Abstract
Background
Patients hospitalized for suspected acute coronary syndrome (ACS) are at risk for transient myocardial ischemia. During the “rule-out” phase, continuous ECG ST-segment monitoring can identify transient myocardial ischemia, even when asymptomatic. However, current ST-segment monitoring software is vastly underutilized due to false positive alarms, with resultant alarm fatigue. Current ST algorithms may contribute to alarm fatigue because; (1) they are not designed with a delay (minutes), rather alarm to brief spikes (i.e., turning, heart rate changes), and (2) alarm to changes in a single ECG lead, rather than contiguous leads.
Purpose
This study was designed to determine sensitivity, and specificity, of ST algorithms when accounting for; ST magnitude (100 μV vs 200 μV), duration, and changes in contiguousECG leads (i.e., aVL, I, - aVR, II, aVF, III; V1, V2, V3, V4, V5, V6, V6, I).
Methods
This was a secondary analysis from the COMPARE Study, which assessed occurrence rates for transient myocardial ischemia in hospitalized patients with suspected ACS using 12-lead Holter. Transient myocardial ischemia was identified from Holter using > 100 μVST-segment ↑ or ↓, in > 1 ECG lead, > 1 minute. Algorithms tested against Holter transient myocardial ischemia were done using the University of California San Francisco (UCSF) ECG algorithm and included: (1)100 μV vs 200 μV any lead during a 5-minute ST average; (2)100 μV vs 200μV any lead > 5 minutes, (3) 100μV vs 200μV any lead during a 5-minute ST average in contiguous leads, and (4) 100 μV vs 200 μV > 5 minutes in contiguous leads (Table below).
Results
In 361 patients; mean age 63 + 12 years, 63% male, 56% prior CAD, 43 (11%) had transient myocardial ischemia. Of the 43 patients with transient myocardial ischemia, 17 (40%) had ST-segment elevation events, and 26 (60%) ST-segment depression events. A higher proportion of patients with ST segment depression has missed ischemic events.
Table shows sensitivity and specificity for the four algorithms tested
| Alarms Tested | 100 mV ST Deviation (↓ or ↑) | 200 mV ST Deviation (↓ or ↑) | ||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| 1. ST-segment change any ECG lead 5 minute average | 76.7% | 47.8% | 46.5% | 74.2% |
| 2. ST-segment change any ECG lead > 5 minutes | 76.7% | 54.4% | 46.5% | 77.4% |
| 3. ST-segment change contiguous leads 5 minute average | 69.8% | 67.9% | 27.9% | 83.6% |
| 4. ST-segment change contiguous leads > 5 minutes | 62.8% | 73.0% | 20.9% | 86.8% |
Conclusions
Sensitivity was highly variable, due to the ST threshold selected, with the 100 μV measurement point being superior to the 200 μV amplitude threshold. Of all the algorithms tested, there was moderate sensitivity and specificity (70% and 68%) using the 100 μV ST-segment threshold, integrated ST-segment changes in continuous leads during a 5-minute average.
Every year, nearly 7 million Americans seek care for chest pain to rule out possible acute coronary syndrome (ACS), a pathology which claims nearly one life every 2 minutes.1 Early identification of myocardial ischemia (pre-infarction), in order to reduce damage to the heart, is a critical step in the treatment of ACS. The presence of ST elevation on the presenting electrocardiogram (ECG) constitutes the principal ECG feature used to risk-stratify and guide treatment; however, ST elevation is present in less than 25% of ACS patients.2 Hence, the current clinical challenge is directed at the other 75% of patients, those with non-ST elevation-ACS (NSTE-ACS) or unstable angina (UA). These are clinically challenging patients because their initial ECG may be non-diagnostic and biomarkers may take hours to become positive. During the “rule-out” phase, continuous ECG ST-segment monitoring can identify high risk patients with transient myocardial ischemia, even when asymptomatic.3–5 While a continuous ECG approach is ideal using ST-segment monitoring, current ST-segment software is vastly underutilized due to false positive alarms,6, 7 which contribute to alarm fatigue. 8–10 During a one month period, Drew et al., found a total of 6,196 ST alarms occurred in 83 patients who were monitored in a 16 bed coronary care unit with ECG ST-segment monitoring (200 alarms/day).8 Of the 6,196 ECG ST alarms, 4961 (80%) were < 30 seconds in duration following brief ST-segment spikes. When the duration of ST-segment changes was lengthened to > 60 seconds there was a substantial drop in the number of alarms from 4,961 to 542. These findings suggest that ST-segment alarms designed with a delay (i.e., minutes) may reduce the number of ST alarms and lower the burden of alarm fatigue.
Research assessing transient myocardial ischemia in hospitalized patients with ACS have defined its presence using the standard rule of “ones,” ST-segment deviation (elevation or depression) of ≥ 100 μV in ≥ 1 ECG leads ≥ 1 minute,11 which is also the recommendation for in-hospital ECG monitoring.12 However, in-hospital ECG monitor algorithms are not designed with this rule, and may contribute to false ST-segment alarms. This may occur for several reasons. First, as described above, many alarms are generated from brief ST-segment change, which can occur during turning, breathing, heart rate changes or noise.8, 10, 13, 14 Second, ST-segment alarms are generated from ST-segment changes in a single ECG lead, rather than contiguous (side-by-side), as is typical in true transient myocardial ischemia. Lastly, the amplitude of ST-segment changes used to determine the presence of transient myocardial ischemia (i.e., 100 microvolts [μV] versus 200 μV) may contribute to ST-segment alarms. While it is predictable that a 100 μV ST-segment measurement point is more sensitive than 200 μV, currently in clinical practice the 200 μV measurement point is commonly used. This is because many clinicians believe that a 100 μV measurement point is too sensitive for continuous inhospital ECG ST-segment monitoring, and have adjusted their alarm threshold to 200 μV in an effort to reduce the number of ST-segment alarms. However, the μV adjustment from 100 μV to 200 μV has not been assessed using continuously acquired ECG data, neither have other algorithm features (i.e., duration and contiguity). This study was designed to determine the sensitivity, and specificity of four developed ECG ST-segment algorithms designed with three features: (1) ST magnitude (100 μV vs 200 μV), (2) duration (minutes), and (3) contiguous ECG leads (i.e., aVL, I, - aVR, II, aVF, III; V1, V2, V3, V4, V5, V6, V6).
Methods
This study is a secondary analysis using data from the COMPARE study (R21 NR-011202, PI: MMP), the design of which has been described previously.15 Briefly, the COMPARE Study examined the frequency and consequences of transient myocardial ischemia among hospitalized patients presenting for suspected ACS. Approval from local institutional review boards was obtained from participating hospitals, and all patients provided written informed consent prior to participation.
Sample/Settings
Included were English speaking patients presenting for treatment of suspected ACS, specifically, non-ST-elevation acute coronary syndrome (NSTE-ACS), or unstable angina (UA). Patients were excluded if admitted for ST-elevation MI (STEMI), were comatose, diagnosed with a major psychiatric disorder, or in isolation precautions. ECG exclusion criteria included left bundle branch block or ventricular paced rhythm because these conditions distort the ST-segment, making it difficult to reliably interpret the ECG for transient myocardial ischemia.12, 15
Electrocardiographic Data Collection
A research nurse/assistant was present in the hospital during the hours of 7 am to 5 pm, Monday through Friday. A 12-lead ECG Holter recorder (Mortara Instruments, Milwaukee, WI) was applied to patients meeting inclusion criteria. The Holter recorder data was not available to clinicians for decision making; rather off-line analysis (described below) was conducted after hospital discharge. The ECG Holter recorder remained in place until the patient was discharged home. All patients were maintained on the hospital’s ECG monitor system as per the hospital protocol.
Primary Study - ECG Transient Ischemia Analysis
The 12-lead ECG Holter data were downloaded to a research computer and analyzed after hospital discharge using the H-Scribe Analysis System (Mortara Instruments, Milwaukee, WI). Transient myocardial ischemia was defined using the standard definition, ST-segment deviation (elevation or depression) of ≥ 100 μV in ≥ 1 ECG leads ≥ 60 seconds measured at J + 60 milliseconds.11, 12 While the H-Scribe software automatically codes myocardial ischemia, all of the ECG data were manually over read by the principal investigator (MMP), to ensure false-positive ST changes were not counted as transient ischemia (i.e., body position changes, intermittent bundle branch block, left ventricular hypertrophy, or arrhythmias).
Algorithms Tested
While the parent COMPARE Study enrolled 488 patients, this secondary analysis examined a sub-set of 361 (74%), who had high-resolution ECG data acquired with a 1,000 sample/second flash card. This sampling rate was required to examine transient myocardial ischemia using our developed University of California San Francisco (UCSF) ECG algorithm. The UCSF ECG algorithm examines ST-segments at the J-point + 60 milliseconds in all 12-ECG leads using a five minute. We used the first thirty minutes of Holter recording to establish a baseline ST-segment level in each of the 12-leads. Transient ST-segment deviation (elevation or depression) was then determined from the established baseline during the remainder of the Holter recording (average recording time 26 hours). A total of four ST-segment alarms were tested and shown in Table 1.
Table 1.
Shown are the four alarms tested (rows). Columns two and three illustrate the ST-segment amplitude tested for each of the four alarms (i.e., 100 microvolts [μV] versus 200 μV) measured at the J-point + 60 milliseconds. Contiguous ECG leads were as follows: aVL, I, - aVR, II, aVF, III; V1, V2, V3, V4, V5, V6, V6.
| Alarms Tested | 100 μV ST Deviation (↓ or ↑) | 200 μV ST Deviation (↓ or ↑) |
|---|---|---|
| 1. ST-segment change any ECG lead using 5 minute average | ||
| 2. ST-segment change any ECG lead > 5 minutes | ||
| 3. ST-segment change contiguous leads using 5 minute average | ||
| 4. ST-segment change contiguous leads > 5 minutes |
Statistical Analysis
Data were analyzed using SPSS 22.0 (IBM Corporation 1994, 2014). Descriptive statistics were used to report demographic (i.e., age, gender, and ethnicity) and clinical information including medical history (i.e., prior angina, prior MI, hypertension, hyperlipidemia, diabetes, prior cardiac procedures, and coronary artery disease (CAD). These values are expressed as means ± standard deviations and percentages. Sensitivity and specificity were calculated to determine the performance of the four algorithms in identifying transient myocardial ischemia. A p value of < 0.05 was adopted as the critical value to determine statistical significance where appropriate.
Results
As shown in Table 2 the sample was mostly male, White, and nearly half had a prior history of coronary artery disease. Of the 361 total patients, 43 (11%) had transient myocardial ischemia identified during Holter recording.
Table 2.
Sample of patients presenting to the hospital with symptoms suggestive of acute coronary syndrome n = 361.
| Variable | N (%) |
|---|---|
|
| |
| Male Gender | 225 (62) |
|
| |
| Race | |
| American Indian/Alaska Native | 6 (2) |
| Asian | 5 (1) |
| Black | 11 (3) |
| Pacific Islander | 5 (1) |
| White | 357 (93) |
|
| |
| Clinical History | |
| Coronary Artery Disease | 165 (46) |
| Myocardial Infarction | 108 (30) |
| Percutaneous Coronary Intervention | 104 (29) |
| Coronary Bypass Graph Surgery | 54 (15) |
| Diabetes | 94 (26) |
| Hypertension | 242 (67) |
| Dyslipidemia | 216 (60) |
|
| |
| Mean Time from Hospital Presentation to Holter (Hours) | 5:30 (± 4) |
|
| |
| Total Holter Recording Time (Hours) | 26 (± 18) |
|
| |
| Discharge Diagnosis of Acute Coronary Syndrome | 147 (38) |
|
| |
| Transient Myocardial Ischemia | 43 (11) |
Tested Algorithms
Table 3 shows the sensitivity and specificity of the four tested algorithms, with comparisons using 100 μV versus 200 μV as the ST threshold. Overall, the algorithm with equivalent sensitivity and specificity (69.8%/67.9%) was #3, which used the 100 μV measurement point in contiguous leads using a 5-minute average.
Table 3.
Sensitivity and specificity for the four alarms tested using ST deviation (elevation or depression) comparing 100 microvolts (μV) to 200 μV measured at the J-point + 60 milliseconds. Contiguous ECG leads were as follows: aVL, I, - aVR, II, aVF, III; V1, V2, V3, V4, V5, V6, V6.
| Alarms Tested | 100 mV ST Deviation (↓ or ↑) | 200 mV ST Deviation (↓ or ↑) | ||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| 1. ST-segment change any ECG lead using 5 minute average | 76.7% | 47.8% | 46.5% | 74.2% |
| 2. ST-segment change any ECG lead > 5 minutes | 76.7% | 54.4% | 46.5% | 77.4% |
| 3. ST-segment change contiguous leads using 5 minute average | 69.8% | 67.9% | 27.9% | 83.6% |
| 4. ST-segment change contiguous leads > 5 minutes | 62.8% | 73.0% | 20.9% | 86.8% |
Algorithm Performance by ST-Segment Amplitude and Direction
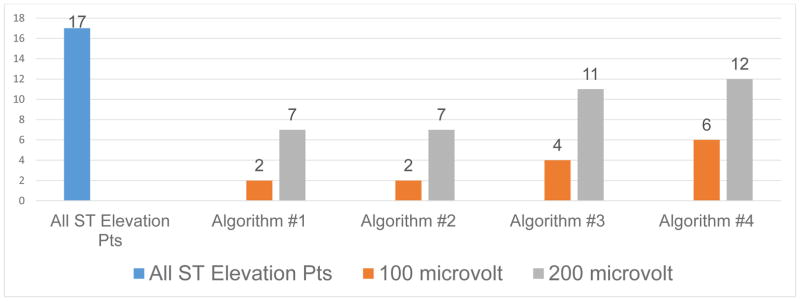
Of the 43 patients with transient myocardial ischemia, 17 (40%) had ST-segment elevation events, and 26 (60%) ST-segment depression events. Figure 1 shows the 17 patients with ST-segment elevation events, and the number of patients missed by each of the four algorithms compared by ST-segment amplitude (100 μV versus 200 μV). As illustrated, for all four algorithms, a higher number of patients with ST elevation events are missed when an amplitude of 200 μV is used. Figure 2 shows ECG’s to illustrate a missed ST-segment elevation event when using the 200 μV amplitude threshold.
Figure 1.
Shows 17 patients with ST-segment elevation events (blue bar) and number of patients missed by each of the four algorithms using ST amplitude of 100 μV (orange) versus 200 μV (grey).
| Algorithms Tested |
|---|
| 1. ST-segment change any ECG lead using 5 minute average |
| 2. ST-segment change any ECG lead > 5 minutes |
| 3. ST-segment change contiguous leads using 5 minute average |
| 4. ST-segment change contiguous leads > 5 minutes |
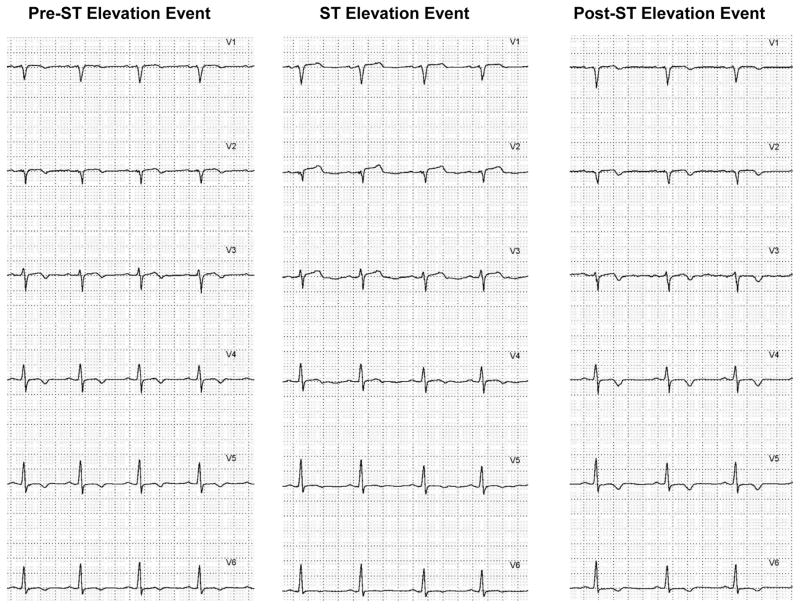
Figure 2.
Shown are the six precordial leads (V1 to V6) in a missed ST-segment elevation event when using the 200 μV amplitude threshold. The panel on the left is the baseline ST-segment level in a 55-year-old male admitted with chest pain rule out acute coronary syndrome. The middle panel shows maximal ST-segment elevation during the event, note ST-segment elevation in leads V1 to V3, which does not exceed the 200 μV threshold. T-wave changes are seen in leads V4 to V6. The panel on the right shows the return of the ST-segments to the level prior to the elevation event.
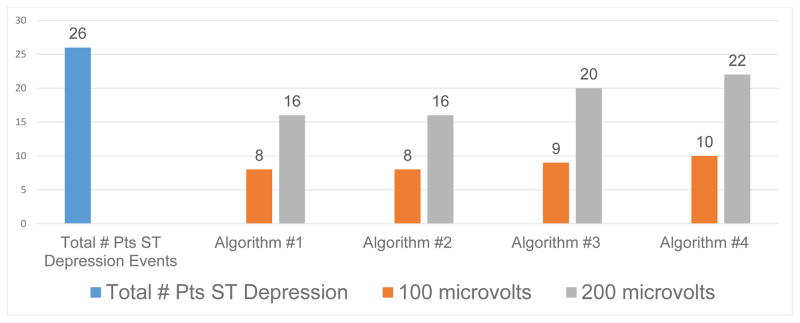
Figure 3 displays the 26 patients with ST-segment depression events, and the number of patients missed by each of the four algorithms comparing the 100 μV versus 200 μV ST amplitude threshold. Similar to patients with elevation events, for all four algorithms, a higher number of patients with ST depression events were missed when an amplitude of 200 μV was used. Figure 4 shows ECG’s to illustrate a missed ST-segment depression event when using the 100 μV amplitude threshold.
Figure 3.
Shows 26 patients with ST-segment depression events (blue bar) and number of patients missed by each of the four algorithms using ST amplitude of 100 μV (orange) versus 200 μV (grey).
| Algorithms Tested |
|---|
| 1. ST-segment change any ECG lead using 5 minute average |
| 2. ST-segment change any ECG lead > 5 minutes |
| 3. ST-segment change contiguous leads using 5 minute average |
| 4. ST-segment change contiguous leads > 5 minutes |
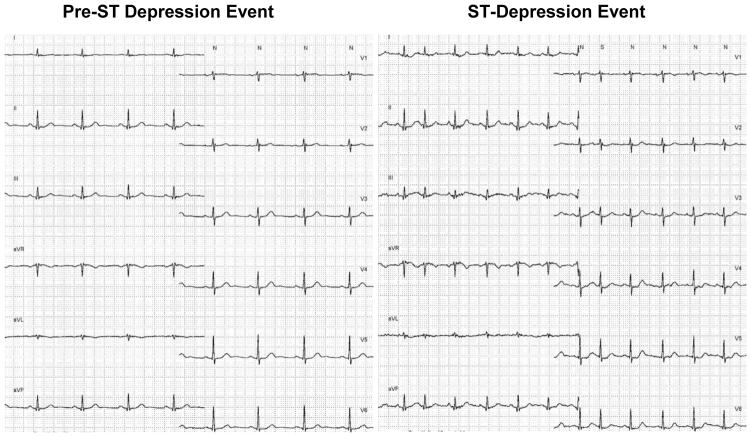
Figure 4.
Shown are two 12-lead ECG’s in a missed ST-segment depression event. The panel on the left is a baseline 12-lead ECG in a 78-year-old male admitted with chest pain rule out acute coronary syndrome. The 12-lead ECG on the right shows an ST-segment depression event, note increased heart rate and ST-segment depression in multiple ECG leads. This event was missed by all tested algorithms since the ST-segment depression only reached −91 μV, and not the −100 μV criteria used in the primary study.
Table 4 shows the 17 patients with ST-segment elevation events and the 26 patients with ST-segment depression events with comparisons by amplitude (100 μV versus 200 μV) for each algorithm. Transient ischemia was missed in a higher proportion of patients when the 200 μV amplitude threshold was used. Due to a small sample size, statistical comparisons should be interpreted with caution.
Table 4.
Of the 43 patients with transient myocardial ischemia, 17 (40%) had ST-segment elevation events, and 26 (60%) ST-segment depression events. Shown are the 17 patients with ST-segment elevation events and the 26 patients with ST-segment depression events. Comparisons by amplitude (100 μV versus 200 μV) are shown for each algorithm. Contiguous ECG leads were as follows: aVL, I, - aVR, II, aVF, III; V1, V2, V3, V4, V5, V6, V6.
| Algorithms Tested | Elevation N = 17 (%) | P-Value χ2* | Depression N = 26 (%) | P-Value χ2* | ||
|---|---|---|---|---|---|---|
| 100 μV | 200 μV | 100 μV | 200 μV | |||
| 1. ST-segment change any ECG lead using 5 minute average | 2 | 7 | 0.154 | 8 | 16 | 0.009 |
| 2. ST-segment change any ECG lead > 5 minutes | 2 | 7 | 0.154 | 8 | 16 | 0.009 |
| 3. ST-segment change contiguous leads using 5 minute average | 4 | 11 | 0.237 | 9 | 20 | 0.063 |
| 4. ST-segment change contiguous leads > 5 minutes | 6 | 12 | 0.102 | 10 | 22 | 0.136 |
Expected cell count less than 5 required for statistical test - Fishers exact test statistics reported
Discussion
This study showed that the sensitivity of ECG algorithms designed to identify transient myocardial ischemia during continuous ECG monitoring is highly variable, ranging from 77% to as low as 21%. This large variation in sensitivity appears to be due to two factors. First, the measurement point used to measure ST-segment deviation. Higher sensitivity was observed when using a 100 μV amplitude change (range 77% to 63%), and considerably lower when using a 200 μV amplitude change (range 47% to 21%). Second, lower sensitivity was seen when using contiguous ECG leads with the lowest sensitivity (21%) seen in the algorithm using ST-segment changes > 5 minutes in continuous leads. While sensitivity was lower when using a 200 μV amplitude change, reasonable specificity was observed (range 74% to 88%). Of all the algorithms tested, the one with equivalent sensitivity and specificity (70% and 68%) used the 100 μV ST-segment threshold, integrated ST-segment changes in continuous leads using a 5-minute average.
One possible explanation for the differences in our study between the cases identified by each algorithm versus those identified in the primary study is likely explained by human/manual over reading. An example of this is shown in figure 4 during an ST-segment depression event. The algorithm identified ST-segment depression of −91 μV, which did not exceed the 100 μV level used in the primary study; hence all of the algorithms missed this ischemic event. Variations in human versus computerized ST-segment measurements have been reported.16, 17, It is also possible in this example that the human reader may have taken into account the concomitant increased heart rate during the ST-segment changes, which when combined with ST-segment depression was determined to be transient ischemia. Current in-hospital ECG monitoring algorithms do not combine other physiologic parameters such as heart rate, which may be useful particularly for ST-segment depression events due to demand related ischemia (i.e., increased heart rate in a partially occluded coronary artery/arteries), and should be tested.
We anticipated improved identification of transient myocardial ischemia by using continuous leads. However, sensitivity was lower in all of the algorithms using this approach, while specificity was somewhat improved. This suggests this approach may reduce false positive alarms, but at the cost of missing true transient ischemia. Wang and co-workers used a “vessel specific lead” approach tested during percutaneous balloon inflation (PCI) and found improved sensitivity of ischemia detection when using a weighted sum of both ST elevation and depression in eight predictor leads.18 However, this study examined ST-segment changes during cardiac catheterization with complete coronary occlusion using the PCI model; hence, there was minimal artifact from patient movement as would be typical during continuous ECG monitoring in patients admitted for rule out ACS. Nevertheless, a weighted sum approach may be useful to test in the future.
Motion artifact from patient movement during continuous ECG monitoring can lead to false alarms and contribute to alarm fatigue. Abdelazez et al., tested an alarm gating approach to determine if their algorithm could identify ST-segment deviation despite the presence of motion artifact.14 Using a signal quality analysis algorithm, the investigators were able to remove 93% of the false positive ST-segment changes caused by motion artifact while maintaining the number of true positive ST events. However, their developed algorithm overestimated ST-segment deviation resulting in 66 false positive events. Firoozabadi and co-workers used a multistep algorithm approach to determine if notifications for ST-segment elevation from the bedside monitor could be lowered by using an algorithm designed for resting 12-lead ECGs.13 Their premise was that current bedside monitoring ST-segment algorithms are prone to false alarms because they are not capable of identifying ECG confounders (i.e., pericarditis, bundle branch block, early repolarization, and left ventricular hypertrophy) as can be done with resting ECG algorithms. In the over 1,000 patients, 25% with continuous monitoring generated at least one ST-elevation notification as compared to only 4% using the developed algorithm. A sensitivity of 100% and specificity of 80% was achieved using a one-minute analysis window. When a 5-minute analysis window was used, sensitivity dropped to 87% sensitivity but specificity improved to 95%. Similarly, we showed that specificity improved when the duration was extended beyond a 5-minute time period, however, sensitivity was unacceptably low.
In many of the studies described above, ST-segment elevation was the focus of analysis; hence algorithms designed to identify dynamic ST-segment deviation with both elevation and depression patterns as is typical in transient myocardial ischemia have not been thoroughly investigated. While one could argue transient ST-segment elevation is the most critical ST pattern to identify in ACS patients since this pattern signifies complete coronary occlusion, the vast majority (two-thirds) of those admitted with suspected ACS (i.e., non-ST elevation ACS, unstable angina) do not present with ST-segment elevation. Rather, these patients are at risk for transient ischemia (i.e., elevation, depression, or both), both of which increase the risk of serious in-hospital and long-term complications.4, 5, 19–21 In our study, a higher proportion of patients experienced ST-segment depression events as compared to elevation events (60% versus 40%). Overall, the algorithms missed more ST-segment depression events, particularly when using the 200 μV measurement point in contiguous leads, which is below the measurement recommended in the Third Universal Definition of MI of > 50 μV in two contiguous leads.22 Hence, it is not surprising so many patients in our study with transient ST-segment depression events were missed. This is an important finding since current bedside ECG ST-segment monitoring algorithms do not allow for adjustments based on ST-segment direction, rather the nurse selects the desired amplitude, typically 100 μV or 200 μV, which is used for both elevation and depression events. Algorithms designed to detect both transient ST-segment elevation and depression using varied ST amplitude, for example 100 mV for ST elevation and 50 mV for ST depression, should be developed and tested.
According to the ECG Practice Standards for in-hospital cardiac monitoring, ST-segment alarm parameters should be set to 100 μV above and below a patients baseline ST-segment level in those considered at high risk (STEMI, NSTEMI-ACS, unstable angina, or rule out MI) and 200 μV in those identified at lower risk.12 Our results corroborate these recommendations as the 100 μV amplitude change was superior to the 200 μV amplitude. However, many hospitals have opted to set the default ST-segment threshold to 200 μV in an attempt to reduce the number of false ECG ST-segment monitoring alarms. While this is a reasonable adjustment to reduce alarm fatigue, our study shows that the 200 μV threshold will miss a substantial number of patients with true transient myocardial ischemia.
Current ECG ST-segment algorithms are ineffective due to high false alarm rates. Clinicians have resorted to making adjustments to bedside ECG monitors that are likely to miss transient ischemic events. There is therefore, an urgent need to develop better ST-segment algorithms designed with the required sensitivity to detect small, but clinically significant ST-segment changes indicative of transient myocardial ischemia, while minimizing false alarms. Suggestions, for algorithm improvements based on our results include: (1) combining ST-segment changes with other physiologic parameters (i.e., heart rate), (2) sum ST segment changes over multiple ECG leads (i.e., ischemic burden), (3) recognize motion artifact, (4) automatically adjust ST-amplitude threshold based on ST-segment direction (i.e., elevation versus depression).
Limitations
Our algorithm uses a 5-minute ST-segment average for classifying transient ischemia, which could potentially limit our ability to identify brief ST-segment changes during true ischemia. However, in prior studies we found that the vast majority of true ischemic events are on average 30 to 60 minutes in duration;3, 4, 23 hence this 5-minute time window is not likely to reduce the ability to identify transient ischemia. Transient myocardial ischemia was diagnosed from Holter recordings by clinical experts; hence, true events may have been missed, or judged to be true when there was not transient ischemia. Only 11% of the sample had transient myocardial ischemia.
Conclusions
This study tested four algorithms, which incorporated ST magnitude, duration, and contiguous ECG leads. Sensitivity was highly variable, due to the ST-segment threshold used, with the 100 μV measurement point being superior to the 200 μV amplitude threshold. Missed ischemic events were higher in patients with ST-segment depression events. Of all the algorithms tested, there was moderate sensitivity and specificity (70% and 68%) using the 100 μV ST-segment threshold, integrated ST-segment changes in continuous leads during a 5-minute ST-segment average. In future work we plan on evaluating other ECG waveforms (i.e., T-wave, QRS width) and combining other physiologic parameters into our algorithm. This study demonstrates that more research and further algorithm development needs to be done to improve detection of transient myocardial ischemia using in-hospital ECG monitoring.
Highlights.
The sensitivity of ECG algorithms designed to identify transient myocardial ischemia in patients with acute coronary syndrome are highly variable when using; (1) amplitude (100 microvolts [μV] versus 200 μV), (2) duration (minutes), and (3) contiguous ECG leads.
Using a 100 μV ST-segment threshold is superior to a 200 μV threshold measured at 60 milliseconds past the J-point.
A higher proportion of ST-segment depression events are missed as compared to ST-segment elevation events.
Moderate sensitivity and specificity (70% and 68%) was achieved using ST-segment deviation of 100 μV, using a five-minute ST-segment average in continuous ECG leads.
Acknowledgments
Funding: This study was supported by grant R21NR011202 (PI - MMP) provided by the National Institutes of Health. The authors have no relationships to disclose with business or industry related to planning, executing, and/or publishing this study.
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation. 2017 doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ C. American College of, G. American Heart Association Task Force on Practice, A. Society for Cardiovascular, Interventions, S. Society of Thoracic, and C. American Association for Clinical 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Drew BJ, Pelter MM, Adams MG. Frequency, characteristics, and clinical significance of transient ST segment elevation in patients with acute coronary syndromes. Eur Heart J. 2002;23(12):941–7. doi: 10.1053/euhj.2001.2987. [DOI] [PubMed] [Google Scholar]
- 4.Pelter MM, Loranger D, Kozik TM, Fidler R, Hu X, Carey MG. Unplanned transfer from the telemetry unit to the intensive care unit in hospitalized patients with suspected acute coronary syndrome. J Electrocardiol. 2016 doi: 10.1016/j.jelectrocard.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelter MM, Loranger DL, Kozik TM, Kedia A, Ganchan RP, Ganchan D, Hu X, Carey MG. Among Unstable Angina and Non-ST-Elevation Myocardial Infarction Patients, Transient Myocardial Ischemia and Early Invasive Treatment Are Predictors of Major In-hospital Complications. J Cardiovasc Nurs. 2016;31(4):E10–9. doi: 10.1097/JCN.0000000000000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel SI, Souter MJ. Equipment-related electrocardiographic artifacts: causes, characteristics, consequences, and correction. Anesthesiology. 2008;108(1):138–48. doi: 10.1097/01.anes.0000296537.62905.25. [DOI] [PubMed] [Google Scholar]
- 7.Sandau KE, Sendelbach S, Frederickson J, Doran K. National survey of cardiologists’ standard of practice for continuous ST-segment monitoring. Am J Crit Care. 2010;19(2):112–23. doi: 10.4037/ajcc2010264. [DOI] [PubMed] [Google Scholar]
- 8.Drew BJ, Harris P, Zegre-Hemsey JK, Mammone T, Schindler D, Salas-Boni R, Bai Y, Tinoco A, Ding Q, Hu X. Insights into the problem of alarm fatigue with physiologic monitor devices: a comprehensive observational study of consecutive intensive care unit patients. PLoS One. 2014;9(10):e110274. doi: 10.1371/journal.pone.0110274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atzema CL, Schull MJ. ALaRMED: adverse events in low-risk chest pain patients receiving continuous ECG monitoring in the emergency department: a survey of Canadian emergency physicians. CJEM. 2008;10(5):413–9. doi: 10.1017/s1481803500010472. [DOI] [PubMed] [Google Scholar]
- 10.Tsimenidis C, Murray A. False alarms during patient monitoring in clinical intensive care units are highly related to poor quality of the monitored electrocardiogram signals. Physiol Meas. 2016;37(8):1383–91. doi: 10.1088/0967-3334/37/8/1383. [DOI] [PubMed] [Google Scholar]
- 11.Crawford MH, Bernstein SJ, Deedwania PC, DiMarco JP, Ferrick KJ, Garson A, Jr, Green LA, Greene HL, Silka MJ, Stone PH, Tracy CM, Gibbons RJ, Alpert JS, Eagle KA, Gardner TJ, Gregoratos G, Russell RO, Ryan TH, Smith SC., Jr ACC/AHA Guidelines for Ambulatory Electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol. 1999;34(3):912–48. doi: 10.1016/s0735-1097(99)00354-x. [DOI] [PubMed] [Google Scholar]
- 12.Drew BJ, Califf RM, Funk M, Kaufman ES, Krucoff MW, Laks MM, Macfarlane PW, Sommargren C, Swiryn S, Van Hare GF. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721–46. doi: 10.1161/01.CIR.0000145144.56673.59. [DOI] [PubMed] [Google Scholar]
- 13.Firoozabadi R, Gregg RE, Babaeizadeh S. Intelligent use of advanced capabilities of diagnostic ECG algorithms in a monitoring environment. J Electrocardiol. 2017 doi: 10.1016/j.jelectrocard.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Abdelazez M, Quesnel PX, Chan ADC, Yang H. Signal Quality Analysis of Ambulatory Electrocardiograms to Gate False Myocardial Ischemia Alarms. IEEE Trans Biomed Eng. 2017;64(6):1318–1325. doi: 10.1109/TBME.2016.2602283. [DOI] [PubMed] [Google Scholar]
- 15.Pelter MM, Kozik TM, Loranger DL, Carey MG. A research method for detecting transient myocardial ischemia in patients with suspected acute coronary syndrome using continuous ST-segment analysis. J Vis Exp. 2012;(70) doi: 10.3791/50124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eskola MJ, Nikus KC, Voipio-Pulkki LM, Huhtala H, Parviainen T, Lund J, Ilva T, Porela P. Comparative accuracy of manual versus computerized electrocardiographic measurement of J-, ST- and T-wave deviations in patients with acute coronary syndrome. Am J Cardiol. 2005;96(11):1584–8. doi: 10.1016/j.amjcard.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 17.Pelter MM, Adams MG, Drew BJ. Computer versus manual measurement of ST-segment deviation. J Electrocardiol. 1996;29(Suppl):78–82. doi: 10.1016/s0022-0736(96)80024-0. [DOI] [PubMed] [Google Scholar]
- 18.Wang JJ, Title LM, Martin TN, Wagner GS, Warren JW, Horacek BM, Sapp JL. Validation of improved vessel-specific leads (VSLs) for detecting acute myocardial ischemia. J Electrocardiol. 2015;48(6):1032–9. doi: 10.1016/j.jelectrocard.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 19.Shusterman V, Goldberg A, Schindler DM, Fleischmann KE, Lux RL, Drew BJ. Dynamic tracking of ischemia in the surface electrocardiogram. J Electrocardiol. 2007;40(6 Suppl):S179–86. doi: 10.1016/j.jelectrocard.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmo P, Ferreira J, Aguiar C, Ferreira A, Raposo L, Goncalves P, Brito J, Silva A. Does continuous ST-segment monitoring add prognostic information to the TIMI, PURSUIT, and GRACE risk scores? Ann Noninvasive Electrocardiol. 2011;16(3):239–49. doi: 10.1111/j.1542-474X.2011.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan AT, Yan RT, Tan M, Senaratne M, Fitchett DH, Langer A, Goodman SG Investigators I. Long-term prognostic value and therapeutic implications of continuous ST-segment monitoring in acute coronary syndrome. Am Heart J. 2007;153(4):500–6. doi: 10.1016/j.ahj.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD I. Task Force for the Universal Definition of Myocardial. Third universal definition of myocardial infarction. Nat Rev Cardiol. 2012;9(11):620–33. doi: 10.1038/nrcardio.2012.122. [DOI] [PubMed] [Google Scholar]
- 23.Drew BJ, Adams MG, McEldowney DK, Lau KY, Wung SF, Wolfe CL, Ports TA, Chou TM. Frequency, duration, magnitude, and consequences of myocardial ischemia during intracoronary ultrasonography. Am Heart J. 1997;134(3):474–8. doi: 10.1016/s0002-8703(97)70084-x. [DOI] [PubMed] [Google Scholar]