Abstract
Mrs3 and Mrs4 are mitochondrial inner membrane proteins that deliver an unidentified cytosolic iron species into the matrix for use in iron-sulfur cluster (ISC) and heme biosynthesis. The Mrs3/4 double-deletion strain (ΔΔ) grew slowly in iron-deficient glycerol/ethanol medium but recovered to wild-type (WT) rates in iron-sufficient medium. ΔΔ cells grown under both iron-deficient and iron-sufficient respiring conditions acquired large amounts of iron relative to WT cells, indicating iron homeostatic dysregulation regardless of nutrient iron status. Biophysical spectroscopy (including Mössbauer, electron paramagnetic resonance, and electronic absorption) and bioanalytical methods (liquid chromatography with online inductively coupled plasma mass spectrometry detection) were used to characterize these phenotypes. Anaerobically isolated mitochondria contained a labile iron pool composed of a nonheme high-spin FeII complex with primarily O and N donor ligands, called Fe580. Fe580 likely serves as feedstock for ISC and heme biosynthesis. Mitochondria from respiring ΔΔ cells grown under iron-deficient conditions were devoid of Fe580, ISCs, and hemes; most iron was present as FeIII nanoparticles. O2 likely penetrates the matrix of slow-growing poorly respiring iron-deficient ΔΔ cells and reacts with Fe580 to form nanoparticles, thereby inhibiting ISC and heme biosynthesis. Mitochondria from iron-sufficient ΔΔ cells contained ISCs, hemes, and Fe580 at concentrations comparable to those of WT mitochondria. The matrix of these mutant cells was probably sufficiently anaerobic to protect Fe580 from degradation by O2. An ~1100 Da manganese complex, an ~1200 Da zinc complex, and an ~5000 Da copper species were also present in ΔΔ and WT mitochondrial flow-through solutions. No lower-mass copper complex was evident.
Graphical Abstract

Mitochondria are the major sites of iron-sulfur cluster (ISC) biosynthesis in eukaryotes and the only sites for the iron-insertion step of heme biosynthesis; thus, large amounts of iron must be imported into the organelle.1,2 Mrs3 and Mrs4 (Mrs3/4) in Saccharomyces cerevisiae are paralogous mitochondrial inner membrane proteins that deliver cytosolic iron into the matrix, presumably for use in both processes.3–7 Mammals have homologues of Mrs3/4 called Mitoferrin1/2.8,9 The iron species imported by these proteins is unknown, but the narrow channels in these proteins imply a small coordination complex.4,10,11
Mössbauer spectra of anaerobically isolated mitochondria reveal a pool of nonheme high-spin (NHHS) FeII in the organelle.12 The concentration of iron in this pool ranges from 60 to 200 μM, whereas the overall iron concentration in mitochondria ranges from 400 to 800 μM.13,14 The FeII complex that composes this pool is selectively chelated when isolated mitochondria are treated with membrane-soluble 1,10-phenanthroline.12 The same treatment inhibits ISC biosynthesis,15–17 suggesting that the FeII complex is feedstock for ISC (and perhaps heme) biosynthesis.18
Size-exclusion chromatograms of low-molecular-mass (LMM) mitochondrial flow-through solutions (defined as solutions that pass through a 10 kDa cutoff membrane) from exponentially growing fermenting yeast cells exhibit a single iron-associated peak with a mass of ~580 Da.18,19 The concentration of the so-called Fe580 complex is on the same order of magnitude as that of the NHHS FeII pool, raising the intriguing possibility that this complex comprises the pool and is the iron-containing substrate for ISC and heme biosynthesis. Flow-through solutions from mammalian mitochondria contain Fe580 along with a few other LMM iron species.19 Mitochondrial flow-through solutions from fermenting yeast cells harvested under stationary-state conditions exhibit a LMM iron species with a mass of ~1100 Da called Fe1100. Anaerobic incubation of such flow-through solutions for a few days caused Fe1100 to disappear and Fe580 to appear; thus, the two species appear to be related.19
Deleting either MRS3 or MRS4 does not afford a growth phenotype, indicating that the two proteins possess redundant functions.3,4 However, deleting both genes simultaneously results in a strain (called ΔΔ) that exhibits a slow-growth phenotype when grown in iron-deficient media. Mitochondria from iron-deficient ΔΔ cells contain low concentrations of hemes and ISC-containing enzymes.4 ΔΔ cells in iron-sufficient medium grow at WT rates and exhibit normal activities of ISC-containing enzymes.3,4,6,7,20 The molecular-level details of how such cells recover remain a puzzle.
A second puzzle is why ΔΔ cells grown under both iron-deficient and iron-sufficient conditions acquire large amounts of iron relative to WT cells grown under the same conditions.3–5 Such iron accumulation indicates that the iron regulon is activated and that iron homeostasis is dysregulated in ΔΔ cells. The iron regulon consists of 20–30 genes, including MRS4, that are involved in iron import, trafficking, and regulation. The iron regulon is activated when WT cells are starved of iron (e.g., grown with the chelator bathophenanthroline disulfonate (BPS) in the medium) or in mutant cells in which mitochondrial ISC biosynthetic activity is defective.1,2 In WT cells, the iron regulon is deactivated under iron-sufficient conditions, whereas in ISC mutant cells (e.g., yfh1Δ), it is activated regardless of the concentration of iron in the medium.21 This results in a massive accumulation of FeIII phosphate oxyhydroxide nanoparticles in mitochondria.22,23 Iron also accumulates in ΔΔ cells but not excessively in mitochondria, as the absence of Mrs3/4 impedes the import of iron into this organelle.3,4 The iron concentration in ΔΔ mitochondria is reportedly approximately half of that in WT mitochondria.3,4 The presence of iron in mitochondria from ΔΔ cells implies the existence of an alternative iron import pathway that does not involve Mrs3/4. This pathway may involve Rim2.20,24,25
Yeast vacuoles are acidic organelles that store and sequester iron; they are another “hub” of iron trafficking. Ccc1 is the only known vacuolar iron importer of cytosolic iron.26 In ΔΔ cells, the rate of vacuolar iron import through Ccc1 is greater than in WT cells, despite lower-than-WT concentrations of the Ccc1 protein.5 Kaplan and co-workers concluded that Ccc1 activity is higher in ΔΔ cells than in WT cells because of a proposed signaling pathway between mitochondria and vacuoles that regulates Ccc1 activity.5,27
In this study, we examined ΔΔ cells using powerful biophysical and bioanalytical methods that allow in-depth characterization of the iron content. These methods included Mössbauer (MB), EPR, and electronic absorption spectroscopies, as well as a liquid chromatography instrument linked to an online inductively coupled plasma mass spectrometer (LC–ICP-MS). These results help explain, on a molecular level, how iron-deficient ΔΔ cells recover in terms of growth and mitochondrial function when placed in iron-sufficient medium. They also suggest how iron might remain dysregulated in ΔΔ cells even under iron-sufficient conditions under which they grow at WT rates. Collectively, this study provides new insights into iron trafficking and regulation in eukaryotic cells.
EXPERIMENTAL PROCEDURES
A γ construct was designed to knock out MRS3 in which 200 bp of the flanking 5′ and 3′ regions of the MRS3 coding sequence were juxtaposed on either side of a BamHI site in the polylinker of vector pRS403. The plasmid was linearized with BamHI and transformed into W303 (MAT α ade2–1 his3–1,15 leu2–3,112 trp1–1 ura3–1) selecting for histidine prototrophy. The correctness of the insertion was confirmed by colony PCR with primers 012302A (5′ aggcgattaagttgggtaac) from the vector and 022314A (5′ gcaatcattaagcaattgggcc) from the MRS3 flanking region generating the expected product of 435 bp. In a second step, 200 bp of 5′ and 3′ flanking regions of MRS4 were juxtaposed on either side of a BamHI site in the polylinker of vector pRS405. The vector was linearized with BamHI and transformed into the mrs3Δ strain, selecting for leucine prototrophy. The correctness of the insertion was confirmed by colony PCR with primers 012302A from the vector and 122314B (5′ tagttattgggtggcatatggg) from the MRS4 flanking region, generating the expected product of 247 bp.
The double mrs3Δmrs4Δ deletion strain (ΔΔ) was identified and further characterized phenotypically. Deletion of MRS3 and MRS4 was confirmed via PCR analysis. To confirm that iron homeostasis was perturbed, nonrepressing ferric reductase activity was assessed using a soft agar overlay assay. Strains were streaked on YPAD agar with 50 μM copper sulfate added and incubated overnight, and then 0.8% soft agar containing 1 mM BPS and 1 mM ferric ammonium sulfate was overlaid. The red color caused by the FeII(BPS)3 complex developed rapidly and was markedly enhanced in the ΔΔ strain compared with that in the W303 parent or mrs3Δ and mrs4Δ single mutants.
WT and ΔΔ cells were grown on YPAD agar plates for 3 days at 30 °C. Cells were taken from plates and grown under respiring conditions in liquid minimal medium containing 3% (v/v) glycerol, 1% (v/v) ethanol, 5 g/L ammonium sulfate, 1.7 g/L YNB that lacked ammonium sulfate and copper sulfate, 100 mg/L leucine, 50 mg/L adenine hemisulfate dihydrate, 20 mg/L histidine, 20 mg/L uracil, 50 mg/L tryptophan, and 1 μM copper sulfate. A 40 mM stock solution of 57FeIII citrate (pH ~5) was prepared as described previously14 and added to the growth medium. For BPS-treated medium, BPS was added to a final concentration of 25 μM followed by supplementation with 1 μM (final concentration) of 57FeIII citrate.
Cell cultures (50 mL and 1 L) were grown in baffled flasks at 30 °C while being constantly shaken. Cell growth was monitored at 600 nm (OD600). For all experiments, single colonies from YPAD plates were inoculated into 50 mL of minimal medium. Cultures (50 mL) were grown to an OD600 of 0.8 and immediately transferred into 1 L of minimal medium. Growth was measured from the time of inoculation. ΔΔ and WT whole-cell samples were harvested at an OD600 of 0.8 by centrifugation at 5000×g for 5 min. Cells were washed three times with unbuffered 1 mM EGTA and then three times with distilled water. Cells were then packed by centrifugation into EPR tubes and Mössbauer cups and frozen in liquid N2 for later analysis.
To prepare mitochondrial samples, 1 L cell cultures (OD600 = 0.8) were used to inoculate 24 L of minimal medium in a custom-built iron-free glass/titanium bioreactor at 30 °C with O2 gas bubbling through the medium at a rate of ~1 L/min. Once the OD600 reached 0.8, cells were harvested and mitochondria were isolated anaerobically in a refrigerated N2 atmosphere glovebox (MBraun, ~10 °C, ~5 ppm of O2) as described previously.19,28,29 Isolated mitochondria were packed into EPR tubes or Mössbauer cups as described above. For ultraviolet–visible studies, mitochondrial samples were thawed anaerobically inside a glovebox and loaded into 2 mm path-length custom quartz cuvettes (NSG Precision Cells, Inc.) as described previously.30
Metal concentrations of whole cells and mitochondria were analyzed by ICP-MS (Agilent model 7700x instrument). After Mössbauer spectra had been collected, samples were thawed and packed into EPR tubes to measure sample volumes (approximately 200–300 μL). Samples were then diluted with 250 μL of high-purity doubly distilled and deionized water generated using a Teflon sub-boiling still (Savillex DST-1000). Aliquots (50, 75, and 100 μL) from the resulting suspensions were transferred to 15 mL screw-top polypropylene Falcon tubes, digested with 250 μL of concentrated trace metal-grade nitric acid, and heated for ~16 h at 90 °C. Digested samples were diluted with high-purity doubly distilled and deionized H2O to a final volume of 8.0 mL. Reported metal concentrations of whole cells and mitochondria were calibrated as described previously19 and corrected using packing efficiencies of 0.70 for whole cells and 0.77 for mitochondria.28,31
For electronic absorption experiments, solutions of isolated mitochondria were packed under anaerobic conditions into EPR tubes by centrifugation, and the volumes of packed organelles were determined. Packed mitochondria were diluted 1:1 with SH buffer [0.6 M sorbitol and 20 mM HEPES (Ph 7.4)] and transferred into cuvettes. Cuvettes were sealed with a rubber septum and removed from the glovebox, and spectra were recorded at room temperature (RT) using a Hitachi U-3310 spectrometer with a head-on photomultiplier tube. Mössbauer spectra were obtained using an MS4 WRC spectrometer (SEE Co., Edina, MN), simulated with WMOSS software, and calibrated at RT with α-iron foil.31 Applied magnetic fields were parallel relative to the γ-radiation. EPR spectra were recorded using an X-band Elexsys spectrometer (Bruker Biospin Corp., Billerica, MA).
For LC–ICP-MS experiments, mitochondrial samples were manipulated anaerobically in refrigerated N2 atmosphere gloveboxes (Mbraun, Labmaster). Isolated mitochondria were washed twice with 20 mM ammonium bicarbonate (pH 8.5) and solubilized with 2% (w/v) Triton X-100 in the same buffer. The resulting suspension was vortexed for 20 min, followed by centrifugation at 12,000×g for 15 min. The soluble mitochondrial extract was passed through a 10 kDa cutoff membrane in an Amicon (Millipore Sigma) stirred-cell concentrator, and then the flow-through solution was injected onto two Superdex peptide 10/300GL columns (GE Healthcare Life Sciences) connected in series and equilibrated with 20 mM ammonium bicarbonate (pH 8.5). Buffer was pumped through the columns at a flow rate of 0.350 mL/min for 166 min using an Agilent (Tokyo, Japan) Bioinert high-performance liquid chromatography instrument. Chromatograms were acquired and analyzed, and columns were calibrated and cleaned as described.19 Metal-containing species that eluted from the column were detected by on-line ICP-MS.
RESULTS
Slow-Growth Phenotype of ΔΔ Cells.
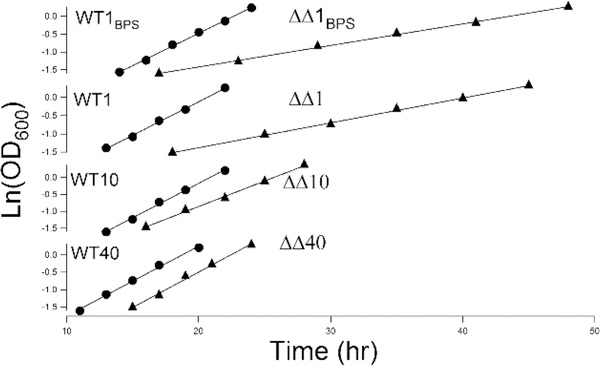
ΔΔ and WT cells were grown in minimal medium containing 1, 10, or 40 μM 57FeIII citrate. Some batches containing 1 μM 57FeIII citrate were pretreated with BPS to chelate endogenous iron and increase the severity of iron deficiency. We will refer to these cells as ΔΔ1 and WT1, ΔΔ10 and WT10, ΔΔ40 and WT40, and ΔΔ1BPS and WT1BPS, respectively. The numbers refer to the concentration of nutrient 57Fe (in micromolar) in the medium, whereas the subscript BPS indicates the presence of the chelator. 57Fe was added to BPS-treated media to enrich cells with a Mössbauer-active isotope. Glycerol or ethanol was exclusively used as the carbon source to force the cells to respire. Cells were harvested while growing exponentially. Exponential growth rates (α) were defined as the slopes of ln(OD600) versus time plots and are listed in Table 1.
Table 1.
Concentrations of Iron and Iron-Containing Species in Whole Cellsa
| sextet from highspin FeIII mainly in vacuoles |
central doublet (CD) due to [Fe4S4]2+ clusters and low-spin FeII hemes in mitochondria and cytosol |
doublet due to NHHS FeII pools in cytosol, mitochondria, and/or vacuoles |
doublet due to high-spin FeII hemes in mitochondria and cytosol |
doublet due to [Fe2S2]2+ clusters mainly in mitochondria |
doublet due to FeIII nanoparticles in mitochondria, vacuoles, and/or cytosol |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sample | growth rate α (h−1) | [Fecell] (μM) | % | μM | % | μM | % | μM | % | μM | % | μM | % | μM |
| WT1BPS | 0.181 ± 0.002 | 120 ± 20 | 0 | 0 | 69 | 84 | 12 | 14 | 12 | 14 | 7 | 8 | 0 | - |
| WT1 | 0.184 ± 0.003 | 200 ± 20 | 39 | 78 | 39 | 78 | 10 | 20 | 4 | 8 | 8 | 16 | 0 | - |
| WT10 | 0.203 ± 0.003 | 480 ± 30 | 39 | 190 | 39 | 190 | 11 | 50 | 5 | 20 | 6 | 30 | 0 | - |
| WT40 | 0.202 ± 0.006 | 880 ± 70 | 52 | 460 | 25 | 220 | 8 | 70 | 6 | 50 | 9 | 80 | 0 | - |
| ΔΔ1BPS | 0.060 ± 0.004 | 360 ± 30 | 11 | 40 | 25 | 90 | 47 | 170 | 6 | 20 | - | - | 11 | 40 |
| ΔΔ1 | 0.068 ± 0.004 | 680 ± 70 | 50 | 340 | 16 | 110 | 20 | 140 | 3 | 20 | - | - | 11 | 70 |
| ΔΔ10 | 0.150 ± 0.006 | 2160 ± 60 | 50 | 1080 | 9 | 190 | 5 | 110 | 5 | 110 | - | - | 31 | 670 |
| ΔΔ40 | 0.204 ± 0.005 | 3920 ± 80 | 57 | 2230 | 4 | 160 | 3 | 120 | 3 | 120 | - | - | 33 | 1290 |
Iron concentrations and growth rates are averages of two independent experiments. Simulation parameters were as follows (δ, ΔEQ, and Γ in units of millimeters per second): NHHS FeIII sextet in ΔΔ spectra, δ = 0.54 ± 0.05, ΔEQ = 0.62 ± 0.08, D = 0.3 ± 0.1 cm–1, E/D = 0.29 ± 0.02, η = 1.5 ± 1, Aiso = –220 ± 8 kG, and Γ = 0.8 ± 0.2; NHHS FeIII sextet in WT spectra, δ = 0.56 ± 0.03, ΔEQ = 0.42 ± 0.05, D = 0.5 cm−1, E/D = 0.33 ± 0.01, η = 1.8 ± 0.5, Aiso = –228 ± 3 kG, and Γ = 0.4; CD, δ = 0.45 ± 0.01, ΔEQ = 1.11 ± 0.05, and Γ = 0.50 ± 0.15; NHHS FeII doublet, δ = 1.23 ± 0.06, ΔEQ = 3.07 ± 0.17, and Γ = 0.59 ± 0.16; high-spin FeII heme doublet, δ = 0.87 ± 0.11, ΔEQ = 2.22 ± 0.04, and Γ = 0.49 ± 0.19; [Fe2S2]2+ doublet, δ = 0.30 ± 0.04, ΔEQ = 0.44 ± 0.07, and Γ = 0.48 ± 0.19; FeIII nanoparticles, δ = 0.50 ± 0.05, ΔEQ = 0.50 ± 0.06, and Γ = 0.4 ± 0.04. The sum of all percentages of Mössbauer components was forced to be equal to 100%; however, 10–15% of overall spectral intensities could not be accounted for by these components.
Under iron-deficient conditions, ΔΔ cells grew substantially slower than WT cells, whereas under iron-sufficient conditions, they grew at WT rates (Figure 1 and Table 1). Thus, the slow-growth phenotype of ΔΔ cells recovered as the iron concentration of the growth medium increased.
Figure 1.
Optical densities of growing WT (●) and ΔΔ (▲) cultures.
Iron Concentrations in ΔΔ Cells.
We determined the absolute iron concentration in ΔΔ and WT cells ([Fecell]) to help quantify the iron-dependent growth phenotype. These determinations are more difficult than measuring the ratio of iron concentration to protein concentration, as is typically reported. To determine absolute concentrations, known volumes of packed cells must be quantitatively transferred, dilution factors must be carefully measured, and packing efficiencies need to be measured and included in calculations.28 However, absolute concentrations once determined allow an analysis that is more in-depth than what is possible using concentration ratios.
[Fecell] in ΔΔ cells was substantially higher than in WT cells grown on the same medium (Table 1). This iron-overload phenotype was observed for all nutrient conditions examined, including those for which the growth rate of ΔΔ cells matched that of WT cells. The iron content of isolated mitochondria was also determined (Table 2). Isolated ΔΔ mitochondria were not iron-overloaded relative to the huge accumulation observed in ISC mutant cells.22,23 This indicates that the excess iron in ΔΔ cells accumulated in non-mitochondrial locations such as vacuoles and/or the cytosol.
Table 2.
Concentrations of Iron and Components of Isolated Mitochondriaa
| sextet, NHHS FeIII |
central doublet, [Fe4S4]2+ clusters and low-spin Fe11 hemes |
doublet, NHHS FeII |
doublet, high-spin FeII hemes |
doublet, [Fe2S2]2+ clusters |
doublet, FeIII nanoparticles |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sample | [Femit] (μM) | % | μM | % | μM | % | μM | % | μM | % | μM | % | μM |
| WT1 | 430 | 15 | 60 | 42 | 180 | 18 | 80 | 4 | 20 | 21 | 90 | - | - |
| WT40 | 690 | 0 | 0 | 57 | 390 | 30 | 210 | 2 | 10 | 11 | 80 | - | - |
| AA1 | 690 | 9 | 60 | 6 | 40 | 8 | 60 | - | - | - | - | 77 | 530 |
| AA40 | 740 | 0 | 0 | 63 | 475 | 15 | 110 | 11 | 80 | - | - | 10 | 75 |
Data are from a single preparation for each strain and condition. Other conditions are described in the footnote of Table 1.
The massive accumulation of iron in ΔΔ cells suggests that the iron regulon is activated under both iron-deficient and iron-sufficient conditions. Ironically, iron-overloaded ΔΔ cells “feel” iron-deficient even when they are grown under iron-sufficient conditions. To explain this, we reasoned that the accumulated iron must not be “sensed” by the cell or be part of the iron regulation mechanism (otherwise, the cell would “feel” iron-overloaded and would shut down excessive iron import). We further reasoned that in iron-sufficient ΔΔ cells the concentration of the sensed iron-containing species, whatever it is, must be below its “set-point” concentration. The set-point concentration is that at which the iron regulatory system would be half-activated. One objective of this study was to identify iron-containing species present at sub-WT concentrations in ΔΔ cells under all growth conditions; this species might be used to sense iron status in the cell.
Mössbauer (MB) Spectra of Whole Cells and Isolated Mitochondria.
We turned to MB spectroscopy to determine the forms of iron that accumulated in ΔΔ cells. The low-temperature low-field MB spectrum of WT1BPS cells was dominated by the central doublet (CD) (Figure 2, simulated by the green line). The CD arises from [Fe4S4]2+ clusters and low-spin FeII hemes; the two types of centers cannot be resolved by MB spectroscopy.29 A minor quadrupole doublet with parameters typical of NHHS FeII with mainly O/N donors was also evident; this feature is simulated by the blue line. All components and parameters used in simulations are listed in Table 1. MB spectra of respiring mitochondria are dominated by the CD. This feature dominates because respiratory complexes and respiration-related proteins contain ISCs and heme centers, and these complexes are strongly expressed in respiring cells.13 Fe2S2 clusters are also prevalent in respiring mitochondria; they contribute a shoulder on the CD.14
Figure 2.
Mössbauer spectra of whole cells (-C ending) and isolated mitochondria (-M ending). Solid lines are simulations for the central doublet (green), NHHS FeII doublet (blue), vacuolar high-spin FeIII sextet (purple), FeIII oxyhydroxide nanoparticles (gold), [Fe2S2]2+ doublet (teal), and high-spin FeII heme doublet (brown). The temperature was 5 K, and a field of 0.05 T was applied parallel to the γ rays. Solid red lines are composite simulations assuming the area percentages listed in Tables 1 and 2.
We previously assumed that all of the CD intensity in whole-cell MB spectra arose from mitochondrial ISCs and low-spin FeII hemes.12 However, non-mitochondrial [Fe4S4]2+ clusters, assembled by the CIA (cytosolic iron-sulfur cluster assembly system),1,2 must contribute to the CD of whole-cell MB spectra; the only issue is how much. We now suggest that they contribute a quarter to half of the overall CD intensity in the WT1BPS spectrum. A proportion of this magnitude is required to explain the presence of CD-like intensity in the MB spectra of iron-deficient ΔΔ cells (Figure 2) even though the spectra of iron-deficient ΔΔ mitochondria are largely devoid of the CD. We have included formation of cytosolic ISCs in the model of Figure 3. Our assumption that a significant portion of the CD intensity in the WT1BPS and ΔΔ1BPS spectra is due to cytosolic [Fe4S4]2+ clusters is also consistent with the distribution of ISCs in human cells.32 Approximately 70 proteins in humans contain Fe4S4 and/or Fe2S2 clusters. If each ISC-containing protein were expressed at the same level (and if the distribution in human and yeast were similar), we calculate from published data32 that ~40% of the iron associated with such clusters would be located in mitochondria and ~60% would be in the cytosol and nucleus (combined). Although our assumptions may not be strictly correct, the calculated 40:60 distribution is remarkably similar to that needed to interpret our MB spectra.
Figure 3.
Model explaining the Mrs3/4ΔΔ phenotype. Nutrient iron enters the cell and becomes cytosolic FeII. Cytosolic FeII can enter vacuoles where most of vacuolar FeII oxidizes to FeIII. In WT cells, very little converts into nanoparticles. Some cytosolic FeII converts into cytosolic [Fe4S4]2+ clusters. The remaining cytosolic FeII enters WT mitochondria through the Mrs3/4 import pathway and through a slow alternative pathway, where it becomes the mitochondrial FeII pool. Cytosolic FeII can enter ΔΔ mitochondria only through the alternative pathway. Mitochondrial FeII is feedstock for the biosynthesis of ISCs and heme centers, the majority of which are installed in respiratory complexes that catalyze the reduction of O2 to water. O2 is constantly diffusing into the matrix. In healthy WT cells, the activity of the respiratory complexes is sufficiently high to prevent O2 from diffusing in, but in iron-deficient ΔΔ cells, the activity is too low to prevent penetration. In that case, O2 reacts with the FeII pool to generate mitochondrial nanoparticles. The cell responds by increasing the level of expression of iron importers on the plasma membrane. Under iron-sufficient conditions, the cytosolic FeII concentration is high, allowing sufficient iron to enter mitochondria and generate sufficient respiration activity to re-establish anaerobic conditions in the matrix. However, the size of the mitochondrial FeII pool and/or heme centers remains subnormal such that the iron regulon remains activated.
The MB spectrum of ΔΔ1BPS cells (Figure 2) was dominated by the same two doublets, albeit with different relative intensities. On a percentage-wise basis, the CD was approximately one-third as intense as it was in the WT1BPS spectrum (Table 1). However, similar CD concentrations are suggested on the basis of absolute iron concentrations. In such cases of apparent discrepancy, we rely more heavily on percentage differences, because fitting MB spectra has fewer sources of error than determining absolute iron concentrations.
Because the MB spectrum of ΔΔ1 mitochondria (Figure 2) was largely devoid of the CD, most ISCs exhibited by the ΔΔ1BPS whole-cell spectrum are probably non-mitochondrial. The model of Figure 3 suggests that the absence of Mrs3/4 should cause NHHS FeII species to accumulate in the cytosol of ΔΔ1BPS cells and cause greater-than-WT rates of cytosolic ISC production. Whether the iron for cytosolic ISC production originates outside the mitochondria is uncertain. The same blockage would cause a deficiency of ISCs and hemes within mitochondria.
The MB spectrum of WT1 cells (Figure 2) exhibited two major features, including the CD and a sextet due to vacuolar NHHS FeIII.33 More intense sextets are evident in the spectrum of WT10 and WT40 cells. This component is simulated by the purple line above the ΔΔ1 spectrum. As confirmation of earlier reports,14 the MB spectrum of mitochondria isolated from WT1 cells was dominated by the CD. As mentioned above, we assumed that approximately half of the CD in the whole-cell WT1 spectrum originates from ISCs in mitochondria and half from ISCs in the cytosol (and nucleus). The MB spectrum of WT1 cells also exhibited a NHHS FeII doublet and a minor sextet suggesting a small amount of NHHS FeIII in WT1 mitochondria. Previous spectra of iron-deficient WT mitochondria also included a semiresolved doublet due to [Fe2S2]2+ clusters.14 Such a doublet is simulated by the solid teal line over the WT1 spectrum.
The MB spectrum of ΔΔ1 cells (Figure 2) was dominated by an intense NHHS FeIII sextet, indicating that the vacuoles are essentially filled with iron under these conditions. On a percentage-wise basis, the intensity of the CD in the ΔΔ1 spectrum was 2.4 times lower than in the WT1 spectrum, while that of the NHHS FeII doublet was 2 times higher. Again, comparison of absolute concentrations differed slightly, with the CD concentration in both ΔΔ1 and WT1 cells being similar (and that of the NHHS FeII doublet for ΔΔ1 cells being 7 times higher than in WT1 cells) (Table 1). According to the model depicted in Figure 3, cytosolic iron should accumulate in ΔΔ1 cells at the expense of mitochondrial ISCs. Higher-thannormal concentrations of cytosolic iron would also be expected to stimulate the import of iron into the vacuoles (and stimulate cytosolic ISC production), consistent with the spectrum. The central region of the ΔΔ1 spectrum was poorly resolved, but the absorption that remained after known features had been removed was a doublet with parameters typical of FeIII oxyhydroxide nanoparticles.
The MB spectrum of mitochondria isolated from ΔΔ1 cells also exhibited a doublet because of such nanoparticles (Figure 2). A low-intensity NHHS FeII doublet was also present, but no CD was evident. This indicates that few [Fe4S4]2+ clusters and/ or low-spin FeII hemes were present in ΔΔ1 mitochondria, and it suggests that most of the CD in the ΔΔ1 whole-cell MB spectrum was due to non-mitochondrial ISCs assembled by the CIA. Similarly, the strong intensity of the NHHS FeII doublet in the ΔΔ1 whole-cell spectrum is incompatible with NHHS FeII species being located in ΔΔ1 mitochondria (too little is present in the mitochondrial spectrum for this species to make a significant contribution to the NHHS FeII doublet in the whole-cell spectrum).
These results support the model of Figure 3 in which iron accumulates in the cytosol of ΔΔ1 cells; the unusually high cytosolic iron concentration should increase the rate of import of iron into vacuoles. Indeed, the concentration of vacuolar FeIII in ΔΔ1 cells is 4 times that of WT1 cells. Most iron in ΔΔ1 mitochondria is present as FeIII nanoparticles. The presence of some CD intensity in the whole-cell ΔΔ1 MB spectrum suggests that cytosolic ISC assembly is functioning in ΔΔ mutant cells grown under our iron-deficient conditions. Although ΔΔ1 cells were iron-overloaded relative to WT1 cells, ΔΔ1 mitochondria contained a ballpark-similar concentration of iron relative to that in WT1 mitochondria (Table 2). Thus, much of the excess iron that entered ΔΔ1 cells did not flow into the mitochondria but rather remained in the cytosol or flowed into vacuoles.
The WT10 whole-cell spectrum (Figure 2) was dominated by the CD and the FeIII sextet; minor doublets due to high-spin FeII hemes and NHHS FeII species were also evident. The corresponding ΔΔ10 whole-cell spectrum was far more intense, reflecting a higher iron concentration in the cell (Table 1). The spectrum was dominated by the FeIII sextet and the nanoparticle doublet. The composite simulation of the ΔΔ10 spectrum included intensity due to the CD, but this doublet was not resolved, which made quantification difficult.
The MB spectrum of WT40 cells (Figure 2) was similar to that of WT10 cells, except that the sextet was more intense and the CD was poorly resolved. The spectrum of mitochondria isolated from WT40 cells exhibited a strong CD and NHHS FeII doublet. This spectrum is generally consistent with previous reports, but the NHHS FeII doublet is more intense than in previous MB of respiring mitochondria.12,13 We previously reported that the size of the NHHS FeII pool was smaller in respiring WT mitochondria than in the organelle from fermenting WT cells; however, the intensity of the NHHS FeII doublet in spectra of recently prepared mitochondria from respiring WT cells is similar to that from fermenting cells.
The MB spectrum of ΔΔ40 cells (Figure 2) was nearly identical to that of ΔΔ10 cells, again indicating the accumulation of nanoparticles and NHHS FeIII. Surprisingly, the spectrum of ΔΔ40 mitochondria was devoid of nanoparticles but rather exhibited an intense CD. Thus, the nanoparticles and NHHS FeIII in ΔΔ40 whole cells must be non-mitochondrial. The spectrum of ΔΔ40 mitochondria also exhibited significant intensity due to the NHHS FeII pool (and perhaps some high-spin FeII hemes), but the spectral intensity of the pool was only approximately half as intense as in the WT40 mitochondrial spectrum. We conclude that ΔΔ40 mitochondria have returned to a “healthy” state (at least from an iron-centric perspective). On the other hand, excessive iron accumulated in ΔΔ40 cells, implying that the iron regulon was activated even though ΔΔ40 mitochondria exhibited a CD with roughly the same intensity as in WT mitochondria. This was unexpected because the iron regulon is thought to be regulated by the ISC activity in mitochondria.1,2 Here, the ISC level in ΔΔ cells is similar to that in WT cells, yet the iron regulon appears to be activated in the mutant cells.
We hypothesize that the slow-growth phenotype of ΔΔ1 cells is due to the absence of respiratory complexes in their mitochondria and that the WT growth rate of ΔΔ40 cells is due to their presence. How this transformation occurs simply with an increase in the nutrient iron concentration is puzzling, as is the reason why the mutant cells continue to be iron dysregulated even though the ISC level has largely recovered. We investigated these issues further using electronic absorption and EPR spectroscopies.
Electronic Absorption and EPR Spectra.
Mitochondria isolated from WT1 and WT40 cells exhibited Soret bands at ~400 nm and α and β bands between approximately 500 and 600 nm (Figure 4). These features are characteristic of cytochromes a, b, and c. Mitochondria isolated from ΔΔ40 cells exhibited similar features, albeit with approximately half of the WT intensity. Mitochondria from ΔΔ1 cells were devoid of such features. This indicates that hemes were not synthesized by ΔΔ cells grown under iron-deficient conditions but were synthesized by such cells under iron-sufficient conditions.
Figure 4.
Electronic absorption spectra of isolated anaerobic mitochondrial suspensions. Packed mitochondria were diluted 1:1 with buffer and transferred to a 2 mm path-length quartz cuvette; the cuvette was sealed with a stopper and removed from the box, and spectra were collected. We estimate a protein concentration of ~80 mg/mL in the sample based on previous results.13 Spectra have been offset for viewing.
Low-temperature X-band EPR spectra of ΔΔ and WT whole cells (Figure 5) were dominated in the g = 2 region by a hyperfine split signal due to mononuclear S = 5/2 MnII species. This signal has been observed previously in spectra of yeast cells14,34 and was found to arise from most of the Mn in the cell (quantified here at ~30 μM (see Table 3)). The presence or absence of Mrs3/4 did not influence the shape or intensity of that signal, consistent with the absence of an observed effect of the deletion of Mrs3/4 on cellular Mn concentration.
Figure 5.
EPR spectra of ΔΔ and WT cells. The temperature for ΔΔ1BPS, ΔΔ10, WT1BPS, and WT10 spectra was 10 K, while that in others was 4.2 K; intensities were temperature-adjusted to allow comparisons. Other parameters: average microwave frequency, 9.373 ± 0.003 GHz; microwave power, 0.2 mW; modulation amplitude, 10 G; gain, 1000; conversion time, 0.3 s. Displayed intensities on the left were adjusted as indicated for ease of viewing. None of the spectra on the right side was adjusted.
Table 3.
Copper, Manganese, and Zinc Concentrations in ΔΔ and WT Cells and Isolated Mitochondriaa
| [copper] (μM) |
[manganese] (μM) |
[zinc] (μM) |
||||
|---|---|---|---|---|---|---|
| sample | cell | mitochondrion | cell | mitochondrion | cell | mitochondrion |
| WT1BPS | 120 ± 10 | – | 21 ± 4 | – | 310 ± 65 | – |
| WT1 | 140 ± 20 | 110 | 24 ± 6 | 22 | 300 ± 20 | 250 |
| WT10 | 130 ± 10 | – | 29 ± 3 | – | 315 ± 60 | – |
| WT40 | 140 ± 20 | 89 | 31 ± 11 | 27 | 330 ± 60 | 320 |
| WT average | 130 ± 8 | 100 ± 15 | 26 ± 5 | 24 ± 4 | 310 ± 10 | 280 ± 50 |
| ΔΔ1BPS | 290 ± 10 | – | 32 ± 5 | – | 240 ± 45 | – |
| ΔΔ1 | 280 ± 30 | 63 | 25 ± 14 | 31 | 200 ± 50 | 180 |
| ΔΔ10 | 320 ± 40 | – | 39 ± 8 | – | 190 ± 30 | – |
| ΔΔ40 | 320 ± 10 | 48 | 29 ± 7 | 34 | 250 ± 25 | 210 |
| ΔΔ average | 300 ± 20 | 56 ± 11 | 31 ± 6 | 32 ± 2 | 220 ± 30 | 200 ± 20 |
Details as in Table 2.
Overlapping the Mn-based signal in all spectra was an isotropic S = 1/2 signal at g = 2.00. This signal was not assigned to a particular radical species, as there were too many candidates. Surprisingly, no g = 1.94-type signals from S = 1/2 [Fe2S2]+ or [Fe4S4]+ clusters were observed. Along with our MB spectra, this demonstrates that the vast majority of such clusters in whole yeast cells are in the oxidized S = 0 [Fe2S2]2+ and [Fe4S4]2+ states. Similarly, there were no EPR signals from low-spin FeIII hemes, suggesting that the majority of such centers in exponentially growing cells are in the FeII state. Some WT EPR spectra exhibited low-intensity signals between g = 4 and g = 6 (weak features between 1000 and 1300 G in Figure 5), which probably arise from cytochrome c oxidase.29
The most prominent signal in the low-field region was at g = 4.3, arising from high-spin S = 5/2 FeIII species with a rhombicity parameter E/D of ~1/3. Most or all of this signal arises from vacuolar FeIII.33 Its intensity in WT cells increased as the nutrient iron concentration increased, consistent with increasing amounts of cellular iron being stored in vacuoles. The intensity of the g = 4.3 signal exhibited by ΔΔ cells was substantially higher than in spectra from comparable WT cells, consistent with the differences observed by MB. The intensity of the g = 4.3 signal from ΔΔ40 cells was defined as 100%. The average intensities of the g = 4.3 signal from ΔΔ1BPS, ΔΔ1, and ΔΔ10 cells were 2, 34, and 53%, respectively. For comparison, the intensities of the same signal in WT1BPS, WT1, WT10, and WT40 cells were 0, 9, 8, and 14%, respectively. We conclude that ΔΔ cells contain substantially more vacuolar FeIII than comparable WT cells do, consistent with our MB analysis (Figure 2 and Table 1).
LC–ICP-MS of Mitochondrial Flow-Through Solutions.
We initially hypothesized that the Fe580 complex passes intact from the cytosol through Mrs3/4 and into the matrix. Thus, we expected to observe Fe580 in flow-through solutions of WT1 and WT40 mitochondria but not in ΔΔ1 or ΔΔ40 mitochondrial samples. Fe580 was indeed observed in flow-through solutions of WT1 and WT40 mitochondrial extracts (Figure 6, top panel) and not in ΔΔ1 extracts (Figure 6, top panel; two independent trials are shown). Unexpectedly, Fe580 was also observed in the ΔΔ40 trace (Figure 6, top panel), albeit with an intensity lower than that in the WT40 trace sample, indicating a lower concentration. Quantification of areas suggests an [Fe580] concentration of 180 μM in WT40 mitochondria and 100 μM in ΔΔ40 mitochondria. The presence of Fe580 in ΔΔ40 mitochondria supports the idea that ΔΔ40 mitochondria have recovered under iron-sufficient conditions and are “healthy”. The trace of the ΔΔ1 flow-through solution exhibited two weak LMM iron peaks, at 2700 and 2100 Da, but they were not investigated further.
Figure 6.
LC–ICP-MS chromatograms of LMM flow-through solutions prepared from the soluble fractions of WT and ΔΔ mitochondrial detergent extracts. The top panel shows 56Fe detection. Trace intensities were adjusted as indicated for ease of viewing. The bottom panel shows 56Fe, 34S, and 31P detection of flow-through solutions from mitochondria harvested from WT40 cells harvested as cells were undergoing the transition to the stationary state. Traces collected immediately are labeled –0D, while those collected after a 5 day incubation are labeled –5D.
Relationship of Fe1100 to Fe580.
Mitochondrial extracts from fermenting yeast harvested at or near stationary state contain an iron species called Fe1100.19 This species converted into Fe580 in mitochondrial extracts that were allowed to sit in a refrigerated anaerobic glovebox for 5 days. The same phenomenon occurred in our current studies involving respiring yeast cells. One batch was harvested at an OD600 of 1.0 (typically we harvest at an OD600 of 0.8). The higher OD suggests that cells were undergoing a transition from the exponential to stationary state when they were harvested. The resulting LC–ICP-MS trace of the flow-through solution from mitochondria isolated from these cells was dominated by Fe1100 (Figure 6, bottom panel); some minor Fe580 intensity is evident. After 5 days in a refrigerated box, the same solution exhibited a strong Fe580 peak and no Fe1100 peak. This behavior indicates that the two LMM iron species are related; perhaps Fe1100 is a dimer of Fe580. Corresponding S and P traces did not exhibit peaks that co-migrated with either iron peak, suggesting that neither P nor S is associated with the iron complexes. The donor atoms coordinating the iron in these complexes are probably O and/or N.
Effect of Deleting Mrs3/4 on Copper, Manganese, and Zinc.
The average manganese and zinc concentrations in ΔΔ cells showed little difference, relative to those of WT cells, across a range of nutrient iron concentrations; no trends were evident (Table 3). The average copper concentration in ΔΔ cells was 2.3-fold higher than in WT cells, regardless of the iron concentration in the medium. In contrast, the average copper concentration in ΔΔ mitochondria was approximately half of that in WT mitochondria. This suggests that copper is dysregulated in ΔΔ cells and that a deficiency of copper in ΔΔ mitochondria either increases the rate of import of copper into the cell or decreases the rate of copper export.
LC–ICP-MS traces of flow-through solutions from mitochondria isolated from respiring WT and ΔΔ cells exhibited the same LMM copper, manganese, and zinc species as reported previously from mitochondrial flow-through solutions from fermenting cells,19 including Cu5000, Mn1100, and Zn1200 (Figure 7). These species are probably involved in metallating apo-metalloproteins in the mitochondria19,35 and are generated at relatively constant levels regardless of metabolic state. Although not the main focus of this study, Mn and Zn traces serve as “anchors” that demonstrate the consistency of our results in contrast to the observed shifts in iron traces. Cu5000 intensities were reduced in ΔΔ traces, relative to what they were in WT traces, consistent with the lower copper concentration in ΔΔ mitochondria. Minor copper species with masses between 200 and 2000 Da were present in some traces but not in others (Figure 7, bottom panel, elution volumes of 27–42 mL), suggesting that such species are artifacts. In contrast, Cu5000 was routinely present. The vast majority of copper in mitochondria has been proposed to be in the form of a labile nonproteinaceous LMM copper species called CuL.36 CuL is thought to be imported from the cytosol through Mrs3 and another IM protein (Pic2).37 CuL is thought to be stored in the matrix and transported back to the IMS through an unidentified copper exporter on the IM. Our results provide no evidence supporting this concept. We are currently trying to identify Cu5000 and probe its physiological function.
Figure 7.
Mn (top), Zn (middle), and Cu (bottom) LC–ICP-MS traces of flow-through solutions of soluble extracts of mitochondria isolated from WT and ΔΔ cells.
DISCUSSION
In this study, we used biophysical and bioanalytical methods to help understand the phenotype of yeast cells in which Mrs3 and Mrs4, the high-affinity iron importers on the mitochondrial IM, were both deleted. Our results can be explained using the model depicted in Figure 3. Under iron-deficient conditions, ΔΔ cells grow slowly relative to WT cells, whereas under iron sufficiency, they grow at WT rates. Also, the rate of import of iron into mitochondria is too slow to prevent O2 from diffusing into the matrix. This is caused by an insufficiently large FeII pool in the matrix that causes insufficient ISCs, hemes, and thus respiratory complexes to be assembled. Insufficient respiratory complexes allow diffusing cellular O2 to penetrate the matrix. Once in the matrix, the O2 reacts with the FeII pool to generate FeIII nanoparticles and ROS, thereby reducing the size of the pool further. This leads to a vicious cycle culminating in ROS-damaged mitochondria that contain few active holo-respiratory complexes and iron mostly in the form of nanoparticles. Mitochondrial membrane potential is probably also affected.
According to the model, in iron-sufficient ΔΔ cells, the process is reversed. Higher cytosolic iron concentrations increase the rate of import of iron into mitochondria through the alternative iron import pathway. This increases the size of the FeII pool as well as the rate of ISC and heme biosynthesis. This allows respiratory complexes to be metallated and become active. Normal respiratory activity blocks O2 from entering the matrix. The anaerobicity of the matrix and the membrane potential are re-established. Our results and analysis illustrate the complex interrelationships between iron metabolism, oxygen, and respiration.
Our results also offer some new insights into why the iron regulon is activated in ΔΔ cells under iron-sufficient conditions, even though such cells grow at normal rates and their mitochondria appear to be relatively “healthy” (from an iron-centric perspective). According to regulatory control theory, the cell should contain a pool of iron that varies with the iron status of the cell and helps regulate cellular iron import.41,42 In WT cells, the concentration of the sensed iron pool should be below its normal set-point concentration under iron-deficient conditions and above the set point under iron-sufficient conditions. Because we observed greater-than-WT iron levels in ΔΔ cells grown under both iron-deficient and iron-sufficient conditions, the sensed form of iron (whatever it is) should be below its set-point concentration in these mutant cells grown under both iron-deficient and iron-sufficient conditions.
Thus, we sought to identify iron species whose concentration was lower in mutant cells than in WT cells grown under equivalent conditions. We could eliminate nanoparticles as the sensed form of iron because these particles were present at higher-than-WT levels in ΔΔ cells. Similarly, cytosolic iron concentrations in mutant cells appear to be higher than in WT cells, as suggested by more intense NHHS FeII quadrupole doublet in comparative Mössbauer spectra and rationalized in the model of Figure 3.
The cytosolic concentration of the hypothetical sulfur-containing species X-S is thought to reflect the level of ISC activity in mitochondria. We previously used the concentration of mitochondrial ISCs as a proxy for X-S in a math model of iron regulation.38 However, our current results do not favor using mitochondrial ISCs as a proxy for X-S-based regulation because mitochondria isolated from ΔΔ cells grown under high-iron conditions contained near normal levels of ISCs.
The only Fe-containing species (observable in our investigation) at lower-than-WT concentrations in mutant cells were the mitochondrial FeII pool and mitochondrial heme centers. The FeII pool could conceivably work together with X-S to regulate (e.g., as substrates for the Fe2S2 cluster that eventually is transferred onto Aft1/2). We previously proposed a similar scenario, except that cytosolic (rather than mitochondrial) FeII was considered to be the sensed pool of iron.38 Our current perspective disfavors this because cytosolic FeII concentrations in ΔΔ cells are probably higher than in WT cells (Figure 3). Understanding iron regulation in eukaryotic cells is complex but also important because dysregulation is associated with numerous diseases such as Friedreich’s ataxia.
Composition of the Mitochondrial NHHS FeII Pool.
Our results provide strong evidence that the mitochondrial NHHS FeII pool in exponentially growing yeast cells is composed exclusively of Fe580 (no other LMM Fe complex was present). This species also appears to be present in mitochondria from mammalian sources.19 The iron concentration associated with the FeII pool in yeast mitochondria ranges from 60 to 200 μM (Table 1), depending on strain and growth conditions. We previously estimated the concentration of Fe580 in mitochondria to be in the same ballpark, ~100 μM.19 Our current LC–ICP-MS results suggest that the irons of Fe580 (and Fe1100) are not coordinated by S donor ligands. The ΔEQ and δ of the NHHS FeII doublet in MB spectra of isolated mitochondria (3.07 and 1.23 mm/s, respectively) are typical of FeII complexes with four-six O and 0–2 N donor ligands.39 These parameters are not typical of FeII complexes dominated by sulfur-based ligands. We are currently attempting to identify this species by high-resolution ESI mass spectrometry.
Mechanisms for the Passage of Iron through Importers.
Cytosolic iron could pass into the mitochondrial matrix using two possible mechanisms. In the “intact channeling” mechanism, Fe580 in the cytosol passes intact through Mrs3/4 channels. In the “unwrapping/rewrapping” mechanism, cytosolic iron enters the IMS through porins on the outer membrane and then docks on Mrs3/4. The iron dissociates from its coordinating ligands, and a “bare” FeII ion passes through the Mrs3/4 channels. Indeed, there are three conserved histidine residues in Mrs3/4 that seem to be poised to transport such an ion.40 When the FeII ion reaches the end of the channel on the matrix side, it coordinates with other ligands to generate the Fe580 complex.
If intact channeling were operative, Fe580 should not have been observed in the flow-through solution of ΔΔ40 mitochondria (but it was). If Fe580 was also channeled intact through the alternative import transporter, then Fe580 should have been present in the flow-through solution of ΔΔ1 mitochondria (but it was not). Thus, we favor the unwrapping/rewrapping mechanism. One complication is that loss of membrane potential in ΔΔ1 mitochondria might have prevented the import of Fe580 through the alternative importer; thus, the intact channeling mechanism cannot be eliminated cleanly. However, we find it unlikely that both Mrs3/4 and the alternative iron import pathway import the same complex (because different proteins would be expected to have different channels). Also, the specificity implied by intact channeling seems to be contradicted by the ability of simple hexaqua FeII ions to enter isolated mitochondria for use in heme and ISC biosynthesis.6,7
The unwrapping/rewrapping mechanism implies the opposite in terms of specificity (i.e., many FeII complexes could serve as cytosolic FeII donors as long as the coordinating ligands were not bound so tightly that they could not dissociate within a reasonable timeframe). Whether Fe580 assembles in the matrix upon exiting the channel should depend, according to the unwrapping/rewrapping mechanism, on the metabolic state of the organelle. Fe580 may not form if the matrix is devoid of the appropriate coordinating ligand(s) or if it is not sufficiently anaerobic to maintain the FeII state. Under stationary-state conditions, the metabolic state of the matrix may be different than under exponential growth conditions (e.g., the concentrations of potential coordinating ligands might vary) such that Fe1100 (or Fe2700 or Fe2100) might form instead of Fe580. Viewed collectively, these considerations support the unwrapping/ rewrapping mechanism, but further studies are required to establish this.
Physiological Function of the Fe580 Mitochondrial FeII Pool.
Our results confirm and extend previous studies showing that there is a pool of NHHS FeII in mitochondria,12 that this pool is feedstock for ISC and heme biosynthesis in the organelle,15–17 and that this pool is composed of Fe580.18,19 Although iron accumulates in mitochondria of cells from which YFH1 has been deleted,22 iron does not accumulate in mitochondria of cells in which YFH1, MRS3, and MRS4 have all been deleted.3,4,6,7 This implies that the iron that accumulates in mitochondria of Friedreich’s ataxia patients (deficient in frataxin, the human homologue of Yfh1) passes through mitoferrins1/2 (the human homologues of Mrs3/4). The iron oxidation state in nanoparticles is FeIII, whereas that for the mitochondrial iron pool is FeII. This implies that Fe580 is an FeII complex that reacts with O2 (in diseased mitochondria) to generate nanoparticles.
Nanoparticles are often described as being toxic to the cell, because ROS is formed in association with them. However, from a chemical perspective, nanoparticles should be benign and unreactive with O2. The reaction of Fe580 with O2 is more likely to be toxic and to generate ROS. Thus, the reaction chemistry of Fe580 is probably essential for understanding the pathophysiology of Friedreich’s ataxia and perhaps other iron-associated mitochondrial diseases. Our study is significant because it further characterizes Fe580 and provides evidence of a model in which O2 is critically important for the iron-related reaction chemistry occurring within mitochondria.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (GM084266 to P.A.L. and DK53953 to A.D.) and the Robert A. Welch Foundation (A1170).
ABBREVIATIONS
- BPS
bathophenanthroline disulfonate
- CD
central doublet
- CIA
cytosolic iron-sulfur assembly complex
- δ
isomer shift
- ΔΔ
Mrs3/4ΔΔ
- ΔEQ
quadrupole splitting
- EPR
electron paramagnetic resonance
- Γ
line width
- ICP-MS
inductively coupled plasma mass spectrometry
- ISC
iron-sulfur cluster
- LMM
low-molecular-mass
- MB
Mössbauer
- NHHS
nonheme high-spin
- PCR
polymerase chain reaction
- ROS
reactive oxygen species
- WT
wild type
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Lill R, Dutkiewicz R, Freibert SA, Heidenreich T, Mascarenhas J, Netz DJ, Paul VD, Pierik AJ, Richter N, Stuempfig M, Srinivasan V, Stehling O, and Mühlenhoff U (2015) The role of mitochondria and the CIA machinery in the maturation of cytosolic and nuclear iron-sulfur proteins. Eur. J. Cell Biol 94, 280–291. [DOI] [PubMed] [Google Scholar]
- (2).Mühlenhoff U, Hoffmann B, Richter N, Rietzschel N, Spantgar F, Stehling O, Uzarska MA, and Lill R (2015) Compartmentalization of iron between mitochondria and the cytosol and its regulation. Eur. J. Cell Biol 94, 292–308. [DOI] [PubMed] [Google Scholar]
- (3).Foury F, and Roganti T (2002) Deletion of the mitochondrial carrier genes MRS3 and MRS4 suppresses mitochondrial iron accumulation in a yeast frataxin-deficient strain. J. Biol. Chem 277, 24475–24483. [DOI] [PubMed] [Google Scholar]
- (4).Mühlenhoff U, Stadler JA, Richhardt N, Seubert A, Eickhorst T, Schweyen RJ, Lill R, and Wiesenberger G (2003) A specific role of the yeast mitochondrial carriers Mrs3/4p in mitochondrial iron acquisition under iron-limiting conditions. J. Biol. Chem 278, 40612–40620. [DOI] [PubMed] [Google Scholar]
- (5).Li LT, and Kaplan J (2004) A mitochondrial-vacuolar signaling pathway in yeast that affects iron and copper metabolism. J. Biol. Chem 279, 33653–33661. [DOI] [PubMed] [Google Scholar]
- (6).Zhang Y, Lyver ER, Knight SAB, Lesuisse E, and Dancis A (2005) Frataxin and mitochondrial carrier proteins, Mrs3p and Mrs4p, cooperate in providing iron for heme synthesis. J. Biol. Chem 280, 19794–19807. [DOI] [PubMed] [Google Scholar]
- (7).Zhang Y, Lyver ER, Knight SAB, Pain D, Lesuisse E, and Dancis A (2006) Mrs3p, Mrs4p, and frataxin provide iron for Fe-S cluster synthesis in mitochondria. J. Biol. Chem 281, 22493–22502. [DOI] [PubMed] [Google Scholar]
- (8).Shaw GC, Cope JJ, Li LT, Corson K, Hersey C, Ackermann GE, Gwynn B, Lambert AJ, Wingert RA, Traver D, Trede NS, Barut BA, Zhou Y, Minet E, Donovan A, Brownlie A, Balzan R, Weiss MJ, Peters LL, Kaplan J, Zon LI, and Paw BH (2006) Mitoferrin is essential for erythroid iron assimilation. Nature 440, 96–100. [DOI] [PubMed] [Google Scholar]
- (9).Paradkar PN, Zumbrennen KB, Paw BH, Ward DM, and Kaplan J (2009) Regulation of Mitochondrial Iron Import through Differential Turnover of Mitoferrin 1 and Mitoferrin 2. Mol. Cell. Biol 29, 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Shvartsman M, and Cabantchik ZI (2012) Intracellular iron trafficking: role of cytosolic ligands. BioMetals 25, 711–723. [DOI] [PubMed] [Google Scholar]
- (11).Robinson AJ, Overy C, and Kunji ERS (2008) The mechanism of transport by mitochondrial carriers based on analysis of symmetry. Proc. Natl. Acad. Sci. U. S. A 105, 17766–17771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Holmes-Hampton GP, Miao R, Garber Morales J, Guo Y, Münck E, and Lindahl PA (2010) A nonheme high-spin ferrous pool in mitochondria isolated from fermenting Saccharomyces cerevisiae. Biochemistry 49, 4227–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Garber Morales J, Holmes-Hampton GP, Miao R, Guo Y, Münck E, and Lindahl PA (2010) Biophysical characterization of iron in mitochondria isolated from respiring and fermenting yeast. Biochemistry 49, 5436–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Holmes-Hampton GP, Jhurry ND, McCormick SP, and Lindahl PA (2013) Iron content of Saccharomyces cerevisiae cells grown under iron-deficient and iron-overload conditions. Biochemistry 52, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lutz T, Westermann B, Neupert W, and Herrmann JM (2001) The mitochondrial proteins Ssq1 and Jac1 are required for the assembly of iron sulfur clusters in mitochondria. J. Mol. Biol 307, 815–825. [DOI] [PubMed] [Google Scholar]
- (16).Amutha B, Gordon DM, Gu YJ, Lyver ER, Dancis A, and Pain D (2008) GTP Is Required for Iron-Sulfur Cluster Biogenesis in Mitochondria. J. Biol. Chem 283, 1362–1371. [DOI] [PubMed] [Google Scholar]
- (17).Pandey A, Pain J, Ghosh AK, Dancis A, and Pain D (2015) Fe-S cluster biogenesis in isolated mammalian mitochondria: coordinated use of persulfide sulfur and iron and requirements for GTP, NADH, and ATP. J. Biol. Chem 290, 640–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).McCormick SP, Moore MJ, and Lindahl PA (2015) Labile Low-Molecular-Mass Metal Complexes in Mitochondria: Trials and Tribulations of a Burgeoning Field. Biochemistry 54, 3442–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).McCormick SP, Moore MJ, and Lindahl PA (2015) Detection of labile low-molecular-mass transition metal complexes in mitochondria. Biochemistry 54, 3442–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Yoon H, Zhang Y, Pain J, Lyver ER, Lesuisse E, Pain D, and Dancis A (2011) Rim2, a pyrimidine nucleotide exchanger, is needed for iron utilization in mitochondria. Biochem. J. 440, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Chen OS, Crisp RJ, Valachovic M, Bard M, Winge DR, and Kaplan J (2004) Transcription of the Yeast iron regulon Does Not Respond Directly to Iron but Rather to Iron-Sulfur Cluster Biosynthesis. J. Biol. Chem 279, 29513–29518. [DOI] [PubMed] [Google Scholar]
- (22).Lesuisse E, Santos R, Matzanke BF, Knight SAB, Camadro JM, and Dancis A (2003) Iron use for haeme synthesis is under control of the yeast frataxin homologue (Yfh1). Hum. Mol. Genet 12, 879–889. [DOI] [PubMed] [Google Scholar]
- (23).Miao R, Kim H, Koppolu UMK, Ellis EA, Scott RA, and Lindahl PA (2009) Biophysical characterization of the iron in mitochondria from Atm1p-depleted Saccharomyces cerevisiae. Biochemistry 48, 9556–9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lin HL, Li LT, Jia XA, Ward DM, and Kaplan J (2011) Genetic and Biochemical Analysis of High Iron Toxicity in Yeast: iron toxicity is due to the accumulation of cytosolic iron and occurs under both aerobic and anaerobic conditions. J. Biol. Chem 286, 3851–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Froschauer EM, Rietzschel N, Hassler MR, Binder M, Schweyen RJ, Lill R, Mühlenhoff U, and Wiesenberger G (2013) The mitochondrial carrier Rim2 co-imports pyrimidine nucleotides and iron. Biochem. J 455, 57–65. [DOI] [PubMed] [Google Scholar]
- (26).Li LT, Chen OS, Ward DM, and Kaplan J (2001) CCC1 is a transporter that mediates vacuolar iron storage in yeast. J. Biol. Chem 276, 29515–29519. [DOI] [PubMed] [Google Scholar]
- (27).Li LT, Murdock G, Bagley D, Jia XA, Ward DM, and Kaplan J (2010) Genetic Dissection of a Mitochondria-Vacuole Signaling Pathway in Yeast Reveals a Link between Chronic Oxidative Stress and Vacuolar Iron Transport. J. Biol. Chem 285, 10232–10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Lindahl PA, Morales JG, Miao R, and Holmes-Hampton GP (2009) Isolation of Saccharomyces cerevisiae mitochondria for Mössbauer, EPR, and electronic absorption spectroscopic analyses. Methods Enzymol. 456, 267–285. [DOI] [PubMed] [Google Scholar]
- (29).Hudder BN, Morales JG, Stubna AA, Münck A, Hendrich MP, and Lindahl PA (2007) Electron paramagnetic resonance and Mössbauer spectroscopy of intact mitochondria from respiring Saccharomyces cerevisiae. J. Biol. Inorg. Chem 12, 1029–1053. [DOI] [PubMed] [Google Scholar]
- (30).Cockrell A, McCormick SP, Moore MJ, Chakrabarti M, and Lindahl PA (2014) Mössbauer, EPR, and modeling study of iron trafficking and regulation in Δccc1 and CCC1-up Saccharomyces cerevisiae. Biochemistry 53, 2926–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Miao R, Holmes-Hampton GP, and Lindahl PA (2011) Biophysical investigation of the iron in Aft1–1(up) and Gal-YAH1 Saccharomyces cerevisiae. Biochemistry 50, 2660–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Andreini C, Banci L, and Rosato A (2016) Exploiting bacterial operons to illuminate human iron-sulfur protein. J. Proteome Research 15, 1308–1322. [DOI] [PubMed] [Google Scholar]
- (33).Cockrell AL, Holmes-Hampton GP, McCormick SP, Chakrabarti M, and Lindahl PA (2011) Mössbauer and EPR study of iron in vacuoles from fermenting Saccharomyces cerevisiae. Biochemistry 50, 10275–10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).McNaughton RL, Reddi AR, Clement MHS, Sharma A, Barnese K, Rosenfeld L, Gralla EB, Valentine JS, Culotta VC, and Hoffman BM (2010) Probing in vivo Mn2+ speciation and oxidative stress resistance in yeast cells with electron-nuclear double resonance spectroscopy. Proc. Natl. Acad. Sci. U. S. A 107, 15335–15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Atkinson A, and Winge DR (2009) Metal Acquisition and Availability in the Mitochondria. Chem. Rev 109, 4708–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Cobine PA, Ojeda LD, Rigby KM, and Winge DR (2004) Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J. Biol. Chem 279, 14447–14455. [DOI] [PubMed] [Google Scholar]
- (37).Vest KE, Wang J, Gammon MG, Maynard MK, White OL, Cobine JA, Mahone WK, and Cobine PA (2016) Overlap of copper and iron uptake systems in mitochondria in Saccharomyces cerevisiae. Open Biol 6, 150223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Wofford JD, and Lindahl PA (2015) Mitochondrial iron-sulfur-cluster activity and cytosolic iron regulate iron traffic in Saccharomyces cerevisiae. J. Biol. Chem 290, 26968–26977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Reisner E, Telser J, and Lippard SJ (2007) A planar carboxylate-rich tetrairon(II) complex and its conversion to linear triiron(II) and paddlewheel diiron(II) complexes. Inorg. Chem 46, 10754–10770. [DOI] [PubMed] [Google Scholar]
- (40).Brazzolotto X, Pierrel F, and Pelosi L (2014) Three conserved histidine residues contribute to mitochondrial iron transport through mitoferrins. Biochem. J 460, 79–89. [DOI] [PubMed] [Google Scholar]
- (41).Sewell C, Morgan JJ, and Lindahl PA (2002) Analysis of Protein Homeostatic Regulatory Mechanisms in Perturbed Environments at Steady-State. J. Theor. Biol 215, 151–167. [DOI] [PubMed] [Google Scholar]
- (42).Yang Q, Lindahl PA, and Morgan JJ (2003) Dynamic Responses of Protein Homeostatic Regulatory Mechanisms to Perturbations from Steady State. J. Theor. Biol 222, 407–423. [DOI] [PubMed] [Google Scholar]