Abstract
Testosterone (T) concentration is a useful indicator of reproductive function in male animals. However, T concentration is not usually measured in veterinary clinics, partly due to the unavailability of reliable and rapid assays for animal samples. In this study, a rapid chemiluminescent enzyme immunoassay system (CLEIA system) that was developed for the measurement of T concentration in humans use was validated for stallion blood samples. First, serum T concentrations were measured using the CLEIA system and compared with those measured by a fluoroimmunoassay that has been validated for use in stallions. The serum T concentrations measured by the two methods were highly correlated (r = 0.9865, n = 56). Second, to validate the use of whole blood as assay samples, T concentrations in whole blood and in the serum were measured by the CLEIA system. T concentrations in both samples were highly correlated (r = 0.9665, n = 64). Finally, to evaluate the practical value of the CLEIA system in clinical settings, T concentrations were measured in three stallions with reproductive abnormalities after the administration of human chorionic gonadotropin (hCG). Two stallions with small or absent testes in the scrotum showed an increase in T production in response to hCG administration and one stallion with seminoma did not. In conclusion, the CLEIA system was found to be a rapid and reliable tool for measuring T concentrations in stallions and may improve reproductive management in clinical settings and in breeding studs.
Keywords: Chemiluminescent enzyme immunoassay, Cryptorchidism, Reproductive abnormalities, Stallions, Testosterone
Testosterone (T), an androgenic hormone, plays an important role in various aspects of male reproduction, including sexual behavior [1], spermatogenesis [2, 3], and the construction of the blood-testis barrier [4]. Thus, T concentrations can be used as an indicator of normal or abnormal reproductive function in male animals. Typically, T response to gonadotropin-releasing hormone (GnRH) or human chorionic gonadotropin (hCG) is used to evaluate fertility in males of many species, including men [5], bulls [6], goat bucks [7], elephant bulls [8] and stallions [9,10,11,12]. While the GnRH challenge test enables the evaluation of pituitary responsiveness in addition to testicular function by measuring luteinizing hormone (LH) or T concentrations, only testicular function can be assessed by the hCG challenge test. In clinical settings, the hCG challenge test seems a more logical choice than the GnRH challenge test for the evaluation of the testicular response in terms of T secretion. The hCG challenge test can be performed in both breeding and non-breeding seasons [13]. In stallions, for example, the hCG challenge test may be used to determine whether a stallion has cryptorchid testes (cryptorchidism) or has been castrated when testes are not found in the scrotum [14, 15].
Treatment with T products is also done on stallions with decreased libido [16]. When T products are used, monitoring of circulating T concentrations is strongly recommended in order to avoid an overdose, which may result in testicular degeneration [16, 17]. However, it usually takes a few days to obtain results for T concentrations since blood samples are sent to a diagnostic laboratory for assays. Therefore, measuring T concentrations may not be an option for most studs and veterinarians in the field.
Some automated benchtop analyzers are available for measuring steroid hormone concentrations in humans. However, there has been no report verifying the use of these systems for stallions. In the present study, one of these systems, a chemiluminescent enzyme immunoassay (CLEIA) system (PATHFAST, LSI Medience, Tokyo, Japan) originally developed for humans [18,19,20] was verified for stallions. This system can measure sex steroid hormones in sera and whole blood within 30 min without extraction using a dedicated cartridge. The cost of using the CLEIA system is equal to or lower than that of the fluoroimmunoassay (FIA). Unlike proteinaceous gonadotropins, structures of steroid hormones are not different between species. However, binding proteins and other factors in sera often interfere in immuno-assay systems; thus, an immuno-assay system developed for one species needs to be verified for other species and, sometimes, for other applications [21]. In the present study, the usefulness of the CLEIA system as a serum T assay in stallions was verified by comparing the T concentrations measured by this system with those measured by FIA, which has been used in stallions [22]. Then, T measurement by the CLEIA system using whole blood as a sample was verified for field use. Finally, the usefulness of the CLEIA system for evaluating testicular function in three typical cases found in the management of stallions was examined.
Materials and Methods
Animals
Eleven stallions (No. 1 to 11) of various breeds in Hokkaido, Japan that were aged from 2 to 26 years were used in the present study (Tables 1, 2, and 3). They were kept in a pasture during the day and were housed individually in stalls at night or they were kept in individual stalls with a paddock. They were fed a maximum of 2 kg of concentrated feed per day with hay and water provided ad libitum.
Table 1. Breed, age, clinical signs, and hCG challenge protocol of stallions in Study 1.
| Stallion No. | Breed | Age | hCG Dosage (IU) | Clinical findings | Date |
| 1 | Warmblood | 2 | 10,000 | No clinical symptom | 2010/9/23 |
| 2 | Warmblood | 2 | 10,000 | No clinical symptom | 2010/9/23 |
| 3 | Warmblood | 2 | 10,000 | No clinical symptom | 2010/10/4 |
| 4 | Halfbred | 3 | 10,000 | No clinical symptom | 2010/10/7 |
Table 2. Breed, age, and T administration protocol of stallions in Study 2.
| Stallion No. | Breed | Age | Dosage per treatment | No. of days for daily treatments | Period of daily blood collection | Date |
| 5 | Halfbred | 7 | 100 mg | 1 | Day 0 to 7 | 2013/1/7 |
| 6 | Warmblood | 4 | 100 mg | 2 | Day 0 to 7 | 2013/1/7 |
| 7-1st | Warmblood | 4 | 250 mg | 1 | Day 0 to 8 | 2013/1/7 |
| 7-2nd a | 250 mg | 4 | Day 0 to 14 | 2013/2/23 | ||
| 8-1st | Halfbred | 6 | 250 mg | 2 | Day 0 to 8 | 2013/1/7 |
| 8-2nd a | 250 mg | 4 | Day 0 to 14 | 2013/2/23 |
a The second T enanthate treatment for both stallions 7 and 8 were started 47 days after the first day of the first treatment.
Table 3. Breed, age, clinical signs and hCG challenge protocol of stallions in Study 3.
| Stallion No. | Breed | Age | hCG Dosage (IU) |
Clinical findings | Date |
| 9 | Thoroughbred | 9 | 5,000 | Small size testes, left: 6.4 × 2.9 × 2.7 (cm), right: 7.6 × 4.1 × 2.7 (cm) a | 2011/1/8 |
| 10 | Haflinger | 26 | 10,000 | Enlarged testes suspected tumor, left: 19.4 × 13.0 × 10.2 (cm), right: 20.8 × 14.0 × 12.0 (cm) a | 2013/1/29 |
| 11 b | Warmblood | 3 | 10,000 | Absence of testes in the scrotal, suspected cryptorchidism or castrated | 2015/9/4 and 2015/11/17 |
a Testicular sizes are indicated as length × height × width (cm). b Stallion 11 was administered 10,000 IU of hCG twice with an interval of two months.
Sample collection
Blood samples were taken from the jugular vein and collected in plain tubes for serum samples and in heparin sodium-loaded tubes for whole blood samples. The plain tubes were centrifuged at 1,700 × g for 10 min to separate the serum, and the serum samples were then stored at –20°C until assay. The assay was performed on the same day as the collection of whole blood. All procedures were carried out in accordance with the guidelines established by the institutional Animal Care and Use Committee of Tokyo University of Agriculture and Technology.
CLEIA System
T concentrations in serum and whole blood were measured by a CLEIA system (PATHFAST, LSI Medience) that was developed and verified for human sera, plasma, and heparinized whole blood. One hundred microliters of either serum or whole blood was applied to a reagent cartridge for the T assay (PF0191-K, LSI Medience) without extraction. The reagent cartridge was set into a CLEIA analyzer to run assays. All reagents were contained in the cartridge, and all of the procedures of the assay were performed inside it. The CLEIA system is based on a one-step competitive immunoassay system method. Using this procedure, samples were first mixed with an alkaline phosphatase (ALP)-labeled antibody and a magnetic latex reagent. Magtration® was then used to remove excess reagents and residual materials not bound to the magnetic latex. A chemiluminescent substrate (CDP-Star®) was added, and luminescence was emitted upon binding to the ALP. Luminescence was then measured and hormonal concentrations were determined. The range of T concentrations measured by CLEIA was between 0.1 and 16.0 ng/ml. Based on the manufacturer’s catalogue, the intra-assay coefficients of variation were determined to be 8.9% at 0.87 ng/ml and 5.6% at 11.60 ng/ml for the serum, and 11.1% at 0.90 ng/ml and 4.2% at 14.60 ng/ml for whole blood.
FIA
Serum T concentrations were measured using a time-resolved FIA kit (DELFIA, PerkinElmer, Walthan, MA, USA) as described previously [23]. Samples were added to a plate coated with an anti-rabbit IgG. A europium-labeled testosterone and testosterone antiserum were then added. After incubation and washing, enhancement solution was added and fluorescence was measured by a time-resolved fluorometer (ARVO X4, PerkinElmer). This system uses serum or plasma without the need for extraction, and the assay takes approximately 3 h. The measurement was performed without extraction. The range of T concentrations measured by FIA was between 0.4 and 50 nmol/l (0.115 and 14.421 ng/ml). Based on the manufacturer’s catalogue, the intra- and inter-assay coefficients of variance were determined to be 6.0% and 12.9% at 1.20 ng/ml, 5.5% and 6.8% at 2.94 ng/ml, and 5.6% and 5.6% at 7.95 ng/ml, respectively.
Experimental design
Study 1: To determine whether the CLEIA system can be used for stallion serum, serum T concentrations measured by the CLEIA system were compared with those measured by FIA. To create a broad range of circulating T concentrations, stallions 1 to 4, which presented no clinical signs, were administered a single dose of 10,000 IU of hCG (GESTORON 5000, Kyoritsu Seiyaku, Tokyo, Japan) intramuscularly (Table 1). Blood samples were taken immediately before hCG administration, and then hourly until 6 h and daily for 7 days afterwards. Serum T concentrations were measured by the CLEIA system and FIA.
Study 2: To validate direct use of whole blood samples for the CLEIA system, T concentrations in whole blood and serum samples were determined using the CLEIA system. The system normalizes T concentrations in whole blood using hematocrit levels. In this study, the hematocrit level was fixed to 40%. Stallions 5 to 8 were administered T enanthate (Enarmon Depot, ASKA Pharmacoutical, Tokyo, Japan) intramuscularly. Doses and protocols for administration as well as blood sampling schedules are shown in Table 2. Stallions 7 and 8 were administered T enanthate twice using different protocols at an interval of 47 days.
Study 3: To evaluate the practical value of the CLEIA system, three stallions with reproductive abnormalities (Table 3) were subjected to hCG challenge tests.
When stallion 9 was introduced to the stud after retiring from racing, both of its testes were found to be smaller than the normal size reported previously [24]. Stallion 10 had bilaterally enlarged testes compared with normally sized testicles [24] and tumors were suspected to be present in the testes. Stallion 11 had no palpable testes in the scrotum and no record of castration when the animal was introduced to our stud. When a mare at estrus came close, stallion 11 mounted and ejaculated as indicated by the observation of tail flagging, but no spermatozoa was observed in the dismount semen. Stallion 9 was administered 5,000 IU of hCG intravenously [11] and stallions 10 and 11 were administered 10,000 IU of hCG [25]. After administration of hCG, serum samples were obtained as in Study 1. Serum T concentrations were measured by the CLEIA system. In stallion 11, hCG challenge tests were performed twice at an interval of two months.
Statistics
In Studies 1 and 2, Pearson’s correlation (r) was calculated using GraphPad Prism 5 for Windows (GraphPad Software, San Diego, CA, USA). Bland-Altman analysis was also performed to assess bias and agreement. In Study 1, Dunnett’s multiple comparison test was performed to compare the T concentrations measured after hCG administration with those measured before administration using GraphPad Prism 5 for Windows.
Results
Study 1
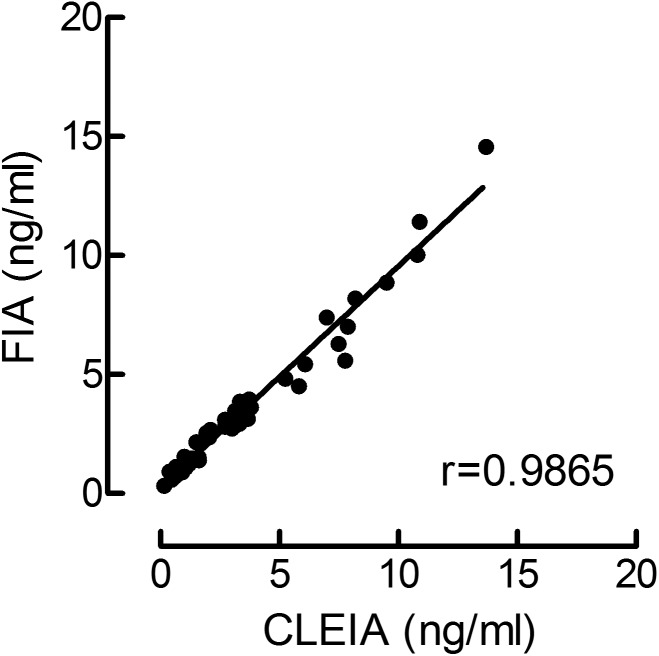
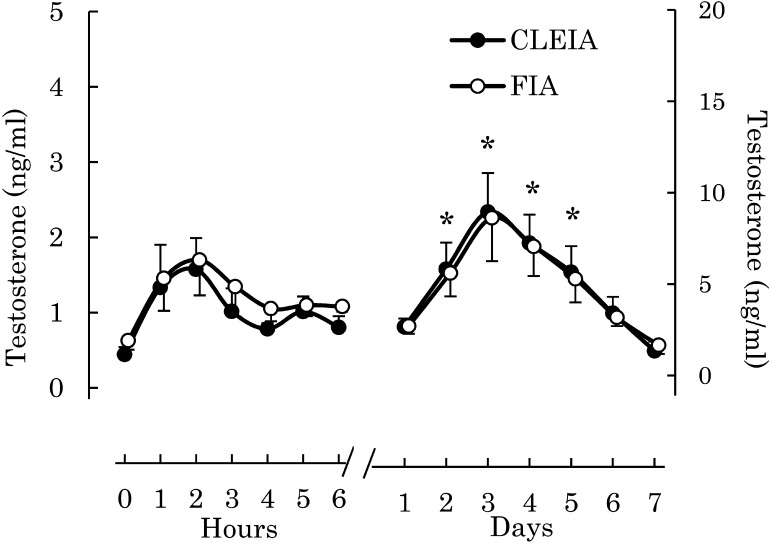
Serum T concentrations measured by the CLEIA system and FIA showed a strong correlation (r = 0.9865, P < 0.01, n = 56, Fig. 1). Values from the two assays also showed good agreement, with a bias of –0.030 ng/ml, with 95% limits of agreement of –1.087~1.026. Changes in serum T concentrations after hCG administration as measured by the CLEIA system and FIA were similar (Fig. 2). Serum T concentrations showed a bimodal peak. The first peak appeared within 3 h after hCG administration, and the second one appeared 3 or 4 days after the administration of hCG. T concentrations between 2 and 5 days after the administration of hCG were significantly higher than those before administration.
Fig. 1.
Correlation of serum testosterone (T) concentrations measured by the chemiluminescent enzyme immunoassay (CLEIA) system and by fluoroimmunoassay (FIA). Stallions 1 to 4 were administered 10,000 IU of human chorionic gonadotrophin (hCG) intravenously, and T concentrations in blood samples (n = 56) were measured by the CLEIA system and FIA. T concentrations measured by the two methods were strongly correlated (r = 0.9865).
Fig. 2.
Comparison of testosterone (T) concentrations measured by the chemiluminescent enzyme immunoassay (CLEIA) system and by fluoroimmunoassay (FIA). Four stallions (1 to 4) that presented no clinical signs were administered 10,000 IU of human chorionic gonadotrophin (hCG). T concentrations in serum samples were measured by the CLEIA system and by FIA. In the figures, T concentrations are shown as averages ± standard error of the mean. The left vertical axis shows hourly data and the right vertical axis shows daily data. “*” indicates that the mean of T concentrations is significantly higher than that before administration.
Study 2
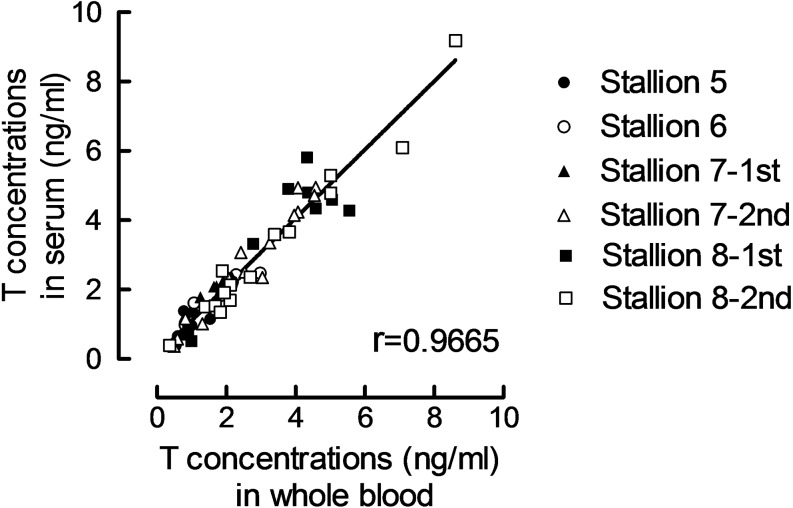
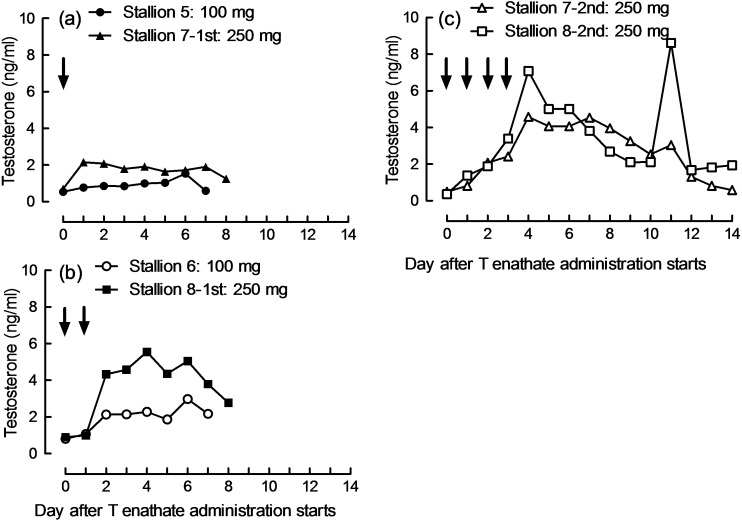
Whole blood T concentrations and serum T concentrations measured by the CLEIA system showed a strong correlation (r = 0.9665, P < 0.01, n = 64, Fig. 3). T concentrations in whole blood and serum samples showed good agreement, with a bias of –0.085 ng/ml and 95% limits of agreement of –0.957~0.787. The changes in T concentrations in whole blood after T product administration for each stallion are shown in Fig. 4. T concentrations increased after single administration of 100 mg or 250 mg, or double administrations of 100 mg T enanthate; however, the increase was insignificant. T concentrations after double or quarterly administrations or T enanthate clearly increased.
Fig. 3.
Correlation of testosterone (T) concentrations in whole blood and serum as measured by the chemiluminescent enzyme immunoassay (CLEIA) system. Stallions 5 to 8 were administered (i. m.) T enanthate (100 or 250 mg) for one to four days (daily). Blood samples (n = 64) were collected before and after T enanthate administration for 5 to 8 consecutive days. See Table 2 for details of the T enanthate treatment protocol in each stallion.
Fig. 4.
Concentrations of testosterone (T) in whole blood after T enanthate administration. Stallions 5 to 8 were administered T enanthate as shown in Table 2. Arrows indicate administration of T enanthate.
Study 3
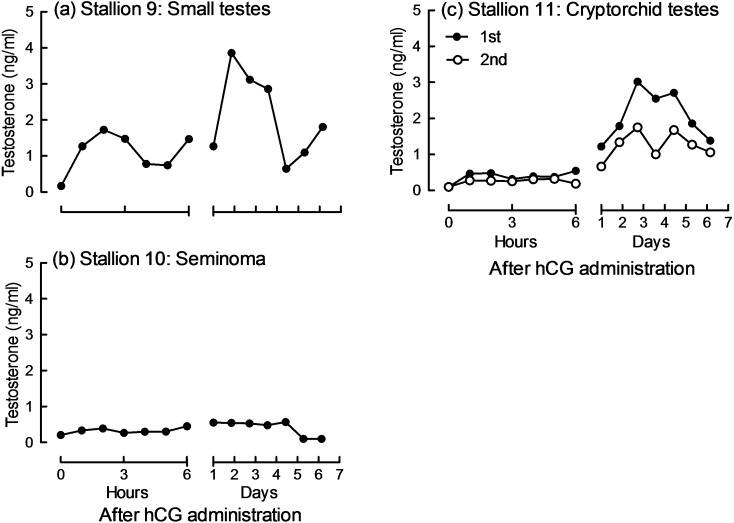
In stallion 9, serum T concentrations showed bimodal changes similar to those in the normal stallions 1 to 4 (Figs. 2 and 5a). Stallion 10 showed no obvious increase in serum T concentration during the seven-day study period after hCG administration (Fig. 5b). In stallion 11, serum T concentration showed no increase for the first 6 h, but increased after 24 h. Serum T concentrations after the second administration tended to be lower than those after the first administration (Fig. 5c).
Fig. 5.
Changes in serum testosterone (T) concentrations (ng/ml) after human chorionic gonadotropin (hCG) administration in stallions with reproductive abnormalities. Stallion 9 (5,000 IU) and stallions 10 and 11 (10,000 IU) were administered hCG to determine T responses to hCG administration. See Table 3 for clinical findings of each animal. Stallion 11 was administered hCG twice with a two-month interval.
Discussion
The present study demonstrated that the CLEIA system can be used to measure T concentrations in stallions. First, serum T concentrations measured by the CLEIA system were compared with those measured by FIA, an established assay system for stallion serum T measurement [22]. T concentrations measured by the CLEIA system were almost the same as those measured by FIA (r = 0.9867). Furthermore, the observed T secretion pattern was very similar to that reported previously [11]. These results, together with the rapidity of the CLEIA system, indicate that the system is a valuable and practical system for measuring T concentrations in stallions as an alternative to FIA. T concentrations can be measured in a much shorter time by the CLEIA system than by FIA (30 min vs. 3 h). Furthermore, the CLEIA system allows us to obtain results with a single step consisting of the application of a sample to a reagent cartridge, while the FIA system requires multiple complex manipulations.
T concentrations in whole blood measured by the CLEIA system were almost the same as those in the serum (r = 0.9665). This system normalizes T concentrations in whole blood by using hematocrit levels. In a previous study conducted in human subjects, T concentrations in whole blood were found to be strongly correlated with those in serum when hematocrit levels were fixed at 40% [18]. In the present study, hematocrit levels were also fixed at 40%, based on that previous study, and T concentrations in whole blood were found to be highly correlated with those in serum in stallions. The rapidity and compatibility of whole blood as samples for the CLEIA system may make the system a useful tool and enable better T therapy for decreased libido in stallions. It is difficult to determine the dose and timing of T administration needed to achieve effective blood T levels and to avoid overdosing. In this study, a single administration of 100 or 250 mg and double administrations of 100 mg of T enanthate did not result in high T concentrations in circulation. Administration of 250 mg T enanthate for more than two days resulted in an increase in T concentrations for several days. Administration of T enanthate is used for stallions with decreased libido; however, it has been shown that the administration of an excess amount of T impairs spermatogenesis and decreases testicular volume in stallions [17]. In our experience, the administration of T enanthate is an option for treatment of low libido. Previously, one stallion in our stud with low libido was able to mount and had an increase in T concentrations after the administration of T enanthate. However, the blood T level that needs to be attained to restore libido is unknown, and an appropriate T administration protocol has not been established [16]. Clearly, further research is needed, and the CLEIA system used in the present study would be a useful tool.
Measurement of T concentrations is often needed in a stud. Popular stallions continue to breed over a lifetime and some of them are over 20 years of age. The risk of reproductive abnormalities, such as testicular degeneration and testicular tumor, increases with aging [26, 27]. When stallions are moved from one farm to another, their clinical records sometimes become uncertain. If a stallion shows an absence of testes in the scrotum, it must be determined whether the stallion was castrated or has cryptorchid testes. In a Thoroughbred stud, some stallions would have been retired from racing at the end of the year, and the period between retirement and the start of breeding would be only two to three months. Stallions are trained to mate with mares during this short period. Some stallions may take a while to start mounting mares and may have low libido. Although the frequency may be low, some new young stallions retiring from racing have small testes compared with the testes of stallions of an equivalent age. In such cases, veterinarians in the stud must evaluate the testicular function of these stallions. Measurement of T concentration with or without hCG challenge may be useful for distinguishing cases of cryptorchidism and castration. An hCG challenge is also useful for distinguishing between infertile and normal stallions. In this study, three stallions with suspected reproductive abnormalities were subjected to the hCG challenge test, and the changes in T concentrations in response to the challenge were determined using the CLEIA system.
Stallion 9, which had small testes, showed a T response to hCG that was similar to the response found in normal stallions in Study 1 (Fig. 2). This suggested that stallion 9 had normal reproductive ability. The conception rate of stallion 9 in the first breeding season was more than 70%, indicating that its testes were small because of individual differences, and that spermatogenesis in stallion 9 was normal. In stallion 10, which had enlarged testes, T concentrations did not increase after hCG administration, indicating the possibility that Leydig cells had lost their steroidogenic capacity. The stallion died one year and four months after the hCG challenge test. The testes were examined histologically and a diagnosis of seminoma, which generally does not affect T production [28] was made. The testicular sizes of stallion 10 were larger upon death (left: 24.0 × 17.0 × 11.0 cm, right: 26.0 × 14.5 × 9.0 cm) than when the hCG challenge test was performed. It is not clear whether seminoma influenced the ability of T secretion. In stallion 11, an observed increase in T concentrations after hCG administration indicated the presence of testes [14, 15]; thus, stallion 11 was diagnosed as having cryptorchid testes and was determined to not have been castrated. Stallion 11 was castrated using laparoscopy to prevent cryptorchidism from developing into tumors [29, 30]. Both testes were found in the abdominal cavity. T concentrations after the second administration tended to be lower than those after the first administration. It is unlikely that antibodies against hCG were produced after a single administration of hCG [31]. It was not clear why T concentrations after the second experiment were lower than those after the first experiment in this study. In these clinical cases, T measurement by the CLEIA system was useful for the evaluation of testicular function and for the assessment of the presence of normal testicular tissue.
The minimum detection level of the CLEIA system is 0.1 ng/ml. Thus, the system may not be sensitive enough to diagnose inactivity of testicular function since basal T concentrations in stallions at rest are as low as 300 and 402 pg/ml in the non-breeding and breeding seasons, respectively [32]. Therefore, a sample may need to be concentrated to ten times, for example, to determine the actual blood concentration. However, this may jeopardize the rapidity and simplicity of the system.
In conclusion, it was shown that the CLEIA system can be used to measure T concentrations in both serum and whole blood of stallions. Using the CLEIA system, it is possible to measure T concentrations rapidly and easily. The system is useful for the diagnosis of reproductive abnormalities by an hCG or GnRH challenge test, and for the treatment of stallions with low libido by administration of a T product in Thoroughbreds.
References
- 1.Mcdonnell SM. Stallion behavior and endocrinology: what do we really know? In: AAEP; 1995; Kentucky, USA. 41: 18−19.
- 2.Dohle GR, Smit M, Weber RFA. Androgens and male fertility. World J Urol 2003; 21: 341–345. [DOI] [PubMed] [Google Scholar]
- 3.Holdcraft RW, Braun RE. Hormonal regulation of spermatogenesis. Int J Androl 2004; 27: 335–342. [DOI] [PubMed] [Google Scholar]
- 4.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci USA 2005; 102: 16696–16700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazici M, Sahin M, Bolu E, Uckaya G, Gok DE, Taslipinar A, Ozgurtas T, Kutlu M. Prediction of testosterone response to human chorionic gonadotrophin in idiopathic hypogonadotropic hypogonadism patients. J Natl Med Assoc 2009; 101: 71–76. [DOI] [PubMed] [Google Scholar]
- 6.Devkota B, Takahashi K, Matsuzaki S, Matsui M, Miyamoto A, Yamagishi N, Osawa T, Hashizume T, Izaike Y, Miyake Y. Basal levels and GnRH-induced responses of peripheral testosterone and estrogen in Holstein bulls with poor semen quality. J Reprod Dev 2011; 57: 373–378. [DOI] [PubMed] [Google Scholar]
- 7.Samir H, Sasaki K, Ahmed E, Karen A, Nagaoka K, El Sayed M, Taya K, Watanabe G. Effect of a single injection of gonadotropin-releasing hormone (GnRH) and human chorionic gonadotropin (hCG) on testicular blood flow measured by color doppler ultrasonography in male Shiba goats. J Vet Med Sci 2015; 77: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Somgird C, Sripiboon S, Mahasawangkul S, Boonprasert K, Brown JL, Stout TA, Colenbrander B, Thitaram C. Differential testosterone response to GnRH-induced LH release before and after musth in adult Asian elephant (Elephas maximus) bulls. Theriogenology 2016; 85: 1225–1232. [DOI] [PubMed] [Google Scholar]
- 9.Roser JF, Hughes JP. Seasonal effects on seminal quality, plasma hormone concentrations, and GnRH-induced LH response in fertile and subfertile stallions. J Androl 1992; 13: 214–223. [PubMed] [Google Scholar]
- 10.Roser JF, Hughes JP. Dose-response effects of gonadotropin-releasing hormone on plasma concentrations of gonadotropins and testosterone in fertile and subfertile stallions. J Androl 1992; 13: 543–550. [PubMed] [Google Scholar]
- 11.Bollwein H, Schulze JJ, Miyamoto A, Sieme H. Testicular blood flow and plasma concentrations of testosterone and total estrogen in the stallion after the administration of human chorionic gonadotropin. J Reprod Dev 2008; 54: 335–339. [DOI] [PubMed] [Google Scholar]
- 12.Parlevliet JM, Bevers MM, van de Broek J, Colenbrander B. Effect of GnRH and hCG administration on plasma LH and testosterone concentrations in normal stallions, aged stallions and stallions with lack of libido. Vet Q 2001; 23: 84–87. [DOI] [PubMed] [Google Scholar]
- 13.Roser JF. Diagnostics and therapeutics for stallions with declining fertility: an endocrine-paracrine-autocrine appproach. In: McKinnon AO, Squires, EL, Vaala WE, Varner DD (eds.), Equine Reproduction Second Edition. West Sussex: Wiley-Blackwell; 2011: 1436−1447.
- 14.Arighi M, Bosu WTK. Comparison of hormonal methods for diagnosis of cryptorchidism in horses. J Equine Vet Sci 1989; 9: 20–26. [Google Scholar]
- 15.Raś A, Rapacz A, Raś-Noryńska M, Janowski TE. Clinical, hormonal and ultrasonograph approaches to diagnosing cryptorchidism in horses. Pol J Vet Sci 2010; 13: 473–477. [PubMed] [Google Scholar]
- 16.McDonnell SM. Ejaculation. Physiology and dysfunction. Vet Clin North Am Equine Pract 1992; 8: 57–70. [DOI] [PubMed] [Google Scholar]
- 17.Berndtson WE, Hoyer JH, Squires EL, Pickett BW. Influence of exogenous testosterone on sperm production, seminal quality and libido of stallions. J Reprod Fertil Suppl 1979; 27: 19–23. [PubMed] [Google Scholar]
- 18.Chuko M, Otsuki J, Nagai Y. Rapid whole blood assay on PATHFAST for quantification of gonadal hormones and the clinical evaluation in assisted reproductive technology. Jpn J Med Pharm Sci 2010; 63: 111–119. [Google Scholar]
- 19.Maeda K, Otsuki J, Terai K, Nagai Y. Rapid whole blood assay on PATHFAST for quantification of testosterone and the clinical evaluation in assisted reproductive technology. Jpn J Med Pharm Sci 2011; 66: 693–700. [Google Scholar]
- 20.Sugie Y, Igami K, Shoji K, Arai N, Tazaki Y, Kouta H, Okamura Y, Tashiro S, Yokoi H. Performance evaluation of the new rapid fertility assays in whole blood and plasma on PATHFAST. Clin Lab 2011; 57: 99–106. [PubMed] [Google Scholar]
- 21.Davies C. Principles of competitive and immunometric assays (Including ELISA). In: Wild D (ed.), The Immunoassay Handbook (Fourth Edition) —Theory and Applications of Ligand Binding, ELISA and Related Techniques. Oxford: Elsevier; 2013: 29−89.
- 22.Kunii H, Nambo Y, Okano A, Matsui A, Ishimaru M, Asai Y, Sato F, Fujii K, Nagaoka K, Watanabe G, Taya K. Effects of an extended photoperiod on gonadal function and condition of hair coats in Thoroughbred colts and fillies. J Equine Sci 2015; 26: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliott CT, Francis KS, Shortt HD, McCaughey WJ. Determination of the concentrations of the steroids estradiol, progesterone and testosterone in bovine sera: comparison of commercial dissociation enhanced lanthanide fluorescence immunoassay kits with conventional radio and enzyme immunoassays. Analyst (Lond) 1995; 120: 1827–1830. [DOI] [PubMed] [Google Scholar]
- 24.Thompson DL, Jr, Pickett BW, Squires EL, Amann RP. Testicular measurements and reproductive characteristics in stallions. J Reprod Fertil Suppl 1979; 27: 13–17. [PubMed] [Google Scholar]
- 25.Silberzahn P, Zwain I, Guerin P, Benoit E, Jouany JM, Bonnaire Y. Testosterone response to human chorionic gonadotropin injection in the stallion. Equine Vet J 1988; 20: 61–63. [DOI] [PubMed] [Google Scholar]
- 26.Stewart BL, Roser JF. Effects of age, season, and fertility status on plasma and intratesticular immunoreactive (IR) inhibin concentrations in stallions. Domest Anim Endocrinol 1998; 15: 129–139. [DOI] [PubMed] [Google Scholar]
- 27.Turner RM, Zeng W. The emerging pathophysiology of age-related testicular degeneration with a focus on the stallion and an update on potential therapies. Reprod Domest Anim 2012; 47(Suppl 4): 178–186. [DOI] [PubMed] [Google Scholar]
- 28.Fung LC, Honey RJ, Gardiner GW. Testicular seminoma presenting with features of androgen excess. Urology 1994; 44: 927–929. [DOI] [PubMed] [Google Scholar]
- 29.Stick JA. Teratoma and cyst formation of the equine cryptorchid testicle. J Am Vet Med Assoc 1980; 176: 211–214. [PubMed] [Google Scholar]
- 30.De Lange V, Chiers K, Lefère L, Cools M, Ververs C, Govaere J. Malignant seminoma in two unilaterally cryptorchid stallions. Reprod Domest Anim 2015; 50: 510–513. [DOI] [PubMed] [Google Scholar]
- 31.Roser JF, Kiefer BL, Evans JW, Neely DP, Pacheco DA. The development of antibodies to human chorionic gonadotrophin following its repeated injection in the cyclic mare. J Reprod Fertil Suppl 1979; 27: 173–179. [PubMed] [Google Scholar]
- 32.Johnson L, Thompson DL., Jr.Effect of seasonal changes in Leydig cell number on the volume of smooth endoplasmic reticulum in Leydig cells and intratesticular testosterone content in stallions. J Reprod Fertil 1987; 81: 227–232. [DOI] [PubMed] [Google Scholar]