Abstract
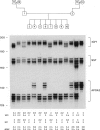
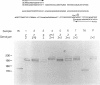
Interspersed DNA elements of the form (dC-dA)n.(dG-dT)n constitute one of the most abundant human repetitive DNA families. We report that specific human (dC-dA)n.(dG-dT)n blocks are polymorphic in length among individuals and therefore represent a vast new pool of potential genetic markers. Comparison of sequences from the literature for (dC-dA)n.(dG-dT)n blocks cloned two or more times revealed length polymorphisms in seven of eight cases. Variations in the lengths of 10 (dC-dA)n.(dG-dT)n blocks were directly demonstrated by amplifying the DNA within and immediately flanking the repeat blocks by using the polymerase chain reaction and then resolving the amplified DNA on polyacrylamide DNA sequencing gels. Use of the polymerase chain reaction to detect DNA polymorphisms offers improved sensitivity and speed compared with standard blotting and hybridization.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge J., Kunkel L., Bruns G., Tantravahi U., Lalande M., Brewster T., Moreau E., Wilson M., Bromley W., Roderick T. A strategy to reveal high-frequency RFLPs along the human X chromosome. Am J Hum Genet. 1984 May;36(3):546–564. [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Braaten D. C., Thomas J. R., Little R. D., Dickson K. R., Goldberg I., Schlessinger D., Ciccodicola A., D'Urso M. Locations and contexts of sequences that hybridize to poly(dG-dT).(dC-dA) in mammalian ribosomal DNAs and two X-linked genes. Nucleic Acids Res. 1988 Feb 11;16(3):865–881. doi: 10.1093/nar/16.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das H. K., Jackson C. L., Miller D. A., Leff T., Breslow J. L. The human apolipoprotein C-II gene sequence contains a novel chromosome 19-specific minisatellite in its third intron. J Biol Chem. 1987 Apr 5;262(10):4787–4793. [PubMed] [Google Scholar]
- Gilliam T. C., Healey S. T., MacDonald M. E., Stewart G. D., Wasmuth J. J., Tanzi R. E., Roy J. C., Gusella J. F. Isolation of polymorphic DNA fragments from human chromosome 4. Nucleic Acids Res. 1987 Feb 25;15(4):1445–1458. doi: 10.1093/nar/15.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D. S., Garrard W. T. The ubiquitous potential Z-forming sequence of eucaryotes, (dT-dG)n . (dC-dA)n, is not detectable in the genomes of eubacteria, archaebacteria, or mitochondria. Mol Cell Biol. 1986 Aug;6(8):3010–3013. doi: 10.1128/mcb.6.8.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D. S., Huang S. Y., Garrard W. T. Chromatin structure of the potential Z-forming sequence (dT-dG)n X (dC-dA)n. Evidence for an "alternating-B" conformation. J Mol Biol. 1985 May 25;183(2):251–265. doi: 10.1016/0022-2836(85)90218-9. [DOI] [PubMed] [Google Scholar]
- Hamada H., Kakunaga T. Potential Z-DNA forming sequences are highly dispersed in the human genome. Nature. 1982 Jul 22;298(5872):396–398. doi: 10.1038/298396a0. [DOI] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6465–6469. doi: 10.1073/pnas.79.21.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T., Seidman M., Stollar B. D. Characterization of genomic poly(dT-dG).poly(dC-dA) sequences: structure, organization, and conformation. Mol Cell Biol. 1984 Dec;4(12):2610–2621. doi: 10.1128/mcb.4.12.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Seidman M., Howard B. H., Gorman C. M. Enhanced gene expression by the poly(dT-dG).poly(dC-dA) sequence. Mol Cell Biol. 1984 Dec;4(12):2622–2630. doi: 10.1128/mcb.4.12.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Royle N. J., Wilson V., Wong Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988 Mar 17;332(6161):278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Coppel R. L., Anders R. F. Repetitive proteins and genes of malaria. Annu Rev Microbiol. 1987;41:181–208. doi: 10.1146/annurev.mi.41.100187.001145. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Krystal M., Arnheim N. A member of a new repeated sequence family which is conserved throughout eucaryotic evolution is found between the human delta and beta globin genes. Nucleic Acids Res. 1981 Nov 25;9(22):5931–5947. doi: 10.1093/nar/9.22.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky A. G. Normal and abnormal color-vision genes. Am J Hum Genet. 1988 Mar;42(3):405–407. [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Leppert M., O'Connell P., Wolff R., Holm T., Culver M., Martin C., Fujimoto E., Hoff M., Kumlin E. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987 Mar 27;235(4796):1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- Nicklas J. A., Hunter T. C., Sullivan L. M., Berman J. K., O'Neill J. P., Albertini R. J. Molecular analyses of in vivo hprt mutations in human T-lymphocytes. I. Studies of low frequency 'spontaneous' mutants by Southern blots. Mutagenesis. 1987 Sep;2(5):341–347. doi: 10.1093/mutage/2.5.341. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. The sequence (dC-dA)n X (dG-dT)n forms left-handed Z-DNA in negatively supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1821–1825. doi: 10.1073/pnas.80.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overhauser J., McMahan J., Wasmuth J. J. Identification of 28 DNA fragments that detect RFLPs in 13 distinct physical regions of the short arm of chromosome 5. Nucleic Acids Res. 1987 Jun 11;15(11):4617–4627. doi: 10.1093/nar/15.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M. L., Lowenhaupt K., Rich A., Nordheim A. (dC-dA)n.(dG-dT)n sequences have evolutionarily conserved chromosomal locations in Drosophila with implications for roles in chromosome structure and function. EMBO J. 1987 Jun;6(6):1781–1789. doi: 10.1002/j.1460-2075.1987.tb02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Schumm J. W., Knowlton R. G., Braman J. C., Barker D. F., Botstein D., Akots G., Brown V. A., Gravius T. C., Helms C., Hsiao K. Identification of more than 500 RFLPs by screening random genomic clones. Am J Hum Genet. 1988 Jan;42(1):143–159. [PMC free article] [PubMed] [Google Scholar]
- Shen L. P., Rutter W. J. Sequence of the human somatostatin I gene. Science. 1984 Apr 13;224(4645):168–171. doi: 10.1126/science.6142531. [DOI] [PubMed] [Google Scholar]
- Skolnick M. H., Wallace R. B. Simultaneous analysis of multiple polymorphic loci using amplified sequence polymorphisms (ASPs). Genomics. 1988 May;2(4):273–279. doi: 10.1016/0888-7543(88)90014-6. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Sun L., Paulson K. E., Schmid C. W., Kadyk L., Leinwand L. Non-Alu family interspersed repeats in human DNA and their transcriptional activity. Nucleic Acids Res. 1984 Mar 26;12(6):2669–2690. doi: 10.1093/nar/12.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Renz M. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res. 1984 May 25;12(10):4127–4138. doi: 10.1093/nar/12.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler-Smith C., Brown W. R. Structure of the major block of alphoid satellite DNA on the human Y chromosome. J Mol Biol. 1987 Jun 5;195(3):457–470. doi: 10.1016/0022-2836(87)90175-6. [DOI] [PubMed] [Google Scholar]
- Waye J. S., Durfy S. J., Pinkel D., Kenwrick S., Patterson M., Davies K. E., Willard H. F. Chromosome-specific alpha satellite DNA from human chromosome 1: hierarchical structure and genomic organization of a polymorphic domain spanning several hundred kilobase pairs of centromeric DNA. Genomics. 1987 Sep;1(1):43–51. doi: 10.1016/0888-7543(87)90103-0. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Lyon J. A., Wolff R. H., Hall T., Lowell G. H., Chulay J. D. Primary structure of a Plasmodium falciparum malaria antigen located at the merozoite surface and within the parasitophorous vacuole. J Biol Chem. 1988 Aug 15;263(23):11421–11425. [PubMed] [Google Scholar]
- Weber J. L. Molecular biology of malaria parasites. Exp Parasitol. 1988 Aug;66(2):143–170. doi: 10.1016/0014-4894(88)90087-2. [DOI] [PubMed] [Google Scholar]