Abstract
Directed C–H activation has emerged as a major approach for developing synthetically useful reactions, owing to the proximity-induced reactivity and selectivity enabled by coordinating functional groups1–6. In contrast, development of palladium-catalyzed non-directed C–H activation has faced significant challenges associated with the lack of sufficiently active palladium catalysts7–8. Current palladium catalysts are only reactive with electron-rich arenes unless an excess of arene is used9–18, which limits synthetic applications. Herein, we disclose a 2-pyridone ligand that significantly enhances the reactivity of a palladium catalyst, allowing for Pd(II)-catalyzed non-directed C–H activation of a broad range of aromatic substrates using the various arenes as the limiting reagent. The significance of this finding is demonstrated by the direct functionalization of advanced synthetic intermediates, drug molecules, and natural products that cannot be utilized in excessive quantities. The potential of this methodology to be expanded to a variety of transformations is indicated by the development of both C–H olefination and C–H carboxylation protocols. Furthermore, the site selectivity in this transformation is governed by a combination of steric and electronic effects, with the pyridone ligand enhancing the influence of sterics on the selectivity, thus providing complementary selectivity to directed C–H functionalization.
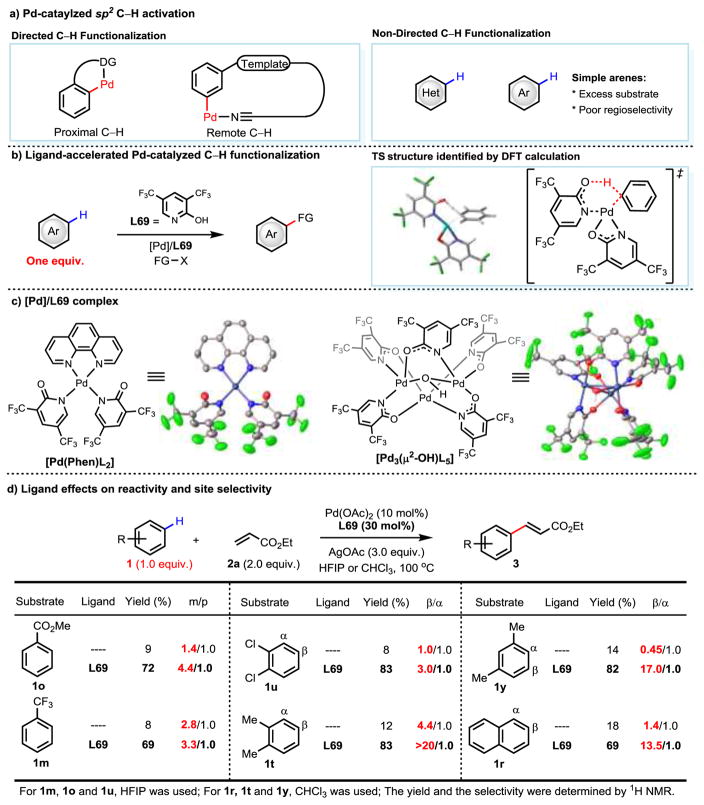
The development of palladium-catalyzed synthetically useful C–H activation reactions faces two well-known challenges: the poor reactivity of palladium catalysts towards C–H bonds and the difficulty in discerning multiple C–H bonds within a given substrate. Typically, directing groups are employed to address these two problems simultaneously by exploiting a complex-induced proximity effect1–6. Extensive research on various directing group designs1–6 and strategies19–21, as well as the development of ligands which accelerate C–H functionalization22, has significantly improved the practicality and utility of this approach. Ligand-acceleration has enabled palladium catalysts to functionalize remote C–H bonds of aromatic substrates by recognition of distance and geometry23–24. (Figure 1a). Despite the tremendous potential of directed C–H activation where proximal or distal site-selectivity can be controlled, the importance of non-directed C–H activation reactions using one equivalent of arenes cannot be overemphasized. Non-directed C–H functionalization may reach sites that are currently not accessible by a directed approach as demonstrated by Ir(III)-catalyzed C–H borylation chemistry25–26. Furthermore, substrates that do not contain appropriate directing groups can only be functionalized using a non-directed approach.
Figure 1. C–H functionalization of arenes.
a, Pd-catalyzed Csp2–H functionalization. DG, directing group. b, Ligand-accelerated Pd-catalyzed C–H functionalization. L; Ligand. DFT-optimized C–H activation transition state at the M06/SDD,6-311+G(d,p)(SMD)//B3LYP/LANL2DZ,6-31G(d) level of theory. c, Crystal structure of Pd/L69. d, Ligand effects on reactivity and site selectivity for selected substrates. HFIP, hexafluoroisopropanol. CHCl3, chloroform.
The first hurdle along the path towards developing Pd(II)-catalyzed non-directed C–H activation reactions is the inherently low reactivity of C–H bonds with palladium catalysts. The significance of this challenge is best illustrated by the lack of progress in the Fujiwara-Moritani olefination reaction of arenes, initially observed in 19679. Sixty years after this initial observation, a large excess of arene substrate is still required to achieve sufficient reactivity with palladium catalysts in a general manner by creating a high molarity of the substrate9–14 (Figure 1a). Furthermore, such electrophilic palladation reactions, analogous to SEAr, are also limited to electron-rich arenes27–28 which prevents the use of a wide range of synthetically useful electron-deficient arenes9. Herein, we report the discovery of an electron-deficient 2-pyridone ligand that promotes palladium-catalyzed non-directed C–H olefination and carboxylation using both electron-deficient and -rich arene as the limiting reagent (Figure 1b). The structures of a pre-catalyst [Pd3(μ2-OH)(L69)5] as well as of a 1,10-phenanthroline stablilized Pd(Phen)(L69)2 complex are characterized by X-ray crystallography (Figure 1c), which provide insight into the coordination mode of these ligands with palladium catalyst. Comprehensive kinetic and DFT studies indicate that the pyridone ligand is likely to serve as an X-type ligand to palladium and can also act as internal base to cleave the C–H bond via concerted metalation deprotonation (CMD) mechanism (Figure 1b). Both simple and heterocyclic arenes are compatible with this protocol. These reactions are also applied to late-stage modification of amino acids, dyes, and pharmaceuticals. While site selectivity is predominantly dictated by the intrinsic electronic and steric bias, ligand influence is also shown to enhance the selectivity with a few classes of arenes that are not possible previously (Figure 1d).
Guided by our recent success in developing ligand-accelerated, directed C–H activation, we embarked on a quest to discover and develop highly active ligands to achieve non-directed C–H activation with Pd(II) catalysts using arene substrates as the limiting reagent. To best evaluate the ligand effects on both reactivity and site selectivity, we selected 1,2-dichlorobenzene (1u) as a model substrate because it displayed poor reactivity and low β/α selectivity under previously reported conditions13. In the absence of ligand, olefination of 1,2-dichlorobenzene was found to proceed in the presence of a catalytic amount of Pd(OAc)2 and 3.0 equivalents of silver acetate in HFIP to provide the olefinated products in a mere 8% yield with poor site selectivity (β/α = 1.0/1.0). With these established initial conditions, a wide range of ligands commonly used in catalysis were evaluated. Phosphine ligands, N-heterocyclic carbene ligands (NHC), oxazoline ligands, pyridine ligands, phenanthroline and 1,10-phenanthrolin-2-ol did not display an improvement in reactivity for this reaction (see supplementary information). The first noticeable ligand enhancement was observed with mono-protected amino acid (MPAA) ligands, which could improve the yield twofold (16% combined yield). The improved selectivity (α/β = 1.6/1.0) is important evidence for ligand involvement in the C–H activation step. Although further screening of other MPAA ligands did not improve the yield, we found that 3-acetylamino-2-hydroxypyridine ligand (L19), previously disclosed for meta-C–H functionalization29, provided a nearly threefold improvement of the reactivity (21% vs. 8% yield). Following this lead, we extensively evaluated a variety of mono-protected 3-amino-2-hydroxypyridine ligands (L20–L50), and found that TFA-protected 3-amino-5-(trifluoromethyl)pyridin-2-ol (L31) afforded a significant improvement in both yield (62%) and selectivity (β/α = 2.3/1.0). Interestingly, ligand L51 (5-trifluoromethyl-2-pyridone) which is devoid of the TFA-protected amino moiety, also imparts reactivity to the palladium catalyst, albeit less significantly than L31 (24% yield). This finding is in line with our previous report on the use of 3-acetylamino-2-hydroxypyridine ligands wherein we showed that the 3-acetylamino group has a positive influence on the reaction, but the pyridone moiety is crucial to the reactivity29. This prompted us to perform further ligand screening with a focus on evaluating a diverse range of substituted 2-pyridones. To our delight, we found that the highly electron-deficient 3,5-bis(trifluoromethyl)-pyridin-2(1H)-one (L69) significantly promoted the reaction, allowing formation of 3u in 70% yield with improved site selectivity (β/α = 3.0/1.0). The yield was further improved to 85% (82% isolated yield) by employing 30 mol% ligand L69 and 2.0 equivalents of ethyl acrylate.
To elucidate the role of the ligand L69 and the origin of this unprecedented reactivity using arene as the limiting reagent, we carried out comprehensive structural characterization, kinetic studies and DFT studies. We first obtained and characterized [Pd(Phen)(L69)2] and the trimeric [Pd3(μ2-OH)(L69)5] complexes through X-ray crystallography (Figure 1c). The coordination mode of L69 in these complexes is shown to be analogous to that found with carboxylates30, which is also consistent with the most favorable transition state proposed by our DFT studies (Figure 1b). The KIE experiments (kH/kD = 2.9) using benzene as a model substrate with ethyl acrylate indicates that the C–H bond cleavage is the rate-limiting step. Further kinetic investigation shows that the ligand L69 increases the initial rate by a factor of 1.4 (see supplementary information). In addition, the reaction profile using 1,2-dichlorobenzene as substrate indicates that the ligand plays a dual role in this reaction. The ligand not only accelerates the initial rate of this reaction by 3-fold but also prevents catalyst decomposition by forming a more stable complex with palladium. Such stabilizing effect is crucial for maintaining the catalyst efficiency in this non-directed C–H activation reaction with arene as the limiting reagent. Notably, electron-deficient arenes are nearly unreactive in the absence of L69. With C–H cleavage being the rate-limiting step in this reaction, it is plausible that the observed rate enhancement is due to the involvement of pyridone ligands in the C–H cleavage step. To support this hypothesis, we performed DFT studies to calculate transition state energies for the C–H cleavage step using palladium complexes containing two acetates, one acetate and one pyridone, and two pyridone ligands respectively. We observed that with each successive replacement of an acetate with the pyridone ligand, the relative Gibbs free energy as well as the Gibbs free energy of activation for the C–H activation transition state decreases. The most favorable transition state (as shown in Figure 1b) consists of palladium coordinated to two pyridone ligands, one of which serves as an X-type ligand and binds in a κ-2 fashion. The second pyridone ligand coordinates to palladium through the nitrogen center and serves as an internal base to cleave the C–H bond via concerted metalation deprotonation (CMD) mechanism (see supplementary information). This transition state has a Gibbs free energy of activation which is 4.8 kcal/mol lower than the one without pyridone ligand. Although our preliminary DFT studies overestimate the rate enhancement of relatively reactive benzene by the new ligand, the rate enhancement observed with electron-deficient 1,2-dichlorobenzene is more dramatic. Detailed studies are underway to understand the effect of HFIP and silver acetate on the reaction rate to get a more quantitative prediction that can be used for further catalyst design and development. Finally, the compelling site-selectivity favoring the β-position observed with the naphthalene contrasts the α-selectivity with the conventional Friedel-Crafts type palladation pathway (see supplementary information).
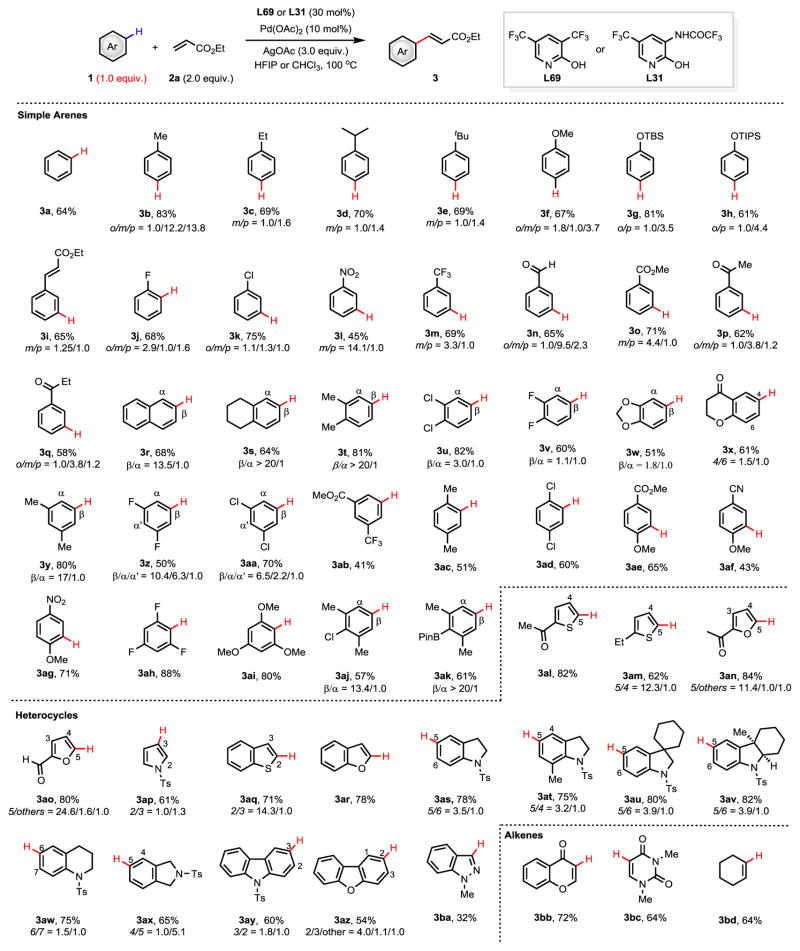
With the optimal conditions in hand, we next examined the substrate generality using ethyl acrylate as the coupling partner. As summarized in Figure 2, both electron-rich and electron-deficient arenes are suitable substrates, delivering the mono-olefinated products in moderate to high yield (3a–3ak). Notably, di-olefinated products were also formed as minor products when employing highly reactive substrates (see supporting information for details). Gratifyingly, the use of chloroform as solvent reduces the amount of di-olefination products. For example, benzene afforded the mono-olefinated product 3a in 64% yield and a mixture of di-olefinated products in 18% yield in HCCl3 (45% mono and 36% di in HFIP). Mono-alkylated arenes were olefinated at the less sterically hindered meta- and para-positions in good yields (3b–3e). Anisole provided the ortho- and para-isomers as the major products due to electronic effects (3f), while the bulkier silyl protected phenol gave the para-olefinated product as the major isomer (3g and 3h). Notably, less reactive electron-deficient arenes are also olefinated using this new catalytic system, highlighting the significance of the ligand acceleration. Subjecting substrates bearing strongly electron-withdrawing groups, such as nitro, trifluoromethyl, aldehyde, ester, ketone, and nitrile to these olefination conditions afforded the corresponding products in moderate to good yields with synthetically useful meta-selectivity (3l–3q, and 3af). Naphthalene also provided the mono-olefinated products (3r) in 68% yield with a 13.5/1.0 selectivity ratio (β/α). The significant improvement of the regioselectivity by the ligand is particularly noteworthy (Figure 1d.) and it appears that the ligand enhances the contribution of sterics to determining the selectivity. Di-substituted, and tri-substituted aromatics were also examined (3s–3ag and 3ah–3ak). The selectivity of symmetrical 1,2-disubstituted arenes, as well as 1,3-disubstituted arenes is primarily governed by sterics. For example, 1,2,3,4-tetrahydronaphthalene, o-xylene, and m-xylene afforded the β-olefinated products (3s, 3t, and 3y) in excellent selectivity, respectively. Substrates with less sterically hindered substituents such as chloro- and fluoro-reacted to provide a mixture of α- and β-olefinated products (3u and 3v), though the β-olefinated products predominate as the major isomer. Methyl 3-(trifluoromethyl)benzoate was transformed to the meta-product (3ab) as a single isomer due to a combination of electronic and steric influence. Symmetrical 1,4-disubstituted aromatics are also suitable substrates for this reaction and generally undergo mono-functionalization in high yields (3ac and 3ad). Notably, 4-substituted anisoles are olefinated at the ortho-position exclusively, due to electronic effects (3ae–3ag). 1,3,5- and 1,2,3-trisubstituted arenes also react smoothly, providing the desired products in high yields (3ah–3ak). Interestingly, 2,6-disubstituted aryl boronate ester is also compatible under the conditions (3ak). Overall, a variety of heterocyclic aromatics were then investigated. Olefination of thiophene, furan, benzothiophene, and benzofuran occured at the electron-rich positions to give the desired products selectively (3al–3ar). Use of pyrrole led to a mixture of 2- and 3-olefinated products (3ap), likely due to the high reactivity of the pyrrole. Indolines, 1,2,3,4-tetrahydroquinoline, isoindoline, carbazole,dibenzofuran, and indazole are also compatible with this non-directed C–H activation generating the desired products in moderate to good yields (3as–3ba). Furthermore, vinylic C–H bonds in cyclic alkenes were also olefinated to provide the corresponding diene products (3bb–3bd).
Figure 2. The C–H olefination of arenes and heterocycles.
TBS, tert-butyldimethylsilyl; TIPS, triisopropylsilyl; Pin, pinacolate; Ts, 4-toluenesulfonyl. The values under each structure indicate isolated yields. Reaction conditions: Pd(OAc)2 (10 mol%), L69 (30 mol%), AgOAc (3.0 equiv.), HFIP (0.5 mL), 100 °C, 24 h; For 3a, 3r, 3t, 3w, 3y, 3ai, 3aj, 3ak, 3an, 3ao, 3ay, 3az and 3bb, CHCl3 (0.5 mL) was used instead of HFIP; For 3j and 3k, L31 (20 mol%) was used instead of L69; For 3l and 3ab, Pd(OAc)2 (20 mol%), L69 (60 mol%), and ethyl acrylate (1.2 equiv.) were used; For 3as–3ax, the reaction was conducted at 90 °C; For 3al, 3am, 3aq and 3ar, Ag2CO3 (1.5 equiv.) was used instead of AgOAc; For 3am, the reaction time was shortened to 12 h; For 3ap, the reaction was conducted at 60 °C; For 3ar, the reaction time was shortened to 8 h. For 3bd, substrates (0.2 mmol), ethyl acrylate (0.1 mmol) were used in CHCl3.
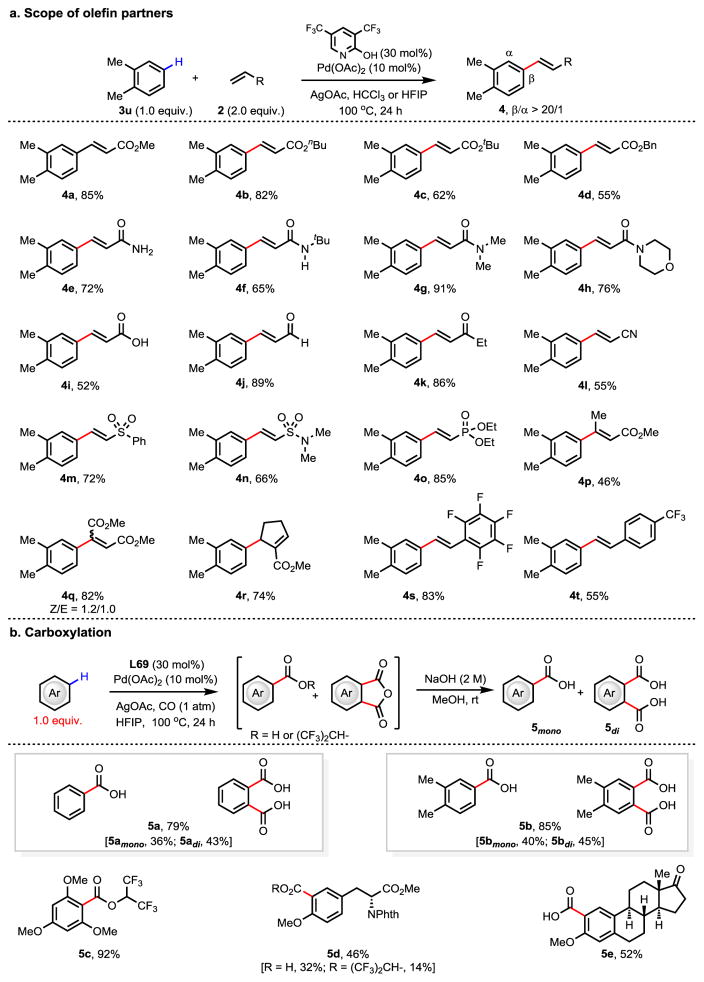
The scope of the olefin coupling partners was evaluated using o-xylene as the model substrate (Figure 3a). α, β-Unsaturated olefins served as particularly effective coupling partners for this Pd-catalyzed olefination reaction (4a–4r). For example, the reactions with acrylate derivatives proceeded in 55–85% yields (4a–4d). Other electron-withdrawing groups attached to the olefins including carboxylic acid, amide, aldehyde, ketone, nitrile, sulfone, sulfonamide, and phosphonate are all compatible with these reaction conditions conditions providing the desired olefinated products in moderate to excellent yields (4e–4o). It is worth noting that acrylic acid, acrylamide and acrolein are often incompatible with Pd-catalyzed C–H olefination reactions. 1,2-Disubstituted α,β-unsaturated olefins including crotonate (4p), maleate (4q) and cyclic tri-substituted olefin (4r) were also found to be suitable coupling partners. While both the aryl C–H bonds and vinylic C–H bonds of styrenes are reactive under these conditions, electron deficient styrene derivatives can be used as coupling partners (4s and 4t). The gram-scale C–H olefination of o-xylene with acrolein using 5 mol% Pd(OAc)2 and 15 mol% L69 was also performed, and provided the desired product in 83% yield (see Supplementary Information). It is noteworthy that the ligand L69 was recovered in 87% yield.
Figure 3. The scope of olefin partners and carboxylation.
a, Scope of olefin coupling partners. HFIP, hexafluoroisopropanol. Reaction conditions: o-xylene (0.1 mmol), olefin (2.0 equiv.), Pd(OAc)2 (10 mol%), L69 (30 mol%), AgOAc (3.0 equiv.), HFIP (0.5 mL), 100 °C, 24 h. For 4a–4d, 4i, 4l, 4p, the reaction was conducted in HCCl3; for 4c, the reaction time was shortened to 16 h. b, Carboxylation of simple arenes. Phth, Phthaloyl. Reaction conditions: Substrate (0.2 mmol), CO (1 atm), Pd(OAc)2 (10 mol%), L (30 mol%), AgOAc (3.0 equiv.), HFIP (2.0 mL), 100 °C, 24 h; then NaOH (1.5 mL, 2 M), MeOH (2.0 mL), 12 h. For 5d and 5e, substrate (0.1 mmol) was used; for 5c and 5d, the products were isolated before hydrolysis.
To demonstrate the potential generality of ligand-accelerated non-directed C–H activation to provide a wide range of transformations, we proceeded to develop a challenging non-directed C–H carboxylation reaction (Figure 3b). Gratifyingly, we found that ligand L69 can promote the C–H carboxylation of arenes using the arene as the limiting reagent under an atmosphere of carbon monoxide. The reaction produces a mixture of the mono-HFIP ester and phthalic anhydride derivatives. The phthalic anhydride derivatives arise from an ortho-C–H carboxylation reaction directed by the product of the first carboxylation. A mixture of mono-acid and phthalic acid derivatives can be obtained after treatment with an aqueous NaOH solution in methanol. Benzene and o-xylene were tested under the standard conditions and were transformed to the mono- and di-acids in 79% and 85% combined yield, respectively (5a and 5b). Direct carboxylation of 1,3,5-trimethoxybenzene gave the corresponding HFIP-ester in 92% yield (5c). Bioactive molecules O-methyl-tyrosine derivative (5d) and estrone 3-methyl ether (5e) were also subjected to the carboxylation procedure, affording the desired products in moderate yields.
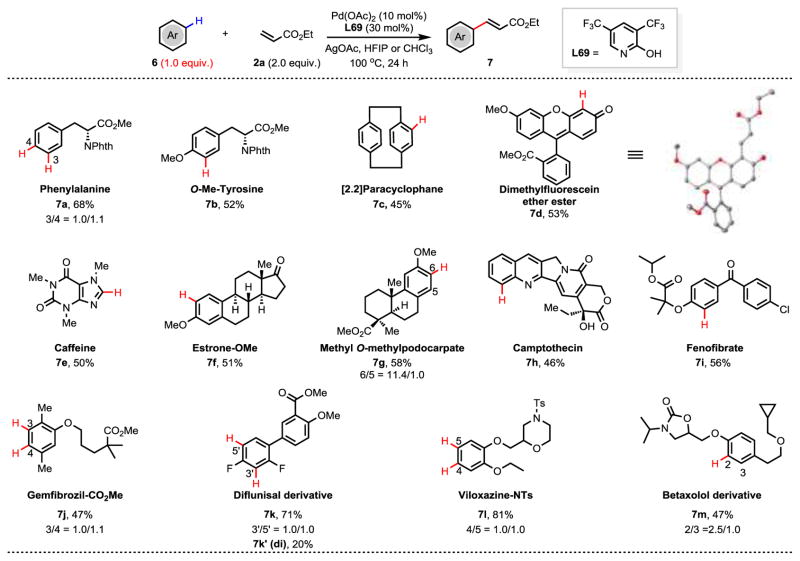
The development of ligand-accelerated, non-directed C–H functionalization using one equivalent of arene presents an opportunity for late-stage functionalization of C–H bonds not accessible by directing group strategies due to distance, geometry, or compatibility (Figure 4). C–H olefination of amino acid derivatives proceeded smoothly to give the desired products in 68% and 52% yield, respectively (7a and 7b). [2.2]Paracyclophane, a commonly used ligand scaffold, was olefinated in 45% yield (7c). Fluorescein derivative (6d), a well-known dye and fluorescence probe, was olefinated in 53% yield (7d). Interestingly, the most active site for 6d is the α-position of the carbonyl group adjacent to the oxo-bridge. In addition, natural products including caffeine, estrone, podocarpic acid derivatives, and camptothecin were tested under the standard conditions delivering the targets in moderate yields (7e–h). A variety of pharmaceuticals including fenofibrate, gemfibrozil, diflunisal, viloxazine, and betaxolol were also olefinated to give the products in 47–91% yields (7i–m).
Figure 4. Late-stage functionalization of natural products and drug molecules.
Phth, Phthaloyl; TBS, tert-butyldimethylsilyl; Ts, 4-toluenesulfonyl; HFIP, hexafluoroisopropanol. The values under each structure indicate isolated yields. Reaction conditions: Substrate (0.1 mmol), Ethyl acrylate (0.2 mmol), Pd(OAc)2 (10 mol%), L69 (30 mol%), AgOAc (3.0 equiv.), HFIP (0.5 mL), 100 °C, 24 h. For 7d, 7f, and 7i, the reaction was conducted for 16 h. For 7g, chloroform was used instead of HFIP.
In summary, the discovery of 2-pyridone ligand scaffold has afforded a significantly more reactive Pd(II) catalyst for non-directed C–H functionalizations. DFT studies have proposed that the ligand serves as an X-type ligand as well as an internal base to accelerate the C–H cleavage step via concerted metalation deprotonation (CMD) mechanism. The structural characterization and kinetic behavior of the precatalyst also sheds light on the structure of the active catalyst which, in turn, is supported by DFT calculations. Owing to the development of the ligand L69, non-directed C–H olefination and carboxylation has been achieved for the first time using the arene as the limiting reagent in the absence of a directing group. A substantial influence of the ligand on site selectivity has also been observed in a few classes of arene substrates. Due to the compatibility of both electron-rich and electron-deficient arenes, this reaction opens an avenue for the diversification of advanced synthetic intermediates, natural products, dyes, and drug molecules.
Methods Summary
General procedure for the Pd/pyridone promoted non-directed C–H activation of simple arenes
Substrate (0.1 mmol), ethyl acrylate (0.2 mmol), Pd(OAc)2 (2.2 mg, 10 mol%), L69 (3.0 mg, 20 mol%), AgOAc (50.1 mg, 0.3 mmol), and HFIP or CHCl3 (0.5 mL) were added to a 2-dram vial. The vial was capped and closed tightly, then the reaction mixture was stirred at 100 °C for 24 h. After cooling to room temperature, the mixture was filtered through a pad of Celite and washed with dichloromethane as the eluent to remove the insoluble precipitate. The resulting solution was concentrated and purified by preparative TLC to afford the desired arylated product. Full experimental details and characterization of new compounds can be found in the Supplementary Information.
Data Availability
The data supporting the findings of this study are available within the article and its Supplementary Information files. Metrical parameters for the structure of Pd(Phen)(L69)2, Pd3(μ2-OH)(L69)5 complex and 7d (see Supplementary Information) are available free of charge from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/) under reference numbers CCDC 1552055, CCDC 1538821, and CCDC 1538820, respectively.
Supplementary Material
Acknowledgments
We gratefully acknowledge The Scripps Research Institute, the NIH (NIGMS, 2R01 GM102265), and Bristol-Myers Squibb for their financial support. We also thank Novartis for providing the drug molecules.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions P.W. developed the ligands and the reactions. P.V. performed the DFT calculations. G.X. performed the kinetic study. J.S., S.T. and P.T.W.C separated the isomers using preparative HPLC. J.X.Q. and M.A.P. participated in the screening of acrylamide derived coupling partners and investigation of the C–H olefination reaction for amino acid substrates. M.E.F. performed preliminary studies on 2-hydroxypyridine ligands. K.-S.Y. helped the screening of sulfonamide derived coupling partners. J.-Q.Y. conceived this concept and prepared this manuscript with feedback from P.W., P.V. and G.X.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of this article.
References
- 1.Whisler MC, MacNeil S, Snieckus V, Beak P. Beyond thermodynamic acidity: a perspective on the complex-induced proximity effect (CIPE) in deprotonation reactions. Angew Chem Int Ed. 2004;43:2206–2225. doi: 10.1002/anie.200300590. [DOI] [PubMed] [Google Scholar]
- 2.Kakiuchi F, Sekine S, Tanaka Y, Kamatani A, Sonoda M, Chatani N, Murai S. Catalytic addition of aromatic carbon–hydrogen bonds to olefins with the aid of ruthenium complexes. Bull Chem Soc Jpn. 1995;68:62–83. [Google Scholar]
- 3.Daugulis O, Do HQ, Shabashov D. Palladium- and copper-catalyzed arylation of carbon–hydrogen bonds. Acc Chem Res. 2009;42:1074–1086. doi: 10.1021/ar9000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engle KM, Mei TS, Wasa M, Yu JQ. Weak coordination as powerful means for developing broadly useful C–H functionalization reactions. Acc Chem Res. 2012;45:788–802. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons TW, Sanford MS. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colby DA, Bergman RG, Ellman JA. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem Rev. 2010;110:624–655. doi: 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhl N, Hopkinson MN, Wencel-Delord J, Glorius F. Beyond directing groups: transition-metal-catalyzed C–H activation of simple arenes. Angew Chem Int Ed. 2012;51:10236–10254. doi: 10.1002/anie.201203269. [DOI] [PubMed] [Google Scholar]
- 8.Hartwig JF, Larsen MA. Undirected, homogeneous C–H bond functionalization: challenges and opportunities. ACS Cent Sci. 2016;2:281–292. doi: 10.1021/acscentsci.6b00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moritani I, Fujiwara Y. Aromatic substitution of styrene-palladium chloride complex. Tetrahedron Lett. 1967;8:1119–1122. [Google Scholar]
- 10.Dams M, De Vos DE, Celen S, Jacobs PA. Toward waste-free production of Heck products with a catalytic palladium system under oxygen. Angew Chem Int Chem. 2003;42:3512–3515. doi: 10.1002/anie.200351524. [DOI] [PubMed] [Google Scholar]
- 11.Yokota T, Tani M, Sakaguchi S, Ishii Y. Direct coupling of benzene with olefin catalyzed by Pd(OAc)2 combined with heteropolyoxometalate under dioxygen. J Am Chem Soc. 2003;125:1476–1477. doi: 10.1021/ja028903a. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YH, Shi BF, Yu JQ. Pd(II)-catalyzed olefination of electron-deficient arenes using 2,6-dialkylpyridine ligands. J Am Chem Soc. 2009;131:5072–5074. doi: 10.1021/ja900327e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubota A, Emmert MH, Sanford MS. Pyridine ligands as promoters in PdII/0-catalyzed C–H olefination reactions. Org Lett. 2012;14:1760–1763. doi: 10.1021/ol300281p. [DOI] [PubMed] [Google Scholar]
- 14.Ying CH, Yan SB, Duan WL. 2-Hydroxy-1,10-phenanthroline vs 1,10-phenanthroline: significant ligand acceleration effects in the Palladium-catalyzed oxidative Heck reaction of arenes. Org Lett. 2014;16:500–503. doi: 10.1021/ol4033804. [DOI] [PubMed] [Google Scholar]
- 15.Li R, Jiang L, Lu W. Intermolecular cross-coupling of simple arenes via C–H activation by tuning concentrations of arenes and TFA. Organometallics. 2006;25:5973–5975. [Google Scholar]
- 16.Shrestha R, Mukherjee P, Tan Y, Litman ZC, Hartwig JF. Sterically controlled, Palladium-catalyzed intermolecular amination of arenes. J Am Chem Soc. 2013;135:8480–8483. doi: 10.1021/ja4032677. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara Y, Taniguchi H, Taniguchi H. Palladium-promoted one-step carboxylation of aromatic compounds with carbon monoxide. J Chem Soc Chem Commun. 1980:220–221. [Google Scholar]
- 18.Yoneyama T, Crabtree RH. Pd(II) catalyzed acetoxylation of arenes with iodosyl acetate. J Mol Catal A. 1996;108:35–40. [Google Scholar]
- 19.Huang C, Chattopadhyay B, Gevorgyan V. Silanol: a traceless directing group for Pd-catalyzed o-alkenylation of phenols. J Am Chem Soc. 2011;133:12406–12409. doi: 10.1021/ja204924j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedford RB, Coles SJ, Hursthouse MB, Limmert ME. The catalytic intermolecular orthoarylation of phenols. Angew Chem Int Ed. 2003;42:112–114. doi: 10.1002/anie.200390037. [DOI] [PubMed] [Google Scholar]
- 21.Zhang FL, Hong K, Li TJ, Park H, Yu J-Q. Functionalization of C(sp3)–H bonds using a transient directing group. Science. 2016;351:252–256. doi: 10.1126/science.aad7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engle KM, Yu JQ. Developing ligands for palladium(II)-catalyzed C–H functionalization: intimate dialogue between ligand and substrate. J Org Chem. 2013;78:8927–8955. doi: 10.1021/jo400159y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leow D, Li G, Mei TS, Yu JQ. Activation of remote meta-C–H bond assisted by an end-on template. Nature. 2012;486:518–522. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu L, Shang M, Tanaka K, Chen Q, Pissarnitski N, Streckfuss E, Yu JQ. Remote meta-C–H activation using a pyridine-based template: achieving site-selectivity via the recognition of distance and geometry. ACS Cent Sci. 2015;1:394–399. doi: 10.1021/acscentsci.5b00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. C–H activation for the construction of C–B bonds. Chem Rev. 2010;110:890–931. doi: 10.1021/cr900206p. [DOI] [PubMed] [Google Scholar]
- 26.Cho JY, Tse MK, Holmes D, Maleczka RE, Jr, Smith MR., III Remarkably selective iridium catalysts for the elaboration of aromatic C–H bonds. Science. 2002;295:305–308. doi: 10.1126/science.1067074. [DOI] [PubMed] [Google Scholar]
- 27.Grimster NP, Gauntlett C, Godfrey CRA, Gaunt MJ. Palladium-catalyzed intermolecular alkenylation of indoles by solvent-controlled regioselective C–H functionalization. Angew Chem Int Ed. 2005;44:3125–3129. doi: 10.1002/anie.200500468. [DOI] [PubMed] [Google Scholar]
- 28.Ueda K, Yanagisawa S, Yamaguchi J, Itami K. A general catalyst for the β-selective C–H Bond arylation of thiophenes with iodoarenes. Angew Chem Int Ed. 2010;49:8946–8949. doi: 10.1002/anie.201005082. [DOI] [PubMed] [Google Scholar]
- 29.Wang P, Farmer ME, Huo X, Jain P, Shen PX, Ishoey M, Bradner JE, Wisniewski SR, Eastgate MD, Yu JQ. Ligand-promoted meta-C–H arylation of anilines, phenols, and heterocycles. J Am Chem Soc. 2016;138:9269–9276. doi: 10.1021/jacs.6b04966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bedford RB, Bowen JG, Davidson RB, Haddow MF, Seymour-Julen AE, Sparkes HA, Webster RL. Facile hydrolysis and alcoholysis of palladium acetate. Angew Chem Int Ed. 2015;54:6591–6594. doi: 10.1002/anie.201500620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Information files. Metrical parameters for the structure of Pd(Phen)(L69)2, Pd3(μ2-OH)(L69)5 complex and 7d (see Supplementary Information) are available free of charge from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/) under reference numbers CCDC 1552055, CCDC 1538821, and CCDC 1538820, respectively.