Abstract
Previous DTI studies have reported associations between white matter integrity and performance on the Stroop interference task. The current study aimed to add to these studies of inhibitory control by investigating how the differences in age and in white matter integrity relate to Stroop performance, and to examine whether the effect of age on Stroop performance is mediated by white matter integrity. 179 healthy adults from 20–80 years old were recruited in the study. DTI data were processed through TRACULA and the mean fractional anisotropy (FA) of 18 major white matter tracts were extracted and used for statistical analysis. Correlation analysis showed a strong negative relationship between age and the Stroop interference score (IG). Higher IG indicated better inhibitory control. Simple linear regression analyses indicated that most of the tracts showed negative relationships with age, and positive relationships with IG. Moderation effect of age on the relationship between FA and IG was tested on tracts that significantly predicted IG after multiple comparison corrections, but none of these moderations were significant. Then we tested if these tracts mediated the effect of age on IG and found significant indirect effects of age on IG through the FA of the left corticospinal tract and through the right inferior longitudinal fasciculus. Our results highlight the role of a number of major white matter tracts in the processes supporting the Stroop inhibitory performance and further pinpointed the lower white matter integrity of specific tracts as contributors to the decrease in inhibitory control ability associated with the Stroop test in older age.
Keywords: Inhibitory control, White matter Integrity, Aging, Stroop Interference, DTI, Mediation effect
1. Introduction
Inhibitory control entails important subcomponents, including attentional control and response inhibition. Attentional control, also known as selective attention, is the capacity to choose what to pay attention to and what to ignore (Posner, 1990). Response inhibition is the ability to inhibit an inappropriate response (prepotent or automatic) in a given context and respond appropriately in a goal-directed behavior (Mostofsky & Simmonds, 2008). Examples of tasks that engage both attentional control and response inhibition include the Stroop task (Stroop, 1935), the go/no-go task (Donders, 1969), and the Simon task (Simon, 1989), all of which have been examined in functional activation tasks to evaluate the associated brain regions activated.The Stroop task is associated with increased activation in the dorsolateral prefrontal cortex (PFC), the anterior cingulate cortex (ACC), the inferior frontal gyrus (IFG), the inferior parietal gyrus (IPG), and the inferior temporal cortex, whereas the Simon task involves the dorsal premotor and the posterior and superior parietal cortices (Liu et al., 2004). The go/no-go task has been shown to activate the pre-supplementary motor area (pre-SMA) and the fusiform gyrus (Simmonds et al., 2008). The prefrontal gyrus is recruited in all of these tasks while the parietal and temporal lobes are also recruited in some of these tasks.
With such a widespread network of regions coordinating the processing of inhibitory control tasks, long-distance axonal tracts are crucial for inter-cortical communication. Integrity of the axonal tracts can be indirectly measured with diffusion tensor imaging (DTI), which detects the diffusion of water molecules by modeling the anisotropy of the diffusion gradient. Water diffusion is more anisotropic in intact axons than in damaged ones (Wakana et al., 2004; Mori, 2007; Mori & Zhang, 2006), and thus, intact white matter microstructure is associated with higher fractional anisotropy (FA) and lower diffusivity values than damaged white matter.
Previous aging studies have found associations between white matter integrity and age-related decline in performance on inhibitory control tasks such as the Stroop test. Wolf et al. (2014) collected Stroop test performance from 49 healthy subjects consisting of 13 younger adults, 20 younger elders and 16 advanced elders. Their data showed a strong positive correlation between Stroop interference and white matter integrity of genu of the corpus callosum, bilateral anterior corona radiata, and bilateral anterior limb of internal capsule, all of which connect frontal regions with other parts of the brain. In particular, anterior corona radiata and the anterior limb of internal capsule connect the frontal regions with parts of the thalamus. Better Stroop performance was also associated with higher FA in thalamic-frontal connections in a number of other studies (Greive et al., 2007; Hughes et al., 2012; Reginold et al., 2015). Reginold et al. (2015) tested 33 healthy subjects age 54 to 76 years old using a set of neuropsychological tests that included the Stroop test. They found that higher FA in the corpus callosum, corticospinal/bulbar tract, and thalamic projection tracts were associated with better Stroop performance. Hughes et al. (2012) showed that mean FA values in the thalamo-frontal tracts decreased significantly with increasing age and the volume of the thalamo-frontal projections was associated with Stroop test performance.
Aging studies conducted on tasks that recruit similar cognitive processes as the Stroop further showed fronto-parietal involvement. Greive et al. (2007) reported associations between executive control and FA of bilateral regions from the prefrontal cortex to the parietal lobe and extended to anterior portions of the thalamus by using executive maze and attention switching tasks. Radial diffusivity, another measure of white matter integrity, of frontoparietal white matter tracts were also correlated with task switching processes in older adults (Jolly et.al, 2017). Overall, integrity of axonal tracts connecting frontal brain regions with thalamic projections and with the parietal lobes were most frequently found to be correlated with age-related decline in inhibitory control tasks requiring attentional control and response inhibition.
While a number of studies have examined the association between white matter integrity and inhibitory control tasks (Albert, 1993; Bennett, 2006; Buchner, 2004; Gazes et al., 2016; Guttmann et al., 1998; Kohama, et al., 2011; Madden et al., 2009; Bendlin et al., 2010; Jacobs et al., 2013; Ystad et al., 2011), few studies have directly examined if the integrity of white matter tracts quantified with DTI measures explain the age-related decline in inhibitory control tasks (Wolf et al., 2014) using mediation model, which is a more stringent test of directional relationships than simple regression. Mediation analysis conducts a series of regression models to evaluate whether the data is consistent with a directional hypothesis. Thus, while it does rely on regression and the data still has the limitation of being cross-sectional, the models do test for directionality of effects (Hayes, 2013).
Our study administered the Stroop task to a large group of healthy adults ranging from 20 to 80 years old, and used mediation analysis to determine whether the variability observed with aging in Stroop performance is mediated (or explained) by the variability in the FA of 18 major white matter tracts, which were automatically generated by the software Tracts Constrained by Underlying Anatomy (TRACULA; Yendiki et al., 2011) that is part of FreeSurfer (https://surfer.nmr.mgh.harvard.edu). These tracts consisted of major pathways connecting prefrontal regions to other lobes such as the bilateral superior longitudinal fasciculus (SLF) as well as pathways connecting bilateral temporal lobes with posterior regions such as the inferior longitudinal fasciculus (ILF). Other tracts found to be associated with inhibitory control in previous studies were also extracted, including the anterior thalamic radiation (ATR) and the corpus callosum. Given that anterior regions suffer greater age-related atrophy than posterior regions (Sullivan et al., 2010), age-related variability in performance for inhibitory control tasks should be explained by variability in white matter integrity of tracts connecting prefrontal regions with other brain regions, such as the SLF, whereas there should be little association between age-related Stroop performance and white matter tracts connecting posterior regions, such as the ILF. Given the large age range in our sample, we tested for both linear and quadratic age trends in the white matter integrity of tracts as well as the interaction between age and white matter integrity on Stroop performance to examine if the white matter to Stroop performance differs by age.
2. Experimental Procedure
2.1 Participants
Participants were taken from a larger study, Reference Ability Neural Networks (RANN study), but the data presented here have not been published before. Actual sample size and subject exclusion is described in section 2.5. Participants were recruited using established market mailing procedures to equate the recruitment procedures of all participants. Participants who responded to the mailing were screened for inclusion (right handed and English speaking) and exclusion criteria (myocardial Infarction, congestive heart failure or any other heart disease, brain disorder such as stroke, tumor, infection, epilepsy, multiple sclerosis, degenerative diseases, head injury (loss of consciousness > 5mins), mental retardation, seizure, Parkinson’s disease, Huntington’s disease, normal pressure hydrocephalus, essential/familial tremor, Down Syndrome, HIV Infection or AIDS diagnosis, learning disability/dyslexia, ADHD or ADD, uncontrolled hypertension, uncontrolled diabetes mellitus, uncontrolled thyroid or other endocrine disease, uncorrectable vision, color blindness, uncorrectable hearing and implant, pregnancy, lactating, any medication targeting central nervous system, cancer within last five years, renal insufficiency, untreated neurosyphillis, any alcohol and drug abuse within last 12 months, recent non-skin neoplastic disease or melanoma, active hepatic disease, insulin dependent diabetes, history of psychosis or ECT, major depressive, bipolar, or anxiety disorder within the past 5 years). Individuals that passed the telephone screen were further screened in person and a Mattis Dementia Rating Scale (DRS) score of at least 130 was required for inclusion in the study. DRS had a mean=140, with SD=2.9, and ranged from 130 to 144. Informed consent, as approved by the Internal Review Board of the College of Physicians and Surgeons of Columbia University, was obtained prior to study participation, and after the nature and risks of the study were explained. Participants were paid for their participation in the study.
2.2 Behavioral measure: Stroop task
Inhibitory control was assessed by the Golden Stroop task (Golden, 1978). There were three types of cards in the Stroop test: (1) word cards contain black color words and the word is to be read; (2) color cards contain solid squares or XXXXXs in different colors and the color is to be named; and (3) color-word cards contain incongruent color words (for example, the word ‘red’ printed in the color blue) and the color of the printed text is to be named while suppressing automatic reading of the word. For the Golden Stroop task, participants have to name as many items as they can in 45s for each card. The outcome variables are the number of items completed for the word card (W raw word score), the color card (C raw color score), and the color–word card (CW raw color–word score), respectively. The predicted color-word score (Pcw) is calculated based on the raw word (W) and raw color (C) scores. The predicted numbers of items named in 45s in the color-word condition (Pcw) is calculated as Pcw=45/{[(45 * W) + (45 * C)]/(W * C)}=(W*C)/(W+C).
Golden’s interference score (IG) is calculated by subtracting this score from the actual color–word score (IG = CW-Pcw) (Golden, 1978). Pcw is an estimate of the number of words read if there were no interference from reading of the word and is based on the number of words read in the word and the color conditions. CW is the actual number of items named on incongruent colored words which requires inhibition of word reading. If inhibition is impaired, then the number of items named in the color word condition should be less than the average number of items named in the word and the color conditions, resulting in a negative IG score. Therefore, the lower the IG score, the greater the impairment in inhibitory control.
2.3 MRI acquisition
MRI images were acquired in a 3.0T Philips Achieva Magnet using a standard quadrature head coil. A T1-weighted scout image was acquired to determine subject position. One hundred sixty-five contiguous 1 mm T1-weighted images of the whole brain were acquired for each subject with an MPRAGE sequence using the following parameters: TR 6.5 ms, TE 3 ms; flip angle 8°, acquisition matrix 256 × 256, and 240 mm field of view. Two sets of DTI images were acquired in 55 directions with b value of 800 s/mm2 and one non diffusion weighted image using these parameters: TE = 69 ms, TR = 11032 ms, Flip Angle = 90°, in-plane resolution 112 × 112 voxels, acquisition time 12 min 56 sec, slice thickness = 2 mm (no gap), 75 slices.
Any T1 scans with potential clinically significant findings, such as abnormal neural structure, were reviewed by a neuroradiologist and removed from the sample prior to the current analysis. However, no clinically significant findings were identified.
2.4 DTI analysis
DTI data were processed with TRACULA (Tracts Constrained by Underlying Anatomy) distributed as part of the FreeSurfer v. 5.3 library (Yendiki et al., 2011) which produces 18 major white matter tracts as listed below:
bilateral corticospinal tract (CST)
bilateral inferior longitudinal fasciculus (ILF)
bilateral uncinate fasciculus (UNC)
bilateral anterior thalamic radiation (ATR)
bilateral cingulum–cingulate gyrus (supracallosal) bundle (CCG)
bilateral cingulum–angular (infracallosal) bundle (CAB)
bilateral superior longitudinal fasciculus–parietal bundle (SLFP)
bilateral Superior longitudinal fasciculus–temporal bundle (SLFT)
forceps major, which passes through splenium of corpus callosum (FMAJ)
forceps minor, which passes through genu of corpus callosum (FMIN).
The software performs informed automatic tractography by incorporating anatomical information from a training data set, provided by the software, with the anatomical segmentation of the T1 image of the current data set also using FreeSurfer. Thus increasing the accuracy of the WM tract placement for each participant. Standard DTI preprocessing steps using the FMRIB’s Diffusion Toolbox (FMRIB’s Software Library v. 4.1.5) (Smith et al., 2004) including movement and eddy current correction, tensor estimation, and bedpostx were performed prior to tractography. See Yendiki et al. (2011) for detailed steps performed by the TRACULA software. For each participant, the means of fractional anisotropy (FA) for each of the 18 tracts were entered into subsequent analyses. FA ranges from 0 to 1 with higher number representing more intact white matter integrity.
2.5 Participant exclusion
Two hundred twenty-eight healthy adults 20–80 years old from the RANN study were administered the Stroop task and had DTI images processed through TRACULA, but 49 were excluded for poor data quality from TRACULA, with 179 included in the analyses. Since there is no official guideline on quality checks for TRACULA output, we adopted stringent inclusion criteria for tracts generated from TRACULA in order to ensure only properly generated tracts were included in the analysis. age2 to investigate if there are linear and quadratic effects of age on FA Thus, anyone with at least one tract that was either just one control point, extended to incorrect directions, or that was not well defined (having many large protrusion rather than a smooth surface along the tract) was excluded from the analysis. We tested to see if there were systematic differences between the excluded and the included groups in terms of age and IG, and did not see significant effects for either variable (age: t=1.703, p>.05; IG: t=1.605, p>.05). The 179 participants had mean age of 49.1 years with SD of 16.6 years, with 51.4% females (92 out of 179) and a mean length of education for 16.1 years with SD of 2.4 years, and ranged from 12 to 22 years.
2.6 Statistical Analysis
Correlation analysis was performed to examine the relationship between age and IG. For each of the 18 white matter tracts, simple linear regressions were performed between the mean FA of the tract and age and age2 to investigate if there are linear and quadratic effects of age on FA, and between FA and IG to examine if FA is a predictor IG. Results were corrected for multiple comparisons (p < .05/18 tracts = .0027). For significant results exceeding the multiple comparison correction, a moderation analysis was performed to examine if there is an interaction effect of age and FA on IG. Moderation must be tested before mediation because it tests if there is an interaction between the independent variable and the mediating variable. Mediation results are valid only if the moderation is not significant. Thus, for tracts with non-significant moderation effect, indicating that the relationship between FA and IG does not differ by age, we investigated if FA mediated the effect of age on IG.
For tracts that did not show a significant moderation effect of age, indicating that the relationship between FA and IG does not differ by age, we investigated if FA mediated the effect of age on IG. The moderation and mediation analysis were performed using the Process macro in SPSS v22 (Hayes, 2013). All statistical analyses were carried out two-tailed.
3. Results
3.1 Regression and correlation analysis
Correlation analysis showed a strong negative relationship between age and IG (r= −0.404, p<0.001). Simple linear regression analyses were performed between linear and quadratic age effects on FA of 18 individual tracts, and between FA and IG. Results are shown in table 1. Some of the white matter tracts showed negative relationships with age such that older participants showed lower FA, and positive relationships with IG, such that better FA is associated with better Stroop inhibitory performance. Four white matter tracts also showed quadratic effects of age but none exceeded correction for multiple correction.
Table 1.
Regression statistics for Age predicting FA, Age2 predicting FA, and FA predicting IG.
| Age (IV)
|
Age2 (IV)
|
IG (DV)
|
||||
|---|---|---|---|---|---|---|
| Beta | t | Beta | t | Beta | t | |
| FMAJ | −0.210 | −2.853** | 0.594 | 1.173 | 0.174 | 2.352* |
| FMIN | −0.332 | −4.675** | 0.140 | 0.286 | 0.097 | 1.302 |
| L.ATR | −0.202 | −2.747** | −0.014 | −0.028 | 0.203 | 2.762** |
| L.CAB | 0.172 | 2.322* | −0.400 | −0.782 | −0.066 | −0.886 |
| L.CCG | −0.300 | −4.177** | −1.188 | −2.434* | 0.129 | 1.727 |
| L.CST | −0.330 | −4.648** | 0.338 | 0.690 | 0.275 | 3.806** |
| L.ILF | −0.275 | −3.804** | 0.017 | 0.034 | 0.219 | 2.987** |
| L.SLFP | −0.213 | −2.906** | −1.422 | −2.863** | 0.172 | 2.323* |
| L.SLFT | −0.093 | −1.248 | −0.829 | −1.614 | 0.173 | 2.336* |
| L.UNC | −0.068 | −0.908 | −0.262 | −0.505 | 0.079 | 1.058 |
| R.ATR | −0.157 | −2.119* | −0.590 | −1.153 | 0.212 | 2.886** |
| R.CAB | 0.068 | 0.905 | 0.130 | 0.250 | 0.100 | 1.343 |
| R.CCG | −0.140 | −1.878 | −1.074 | −2.111* | 0.063 | 0.837 |
| R.CST | −0.093 | −1.241 | 1.438 | 2.839** | 0.228 | 3.114** |
| R.ILF | −0.280 | −3.885** | 0.404 | 0.811 | 0.259 | 3.568** |
| R.SLFP | 0.037 | 0.496 | −0.075 | −0.145 | 0.062 | 0.830 |
| R.SLFT | −0.030 | −0.395 | 0.147 | 0.284 | 0.098 | 1.310 |
| R.UNC | −0.057 | −0.758 | 0.247 | 0.476 | 0.098 | 1.308 |
Notes. Significant results with p<0.0027, corrected for multiple comparisons, are bolded and marked with **. Uncorrected significance levels at p<0.05 are marked with * and p<0.01 with **. Tract acronyms are defined in the Methods section. IV = independent variable, DV = dependent variable, R = right, L = left. Beta = standardized beta, t= t value.
3.2 Moderation and Mediation analyses
Moderation of age on the relationship between FA and IG was tested on all tracts that significantly predicted IG after multiple comparison corrections, which consisted of bilateral corticospinal tract and the right inferior longitudinal fasciculus but none of these moderations were significant (L.CST: t=−1.20; R.CST: t=−0.81; R.ILF: t=− 0.34, all p>0.05) which suggests that the relationship between FA and IG are similar across the age range. Then we tested if these three tracts mediated the effect of age on IG and found significant indirect effects of age on IG through the FA of the left corticospinal tract and independently through the right inferior longitudinal fasciculus. In both mediation results, age was negatively associated with FA of the tract, consistent with the decline of white matter integrity in older age, and the FA was positively associated with IG, demonstrating better inhibitory performance with more intact white matter integrity. Detailed statistics of the mediation analyses are shown in Figure 1A. Total effect (c) consists of direct (c′) and indirect effects (IE). Mediation is significant when the 95% confidence interval for IE does not contain zero. There was a significant indirect effect of age on IG through the FA of the left corticospinal tract, c′= −0.177, IE 95% CI [−.0550, −.0071]. There was also a significant indirect effect of age on IG through FA of the right inferior longitudinal fasciculus, c′= −0.1812, IE 95% CI [−.0534, −.0061]. Figure 1B shows scatterplots of IG against FA values of the two tracts that have significant mediation effect to illustrate the positive relationship between the two factors. After checking the two outliers on the scatterplots, we found that they have very low raw color-word scores. The results did not change even if removing them from the sample, thus we kept them in the sample. Figure 1C shows the tracts with significant mediation effects.
Figure 1.
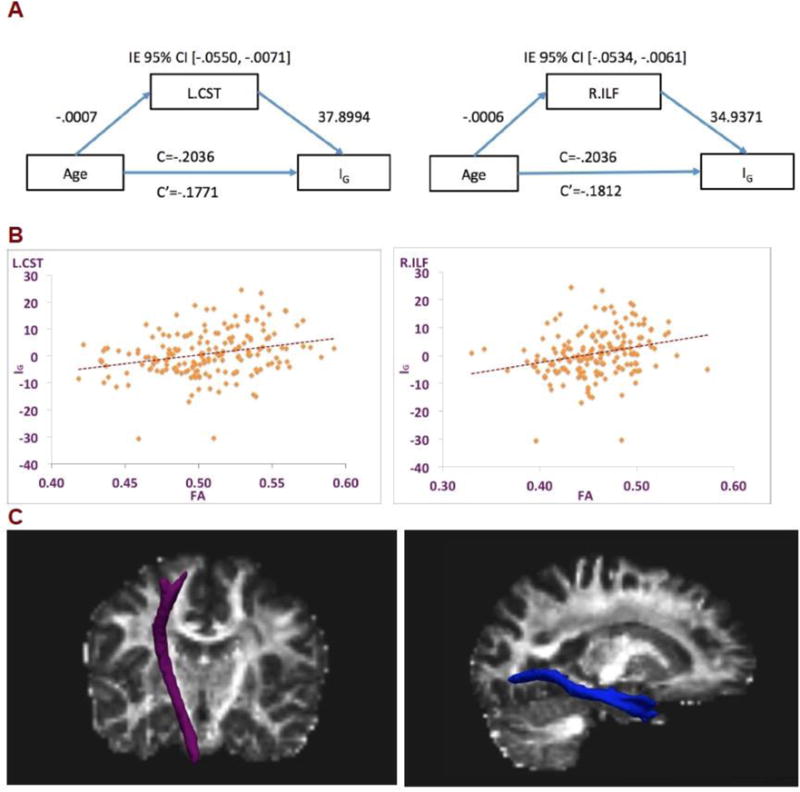
A. Mediation effects of age in Stroop interference score through FA of white matter tracts. IG=Stroop interference score. L.CST=left hemisphere corticospinal tracts; R.ILF=right hemisphere inferior longitudinal fasciculus. IE 95% CL=95% confidential interval of indirect effect. c=total effect, c′=direct effect.
B. Scatterplots of FA with IG. left: L.CST=left corticospinal tract; right: R.ILF=right inferior longitudinal fasciculus; IG=Stroop Interference Score; FA=fractional ansitropy. C. White matter tracts that show significant mediation effects. The left figure shows the left corticospinal tract (Y=16). The right figure shows the right inferior longitudinal fasciculus (X=25).
4. Discussion
This study examined whether integrity for 18 major white matter tracts explains the effect of age on the performance in an inhibitory control paradigm, the Stroop task, which involves both response inhibition and attentional control processes. We tested whether the FA of 18 axonal tracts mediated the effect of age on the Stroop interference score (IG). While none of the tracts showed differential relationship with IG across age, FA of the left corticospinal tract (CST) and the right inferior longitudinal fasciculus (ILF) mediated the effect of age on IG such that older age was associated with lower FA, which in turn was associated with lower IG, indicating worse Stroop performance.
Consistent with previous studies, we observed a number of major white matter tracts in which the mean FA was positively correlated with IG. Higher IG score suggests better inhibitory control and higher FA is indicative of better preserved white matter integrity because damaged myelination and axons would result in lower anisotropy in white matter tracts (Voineskos, et al., 2012). The tracts showing this positive association included posterior section of the corpus callosum (Splenium), the bilateral anterior thalamic radiation (ATR), the bilateral cortical spinal tract (CST), the bilateral inferior longitudinal fasciculus (ILF), and the parietal and temporal portions of the left superior longitudinal fasciculus (SLF). Even though some of these tracts did not survive the p-value correction for multiple comparisons, each of the tracts was associated with processes essential to response inhibition and attentional control in previous studies (Wolf et al., 2014). Splenium plays a role in disengagement and shifting of attention away from the invalidly cued color to the correct target color in the incongruent condition of the Stroop task (Christman 2001; Schulte et al., 2006; Kennedy & Raz, 2009). The tracts connecting anterior regions overlap with those reported by Wolf et al. (2014) such as the bilateral ATR, which is part of the anterior corona radiata, and bilateral CST, which connect to the internal capsule. The internal capsule, in turn, carries nerve fibers between the prefrontal cortex and the thalamus (Mamah et al., 2010).
Tracts that exceeded multiple comparison correction were further examined with mediation analysis. The mediation results provided stronger evidence than the simple regression results, supporting the role white matter plays in the differences observed across age along with decreases in Stroop performance. While this only provides a cross-sectional relationship, it nevertheless is consistent with a central role that certain white matter tracts play in the aging decline observed with inhibition control. Both the right inferior longitudinal fasciculus (ILF) and the left corticospinal tract (CST) mediated the effect of age on Stroop interference performance. ILF connects the occipital and the temporal lobes intrahemispherically (Nieuwenhuys et al., 2007). The temporal lobe is significant in processing semantic information of both verbal and visual language, while the occipital lobe serves as the core visual processing center of the human brain and is important in processing visual information (Salvan et al., 2004). As the major white matter tracts connecting the temporal and the occipital lobes, ILF facilitates the communication between the two lobes, and should thus play an important role in facilitating the identification of colors and inhibiting the meaning of the words in the Stroop test. Furthermore, the attentional network is right hemisphere dominant (Shulman et al., 2010), which is consistent with our result that the right ILF was a significant mediator.
The left corticospinal tract (CST) was also shown to be a significant mediator of age effects on Stroop performance. The likely role of this tract in Stroop performance is cortical-subcortical regulation of inhibitory control. CST originates from regions of the cerebral cortex, including the precentral gyrus (premotor area), postcentral gyrus (primary motor cortex), anterior cingulate cortex (ACC), and parietal cortices, and enters the internal capsule, which carries information to the basal ganglia (Catani & Thiebaut De Schotten, 2012). The frontal cortex send afferent signals to the subthalamic nucleus (STN; Nambu et al., 2000) such that the excitatory signals from the pre-SMA to STN is modulated by the inferior frontal gyrus, from which STN further inhibits the motor cortex (Rae et al., 2014). Aron and Poldrack (2006) showed that the modulation by the IFG on the pre-SMA and STN predicted performance in an inhibition task. Tractography data also indicated that the tracts between pre-SMA and STN and between IFG and STN predicted interindividual difference in an inhibition task (Rae et al., 2015). Since communication between the cortex and the subcortical structures are essential in inhibitory control, integrity of CST should play a role in modulating inhibitory control. The fact that only the left CST was found to be a significant mediator may be attributed to the inhibition of the word reading, a left-lateralized task.
It was hypothesized that white matter tracts connecting anterior regions such as SLF would be significant mediators of the age-related effects on IG, but the two mediating tracts observed in our study do not directly connect with anterior regions. As shown above, CST does consist of tracts originating from frontal regions such as the premotor cortices, but some of the tracts also originate from other regions of the brain such as the parietal cortices (Catani & Thiebaut De Schotten, 2012). ILF only connects between temporal and occipital lobes and does not traverse to the frontal regions (Nieuwenhuys et al., 2007).
This discrepancy in results between our study and previous studies that did not employ mediation analysis may be attributed to the difference in the questions being examined via mediation and via simple regression. Using simple regression with both age and white matter integrity as predictors for inhibitory control, previous studies did not explicitly test if the effects of age on inhibitory control can be explained by variability in white matter tracts. Rather, previous studies examined if white matter integrity predicts inhibitory control over and above the effect of age on inhibitory control. Mediation uses a series of regression models to test whether the direct effect of age on inhibitory control is reduced with the addition of white matter integrity (Hayes, 2013). Thus, simple regression and mediation analysis test complementary questions: what is the effect of white matter integrity on inhibitory control over and above age, and does age exert some of its effect on inhibitory control via white matter integrity of tracts, respectively. It is not surprising then that our results do not coincide.
The main caveat in this study is the use of cross-sectional data in the mediation analysis, which while tests for causal relationships, did not actually demonstrate causality. Rather, we demonstrated that when examined cross-sectionally, the data is consistent with a mediational role for the white matter tracts in the relationship between age and Stroop inhibitory performance. Longitudinal data are necessary to confirm the causal relationships suggested by our data.
5. Conclusion
Our results highlight the role of a number of major white matter tracts in inhibitory control processes, and more specifically illustrated that lower white matter integrity of the inferior longitudinal fasciculus and the corticospinal tract contribute to the age-related decline in inhibition control associated with the Stroop test.
Highlights.
Better white matter integrity is associated with more intact inhibitory control.
White matter integrity to inhibitory control relationship does not change with age.
Integrity of specific WM tracts mediate age effect on Stroop inhibitory control.
Acknowledgments
This work was supported by NIH K01 AG051777, NIH/NIA R01 AG26158, NIH/NIA R01 AG038465, and NIH UL1 TR000040/NCRR UL1 RR024156.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert M. Neuropsychological and neurophysiological changes in healthy adult humans across the age range. Neurobiology of Aging. 1993;14(6):623–625. doi: 10.1016/0197-4580(93)90049-h. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and Subcortical Contributions to Stop Signal Response Inhibition: Role of the Subthalamic Nucleus. Journal of Neuroscience. 2006;26(9):2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendlin BB, Fitzgerald ME, Ries ML, Xu G, Kastman EK, Thiel BW, Johnson SC. White Matter in Aging and Cognition: A Cross-Sectional Study of Microstructure in Adults Aged Eighteen to Eighty-Three. Developmental Neuropsychology. 2010;35(3):257–277. doi: 10.1080/87565641003696775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA. Postmortem indices linking risk factors to cognition: results from the Religious Order Study and the Memory and Aging Project. Alzheimer Disease and Associated Disorders. 2006;20:S63–S68. doi: 10.1097/00002093-200607001-00009. [DOI] [PubMed] [Google Scholar]
- Buckner R. Memory and Executive Function in Aging and AD. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut De Schotten M. Atlas of Human Brain Connections. Oxford: Oxford University Press; 2012. [Google Scholar]
- Christman SD. Individual differences in stroop and local- global processing: a possible role of interhemispheric interaction. Brain and Cognition. 2001;45(1):97–118. doi: 10.1006/brcg.2000.1259. [DOI] [PubMed] [Google Scholar]
- Donders FC. On the speed of mental processes. Acta Psychologia(Amst) 1969;30:412–431. doi: 10.1016/0001-6918(69)90065-1. [DOI] [PubMed] [Google Scholar]
- Gazes Y, Bowman FD, Razlighi QR, Oshea D, Stern Y, Habeck C. White matter tract covariance patterns predict age-declining cognitive abilities. NeuroImage. 2016;125:53–60. doi: 10.1016/j.neuroimage.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ. Stroop color and word test. Wood Dale, IL: Stoelting Co; 1978. [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive Aging, Executive Function, and Fractional Anisotropy: A Diffusion Tensor MR Imaging Study. American Journal of Neuroradiology. 2007;28(2):226–235. [PMC free article] [PubMed] [Google Scholar]
- Guttmann CRG, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50(4):972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York: Guilford press; 2013. [Google Scholar]
- Hughes EJ, Bond J, Svrckova P, Makropoulos A, Ball G, Sharp DJ, Edwards AD, Hajnal JV, Counsell SJ. Regional changes in thalamic shape and volume with increasing age. NeuroImage. 2012;63:1134–1142. doi: 10.1016/j.neuroimage.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. NeuroImage. 2004;22(3):1097–1106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Jacobs HI, Leritz EC, Williams VJ, Van Boxtel MP, van der Elst W, Jolles J, Verhey FR, McGlinchey RE, Milberg WP, Salat DH. Association between white matter microstructure, executive functions, and processing speed in older adults: the impact of vascular health. Human Brain Mapping. 2013;34(1):77–95. doi: 10.1002/hbm.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly TAD, Cooper PS, Rennie JL, Levi CR, Lenroot R, Parsons MW, Michie PT, Karayanidis F. Age-Related Decline in Task Switching is Linked to Both Global and Tract-Specific Changes in White Matter Microstructure. Human Brain Mapping. 2017;38(3):1588–1603. doi: 10.1002/hbm.23473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47(3):916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama S, Rosene D, Sherman L. Age-related changes in human and non-human primate white matter: From myelination disturbances to cognitive decline. Age. 2011;34(5):1093–1110. doi: 10.1007/s11357-011-9357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden D, Bennett I, Song A. Cerebral White Matter Integrity and Cognitive Aging: Contributions from Diffusion Tensor Imaging. Neuropsychology Review. 2009;19(4):415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, Mcmichael AR, Csernansky JG. Anterior thalamic radiation integrity in schizophrenia: A diffusion-tensor imaging study. Psychiatry Research: Neuroimaging. 2010;183(2):144–150. doi: 10.1016/j.pscychresns.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of Diffusion Tensor Imaging and Its Applications to Basic Neuroscience Research. Neuron. 2006;51(5):527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Mori S. Introduction to Diffusion Tensor Imaging. Amsterdam: Elsevier; 2007. [Google Scholar]
- Mostofsky S, Simmonds D. Response Inhibition and Response Selection: Two Sides of the Same Coin. Journal of Cognitive Neuroscience. 2008;20(5):751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, Hasegawa N. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus. Journal of Neurophysiology. 1998;84(1):289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System: A Synopsis and Atlas. 4th. New York: Springer-Verlag; 2007. [Google Scholar]
- Posner MI. The Attention System of the Human Brain. Annual Review Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Rae CL, Hughes LE, Anderson MC, Rowe JB. The Prefrontal Cortex Achieves Inhibitory Control by Facilitating Subcortical Motor Pathway Connectivity. Journal of Neuroscience. 2015;35(2):786–794. doi: 10.1523/JNEUROSCI.3093-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CL, Hughes LE, Weaver C, Anderson MC, Rowe JB. Selection and stopping in voluntary action: A meta-analysis and combined fMRI study. NeuroImage. 2014;86:381–391. doi: 10.1016/j.neuroimage.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A. Anatomy of attentional networks. The Anatomical Record. 2004;281B(1):21–36. doi: 10.1002/ar.b.20035. [DOI] [PubMed] [Google Scholar]
- Reginold W, Itorralba J, Tam A, Luedke AC, Fernandez-Ruiz J, Reginold J, Garcia A. Correlating quantitative tractography at 3T MRI and cognitive tests in healthy older adults. Brain Imaging and Behavior. 2015;10(4):1223–1230. doi: 10.1007/s11682-015-9495-0. [DOI] [PubMed] [Google Scholar]
- Salvan CV, Ulmer JL, DeYoe EA, Wascher T, Mathews VP, Lewis JW. Visual object agnosia and pure word alexia: correlation of functional magnetic resonance imaging and lesion localization. Journal of Computer Assisted Tomography. 2004;28(1):63–67. doi: 10.1097/00004728-200401000-00010. [DOI] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Pfefferbaum A, Sullivan EV. Callosal involvement in a lateralized Stroop task in alcoholic and healthy subjects. Neuropsychology. 2006;20(6):727–736. doi: 10.1037/0894-4105.20.6.727. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(S1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Simon JR. The Effects of an Irrelevant Directional CUE on Human Information Processing. Advances in Psychology. 1989:31–86. [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46(1):224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Pope DL, Astafiev SV, Mcavoy MP, Snyder AZ, Corbetta M. Right Hemisphere Dominance during Spatial Selective Attention and Target Detection Occurs Outside the Dorsal Frontoparietal Network. Journal of Neuroscience. 2010;30(10):3640–3651. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology: General. 1935;18(6):643–662. [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Longitudinal Study of Callosal Microstructure in the Normal Adult Aging Brain Using Quantitative DTI Fiber Tracking. Developmental Neuropsychology. 2010;35(3):233–256. doi: 10.1080/87565641003689556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Rajji TK, Lobaugh NJ, Miranda D, Shenton ME, Kennedy JL, Pollock BG, Mulsant BH. Age-related decline in white matter tract integrity and cognitive performance: a DTI tractography and structural equation modeling study. Neurobiology of Aging. 2012;33(1):21–34. doi: 10.1016/j.neurobiolaging.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S. Fiber Tract–based Atlas of Human White Matter Anatomy1. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wolf D, Zschutschke L, Scheurich A, Schmitz F, Lieb K, Tüscher O, Fellgiebel A. Age-related increases in stroop interference: Delineation of general slowing based on behavioral and white matter analyses. Human Brain Mapping. 2014;35(5):2448–2458. doi: 10.1002/hbm.22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Panneck P, Srinivasan P, Stevens A, Zöllei L, Augustinack J, Wang R, Salat D, Ehrlich S, Behrens T, Jbabdi S, Gollub R, Fischl B. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in Neuroinformatic. 2011;14(5) doi: 10.3389/fninf.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad M, Hodneland E, Adolfsdottir S, Haasz J, Lundervold AJ, Eichele T, Lun-dervold A. Corticostriatal connectivity and cognition in normal aging: a combined DTI and resting state fMRI study. NeuroImage. 2011;55(1):24–31.a. doi: 10.1016/j.neuroimage.2010.11.016. [DOI] [PubMed] [Google Scholar]


