatory mediators,9 mandating further research on barrier regulation in this age group.
Another epidermal feature of AD is an impaired innate immune response, that results in increased microbial colonization and, consequently, recurrent skin infections.126,127 This process is further pronounced in FLG mutated skin,128 consistent with the observation that filaggrin components do also have antimicrobial activity.129 But irrespective of filaggrin expression, antimicrobial peptides, a key component of skin host defense, show a relative deficiency in AD patients,130 most likely due to down-regulatory effects of IL-4 and IL-13.131–135 IL-22, especially in combination with IL-17, may upregulate antimicrobial peptides,136 which suggests that Th2 activation is primarily involved in antimicrobial peptide deficiency in AD.
The microbiome in shaping the immune system
During the last decade, the interaction between the environmental microflora and the immune system has been demonstrated to regulate the immune response.137–139 Whole metagenome profiling suggests that AD-associated microbiomes can elevate the risk to develop AD flares by influencing the microenvironment of the skin surface as well as through interactions with the host immune system.139 S. aureus seems to play an eminent role in this regard, and AD skin is frequently superinfected with this pathogen.1 Indeed, antibiotics or dilute bleach baths that have antimicrobial activity have become a treatment option.140 In general, AD flares are associated with a decreased diversity of the skin microbiota accompanied by increased abundance of S. aureus.141,142 Species-level investigation of AD flares showed that S. aureus has greater predominance in patients with more severe AD, while S. epidermidis predominance was found in patients with less severe disease.143 Consistently, S. aureus upregulates IL-4, IL-13 and IL-22 expression in skin.144–146 Interestingly, S. aureus strains from more severe AD patients induced epidermal thickening, as well as expansion of Th2 and Th17 cells in a mouse model, that was stronger than S. aureus from less severe AD and healthy controls.143 Importantly, skin commensal bacteria such as S. epidermidis and S. hominis are protective against S. aureus via production of antimicrobial peptides.147 These strain-specific, highly potent antimicrobial peptides that can selectively kill S. aureus were commonly present in healthy control skin, but only rarely found on AD skin.147 Conversely, reintroduction of commensal strains harboring antimicrobial activity to AD patients decreased colonization by S. aureus in vivo.147 This demonstrates that commensal skin bacteria can protect against pathogens, and that dysbiosis can drive disease.147
Recently, bacterial community structures and diversity was shown to shift over time in a birth cohort evaluating skin samples on day 2, month 2, and month 6 of life.148 In this cohort, increased commensal staphylococci early in life reduced the risk of developing AD at month 12, while in infantile onset AD, there was no difference between the AD population and healthy control subjects,148 further adding to the notion that AD is quite a heterogeneous disease.
Given the fact that AD is characterized by a high level of skin barrier defects, accompanied by frequent bacterial and viral superinfection,149 blocking immune pathways might raise the concern of increasing the risk of skin infections. However, dupilumab was not only well tolerated, but also reduced S. aureus abundance on lesional and non-lesional AD skin in a phase II trial.87 Indeed, dupilumab treatment resulted in lower rates of infectious adverse events than in placebo in clinical trials, demonstrating that immune activation directly leads to skin dysbiosis and infection, as suggested by preclinical data.134,135,150
CONCLUSION
The interaction of immune responses in AD with epithelial dysfunction and microbial dysbiosis is complex, but clinical trials are helping to elucidate key steps in this process (Table 2). Future clinical trials with targeted therapies will likely further contribute to the elucidation of AD skin biology. Results might not only optimize treatment approaches, but will most likely also increase our understanding of AD disease heterogeneity.
Table 2:
Key messages on the immunologic, microbial and epithelial interactions in AD.
AD has a complex pathogenesis and considerable phenotypic heterogeneity.
|
Financial support:
PMB was supported in part by grant # UL1TR0001866 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. DYL is supported in part by USPHS grants AR41256, U19 AI11767, and UL1 RR025780.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: PMB received personal fees from LEO Pharma and Sanofi. EGY is a board member for Sanofi Aventis, Regeneron, Stiefel/GlaxoSmithKline, MedImmune, Celgene, Anacor, AnaptysBio, Celsus, Dermira, Galderma, Glenmark, Novartis, Pfizer, Vitae and Leo Pharma; has received consultancy fees from Regeneron, Sanofi, MedImmune, Celgene, Stiefel/GlaxoSmithKline, Celsus, BMS, Amgen, Drais, AbbVie, Anacor, AnaptysBio, Dermira, Galderma, Glenmark, LEO Pharma, Novartis, Pfizer, Vitae, Mitsubishi Tanabe and Eli Lilly; and has received research support from Janssen, Regeneron, Celgene, BMS, Novartis, Merck, LEO Pharma and Dermira. DYML has received research support from Pfizer, and MedImmune; and has received consultancy fees from Aimmune Therapeutics, Regeneron and Sanofi.
REFERENCES
- 1.Weidinger S, Novak N. Atopic dermatitis. Lancet 2016;387:1109–22. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg JI. Public Health Burden and Epidemiology of Atopic Dermatitis. Dermatol Clin 2017;35:283–9. [DOI] [PubMed] [Google Scholar]
- 3.Bonness S, Bieber T. Molecular basis of atopic dermatitis. Curr Opin Allergy Clin Immunol 2007;7:382–6. [DOI] [PubMed] [Google Scholar]
- 4.Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy 2014;69:3–16. [DOI] [PubMed] [Google Scholar]
- 5.Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ. Atopic dermatitis and the atopic march revisited. Allergy 2014;69:17–27. [DOI] [PubMed] [Google Scholar]
- 6.Czarnowicki T, Krueger JG, Guttman-Yassky E. Novel concepts of prevention and treatment of atopic dermatitis through barrier and immune manipulations with implications for the atopic march. J Allergy Clin Immunol 2017;139:1723–34. [DOI] [PubMed] [Google Scholar]
- 7.Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol 2014;134:769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suarez-Farinas M, Dhingra N, Gittler J, et al. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol 2013;132:361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esaki H, Brunner PM, Renert-Yuval Y, et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol 2016;138:1639–51. [DOI] [PubMed] [Google Scholar]
- 10.Noda S, Suarez-Farinas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol 2015;136:1254–64. [DOI] [PubMed] [Google Scholar]
- 11.Napolitano M, Megna M, Patruno C, Gisondi P, Ayala F, Balato N. Adult atopic dermatitis: a review. G Ital Dermatol Venereol 2016;151:403–11. [PubMed] [Google Scholar]
- 12.Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol 2017;139:S65–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon BR. The allergic march: can we prevent allergies and asthma? Otolaryngol Clin North Am 2011;44:765–77, xi. [DOI] [PubMed] [Google Scholar]
- 14.Czarnowicki T, Malajian D, Shemer A, et al. Skin-homing and systemic T-cell subsets show higher activation in atopic dermatitis versus psoriasis. J Allergy Clin Immunol 2015;136:208–11. [DOI] [PubMed] [Google Scholar]
- 15.Czarnowicki T, Esaki H, Gonzalez J, et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)(+) TH2/TH1 cell imbalance, whereas adults acquire CLA(+) TH22/TC22 cell subsets. J Allergy Clin Immunol 2015;136:941–51 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czarnowicki T, Gonzalez J, Shemer A, et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J Allergy Clin Immunol 2015;136:104–15 e7. [DOI] [PubMed] [Google Scholar]
- 17.Suarez-Farinas M, Tintle SJ, Shemer A, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol 2011;127:954–64 e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esaki H, Brunner PM, Renert-Yuval Y, et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol 2016;138:1639–51. [DOI] [PubMed] [Google Scholar]
- 19.Ungar B, Garcet S, Gonzalez J, et al. An Integrated Model of Atopic Dermatitis Biomarkers Highlights the Systemic Nature of the Disease. J Invest Dermatol 2017;137:603–13. [DOI] [PubMed] [Google Scholar]
- 20.Thijs J, Krastev T, Weidinger S, et al. Biomarkers for atopic dermatitis: a systematic review and meta-analysis. Curr Opin Allergy Clin Immunol 2015;15:453–60. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Suarez-Farinas M, Estrada Y, et al. Identification of Unique Proteomic Signatures in Allergic and Non-Allergic Skin Disease. Clin Exp Allergy 2017. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 22.Brunner PM, Suarez-Farinas M, He H, et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci Rep 2017;7:8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deckert S, Kopkow C, Schmitt J. Nonallergic comorbidities of atopic eczema: an overview of systematic reviews. Allergy 2014;69:37–45. [DOI] [PubMed] [Google Scholar]
- 24.Brunner PM, Silverberg JI, Guttman-Yassky E, et al. Increasing Comorbidities Suggest that Atopic Dermatitis Is a Systemic Disorder. J Invest Dermatol 2017;137:18–25. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies. Allergy 2015;70:1300–8. [DOI] [PubMed] [Google Scholar]
- 26.Silverberg JI, Greenland P. Eczema and cardiovascular risk factors in 2 US adult population studies. J Allergy Clin Immunol 2015;135:721–8 e6. [DOI] [PubMed] [Google Scholar]
- 27.Zhang A, Silverberg JI. Association of atopic dermatitis with being overweight and obese: a systematic review and metaanalysis. J Am Acad Dermatol 2015;72:606–16 e4. [DOI] [PubMed] [Google Scholar]
- 28.Silverberg JI, Simpson EL. Association between obesity and eczema prevalence, severity and poorer health in US adolescents. Dermatitis 2014;25:172–81. [DOI] [PubMed] [Google Scholar]
- 29.Silverberg JI, Becker L, Kwasny M, Menter A, Cordoro KM, Paller AS. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol 2015;151:144–52. [DOI] [PubMed] [Google Scholar]
- 30.Strom M, Silverberg JI. Associations of Physical Activity and Sedentary Behavior with Atopic Disease in US Children. J Pediatrics 2016;174:247–253. [DOI] [PubMed] [Google Scholar]
- 31.Hjuler KF, Bottcher M, Vestergaard C, et al. Increased Prevalence of Coronary Artery Disease in Severe Psoriasis and Severe Atopic Dermatitis. Am J Med 2015;128:1325–34. [DOI] [PubMed] [Google Scholar]
- 32.Brunner PM, Silverberg JI, Guttman-Yassky E, et al. Increasing Comorbidities Suggest that Atopic Dermatitis Is a Systemic Disorder. J Invest Dermatol 2017;137:18–25. [DOI] [PubMed] [Google Scholar]
- 33.Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol 2015;135:984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitt J, Buske-Kirschbaum A, Roessner V. Is atopic disease a risk factor for attention-deficit/hyperactivity disorder? A systematic review. Allergy 2010;65:1506–24. [DOI] [PubMed] [Google Scholar]
- 35.Arima M, Shimizu Y, Sowa J, et al. Psychosomatic analysis of atopic dermatitis using a psychological test. J Dermatol 2005;32:160–8. [DOI] [PubMed] [Google Scholar]
- 36.Dieris-Hirche J, Gieler U, Kupfer JP, Milch WE. [Suicidal ideation, anxiety and depression in adult patients with atopic dermatitis]. Hautarzt 2009;60:641–6. [DOI] [PubMed] [Google Scholar]
- 37.Sanna L, Stuart AL, Pasco JA, et al. Atopic disorders and depression: findings from a large, population-based study. J Affect Disord 2014;155:261–5. [DOI] [PubMed] [Google Scholar]
- 38.Schut C, Bosbach S, Gieler U, Kupfer J. Personality traits, depression and itch in patients with atopic dermatitis in an experimental setting: a regression analysis. Acta Derm Venereol 2014;94:20–5. [DOI] [PubMed] [Google Scholar]
- 39.Buske-Kirschbaum A, Schmitt J, Plessow F, Romanos M, Weidinger S, Roessner V. Psychoendocrine and psychoneuroimmunological mechanisms in the comorbidity of atopic eczema and attention deficit/hyperactivity disorder. Psychoneuroendocrinology 2013;38:12–23. [DOI] [PubMed] [Google Scholar]
- 40.Riis JL, Vestergaard C, Deleuran MS, Olsen M. Childhood atopic dermatitis and risk of attention deficit/hyperactivity disorder: A cohort study. J Allergy Clin Immunol 2016;138:608–10. [DOI] [PubMed] [Google Scholar]
- 41.Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol 2013;131:428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chrostowska-Plak D, Reich A, Szepietowski JC. Relationship between itch and psychological status of patients with atopic dermatitis. J Europ Acad Derm Venereol 2013;27:e239–42.. [DOI] [PubMed] [Google Scholar]
- 43.Senra MS, Wollenberg A. Psychodermatological aspects of atopic dermatitis. Br J Dermatol 2014;170 Suppl 1:38–43. [DOI] [PubMed] [Google Scholar]
- 44.Weisshaar E, Diepgen TL, Bruckner T, et al. Itch intensity evaluated in the German Atopic Dermatitis Intervention Study (GADIS): correlations with quality of life, coping behaviour and SCORAD severity in 823 children. Acta Derm Venereol 2008;88:234–9. [DOI] [PubMed] [Google Scholar]
- 45.Zimmerman AW, Jyonouchi H, Comi AM, et al. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol 2005;33:195–201. [DOI] [PubMed] [Google Scholar]
- 46.Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for ‘depression due to a general medical condition’, immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol 2002;5:389–99. [DOI] [PubMed] [Google Scholar]
- 47.Yarlagadda A, Alfson E, Clayton AH. The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry (Edgmont) 2009;6:18–22. [PMC free article] [PubMed] [Google Scholar]
- 48.Ishiuji Y, Coghill RC, Patel TS, Oshiro Y, Kraft RA, Yosipovitch G. Distinct patterns of brain activity evoked by histamine-induced itch reveal an association with itch intensity and disease severity in atopic dermatitis. Br J Dermatol 2009;161:1072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 2006;27:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenkranz MA, Busse WW, Johnstone T, et al. Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. Proc Natl Acad Sci U S A 2005;102:13319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Werfel T, Allam JP, Biedermann T, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol 2016;138:336–49. [DOI] [PubMed] [Google Scholar]
- 52.Biedermann T, Skabytska Y, Kaesler S, Volz T. Regulation of T Cell Immunity in Atopic Dermatitis by Microbes: The Yin and Yang of Cutaneous Inflammation. Front Immunol 2015;6:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon D, Wittwer J, Kostylina G, Buettiker U, Simon HU, Yawalkar N. Alefacept (lymphocyte function-associated molecule 3/IgG fusion protein) treatment for atopic eczema. J Allergy Clin Immunol 2008;122:423–4. [DOI] [PubMed] [Google Scholar]
- 54.Harper EG, Simpson EL, Takiguchi RH, et al. Efalizumab therapy for atopic dermatitis causes marked increases in circulating effector memory CD4+ T cells that express cutaneous lymphocyte antigen. J Invest Dermatol 2008;128:1173–81. [DOI] [PubMed] [Google Scholar]
- 55.Hijnen DJ, ten Berge O, Timmer-de Mik L, Bruijnzeel-Koomen CA, de Bruin-Weller MS. Efficacy and safety of long-term treatment with cyclosporin A for atopic dermatitis. J Eur Acad Dermatol Venereol 2007;21:85–9. [DOI] [PubMed] [Google Scholar]
- 56.Graham MT, Nadeau KC. Lessons learned from mice and man: mimicking human allergy through mouse models. Clin Immunol 2014;155:1–16. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka A, Amagai Y, Oida K, Matsuda H. Recent findings in mouse models for human atopic dermatitis. Exp Anim 2012;61:77–84. [DOI] [PubMed] [Google Scholar]
- 58.Ewald DA, Noda S, Oliva M, et al. Major differences between human atopic dermatitis and murine models, as determined by using global transcriptomic profiling. J Allergy Clin Immunol 2017;139:562–571. [DOI] [PubMed] [Google Scholar]
- 59.Mansouri Y, Guttman-Yassky E. Immune Pathways in Atopic Dermatitis, and Definition of Biomarkers through Broad and Targeted Therapeutics. J Clin Med 2015;4:858–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khattri S, Shemer A, Rozenblit M, et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immunol 2014;133:1626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tintle S, Shemer A, Suarez-Farinas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol 2011;128:583–93 e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: Barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol 2016;137:1091–102. [DOI] [PubMed] [Google Scholar]
- 63.Thaci D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet 2016;387:40–52. [DOI] [PubMed] [Google Scholar]
- 64.Hamilton JD, Suarez-Farinas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 2014;134:1293–300. [DOI] [PubMed] [Google Scholar]
- 65.Beck LA, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med 2014;371:130–9. [DOI] [PubMed] [Google Scholar]
- 66.Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest 1994;94:870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med 2016;375:2335–2348. [DOI] [PubMed] [Google Scholar]
- 68.Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017;389:2287–303. [DOI] [PubMed] [Google Scholar]
- 69.Wollenberg A, Howell MD, Guttman-Yassky E, et al. A Phase 2b Dose-Ranging Efficacy and Safety Study of Tralokinumab in Adult Patients with Moderate to Severe Atopic Dermatitis (AD). American Academy of Dermatology Annual Meeting 2017. [Google Scholar]
- 70.Simpson EL, Flohr C, Eichenfield L. Efficacy and safety of lebrikizumab in patients with atopic dermatitis: a Phase II randomized, controlled trial (TREBLE). 25th European Academy of Dermatology and Venerology Congress 2016. [Google Scholar]
- 71.Brunner PM, Khattri S, Garcet S, et al. A mild topical steroid leads to progressive anti-inflammatory effects in the skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 2016;138:169–78. [DOI] [PubMed] [Google Scholar]
- 72.Khattri S, Brunner PM, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp Dermatol 2017;26:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oldhoff JM, Darsow U, Werfel T, et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy 2005;60:693–6. [DOI] [PubMed] [Google Scholar]
- 74.Furue M, Yamamura K, Kido-Nakahara M, Nakahara T, Fukui Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy 2017. [DOI] [PubMed] [Google Scholar]
- 75.Ruzicka T, Hanifin JM, Furue M, et al. Anti-Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N Engl J Med 2017;376:826–35. [DOI] [PubMed] [Google Scholar]
- 76.Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy 2009;39:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol 2007;120:238–44; quiz 45–6. [DOI] [PubMed] [Google Scholar]
- 78.Webb GJ, Hirschfield GM, Lane PJ. OX40, OX40L and Autoimmunity: a Comprehensive Review. Clin Rev Allergy Immunol 2016;50:312–32. [DOI] [PubMed] [Google Scholar]
- 79.Gittler JK, Shemer A, Suarez-Farinas M, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 2012;130:1344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nograles KE, Zaba LC, Shemer A, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol 2009;123:1244–52 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 2009;119:3573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol 2010;107:1–29. [DOI] [PubMed] [Google Scholar]
- 83.Nograles KE, Zaba LC, Guttman-Yassky E, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol 2008;159:1092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol 2005;174:3695–702. [DOI] [PubMed] [Google Scholar]
- 85.Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med 2010;362:118–28. [DOI] [PubMed] [Google Scholar]
- 86.Saeki H, Kabashima K, Tokura Y, et al. Efficacy and safety of ustekinumab in Japanese patients with severe atopic dermatitis: a randomized, double-blind, placebo-controlled, phase II study. Br J Dermatol 2017;177:419–427. [DOI] [PubMed] [Google Scholar]
- 87.Guttman-Yassky E, Hamilton JD, Bissonnette R, et al. Dupilumab improves clinical atopic dermatitis parameters and modulates specific IgEs and Staphylococcus aureus abundance (ESDR Annual Meeting 2016. Munich, Germany). [Google Scholar]
- 88.Heil PM, Maurer D, Klein B, Hultsch T, Stingl G. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course - a randomized, placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges 2010;8:990–8. [DOI] [PubMed] [Google Scholar]
- 89.Andreae DA, Wang J. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Pediatrics 2014;134 Suppl 3:S160. [DOI] [PubMed] [Google Scholar]
- 90.Maurer M, Rosen K, Hsieh HJ, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med 2013;368:924–35. [DOI] [PubMed] [Google Scholar]
- 91.Toshitani A, Ansel JC, Chan SC, Li SH, Hanifin JM. Increased interleukin 6 production by T cells derived from patients with atopic dermatitis. J Invest Dermatol 1993;100:299–304. [DOI] [PubMed] [Google Scholar]
- 92.Navarini AA, French LE, Hofbauer GF. Interrupting IL-6-receptor signaling improves atopic dermatitis but associates with bacterial superinfection. J Allergy Clin Immunol 2011;128:1128–30. [DOI] [PubMed] [Google Scholar]
- 93.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987–97. [DOI] [PubMed] [Google Scholar]
- 94.Noda S, Krueger JG, Guttman-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol 2015;135:324–36. [DOI] [PubMed] [Google Scholar]
- 95.Sumimoto S, Kawai M, Kasajima Y, Hamamoto T. Increased plasma tumour necrosis factor-alpha concentration in atopic dermatitis. Arch Dis Child 1992;67:277–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Junghans V, Gutgesell C, Jung T, Neumann C. Epidermal cytokines IL-1beta, TNF-alpha, and IL-12 in patients with atopic dermatitis: response to application of house dust mite antigens. J Invest Dermatol 1998;111:1184–8. [DOI] [PubMed] [Google Scholar]
- 97.Jacobi A, Antoni C, Manger B, Schuler G, Hertl M. Infliximab in the treatment of moderate to severe atopic dermatitis. J Am Acad Dermatol 2005;52:522–6. [DOI] [PubMed] [Google Scholar]
- 98.Buka RL, Resh B, Roberts B, Cunningham BB, Friedlander S. Etanercept is minimally effective in 2 children with atopic dermatitis. J Am Acad Dermatol 2005;53:358–9. [DOI] [PubMed] [Google Scholar]
- 99.Malisiewicz B, Murer C, Pachlopnik Schmid J, French LE, Schmid-Grendelmeier P, Navarini AA. Eosinophilia during psoriasis treatment with TNF antagonists. Dermatology 2011;223:311–5. [DOI] [PubMed] [Google Scholar]
- 100.Mangge H, Gindl S, Kenzian H, Schauenstein K. Atopic dermatitis as a side effect of anti-tumor necrosis factor-alpha therapy. J Rheumatol 2003;30:2506–7. [PubMed] [Google Scholar]
- 101.Ruiz-Villaverde R, Galan-Gutierrez M. Exacerbation of atopic dermatitis in a patient treated with infliximab. Actas Dermosifiliogr 2012;103:743–6. [DOI] [PubMed] [Google Scholar]
- 102.Wright RC. Atopic dermatitis-like eruption precipitated by infliximab. J Am Acad Dermatol 2003;49:160–1. [DOI] [PubMed] [Google Scholar]
- 103.Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol 2016;75:494–503 e4. [DOI] [PubMed] [Google Scholar]
- 104.Paton DM. Crisaborole: Phosphodiesterase inhibitor for treatment of atopic dermatitis. Drugs Today (Barc) 2017;53:239–45. [DOI] [PubMed] [Google Scholar]
- 105.Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs 2009;10:1236–42. [PubMed] [Google Scholar]
- 106.Samrao A, Berry TM, Goreshi R, Simpson EL. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol 2012;148:890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Volf EM, Au SC, Dumont N, Scheinman P, Gottlieb AB. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol 2012;11:341–6. [PubMed] [Google Scholar]
- 108.Bissonnette R, Papp KA, Poulin Y, et al. Topical tofacitinib for atopic dermatitis: A Phase 2a randomised trial. Br J Dermatol 2016;175:902–911. [DOI] [PubMed] [Google Scholar]
- 109.Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol 2015;73:395–9. [DOI] [PubMed] [Google Scholar]
- 110.Damsky W, King BA. JAK inhibitors in dermatology: The promise of a new drug class. J Am Acad Dermatol 2017;76:736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Benedetto A, Yoshida T, Fridy S, Park JE, Kuo IH, Beck LA. Histamine and Skin Barrier: Are Histamine Antagonists Useful for the Prevention or Treatment of Atopic Dermatitis? J Clin Med 2015;4:741–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006;38:441–6. [DOI] [PubMed] [Google Scholar]
- 113.van den Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ 2009;339:b2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen H, Common JE, Haines RL, et al. Wide spectrum of filaggrin-null mutations in atopic dermatitis highlights differences between Singaporean Chinese and European populations. Br J Dermatol 2011;165:106–14. [DOI] [PubMed] [Google Scholar]
- 115.Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci 2009;122:1285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oliva M, Renert-Yuval Y, Guttman-Yassky E. The ‘omics’ revolution: redefining the understanding and treatment of allergic skin diseases. Curr Opin Allergy Clin Immunol 2016;16:469–76. [DOI] [PubMed] [Google Scholar]
- 117.Cole C, Kroboth K, Schurch NJ, et al. Filaggrin-stratified transcriptomic analysis of pediatric skin identifies mechanistic pathways in patients with atopic dermatitis. J Allergy Clin Immunol 2014;134:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Czarnowicki T, Krueger JG, Guttman-Yassky E. Skin barrier and immune dysregulation in atopic dermatitis: an evolving story with important clinical implications. J Allergy Clin Immunol Pract 2014;2:371–9; quiz 80–1. [DOI] [PubMed] [Google Scholar]
- 119.Gutowska-Owsiak D, Schaupp AL, Salimi M, et al. IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp Dermatol 2012;21:104–10. [DOI] [PubMed] [Google Scholar]
- 120.Malajian D, Guttman-Yassky E. New pathogenic and therapeutic paradigms in atopic dermatitis. Cytokine 2015;73:311–8. [DOI] [PubMed] [Google Scholar]
- 121.Kezic S, O’Regan GM, Lutter R, et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol 2012;129:1031–9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med 2011;365:1315–27. [DOI] [PubMed] [Google Scholar]
- 123.Kono M, Nomura T, Ohguchi Y, et al. Comprehensive screening for a complete set of Japanese-population-specific filaggrin gene mutations. Allergy 2014;69:537–40. [DOI] [PubMed] [Google Scholar]
- 124.Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol 2012;130:912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol 2007;120:150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bieber T Atopic dermatitis. Ann Dermatol 2010;22:125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zollner TM, Wichelhaus TA, Hartung A, et al. Colonization with superantigen-producing Staphylococcus aureus is associated with increased severity of atopic dermatitis. Clin Exp Allergy 2000;30:994–1000. [DOI] [PubMed] [Google Scholar]
- 128.Cai SC, Chen H, Koh WP, et al. Filaggrin mutations are associated with recurrent skin infection in Singaporean Chinese patients with atopic dermatitis. Br J Dermatol 2012;166:200–3. [DOI] [PubMed] [Google Scholar]
- 129.Miajlovic H, Fallon PG, Irvine AD, Foster TJ. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J Allergy Clin Immunol 2010;126:1184–90 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 2002;347:1151–60. [DOI] [PubMed] [Google Scholar]
- 131.Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol 2003;171:3262–9. [DOI] [PubMed] [Google Scholar]
- 132.Howell MD, Boguniewicz M, Pastore S, et al. Mechanism of HBD-3 deficiency in atopic dermatitis. Clin Immunol 2006;121:332–8. [DOI] [PubMed] [Google Scholar]
- 133.de Jongh GJ, Zeeuwen PL, Kucharekova M, et al. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J Invest Dermatol 2005;125:1163–73. [DOI] [PubMed] [Google Scholar]
- 134.Honzke S, Wallmeyer L, Ostrowski A, et al. Influence of Th2 Cytokines on the Cornified Envelope, Tight Junction Proteins, and ss-Defensins in Filaggrin-Deficient Skin Equivalents. J Invest Dermatol 2016;136:631–9. [DOI] [PubMed] [Google Scholar]
- 135.Albanesi C, Fairchild HR, Madonna S, et al. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol 2007;179:984–92. [DOI] [PubMed] [Google Scholar]
- 136.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006;203:2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bjorksten B Disease outcomes as a consequence of environmental influences on the development of the immune system. Curr Opin Allergy Clin Immunol 2009;9:185–9. [DOI] [PubMed] [Google Scholar]
- 138.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science 2016;352:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chng KR, Tay AS, Li C, et al. Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat Microbiol 2016;1:16106. [DOI] [PubMed] [Google Scholar]
- 140.Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics 2009;123:e808–14. [DOI] [PubMed] [Google Scholar]
- 141.Dybboe R, Bandier J, Skov L, Engstrand L, Johansen JD. The Role of the Skin Microbiome in Atopic Dermatitis: A Systematic Review. Br J Dermatol 2017. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 142.Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 2012;22:850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Byrd AL, Deming C, Cassidy SKB, et al. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nakatsuji T, Chen TH, Two AM, et al. Staphylococcus aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression. J Invest Dermatol 2016;136:2192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Niebuhr M, Scharonow H, Gathmann M, Mamerow D, Werfel T. Staphylococcal exotoxins are strong inducers of IL-22: A potential role in atopic dermatitis. J Allergy Clin Immunol 2010;126:1176–83 e4. [DOI] [PubMed] [Google Scholar]
- 146.Neuber K, Steinrucke K, Ring J. Staphylococcal enterotoxin B affects in vitro IgE synthesis, interferon-gamma, interleukin-4 and interleukin-5 production in atopic eczema. Int Arch Allergy Immunol 1995;107:179–82. [DOI] [PubMed] [Google Scholar]
- 147.Nakatsuji T, Chen TH, Narala S, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kennedy EA, Connolly J, Hourihane JO, et al. Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J Allergy Clin Immunol 2017;139:166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sabin BR, Peters N, Peters AT. Chapter 20: Atopic dermatitis. Allergy Asthma Proc 2012;33 Suppl 1:S67–9. [DOI] [PubMed] [Google Scholar]
- 150.Ong PY, Leung DY. Bacterial and Viral Infections in Atopic Dermatitis: a Comprehensive Review. Clin Rev Allergy Immunol 2016;51:329–37. [DOI] [PubMed] [Google Scholar]


