Abstract
Background & Aims
Recommendations for surveillance after curative surgery for colorectal cancer (CRC) include a 1 year post-resection abdominal-pelvic computed tomography scan and optical colonoscopy (OC). CT colonography (CTC), when used in CRC screening, effectively identifies colorectal polyps ≥10 mm and cancers. We performed a prospective study to determine whether CTC, concurrent with CT, could substitute for OC in CRC surveillance.
Methods
Our study enrolled 231 patients with resected stage 0–III CRC, identified at 5 tertiary care academic centers. Approximately 1 year after surgery, participants underwent outpatient CTC plus CT, followed by same-day OC. CTC results were revealed after endoscopic visualization of sequential colonic segments, which were reexamined for discordant findings. The primary outcome was performance of CTC in detection of colorectal adenomas and cancers, using endoscopy as the reference standard.
Results
Of the 231 participants, 116 (50.2%) had polyps of any size or histology identified by OC, and 15.6% had conventional adenomas and/or serrated polyps ≥6 mm. No intra-luminal cancers were detected. CTC detected patients with polyps of ≥6 mm with 44.0% sensitivity (95% CI, 30.2–57.8) and 93.4% specificity (95% CI, 89.7–97.0). CTC detected polyps ≥10 mm with 76.9% sensitivity (95% CI 54.0–99.8) and 89.0% specificity (95% CI, 84.8–93.1). Similar values were found when only adenomatous polyps were considered. The negative predictive value of CTC for adenomas ≥6 mm was 90.7 (95% CI, 86.7–94.5) and for adenomas ≥10 mm the negative predictive value was 98.6 (95% CI, 97.0–100).
Conclusions
In a CRC surveillance population 1 year following resection, CTC was inferior to OC for detecting patients with polyps ≥6 mm.
Keywords: Colorectal cancer, colonoscopy, CT colonography, Surveillance
Introduction
In the United States, about 100,000 patients annually undergo colorectal cancer (CRC) resection with curative intent. 1 These patients join the roughly 1,000,000 patients eligible for participation in post-resection surveillance. 2 Adherence with widely promulgated surveillance guidelines is uneven. 3,4 These follow-up strategies typically incorporate endoscopic and radiologic imaging, laboratory testing and physical examination. 5, 6 The goal of endoscopic surveillance is primarily to detect adenomatous polyps, as the identification of metachronous cancers or intra-luminal recurrence is infrequent. The complementary goals of cross sectional imaging are to provide information about extra-luminal disease, either local recurrence or more often, distant metastatic disease.
Virtual colonoscopy or computed tomography colonography (CTC) combines CT scanning with image reconstruction based on 2D and 3D visualization modes capable of visualizing the entire colorectum. Like standard optical colonoscopy (OC), CTC typically requires a cathartic bowel preparation. 7 In CRC screening studies, CTC identifies colorectal polyps ≥10mm in diameter and cancers nearly as well as OC. 8, 9, 10 Potential advantages of CTC over OC include shorter procedure times, no sedation requirements or need for a chaperone afterwards, and less risk of procedural complications.
Patients enrolled in post-operative CRC surveillance programs typically undergo abdominal-pelvic CT scan at one year after surgery. 11 We hypothesized that visualizing the colonic lumen with CTC could provide a clinically useful substitute for OC. The combined performance of CT and CTC could improve patient compliance and safety through the potential elimination of a separate colonoscopy. Here, we report the results of a prospective trial comparing the clinical performance of CTC (conducted with standard surveillance CT) to OC in the setting of 1 year post-operative CRC surveillance.
Methods
Patient Recruitment
Participants in this institutional review board approved trial were recruited from the medical and surgical oncology practices of five United States academic medical centers of varied size. We searched the scheduling databases of each, applying electronic filters matching eligibility requirements which included: (a) men and women 18 years and older; (b) personal history of CRC stages 0–III at diagnosis; (c) no overt evidence of distant metastatic disease; (d) completion of acute cancer-specific therapy. Patients with a diverting ileostomy, a history of inflammatory bowel disease or familial adenomatous polyposis were excluded as were patients who were pregnant or had active gastrointestinal symptoms (including gastrointestinal bleed, diarrhea, and/or severe abdominal pain).
The primary study goal was to compare, in the post-CRC resection surveillance setting, the performance of CTC to detect colorectal adenomas and cancers, using OC as the reference standard. Most guidelines recommend OC one year after curative resection. 5,11 To allow for scheduling variability, CTCs and OCs performed 9–16 months following resection were acceptable for this study. All study participants provided informed consent to allow performance of standard abdominal-pelvic CT plus CTC followed by OC on the same day.
Study Procedures
The day before the examinations patients completed any of several cathartic bowel preparations typically used to prepare for OC. All patients were made nothing-per-os (NPO) after midnight before testing. In all cases, CTC was performed first, followed by OC.
CTC
In addition to bowel catharsis, oral contrast tagging was performed for CTC. The colon was distended with automated low-pressure carbon dioxide delivery per rectum or end colostomy in a standard fashion. Following an unenhanced, low-dose prone and/or decubitus series for CTC, a diagnostic, IV contrast-enhanced CT/CTC series of the abdomen and pelvis was performed in the supine position. Imaging in two positions minimizes the likelihood of inadequate visualization due to limited colonic distention. 12 CTC evaluation of the entire colorectum was performed on dedicated workstations using 2D as well as 3D endoluminal imaging. All polyps seen were identified by size, morphology and location. After localization, the anastomotic region (defined as within 5 cm of the surgical anastomosis) was separately evaluated for abnormality. Upon completion and immediate interpretation of the examination, results were collected by a research team member who would be present for the subsequent OC.
OC
OC was performed within several hours (average 1–2 hours) by an experienced endoscopist blinded to the results of the CTC. Once OC results for specific colon segments (cecum/ascending colon, transverse colon, descending colon, sigmoid colon/rectum) were collected, results of the same segment on CTC were revealed. In the event of a discrepancy between the two studies (i.e. lesion seen on CTC but not found on initial OC), the endoscopist re-examined the segment of the colorectum with discordant findings, with the final “enhanced” colonoscopy (EC) considered as the gold standard for lesion identification. This method of segmental unblinding as well as the creation of an enhanced reference standard is described for other CTC studies. 8,13 Polyps detected at OC were digitally photographed prior to polypectomy.
Histology Review and Lesion Matching
All polyps underwent histological analysis by site pathologists. Polyps were classified based upon standard criteria into conventional adenomas, serrated polyps/adenomas (sessile serrated and traditional serrated), polyps with conventional adenoma and serrated components, hyperplastic and inflammatory polyps. Lesion size was determined from the OC (or EC) report. Lesion matching between radiographic and endoscopic examinations was performed based on established algorithms that incorporate lesion location (within one colonic segment) and size (+/−50%). 8
Data Analysis
Standard descriptive statistics were used to characterize the study population. The primary efficacy analyses were at the per person, rather than per polyp level because in standard clinical practice identification of one (or more) ≥6 mm lesions on CTC would trigger OC follow-up. Sensitivity and specificity along with 95% two-sided confidence intervals were computed. We defined sensitivity in terms of patients for whom OC detected at least one ≥6 mm adenoma. Similarly, we defined specificity in terms of patients for whom OC detected no ≥6 mm adenomas. For the person-level analyses, CTC was defined as positive if at least one ≥6mm lesion as measured by CTC was detected.
To evaluate CTC efficacy, we conducted a 1-sided test of an exact binomial proportion (α=0.05) with a null hypothesis that sensitivity was ≥0.9 against the alternative that sensitivity was less than 0.9. We tested specificity using the same approach. Per protocol, we proposed that CTC performance would not represent an adequate replacement for OC if the sensitivity or specificity were significantly less than 0.9 in comparison. We also computed positive predictive value (PPV), negative predictive value (NPV), along with associated two-sided 95% confidence bounds to further characterize the utility of CTC in this population. In secondary analyses we estimated sensitivity, specificity, PPV and NPV for (1) adenomas (conventional, serrated, mixed histology) ≥10 mm, (2) any OC-detected polyp (any histology) ≥6 mm, and (3) any OC-detected polyp ≥10 mm. When required, confidence intervals based on robust standard errors estimated using Generalized Estimating Equations (GEE) methods for binary outcome data were used to account for within-patient correlations across lesions. For these analyses CTC was defined as positive for lesions ≥6mm as measured by CTC.
We computed sensitivity, specificity, NPV and PPV of CTC, along with 95% two-sided confidence intervals, separately for different sub groups (e.g., age, gender, recruiting institution, primary site). Generalized Fisher’s exact tests (two-sided, α=0.05) were used to assess the equality of these statistics across sub groups. We also examined the results of CTC and OC compared to “enhanced colonoscopy” (EC), where EC was defined as the findings on OC after CTC results were revealed during segmental unblinding. All statistics were computed using SAS software version 9.4 (Carey, NC).
Sample size for this study was selected to provide at least 85% power to detect true sensitivities of less than 82% and specificities less than 87%. The available published literature suggested that 15% of study patients would harbor at least 1 adenoma ≥6 mm by OC. 14 The initial sample size target of approximately 1000 participants was not reached because of slower than anticipated enrollment (seen equally at all sites). There were no systematic demographic or clinical features which differentiated participants from those who declined participation. However, following recommendations of the study’s Data and Safety Monitoring Board which conducted planned, blinded, interim data analyses, we continued study enrollment until the end of the funding period.
All authors had access to the study data and reviewed and approved the final manuscript.
Results
A total of 231 participants completed CTC and OC (Figure). Because of larger than anticipated differences in CTC and OC performance, this smaller sample size was adequate to address the primary and secondary study questions. The quality of bowel visualization was subjectively deemed adequate in all cases by experienced radiologists and endoscopists. No formal scoring systems were systematically employed. The oral contrast employed for CTC had no meaningful impact on OC performance. The cecum (or ileo-colonic anastomosis) was visualized endoscopically in all cases.
Figure.
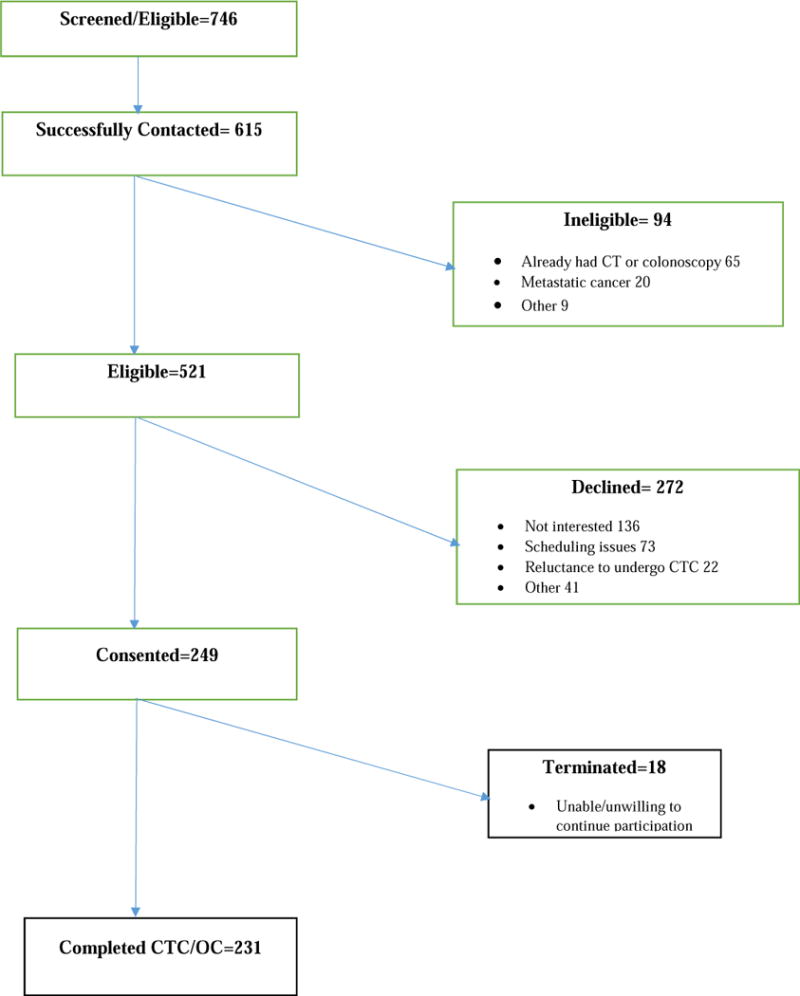
CT Colonography v. Colonoscopy for Post-Operative CRC Surveillance
Table 1 presents baseline demographic and clinical characteristics, including primary tumor location, cancer stage, post-operative treatment and time to CTC/OC follow-up. The median participant age was 58 years (range: 25–89y). The majority of participants were male (60.6%) and white (87.8%). Distant metastatic disease at diagnosis was an exclusion criterion, so all participants had Stage 0–III disease. Slightly less than half (48.1%) of the participants had stage III disease. Tumor site distribution was consistent with historical norms. Roughly 10% of participants had occlusive lesions at diagnosis which precluded full pre-operative colonoscopic evaluation (data not shown).
Table 1.
Demographic and clinical characteristics (N=231)
| Characteristic | |
|---|---|
| Age at enrollment in years | |
| Mean (Standard Deviation) | 58.47 (13.12) |
| Median (Range) | 58 (25–89) |
| Gender number (%) | |
| Male | 140 (60.6) |
| Female | 91 (39.4) |
| Race or ethnic group — number. (%) | |
| White | 203 (87.8) |
| African American | 14 (6.1) |
| Other | 14 (6.1) |
| Hispanic or Latino ethnicity number (%) | |
| Yes | 4 (1.7) |
| No | 224 (97.0) |
| Unknown | 3 (1.3) |
| Stage | |
| 0 | 3 (1.3) |
| I | 46 (19.9) |
| II | 71 (30.7) |
| III | 111 (48.1) |
| Treatment after surgery number (%) | |
| No treatment | 102 (44.1) |
| Chemotherapy | 112 (48.5) |
| Chemotherapy and Radiotherapy | 17 (7.4) |
| Time from surgery to months follow-up CT Colonography/Optical colonoscopy in | |
| Mean (Standard Deviation) | 11.99 (2.83) |
| Median (Range) | 11.9 (2.3–23.9) |
| Primary tumor location | |
| Cecum/Ascending colon | 80 (34.6) |
| Descending colon | 74 (32.0) |
| Transverse colon | 11 (4.8) |
| Rectum/Sigmoid colon | 66 (28.6) |
Overall, 116/231 (50.2%) participants had polyps of any size or histology identified by OC. When considering only lesions ≥6mm or ≥10mm, 50/231 (21.6%) and 13/231 (5.6%) of patients respectively harbored polyps. Conventional and/or serrated polyp/adenomas of ≥6mm were seen in 36/231 (15.6%) of participants, and conventional and/or serrated polyp/adenomas ≥10 mm were seen in 10/231 (4.3%) (Table 2). No intra-luminal cancers were identified.
Table 2.
Distribution of number of lesions per patient for CT Colonography and Optical Colonoscopy by size and pathology.
| Number of Lesions | Optical Colonoscopy Any size | Optical Colonoscopy ≥6mm |
Optical Colonoscopy ≥10mm |
Optical Colonoscopy Any Size | Optical Colonoscopy ≥6mm |
Optical Colonoscopy ≥10mm |
CT Colonography |
|---|---|---|---|---|---|---|---|
| Pathology | All lesions | All lesions | All lesions | Adenomas1 | Adenomas1 | Adenomas1 | All lesions |
| 0 | 115 | 181 | 218 | 160 | 195 | 221 | 172 |
| 1 | 58 | 36 | 9 | 49 | 29 | 8 | 41 |
| 2 | 25 | 11 | 2 | 11 | 6 | 1 | 10 |
| 3 | 15 | 1 | 1 | 3 | 0 | 1 | 4 |
| 4 | 8 | 0 | 1 | 3 | 0 | 0 | 1 |
| 5 | 3 | 1 | 0 | 3 | 1 | 0 | 1 |
| 6 | 4 | 1 | 0 | 1 | 0 | 0 | 1 |
| 7 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 2 | 0 | 0 | 1 | 0 | 0 | 0 |
Adenomas included conventional, serrated and mixed histology
Table 3 demonstrates the comparative performance of CTC for any polyp ≥6mm as measured by OC and also for the smaller group of participants with any polyp ≥10mm. The gold standard for comparison in both cases was the OC. In the per patient analysis (where the identification of one or more polyps is defined as a positive result), the sensitivity and PPV of CTC was limited compared to OC for polyps at the 6mm or the 10mm cutoff. For polyps ≥6mm the sensitivity, specificity and PPV were 44.0% (95% CI 30.2–57.8), 93.4% (95% CI 89.7–97.0) and 64.7% (95% CI 48.6–80.8), respectively. The estimates for sensitivity and positive predictive value were significantly different from the a priori defined null rate of 90% (p<.05). At the 10mm cutoff, sensitivity increased to 76.9% (95% CI 54.0–99.8), however the PPV decreased to 29.4% (95% CI 14.1–44.7).
Table 3.
Performance Characteristics of CT Colonography relative to Optical Colonoscopy for detecting lesions of all types1,2
| Performance Characteristic | Lesion Size Category from Optical Colonoscopy | |
|---|---|---|
| ≥6mm | ≥10mm | |
| Patient-Level Analyses3 | ||
| Sensitivity | 22/50 44.0 (30.2–57.8)* |
10/13 76.9 (54.0–99.8) |
| Specificity | 169/181 93.4 (89.7–97.0) |
194/218 89.0 (84.8–93.1) |
| Negative Predictive Value | 169/197 85.8 (80.9–90.7) |
194/197 98.5 (96.8–100) |
| Positive Predictive Value | 22/34 64.7 (48.6–80.8)* |
10/34 29.4 (14.1–44.7)* |
number/total number, performance characteristic estimate and two-sided 95% Cell values include confidence intervals
Optical colonoscopy results are from initial pass before CTC results were revealed to gastroenterologist. CTC is defined as positive for a patient if at least one ≥6mm lesion as measured by CTC is detected.
Lesion size category represents maximum lesion size per patient as determined by optical colonoscopy.
operating characteristics significantly less than 90% (p<.05).
The CTC performance characteristics were overall lower than those reported in the CRC screening setting. 15 However, because the pre-test probability of non-diminutive (>5mm) polyps in the current patient population was small, the observed negative predictive value of CTC for polyps ≥6mm was 85.8% (95%CI 80.9–90.7) and for polyps ≥10mm was 98.5% (95% CI 96.8–100).
Radiographically, it can be difficult to differentiate adenomatous (conventional or serrated) from non-adenomatous polyps. However, the identification of polyps without cancer risk (e.g. hyperplastic polyps) is of less clinical relevance. Therefore, Table 4 repeats the same analysis as Table 3, but is restricted to those polyps identified at subsequent histologic analysis as conventional or serrated polyp/adenomas. No substantial test performance differences were noted with this added restriction. Sensitivity remained significantly lower than 90% at the 6mm cutoff (p<.05), while NPV was maintained for ≥6mm and ≥10mm adenomas at 90.7 (95%CI 86.7–94.5) and 98.6% (95%CI 97.0–100), respectively.
Table 4.
Performance Characteristics of CT Colonography relative to Optical Colonoscopy for detecting adenomas1,2,3
| Performance Characteristic | Adenoma Size Category from Optical Colonoscopy | |
|---|---|---|
| ≥6mm | ≥10mm | |
| Patient-Level Analyses4 | ||
| Sensitivity | 16/36 44.4 (28.2–60.7)* |
7/10 70.0 (41.6–98.4) |
| Specificity | 193/195 98.9 (97.6–100) |
210/221 95.0 (92.2–97.9) |
| Negative Predictive Value | 193/213 90.7 (86.7–94.5) |
210/213 98.6 (97.0–100) |
| Positive Predictive Value | 16/18 88.9 (74.4–100) |
7/18 38.9 (16.4–61.4)* |
Cell values include number/total number, performance characteristic estimate and two sided 95%-confidence intervals
Optical colonoscopy results are from initial pass before results from virtual colonoscopy were revealed to gastroenterologist. CTC is defined as positive for a patient if at least one ≥6mm lesion as measured by CTC is detected.
Adenomas include serrated polyps
Adenoma size category represents maximum adenoma size per patient as determined by optical colonoscopy.
-operating characteristics significantly less than 90% (p<.05).
Because of the concern that CTC may not adequately detect serrated polyps since such lesions are more often morphologically flat 16, we calculated the patient level test performance of CTC when polyps were further classified into conventional adenomas, serrated or mixed histology subtypes (Table 5). At both the ≥6mm and ≥10mm cutoffs, sensitivity and specificity were similar across subtypes, and to results when “adenomatous polyps” were considered as a single group.
Table 5.
Performance Characteristics of CT Colonography relative to Optical Colonoscopy to detect serrated v. conventional adenomas1,2,
| 1. Serrated only, patient level | ||
|---|---|---|
| Performance Characteristic | Adenoma Size Category from Optical Colonoscopy | |
| ≥6mm | ≥10mm | |
| Patient-Level Analyses3 | ||
| Sensitivity | 6/10 60.0 (29.6–90.4)* |
3/4 75.0 (32.6–100) |
| Specificity | 168/221 76.0 (70.4–81.6)* |
171/227 75.3 (69.7–80.9)* |
| Negative Predicted Value | 168/172 97.7 (95.4–99.9) |
171/172 99.4 (98.3–100) |
| Positive Predicted Value | 6/59 10.2 (2.5–17.9)* |
3/59 5.1 (0–10.7)* |
| 2. Serrated + mixed serrated and conventional adenoma, patient level | ||
|---|---|---|
| Performance Characteristic | ≥6mm | ≥10mm |
| Patient-Level | ||
| Sensitivity | 6/11 54.6 (25.1–84.0)* |
3/4 75.0 (32.6–100) |
| Specificity | 167/220 75.9 (70.3–81.6)* |
171/227 75.3 (69.7–80.9)* |
| Negative Predicted Value | 167/172 97.1 (94.6–99.6) |
171/172 99.4 (98.3–100) |
| Positive Predicted Value | 6/59 10.2 (2.5–17.9)* |
3/59 5.1 (0–10.7)* |
| 3. Conventional adenoma only, patient level. | ||
|---|---|---|
| Performance Characteristic | ≥6mm | ≥10mm |
| Patient-Level | ||
| Sensitivity | 16/25 64.0 (45.2–82.8)* |
5/6 83.3 (53.5–100) |
| Specificity | 163/206 79.1 (73.6–84.7)* |
171/225 76.0 (70.4–81.6)* |
| Negative Predicted Value | 163/172 94.8 (91.4–98.1) |
171/172 99.4 (98.3–100) |
| Positive Predicted Value | 16/59 27.1 (15.8–38.5)* |
5/59 8.5 (1.4–15.6)* |
Cell values include number/total number, performance characteristic estimate and two-sided 95% confidence intervals
Optical colonoscopy results are from initial pass before results from virtual colonoscopy were revealed to gastroenterologist. CTC is defined as positive for a patient if at least one ≥6mm lesion as measured by CTC is detected.
Adenoma size category represents maximum adenoma size per patient as determined by optical colonoscopy.
operating characteristics significantly less than 90% (p<.05).
We performed additional subset analyses exploring the relationships between CTC test performance and patient age, gender, site of primary resection, impact of per-anastomotic lesions and enrollment site. No important variations from the primary results were observed for age sub groups (Supplemental Table 1). However, for adenomas ≥6mm there were significant differences in sensitivity across genders (p<0.05) and primary tumor sites (p<0.05), with diminished test performance values observed in males (32% sensitivity) and patients with initial tumors in the ascending colon (0% sensitivity) (Supplemental Tables 2 and 3). A significant difference in NPV across genders (p<0.05) was also observed. There were no significant differences in test performance across enrollment sites (Supplemental Table 4).
We employed a standard protocol to match polyps detected by CTC or OC. 8 In addition to presence or absence, lesions were matched for size and location. To ensure appropriate classification and matching of lesions seen on CTC, we employed EC as an enhanced gold standard. A total of 8 polyps (including 5 conventional or serrated adenomas, ranging from 4–10mm) were seen on CTC, but not first pass OC. EC detected 2 lesions ≥10 mm observed by CTC that were missed by OC. (Supplemental Table 5) To assess the fidelity of the matching process for polyp size, we reviewed the data from all participants with a lesion ≥6mm in diameter seen on CT, OC and/or EC. The median absolute difference in polyp size measurement of between CTC and OC, and CTC and EC was 1.0mm.
Extra colonic findings
CTC detected extramural peri-anastomotic recurrence in two patients (0.9%). In addition, CT imaging revealed new extra-colonic disease consistent with metastatic CRC in 11 patients.
Adverse Events
No adverse events requiring prolonged observation or hospitalization were reported following OC. Immediately post-CTC, one participant received antibiotics and extended observation for a possible perforation. No surgical intervention was required.
Discussion
Our study compared the clinical performance characteristics of CT colonography (CTC) to optical colonoscopy (OC) to identify colorectal lesions in patients one year after curative intent surgical resection for colorectal cancer. For polyps 6–9 mm, or ≥10mm, the sensitivity and positive predictive value of CTC were inferior relative to OC. Sensitivity of CTC remained suboptimal when the analyses considered conventional adenomas and serrated polyps only. The negative predictive value of CTC at 1 year exceeded 98% for large (≥10mm) polyps of any histology reflecting, in part, the predictably low prevalence of neoplastic polyps in a 1 year post-CRC surveillance population.
Most current CRC surveillance guidelines recommend physical examination, laboratory testing, OC to assess for intra-luminal neoplasia (polyps and/or cancer) and IV contrast-enhanced CT to evaluate for both distant metastatic and local extra-luminal recurrence. Adherence with this multi-component strategy is inconsistent. 3 While potentially attractive, a role for CTC in CRC surveillance is undefined. 11,17 With appropriate planning, CT and CTC can be completed at the same time, raising the possibility that CTC could prove a compelling alternative to the independent performance of CT and OC, especially if the combination improved surveillance compliance.
Recent analyses suggest that patients who adhere to surveillance colonoscopy after CRC resection have lower overall, but not disease-specific mortality. 11 Intra-luminal and anastomotic recurrences detectable by OC are infrequent compared to extra-luminal recurrence that is detectable by standard CT. Therefore, the substitution of CTC for higher risk OC would be desirable if the clinical performance of the former were acceptable. Previous studies of CTC for post-operative surveillance in CRC are limited with no prospective comparison of CTC and OC in all participants. 18, 19, 20 The design of the largest of these retrospective cohort studies (548 patients) reported OC results only in those patients with a positive CTC. 18 The performance characteristics for neoplasia detection in that report are similar to those reported here. A recent meta-analysis reported that CTC was 100% sensitive to detect metachronous CRC in a surveillance population. 21 That same report provided no information about polyp detection rates, the more clinically relevant question for CTC in the surveillance setting.
The most prominent role for CTC to date is following incomplete OC. However, screening is the application with the greatest potential impact. CTC was included as an acceptable screening method for average risk individuals in the 2016 guidelines from the U.S. Preventive Services Task Force (USPSTF). 22 The performance characteristics we report for CTC in the surveillance setting are somewhat lower than those in the screening setting, although the results are more comparable for polyps ≥10mm. The inability of CTC to identify flat polyps is a potential concern in both screening and surveillance settings.23, 24 Our study, in contrast to some recent reports16 did not identify a significant difference in detection rates for serrated versus non-serrated polyps. In addition, it is well described that CTC is not as accurate as OC to identify small (≤5 mm) polyps. 25 In average risk populations missing these diminutive polyps, which rarely harbor advanced histology, is probably of little clinical importance.26 However, in increased-risk patients with a history of resected CRC, risk tolerance may be different, especially in those patients who did not have a complete, pre-resection OC.
Results from the present study shed important light on some potential radiographic challenges seen more frequently in the post-operative setting. Evaluation of the anastomosis by CTC appears comparable to OC. 21,27 However, polyp identification by CTC was relatively better in patients with primary left rather than right-sided cancers suggesting that right hemicolectomy, including ileocecal valve resection, might reduce accuracy perhaps because of impaired insufflation.
Based on our study findings, it is clear that in head-to-head comparison CTC cannot be viewed as a replacement for OC. However, the likelihood of advanced adenoma or cancer in average risk surveillance populations is low. 28 Based on our data, universal application of CTC at year 1 post-CRC resection could reduce OC use by 92.2%. Thus, for every 1000 patients, this approach would eliminate the need for 922 OCs. Of the 78 OCs performed per 1000 we would anticipate 69.3 would identify patients with one or more ≥6 mm adenomas; or 30.3 patients with one or more ≥10 mm adenoma(s). Unfortunately, this strategy would also result in 86.6 patients with one or more ≥6 mm adenomas, and 13.0 patients with one or more ≥10 mm adenomas going undetected at year one post-resection. Additional investigations of the cost-effectiveness, patient preference and compliance with different surveillance strategies may inform these important trade-offs.
Our study has potential limitations. There were two extra-luminal peri-anastomotic lesions detected on CT, but no intra-luminal cancers were identified in this study group. Intra-luminal metachronous cancer 1 year post-operatively would be an unusual event in any population at average risk for CRC, where the majority (90% in this study) had a complete pre-operative colonoscopy presumably excluding synchronous CRCs. The primary goal of intra-luminal colonic visualization, by any post-operative surveillance technique, is to prevent metachronous CRC by adenoma detection and subsequent removal. In our study, about 15% of the study population did have colon adenomas ≥6mm, the same prevalence reported by others. The radiologists involved in our study typically had extensive experience with CTC. Hence, generalizability to other settings where CTC is infrequently performed is unknown. Finally, in the screening setting, patient preference between CTC and OC is mixed, although generally favoring the former. 29–31 As compliance with post-operative surveillance is sub-optimal, a better understanding of preference among CRC patients will be required, especially if CTC were recommended as part of routine post-operative surveillance program. In this context, it is useful to note that the sensitivity and specificity of CTC in our study is comparable to the performance of a universally endorsed surveillance test, mammography for women with a history of breast cancer. 32 In contrast, breast MRI a more sensitive technique, has yet to be adopted because of uncertainty about patient preference and cost-effectiveness. 33
In summary, this prospective, multi-center trial compared CTC and OC to detect colorectal polyps and cancer in patients enrolled in post-operative CRC surveillance programs. The performance of CTC was inferior to OC. While CTC was substantially less sensitive than OC, further study particularly of patient preference and cost-effectiveness, is required to assess whether there is any role for the programmatic use of CTC in patients already receiving CT surveillance for extra-luminal disease.
Supplementary Material
Acknowledgments
Study Funding: NIH RO1 CA155347 (DSW). The funder had no role in any aspect of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No author reports any significant potential conflicts.
Author Contributions: All authors contributed to acquisition of data, analysis and interpretation of data, and critical revision of the manuscript. In addition, Dr Weinberg was responsible for the study concept and design, obtaining funding and along with Ms Keenan, overall study supervision. Dr Ross and Ms Li directed the statistical analyses.
Clinical Trials.gov Registration Number: NCT02143115
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Mariotto AB, Rowland JH, Ries LA, et al. Multiple cancer prevalence: a growing challenge in long-term survivorship. Cancer Epidemiol Biomarkers Prev. 2007;16:566–71. doi: 10.1158/1055-9965.EPI-06-0782. [DOI] [PubMed] [Google Scholar]
- 3.Cooper GS, Kou TD, Reynolds HL., Jr Receipt of guideline-recommended followup in older colorectal cancer survivors : a population-based analysis. Cancer. 2008;113:2029–37. doi: 10.1002/cncr.23823. [DOI] [PubMed] [Google Scholar]
- 4.Hines RB, Barrett A, Twumasi-Ankrah P, et al. Predictors of guideline treatment nonadherence and the impact on survival in patients with colorectal cancer. J Natl Compr Canc Netw. 2015;13:51–60. doi: 10.6004/jnccn.2015.0008. [DOI] [PubMed] [Google Scholar]
- 5.Benson AB, 3rd, Venook AP, Bekaii-Saab T, et al. Colon cancer, version 3.2014. J Natl Compr Canc Netw. 2014;12:1028–59. doi: 10.6004/jnccn.2014.0099. [DOI] [PubMed] [Google Scholar]
- 6.Desch CE, Benson AB, 3rd, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–9. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 7.Neri E, Lefere P, Gryspeerdt S, et al. Bowel preparation for CT colonography. Eur J Radiol. 2013;82:1137–43. doi: 10.1016/j.ejrad.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 9.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207–17. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin JS, Piper MA, Perdue LA, et al. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;315:2576–94. doi: 10.1001/jama.2016.3332. [DOI] [PubMed] [Google Scholar]
- 11.Kahi CJ, Boland CR, Dominitz JA, et al. Colonoscopy surveillance after colorectal cancer resection: recommendations of the US multi-society task force on colorectal cancer. Gastrointest Endosc. 2016;83:489–98 e10. doi: 10.1016/j.gie.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007;357:1403–12. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 13.Cotton PB, Durkalski VL, Pineau BC, et al. Computed tomographic colonography (virtual colonoscopy): a multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA. 2004;291:1713–9. doi: 10.1001/jama.291.14.1713. [DOI] [PubMed] [Google Scholar]
- 14.Cone MM, Beck DE, Hicks TE, et al. Timing of colonoscopy after resection for colorectal cancer: are we looking too soon? Dis Colon Rectum. 2013;56:1233–6. doi: 10.1097/DCR.0b013e3182a228d1. [DOI] [PubMed] [Google Scholar]
- 15.Mulhall BP, Veerappan GR, Jackson JL. Meta-analysis: computed tomographic colonography. Ann Intern Med. 2005;142:635–50. doi: 10.7326/0003-4819-142-8-200504190-00013. [DOI] [PubMed] [Google Scholar]
- 16.JE IJ, Tutein Nolthenius CJ, Kuipers EJ, et al. CT-Colonography vs. Colonoscopy for Detection of High-Risk Sessile Serrated Polyps. Am J Gastroenterol. 2016;111:516–22. doi: 10.1038/ajg.2016.58. [DOI] [PubMed] [Google Scholar]
- 17.Spada C, Stoker J, Alarcon O, et al. Clinical indications for computed tomographic colonography: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline. Eur Radiol. 2015;25:331–45. doi: 10.1007/s00330-014-3435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HJ, Park SH, Pickhardt PJ, et al. CT colonography for combined colonic and extracolonic surveillance after curative resection of colorectal cancer. Radiology. 2010;257:697–704. doi: 10.1148/radiol.10100385. [DOI] [PubMed] [Google Scholar]
- 19.Amitai MM, Fidder H, Avidan B, et al. Contrast-enhanced CT colonography with 64-slice MDCT compared to endoscopic colonoscopy in the follow-up of patients after colorectal cancer resection. Clin Imaging. 2009;33:433–8. doi: 10.1016/j.clinimag.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Choi YJ, Park SH, Lee SS, et al. CT colonography for follow-up after surgery for colorectal cancer. AJR Am J Roentgenol. 2007;189:283–9. doi: 10.2214/AJR.07.2305. [DOI] [PubMed] [Google Scholar]
- 21.Porte F, Uppara M, Malietzis G, et al. CT colonography for surveillance of patients with colorectal cancer: Systematic review and meta-analysis of diagnostic efficacy. Eur Radiol. 2017;27:51–60. doi: 10.1007/s00330-016-4319-1. [DOI] [PubMed] [Google Scholar]
- 22.Force USPST. Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315:2564–75. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 23.Kim DH, Matkowskyj KA, Lubner MG, et al. Serrated Polyps at CT Colonography: Prevalence and Characteristics of the Serrated Polyp Spectrum. Radiology. 2016:151608. doi: 10.1148/radiol.2016151608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickhardt PJ, Kim DH, Robbins JB. Flat (Nonpolypoid) Colorectal Lesions Identified at CT Colonography in a US Screening Population. Academic Radiology. 2010;17:784–790. doi: 10.1016/j.acra.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Pickhardt PJ. Missed lesions at CT colonography: lessons learned. Abdom Imaging. 2013;38:82–97. doi: 10.1007/s00261-012-9897-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickhardt PJ, Kim DH. Performance of CT colonography for detecting small, diminutive, and flat polyps. Gastrointestinal Endoscopy Clinics of North America. 2010;20:209–26. doi: 10.1016/j.giec.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Pickhardt P, Edwards K, Bruining D, et al. Prospective Trial Evaluating the Surgical Anastomosis at One-year Colorectal Cancer Surveillance: CT colonography versus optical colonoscopy and implications for patient care. Diseases of the Colon and Rectum. 2017 doi: 10.1097/DCR.0000000000000845. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rulyak SJ, Lieberman DA, Wagner EH, et al. Outcome of follow-up colon examination among a population-based cohort of colorectal cancer patients. Clin Gastroenterol Hepatol. 2007;5:470–6. doi: 10.1016/j.cgh.2006.11.027. quiz 407. [DOI] [PubMed] [Google Scholar]
- 29.Siewert B, Gareen I, Vanness D, et al. ACRIN 6664: Patient acceptance and preferance of CT colonography compared to optical colonoscopy for colon cancer screening. J Clin Oncol. 2009;27:4034. [Google Scholar]
- 30.Pooler BD, Baumel MJ, Cash BD, et al. Screening CT colonography: multicenter survey of patient experience, preference, and potential impact on adherence. American Journal of Roentgenology. 2012;198:1361–1366. doi: 10.2214/AJR.11.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moawad FJ, Maydonovitch CL, Cullen PA, et al. CT colonography may improve colorectal cancer screening compliance. American Journal of Roentgenology. 2010;195:1118–1123. doi: 10.2214/AJR.10.4921. [DOI] [PubMed] [Google Scholar]
- 32.Robertson C, Ragupathy SK, Boachie C, et al. Surveillance mammography for detecting ipsilateral breast tumour recurrence and metachronous contralateral breast cancer: a systematic review. Eur Radiol. 2011;21:2484–91. doi: 10.1007/s00330-011-2226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam DL, Houssami N, Lee JM. Imaging Surveillance After Primary Breast Cancer Treatment. AJR Am J Roentgenol. 2017;208:676–686. doi: 10.2214/AJR.16.16300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


