Abstract
This retrospective comparative study aims to explore the time courses of serum myoglobin (Mb) changes, and summarize our experience in treating patients with hypermyoglobinemia after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC).
This study covered 60 patients with peritoneal carcinomatosis treated with CRS + HIPEC as the study group, and another 25 cancer patients treated with conventional extensive surgery without HIPEC as the control group from February to October 2016. In the study group, patients with postoperative hypermyoglobinemia were on a comprehensive treatment regimen consisting intravenous injection of sodium bicarbonate solution according to the Mb level. In the control group, patients were recorded and treated with the same regimen except for special sodium bicarbonate solution. The preoperative and postoperative serum Mb, blood urine nitrogen (BUN), and creatinine (Cr) levels were evaluated.
There were no significantly difference between the 2 groups in serum Mb, BUN, and Cr levels before surgery. Postoperative serum Mb levels were elevated in both groups and significantly higher on postoperative 0 to 2 days (P < .05) in the study group than the control group. The peak value of serum Mb levels (426.65 ± 108.386 μg/L) occurred on the surgery day. The serum Mb change rate was much bigger in the study group than the control group. Serum BUN levels in both groups revealed a slow increase during the early postoperative period and were significantly lower in the study group than the control group on days 1 and 2. The serum Cr levels were similar and stable between the 2 groups after surgery. The serum Cr change rates changed synchronously with same tendency in both groups, and on postoperative day 1 the increase rate was bigger in the control group than the study group.
Hypermyoglobinemia is a common and prominent lab abnormality after CRS + HIPEC, and serum Mb levels could be an early and sensitive indicator for dramatic disturbances in the internal milieu after CRS + HIPEC. Adequate treatment with sodium bicarbonate could accelerate the reduction in serum Mb levels and reduce the risk for major organ damages.
Keywords: cytoreductive surgery, hypermyoglobinemia, hyperthermic intraperitoneal chemotherapy
1. Introduction
Cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) is a novel therapy for peritoneal carcinomatosis (PC) that have been well developed over the past 3 decades. This comprehensive treatment modality takes the advantage of extensive surgery to remove the bulky tumor-involved viscerals and peritoneum, and the hyperthermic chemotherapy to eradicate the microscopic residual tumor nodules and free cancer cells.[1] CRS + HIPEC has been established as the standard care for selected patients with pseudomyxoma peritonei,[2] malignant peritoneal mesothelioma, and peritoneal carcinoma from the colorectal cancer.[3] In addition, CRS + HIPEC has also gained increasing clinical evidence to bring significant survival benefits for PC patients from epithelial ovarian carcinoma,[4,5] primary peritoneal carcinoma,[6] and gastric cancer.[7] CRS + HIPEC has been established and promoted in many cancer centers in Europe, America, and Asia-Pacific regions.[1]
Myoglobin (Mb) is a heterodimer composed of a peptide chain and a heme group. The molecular weight is approximately 17.9 kDa.[8] Mb is synthesized and stored by skeletal muscle cells and cardiomyocytes, in other cells in the body it is considered to be nonexistent. Therefore, direct damages to either skeletal muscles or myocardial muscles could release Mb into the circulation. Clinically, serum Mb levels detection is mainly used to diagnose myocardial and skeletal muscle cells injury. Hypermyoglobinemia is mainly seen in traumatic or nontraumatic rhabdomyolysis syndrome, with many potential severe consequences such as acute kidney injury (AKI). The peak creatinine (Cr) values are significantly higher with higher Mb, incidence of Mb-mediated AKI (Mb-AKI) increasing significantly in parallel with Mb in rhabdomyolysis syndrome. Blood Mb level could serve as a valuable early predictor and marker of rhabdomyolysis and Mb-AKI.[9]
For cancer patients with extensive surgery, drastic release of Mb after surgery could cause hypermyoglobinemia and related problems.[10] Hypermyoglobinemia could be found in many cancer patients from the lungs, colon, and rectum, and continued for 3 to 4 days postoperation.[11] Moreover, increased Mb levels may aggravate the injury caused by oxidative stress.[8] In addition, extensive surgery itself also enhances lipid peroxidation and cause cellular injury to promote AKI occur.[10]
As an established cancer center for PC treatment, we have applied CRS + HIPEC as a standardized procedure for PC patients from gastrointestinal[12–14] and gynecological malignancies.[1,5] For such patients, hypermyoglobinemia is an important consideration in the postoperative care period. In this retrospective study, we compared the serum Mb levels after conventional extensive surgery versus CRS + HIPEC, explored the time courses of serum Mb changes, and summarized our experience in treating patients with hypermyoglobinemia after CRS + HIPEC.
2. Patients and methods
2.1. Patient selection
From February to October 2016, 60 PC patients treated by CRS + HIPEC at the Department of Peritoneal Cancer Surgery, Beijing Shijitan Hospital of Capital Medical University, Beijing, China, were enrolled into this study. These patients were pathologically diagnosed as PC, and treated with CRS + HIPEC. They were designated as the study group in this work. The preoperative evaluations, major inclusion criteria, and exclusion criteria were reported previously.[1] Written informed consents were obtained from all patients and the study was approved by the institutional review board and the ethics committee.
In the same time period, we also recorded the serum Mb levels of the control group of 25 cancer patients treated with conventional extensive surgery without HIPEC at our institution. Detailed clinicopathological information of these patients was also summarized.
2.2. CRS + HIPEC procedure
All CRS + HIPEC procedures were conducted as described before by a designed team of surgical oncologists focusing on PC treatment. Briefly, after abdominal exploration, peritoneal cancer index (PCI) was evaluated according to the standardized rating and recording system.[15] Then maximal CRS was performed, including the curative or palliative resection of the primary tumor with acceptable margins, any involved adjacent structures, lymphadenectomy, peritonectomies where peritoneal surfaces were involved by tumor, according to the peritonectomy procedure developed by Sugarbaker.[16] The completeness of cytoreduction (CC) was evaluated with standardized CC scoring system.[17] And after which the HIPEC was implemented by the open coliseum technique with each drug dissolved into 3 L of heated saline with temperature 43 ± 0.5°C, the duration of HIPEC for each drug was 30 min with a flow rate of 400 mL/min. The drugs for HIPEC were docetaxel 120 mg and cisplatin 120 mg for PC patients from gynecological malignancies, mitomycin 30 mg and cisplatin 120 mg for PC patients from gastrointestinal malignancies, docetaxel 120 mg and mitomycin 30 mg for PC patients with high risk for renal damage, or only docetaxel 120 mg for PC patients with only 1 kidney or with lab results suggesting existing renal damage.
2.3. Perioperative care after CRS + HIPEC
Immediately after CRS + HIPEC, the patients were on a comprehensive treatment regimen consisting of hemodynamic stabilizing therapies, nutrition support, antisepsis therapies, and psychophysical therapies. All the vital signs were carefully monitored and the serum levels of key electrolytes and metabolites were daily checked.
2.4. Special treatment for hypermyoglobinemia
Treatment of hypermyoglobinemia was initiated at the operating room immediately after HIPEC, and continued until 3 to 4 days postoperation, depending on daily lab reports on serum Mb levels and renal functions. At the operating room, the patient received intravenous injection of sodium bicarbonate solution 50 to 100 mL once. On days 1 to 4 after surgery, sodium bicarbonate solution 50 to 100 mL was intravenously infused once every 12 h, along with intermittent administering diuretics daily. The daily urine output was adjusted to about 1000 to 2000 mL.
2.5. Parameters studied for efficacy evaluation
The preoperative examinations included blood tests of serum biochemistry, Mb levels, and liver and renal functions. After CRS + HIPEC, routine daily monitoring of serum Mb levels, renal function and urine casts were done at surgery day and 1 to 4 days after surgery. The clinical courses of the patients were closely observed and recorded into the standardize patient files. For the purpose of this study, particular attention was paid to warning symptoms and signs such as low back pain, oliguria, and abnormal urine color.
As hypermyoglobinemia could also be caused by myocardium damage, we also monitored the myocardial parameters, including troponin-I (TnI) and electrocardiogram (ECG).
2.6. Statistical analysis
All the clinicopathological data were systematically collected to construct a comprehensive database. The clinical information, surgical reports, laboratory examination, and pathology reports were analyzed using Statistical Package for Social Science (SPSS, IBM, Armonk, NY). Continuous data were expressed as median and range or mean ± standard error, and category data were presented as number and percentage.
3. Results
3.1. Major clinicopathological characteristics of the patients in both groups
Detailed clinicopathological features of the patients were listed in Table 1. In the study group, there were 60 patients treated with 60 CRS + HIPEC procedures. In the control group, there were 25 patients treated with conventional extensive surgery. Both groups were comparable in most variables. The study group had more patients with massive ascites (>1000 mL) than the control group (31.7% vs. 0, P = .001). The study group also had more blood loss (600 mL vs. 200 mL, P = .004) and more plasma transfusion (800 mL vs. 200 mL, P < .001) than the control group during the operation. Therefore, the study group had more advanced clinical presentation and more complex surgical treatments.
Table 1.
Major clinicopathological characteristics of the patients.

3.2. Comparison in serum Mb levels between the 2 groups
The mean serum Mb levels in the control group versus the study group were 22.89 ± 2.318 μg/L versus 17.99 ± 0.882 μg/L (P = .058) before surgery, 98.08 ± 12.601 μg/L versus 426.65 ± 108.386 μg/L (P = .005) on postoperative day 0, 121.19 ± 21.484 μg/L versus 344.51 ± 54.002 μg/L (P = .0003) on postoperative day 1, 81.20 ± 13.111 μg/L versus 161.42 ± 21.549 μg/L (P = .014) on postoperative day 2, 35.59 ± 6.784 μg/L versus 62.71 ± 10.189 μg/L (P = 0.114) on postoperative day 3, and 37.28 ± 7.773 μg/L versus 38.13 ± 5.870 μg/L (P = .930) on postoperative day 4 (Fig. 1).
Figure 1.
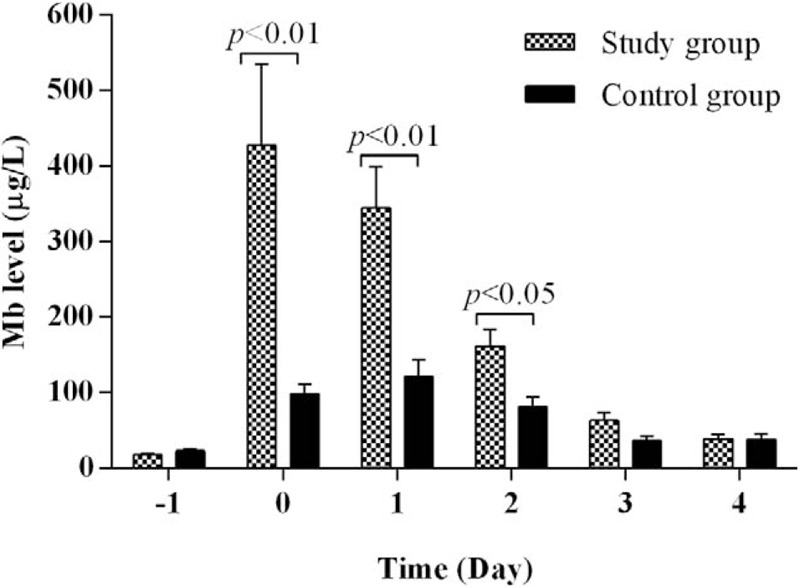
Comparison in perioperative serum Mb levels between the study group and the control group. Serum Mb levels were similar between the 2 groups before surgery (day −1). Immediately after surgery, the study group had significantly higher serum Mb levels than the control groups for 3 days (days 0, 1, and 2). On postoperative days 3 and 4, there were no significant differences in serum Mb levels between the 2 groups.
3.3. Changes in serum Mb levels during the treatment course
Figure 2A shows the differences in the time course of serum Mb changes between the 2 groups. In the study group, there was a sharp and statistically significant increase in serum Mb levels immediately after CRS + HIPEC (day 0). From postoperative days 1 to 4, there was a continuous decrease in serum Mb levels. In the control group, however, there was a slight and gradual increase in serum Mb levels on the day of operation and postoperative day 1. From postoperative days 2 to 4, the serum Mb levels gradually decreased.
Figure 2.
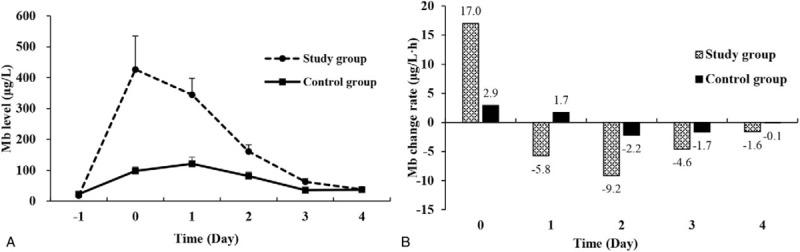
Serum Mb level changes in the study group and the control group. (A) The serum Mb curves showed that the study group had much bigger increases and decreases than the control group. (B) In terms of serum Mb change rates, at each time point, the serum Mb change rates were much bigger in the study group than the control group.
In terms of change rates of serum Mb levels, the differences between the 2 groups become more evident (Fig. 2B). The serum Mb change rates in study group versus control group were 17.0 μg/L h versus 2.9 μg/L h on day 0, −5.8 μg/L h versus 1.7 μg/L h on day 1, −9.2 μg/L h versus −2.2 μg/L h on day 2, −4.6 μg/L h versus −1.7 μg/L h on day 3, and −1.6 μg/L h versus −0.1 μg/L h on day 4 after operation. At each time point, the serum Mb level change rates were bigger in the study group than the control group.
3.4. Comparison in serum BUN levels between the 2 groups
The mean serum blood urine nitrogen (BUN) levels in the control group versus the study group were 5.27 ± 0.573 mmol/L versus 4.71 ± 0.216 mmol/L (P = .265) before surgery, 4.74 ± 0.730 mmol/L versus 3.63 ± 0.170 mmol/L (P = .157) on postoperative day 0, 5.38 ± 0.628 mmol/L versus 4.01 ± 0.164 mmol/L (P = .046) on postoperative day 1, 7.06 ± 0.564 mmol/L versus 5.64 ± 0.251 mmol/L (P = .028) on postoperative day 2, 8.03 ± 0.496 mmol/L versus 7.24 ± 0.311 mmol/L (P = .184) on postoperative day 3, and 7.94 ± 0.544 mmol/L versus 7.96 ± 0.430 mmol/L (P = .970) on postoperative day 4 (Fig. 3).
Figure 3.
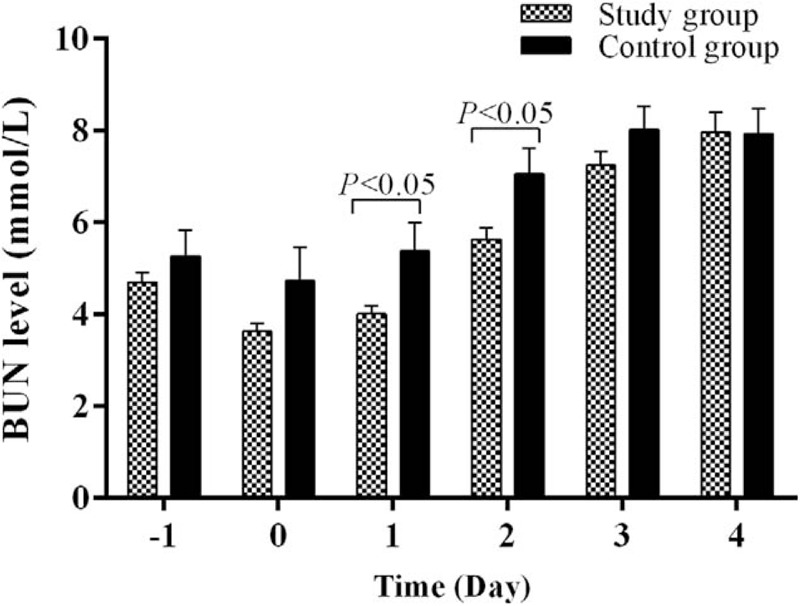
Comparison in perioperative serum BUN levels between the study group and the control group. Serum BUN levels were similar between the 2 groups before surgery (day −1) and surgery day (day 0). After surgery, the study group had significantly lower serum BUN levels than the control groups for 2 days (days 1 and 2). On postoperative days 3 and 4, there were no significant differences in serum BUN levels between the 2 groups. BUN = blood urine nitrogen.
3.5. Changes in serum BUN levels during the treatment course
Figure 4 shows the time course of serum BUN changes in the early postoperative period. On the day of operation (day 0), both groups had slight decrease in serum BUN levels (Fig. 4A), but the decrease was more prominent in the study group than the control group in terms of BUN change rate (Fig. 4B). After operation, both groups had gradual and continuous increases in serum BUN levels (Fig. 4A). On postoperative days 1 and 2, the increase rates were bigger in the control group than the study group (Fig. 4B). On postoperative days 3 and 4, the study group had a bigger increase then the control groups, but the differences were statistically not significant.
Figure 4.
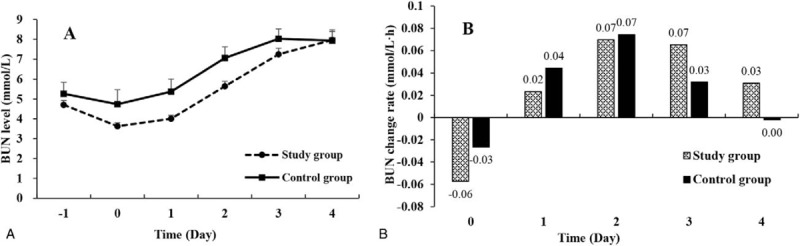
Serum BUN level changes in the study group and the control group. (A) The serum BUN curves showed that both groups had a slow increase during the early postoperative period. (B) In terms of serum BUN change rates, at each time point, the serum BUN change rates increased synchronously but decreased slowly in the study group than the control group after surgery. BUN = blood urine nitrogen.
3.6. Comparison in serum Cr levels between the 2 groups
The mean serum Cr levels in the control group versus study group were 58.56 ± 5.058 μmol/L versus 57.47 ± 2.317 μmol/L (P = .822) before surgery, 58.47 ± 7.536 μmol/L versus 52.07 ± 2.588 μmol/L (P = .431) on postoperative day 0, 64.33 ± 6.587 μmol/L versus 58.15 ± 2.286 μmol/L (P = .383) on postoperative day 1, 64.68 ± 6.936 μmol/L versus 60.56 ± 2.756 μmol/L (P = .585) on postoperative day 2, 63.50 ± 7.082 μmol/L versus 56.47 ± 2.280 μmol/L (P = .354) on postoperative day 3, and 57.70 ± 5.701 μmol/L versus 55.28 ± 3.011 μmol/L (P = .684) on postoperative day 4 (Fig. 5).
Figure 5.
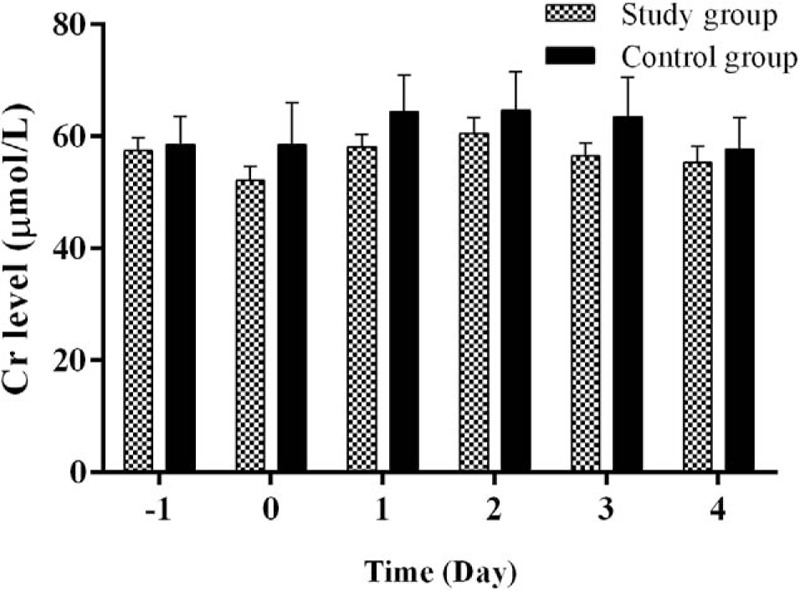
Comparison in perioperative serum Cr levels between the study group and the control group. Serum Cr levels were similar between the 2 groups before surgery (day −1) and postoperation days (days 0–4).
3.7. Changes in serum Cr levels during the treatment course
Figure 6 shows the time course of serum Cr changes during the perioperative period. Both groups had similar and stable Cr levels (Fig. 6A). On the day of operation (day 0), the study group had bigger decrease rate than the control group in terms of serum Cr change rate (Fig. 6B). On postoperative day 1, the increase rate was bigger in the control group than the study group.
Figure 6.
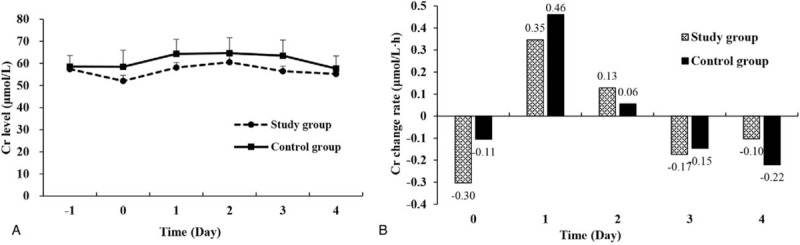
Serum Cr level changes in the study group and the control group. (A) The serum Cr curves showed that both groups had a similar and stable serum Cr levels during the perioperative period. (B) In terms of serum Cr change rates, at each time point, the serum Cr change rates changed synchronously with same tendency in both groups.
3.8. Clinical outcomes in terms of hypermyoglobinemia-related symptoms
All 60 PC patients with CRS + HIPEC had no symptoms of low back pain. The median urine output on 1 to 4 days after surgery was 1300 (400–4000) mL, 1900 (700–5000) mL, 2100 (850–4500) mL, and 2300 (800–4000) mL. All patients had normal urine color. Except for 1 patient developing AKI, all other patients recovered from CRS + HIPEC.
In terms of myocardial function, all patients had normal ECG. However, 10 patients had increased TnI levels, ranging from 0.05 to 2.69 ng/mL. As increased TnI indicates myocardium ischemia, these patients were treated with isosorbide dinitrate, to improve the blood supply to the myocardium. The serum TnI levels returned to normal within 12 days after the treatment. There were no patients with symptomatic heart dysfunction.
AKI developed in 1 patient. This 69-year-old female patient had PC from serous papillary adenocarcinoma of the fallopian tube. She was treated by CRS + HIPEC with cisplatin 120 mg and mitomycin C 30 mg. The patient developed hypermyoglobinemia with serum peak Mb level of 77.7 μg/L. She progressed to renal failure and treated with hemodialysis. However, uncontrolled serious sepsis occurred and the patient died at 26 days after surgery.
In control group, all 25 patients had uneventful clinical course during the postoperative period, and the patients recovered well after operation.
3.9. A typical case presentation
There was a typical case with significantly elevated serum Mb level after CRS + HIPEC. This 54-year-old female patient had pathological diagnosis of PC from malignant peritoneal mesothelioma. Before she was referred to cancer center, the patient suffered from progressive abdominal pain and distension for 6 months, with clinically suspected diagnosis of peritoneal tuberculosis. She was on a 5-month tentative antituberculosis therapy, without any efficacy. The clinical symptoms deteriorated and the patient developed a bulging belly. She received ultrasound-guided omentum biopsy, with pathological diagnosis of malignant peritoneal mesothelioma. Thereafter, the patient was referred to our center for further treatment.
During the 950-min surgery, maximal CRS were performed with extensive peritonectomies, and multivisceral resections including the left colon, right colon, rectum, and uterine appendages. The serum Mb level on surgery day was 3343.8 μg/L (50.8 times the upper limit of the normal value). Sodium bicarbonate 50 mL was immediately infused intravenously. On postoperative day 1, the serum Mb level decreased to 1988.7 μg/L, a decline by 40.5%. Intravenous infusion of sodium bicarbonate 50 mL twice daily and furosemide 10 mg were continued for 2 days. On day 2 the serum Mb level reduced to 507.8 μg/L, a reduction by 74.5%. On postoperative days 3 and 4, it was reduced to 208.9 and 116.3 μg/L. During the 4 days after surgery, the serum Cr level (51–59 μmol/L) was all within normal range, but BUN level (4.97–11.05 mmol/L) slightly increased on days 3 and 4, and there were no clinical symptoms. The patient recovered well and was discharged 15 days after surgery.
4. Discussion
This study has found that in study group, serum Mb levels after CRS + HIPEC are significantly elevated and the peak value occurs on the surgery day. The peak value could be as high as 4 to 6 times normal upper limit. Serum Mb remains at high levels for 2 days. With prompt treatment with intravenous infusion of sodium bicarbonate solution, usually on the 2nd postoperative day, serum Mb levels begin to decline markedly, and decreases to normal range in about 3 to 4 days. It is different from serum Mb, serum BUN, and Cr levels decrease on the surgery day first, and then serum BUN increases parallel with serum Mb after surgery with a slow increasing tendency, serum Cr levels increases (days 1 and 2) and decreases (days 3 and 4) in a narrow range.
Compared to those cancer patients treated with conventional extensive surgery, patients treated by CRS + HIPEC had significantly higher serum Mb levels after surgery. This result could be due to the more extensive resections and additional damages from hyperthermic and cytotoxicity of HIPEC. After CRS + HIPEC, more Mb is released into circulation to cause hypermyoglobinemia, rendering high risk of organic damages in particular kidney injury. All these findings suggest that serum Mb could be an earlier and more sensitive indicator superior to BUN and Cr in patients treated with CRS + HIPEC.
Increased serum Mb levels could lead to major damages via different mechanisms. The key mechanism of Mb-related damages centers on reactive oxygen species (ROS), and the key condition promoting such damages is the acid pH of the internal milieu. Mb is a heme protein-containing ferrous oxide (Fe2+), responsible for oxygen transportation in skeletal muscles. In normal conditions, when the intracellular Mb meets molecular oxygen, the ferrous oxide (Fe2+) is oxidized to ferric oxide (Fe3+), generating hydroxyl radicals, which are effectively eliminated by intracellular antioxidants. No damages could occur due to this balancing mechanism. However, when Mb is released into the blood circulation in abnormal conditions, such as major surgery and injury, similar chemical reactions produce uncontrolled leakage of ROS and free radicles, leading to a variety of damages to major organ systems.[18]
One of the most severe damages is the Mb-AKI. Several mechanisms account for AKI. First, the Mb-mediated peroxidation stress reaction increases vasoconstriction factors and decreases vasodilation factor, directly reducing blood perfusion to the kidneys to cause a series of ischemia-related kidney damages. Second, the circulating Mb becomes concentrated along the renal tubules, a process that is enhanced by volume depletion and renal vasoconstriction, and it precipitates when interacting with the Tamm–Horsfall protein, a process accelerated by acidic urine. This Tamm–Horsfall protein–Mb complex causes direct tubule cytotoxicity to the proximal tubules and obstruction to the distal tubules, leading to tubules necrosis and cast formation.[18] Recent study shows that Mb induced apoptosis in tubular epithelia cells by oxidative stress pathway, in vitro addition of Mb can cause significant apoptosis of renal tubular epithelial cells in animal experiment.[19]
It has been observed that major surgeries could lead to significantly increased serum Mb, enhancing the risks for adverse events such as AKI. In cancer patients from the lungs, the colon and the rectum, hypermyoglobinemia after surgery was correlated with more severe and longer postoperative complications such as peritonitis, pneumonia, and sepsis.[11] Moreover, in patients with sepsis, increased serum Mb levels is associated with in-hospital mortality.[8]
In our series of 60 PC patients undergoing CRS + HIPEC, we also found such increases in serum Mb. But in the patient with renal failure after surgery, Mb level peak value was just 77.7 μg/L, and decreased to normal range on 3rd postoperative day. In the end, the patient was dead due to severe sepsis and irreversible renal failure despite using hemodialysis. As this patient also had cisplatin in HIPEC, it remains doubtful whether hypermyoglobinemia was the direct cause of renal failure for this patient.
To reduce the risk of increase serum Mb, effective measures should be taken in the perioperative period. First is the critical preoperative evaluation. All patients must receive renal function evaluation with detection with serum BUN and Cr levels. In patients with suspected renal dysfunction or surgery involving the kidney, function evaluation of each kidney using renal dynamic imaging must be evaluated paying special attention to blood perfusion, glomerular filtration, and excretion functions. In patients with existing renal problems, chemotherapy drugs and anesthesia drugs with no renal damage should be used. If needed kidney-replacement therapies should also be considered.
Second is the careful maneuver during operation. Several practical techniques could be applied to minimize the damages to the skeletal muscles, including careful separation, avoid tissue and muscle tearing, sharp tissue dissection by appropriate electro-surgery, adequate temperature control during HIPEC, and careful selection of chemotherapeutic drugs during HIPEC.
Third is the minute monitoring during CRS + HIPEC. As the key promoting factor for Mb-mediated damage is acid pH, effective measures must be taken on the part of anesthesiologists to keep a balanced blood pH level. The blood gas should be checked regularly, preferably on real time. Any acidosis during operation and postoperative period should be promptly addressed by intravenous infusion of sodium bicarbonate solutions and other similar measures. The key point is to keep the blood pH in neutral range.
Fourth is the careful postoperative management. The key points are adequate control of the hemodynamic conditions to keep the stable blood pressure and appropriate tissue perfusion. Therefore, hemodynamic monitoring is necessary. And the fluid infusion should be fine-tuned at an hourly infusion rate of about 100 to 120 mL. The blood pressure and heart rate should be kept at normal range as much and as early as possible. Another consideration is the intravenous infusion of sodium bicarbonate solution. A reduction in oxidative stress on renal tubular cells may be a key mechanism in prevention of AKI by sodium bicarbonate. Bicarbonate is able to slow the Haber–Weiss reaction that generates free radicals. Sodium bicarbonate may also directly scavenge peroxynitrite and other reactive species generated from nitric oxide. Moreover, intravenous sodium bicarbonate administration can directly induce urinary alkalization, this effect may reduce the pH-dependent generation of methemoglobin containing tubular casts, ferrous-ion catalyzed production of free radicals as well as proteinuria oxidative damage. Thus, attenuation of oxidative stress through urine alkalization with sodium bicarbonate may attenuate AKI.[20]
In our study to infuse small dose of sodium bicarbonate solution infusion and to keep adequate urine output, there was no Mb-AKI occurred which was defined as Cr > 200 μmol/L.[9] So another important issue is monitoring and maintaining the urine output adequately. There should be an hourly urine output record for at least 4 days after CRS + HIPEC, with key consideration focusing on steady and continuous urine output rather than total daily urine output meeting the required level.
Although the clinical significance of increased Mb levels has been revealed, there are limitations in this study. First is the study design. This is a retrospective comparison study on the prospectively established database. Such study could facilitate to discover potential risk factors. But the identification and validation of such risk factors require prospective study. Second is the diversity of surgical procedures and chemotherapy regimens. As these 60 patients with PC had very different primary cancers, and the CRS + HIPEC procedures were not same, the prognostic value of Mb levels for different primaries could not be the same. Therefore, the results should be interpreted with caution regarding the clinical setting. A future prospective study with more homogeneous patient population and more standardized CRS + HIPEC procedures could help address these limitations.
5. Conclusion
Hypermyoglobinemia is a common and prominent lab abnormality after CRS + HIPEC, and serum Mb levels could be an early and sensitive indicator for dramatic disturbances in the internal milieu after CRS + HIPEC. Adequate treatment with sodium bicarbonate could accelerate the reduction in serum Mb levels and reduce the risk for major organ damages.
Footnotes
Abbreviations: AKI = acute kidney injury, BUN = blood urine nitrogen, CC = completeness of cytoreduction, Cr = creatinine, CRS = cytoreductive surgery, ECG = electrocardiogram, HIPEC = hyperthermic intraperitoneal chemotherapy, Mb = myoglobin, Mb-AKI = Mb-mediated AKI, PC = peritoneal carcinomatosis, PCI = peritoneal cancer index, PMP = pseudomyxoma peritonei, ROS = reactive oxygen species, TnI = troponin-I.
GL and Z-HJ have contributed equally to this work.
Supported by the Key Discipline Development Fund of Beijing Shijitan Hospital, Capital Medical University (2016fmzlwk).
The authors have no conflicts of interest to disclose.
References
- [1].Li Y, Zhou YF, Liang H, et al. Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancies. World J Gastroenterol 2016;22:6906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449–56. [DOI] [PubMed] [Google Scholar]
- [3].Verwaal VJ, Van Ruth S, De Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737–43. [DOI] [PubMed] [Google Scholar]
- [4].Spiliotis J, Halkia E, Lianos E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol 2015;22:1570–5. [DOI] [PubMed] [Google Scholar]
- [5].Sun JH, Ji ZH, Yu Y, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat advanced/recurrent epithelial ovarian cancer: results from a retrospective study on prospectively established database. Transl Oncol 2016;9:130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sun JH, Ji ZH, Peng KW, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy for the treatment of primary peritoneal serous carcinoma: results of a Chinese retrospective study. Int J Hyperthermia 2016;32:289–97. [DOI] [PubMed] [Google Scholar]
- [7].Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yao L, Liu Z, Zhu J, et al. Higher serum level of myoglobin could predict more severity and poor outcome for patients with sepsis. Am J Emerg Med 2016;34:948–52. [DOI] [PubMed] [Google Scholar]
- [9].Premru V, Kovac J, Ponikvar R. Use of myoglobin as a marker and predictor in myoglobinuric acute kidney injury. Ther Apher Dial 2013;17:391–5. [DOI] [PubMed] [Google Scholar]
- [10].Sloventantor V, Poluektova MV, Poverennyi AM, et al. Postoperative hypermyoglobinemia. Mechanism of occurrence. Predictive value. Vopr Med Khim 1995;41:45–9. [PubMed] [Google Scholar]
- [11].Poluektova MV, Sloventantor V, Khmelevskii IaM, et al. Dynamics of the level of plasma myoglobin in oncology patients with and without complications in the postoperative period. Vopr Med Khim 1995;41:47–9. [PubMed] [Google Scholar]
- [12].Wu HT, Peng KW, Ji ZH, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel to treat synchronous peritoneal carcinomatosis from gastric cancer: results from a Chinese center. Eur J Surg Oncol 2016;42:1024–34. [DOI] [PubMed] [Google Scholar]
- [13].Huang CQ, Feng JP, Yang XJ, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from colorectal cancer: a case-control study from a Chinese center. J Surg Oncol 2014;109:730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huang CQ, Yang XJ, Yu Y, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for patients with peritoneal carcinomatosis from colorectal cancer: a phase II study from a Chinese center. PLoS ONE 2014;9:e108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359–74. [DOI] [PubMed] [Google Scholar]
- [16].Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sugarbaker PH. Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol 2001;27:239–43. [DOI] [PubMed] [Google Scholar]
- [18].Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med 2009;361:62–72. [DOI] [PubMed] [Google Scholar]
- [19].Zhang XM, Tang Y, Yang YY, et al. Preliminary study on the pathogenic mechanism of myoglobin-induced endoplasmic reticulum stress and apoptosis in crush syndrome. Sichuan Da Xue Xue Bao Yi Xue Ban 2015;46:22–6. [PubMed] [Google Scholar]
- [20].Schneider AG, Bellomo R, Reade M, et al. Safety evaluation of a trial of lipocalin-directed sodium bicarbonate infusion for renal protection in at-risk critically ill patients. Crit Care Resusc 2013;15:126–33. [PubMed] [Google Scholar]


