Abstract
Background
This study was performed to evaluate the effects and stability of the new hepatitis B immunoglobulin (HBIG), Hepabulin, in patients undergoing liver transplantation for hepatitis B.
Material/Methods
A total of 87 patients undergoing liver transplantation for hepatitis B-related liver disease were enrolled in this multicenter, phase III, open-label, single-arm study. Seventy (80.5%) of the 87 enrolled patients completed the study during the 52-week study period. Hepabulin (10,000 units) was intravenously injected intraoperatively, daily for 1 week, weekly for 1 month, and then once per month. Hepabulin was used as monotherapy without antiviral agents. Hepatitis B recurrence was defined as conversion from negativity for surface antigen after HBIG administration to positivity.
Results
There were no cases of hepatitis B recurrence during the 52-week observation period. A total of 876 adverse events (AEs) that occurred during the study period were observed in 83 (95.4%) of 87 patients, and serious AEs were seen in 119 cases in 44 (50.6%) of the 87 patients. None of the AEs showed a relationship with this drug. Hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) rapidly disappeared within 1 week after HBIG administration, but hepatitis B virus (HBV) DNA persisted for up to 8 weeks after surgery, which was related to HBV viral load. Hepatitis B surface antibody (HBsAb) was correlated with HBIG (Hepabulin) dose.
Conclusions
The new HBIG, Hepabulin, was shown to be safe and effective in preventing the recurrence of HBV after liver transplantation.
MeSH Keywords: Hepatitis B Antibodies, Hepatitis B virus, Liver Transplantation
Background
Liver transplantation (LT) is the definitive treatment for cirrhosis and fulminant liver failure due to hepatitis B virus (HBV) infection [1]. HBV is the most common cause of liver transplantation in endemic areas, and recent reports have indicated that >70% of LT cases are associated with this disease [2]. As recently as 15 years ago, hepatitis B-associated liver cirrhosis was associated with poor liver transplant outcome, which was attributable to reinfection by HBV [3]. The HBV recurrence rate after LT is >80% without any prophylaxis, and HBV reinfection may lead to rapid disease progression and early graft loss [3]. The evolution of effective prophylactic strategies over the past 15 years has markedly improved survival rates, making LT a therapeutic option for patients with hepatitis B-related liver disease [4]. Long-term treatment with hepatitis B immunoglobulin (HBIG) is associated with reduced risk of recurrent HBV infection and reduced mortality. Since the introduction of lamivudine, a nucleoside analog that inhibits HBV replication, the HBV recurrence rate has been reduced to <10%. Recently, new antiviral agents that show better results have been developed. The combination of long-term HBIG and nucleos(t)ide analogs is currently the standard treatment and has been shown to be effective in reducing HBV recurrence rates. Recently, a few centers reported that hepatitis B recurrence was reduced by antiviral agents alone, similar to the results of combination therapy. In lower-risk individuals, HBIG may only be required in the perioperative setting, with lifelong antiviral therapy use thereafter [5]. However, these studies mostly had a short-term follow-up, and the numbers of cases were insufficient to draw definitive conclusions. In addition, the use of antiviral agents only is controversial in endemic areas where viral load is high. Therefore, combination therapy consisting of HBIG and antiviral agents is still the standard treatment.
The use of HBIG for the prevention of HBV reinfection after liver transplantation is necessary. However, HBIG is expensive and requires intravenous injection, which is inconvenient. The development of HBIG by multiple pharmaceutical companies has resolved this economic problem to some extent. Non-clinical studies, virus removal, and fluorination/elimination tests have confirmed the stability of the new HBIG, Hepabulin (SK Plasma, Seoul, South Korea), and its ability to remove viruses. The present study was performed to investigate the efficacy and safety of Hepabulin for the prevention of hepatitis B recurrence in liver transplant patients.
Material and Methods
Study design and population
This prospective, single-arm, multicenter clinical trial was conducted from November 2011 to March 2014. Six transplantation centers participated in the study. Institutional review board approval was obtained for patient enrollment prior to the start of the study. The number of subjects was calculated as 80 considering the one-sample predominant hypothesis, recurrence rate of 7.1% (α=0.025, power of 80%), and dropout rate of 20%. Of the 89 subjects who provided written consent to participate in the trial, 87 fulfilled the selection/exclusion criteria after the end of the observation period within 2 weeks. Two were lost to follow-up and consent was withdrawn. Seven subjects dropped out of the study 1 month before the validity criterion. Therefore, the full analysis set (FAS) consisted of 80 subjects. Ten were unable to complete up to 52 weeks and dropped out of the study. An additional 10 subjects who completed the clinical trials had protocol violations/deviations. Therefore, 60 subjects were left for per protocol (PP) analysis (Figure 1). A dose of 10,000 IU of Hepabulin was diluted in 150 mL of 5% glucose solution, and instilled for about 1 hour. Hepabulin was given in an anhepatic phase during the operation, daily until 1 week after surgery, weekly until 4 weeks after surgery, and monthly until 12 months after surgery. However, patients positive for HBV DNA or hepatitis B e antigen (HBeAg) before surgery received 20,000 IU of Hepabulin during surgery (Figure 2). Blood samples were taken before drug administration and serum was separated and stored for further examination. During this trial period, Hepabulin was used as monotherapy without an antiviral agent. For the clinical study, the visit dates were screening (visit 1), postoperative day 1 (visit 2), weekly until week 4 (visits 3–6), and monthly until 48 weeks (visits 7–17). The last visit at 4 weeks after the last dose (visit 18) was performed for a total of 52 weeks (Figure 2). Immunosuppressive treatment included a regimen featuring a tacrolimus as a component of a double- or triple-drug cocktail [the other 2 drugs were prednisone and mycophenolate mofetil (MMF)].
Figure 1.
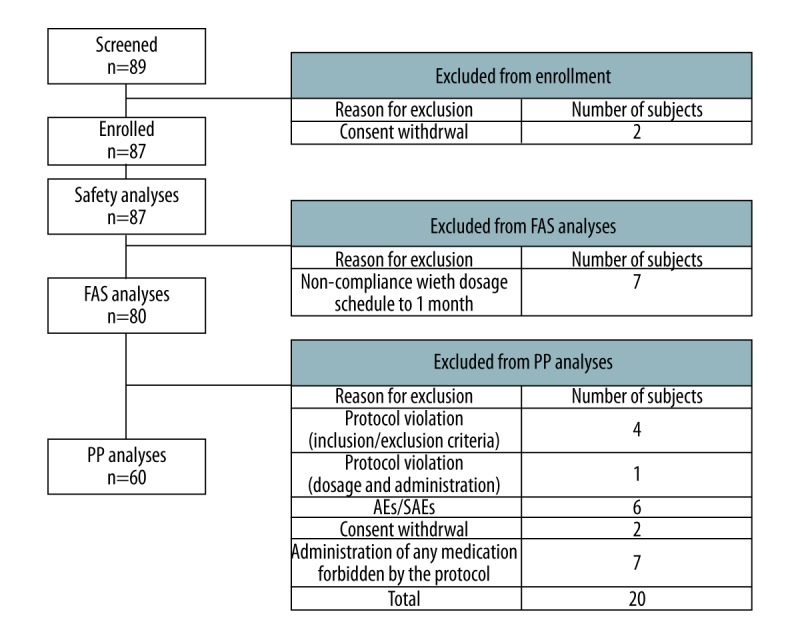
Clinical trial participants.
Figure 2.
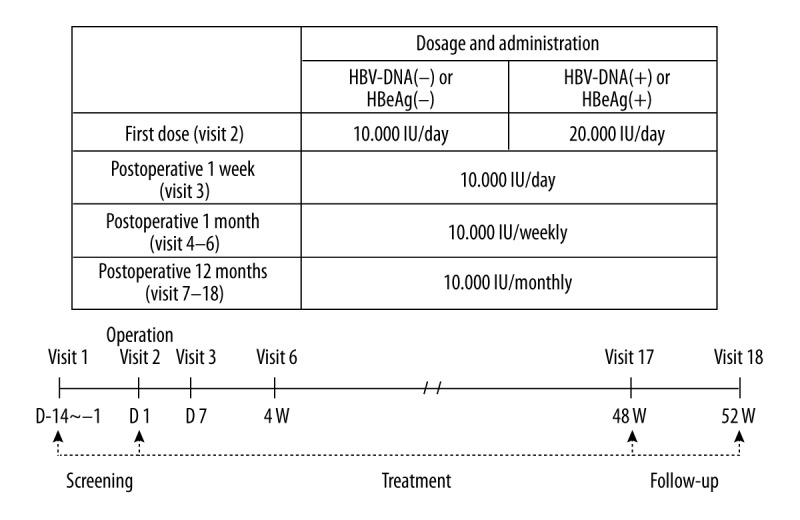
Method of Hepabulin administration: Hepabulin was administered intravenously at a dose of 10,000 IU diluted in 150 mL of 5% dextrose in water according to the following regimen.
Inclusion criteria
Patients matching the following criteria and receiving liver transplantation due to hepatitis B were included in the study:
Patients were at least 18 years of age and younger than 64 years of age.
Patients were positive for hepatitis B surface antigen (HBsAg) before liver transplantation.
Patients with hepatocellular carcinoma that satisfied the Milan criteria.
Exclusion criteria
The exclusion criteria were as follows:
Patients undergoing emergency transplantation in status I or IIA.
Patients who had received at least one organ transplant before screening.
Patients with a history of malignant tumors other than liver disease within 5 years.
Patients with severe renal impairment (e.g., serum creatinine more than twice the upper limit of reference, dialysis patients).
Patients with cardiovascular events, myocardial infarction, coronary angioplasty, cerebral infarction, and cerebral hemorrhage within 6 months.
Study endpoints
The primary endpoint was the rate of hepatitis recurrence, which was evaluated by the percentage of subjects who relapsed from HBsAg-negative to HBsAg-positive after liver transplantation. Secondary endpoints included the HBsAg recurrence period (the period from the time of confirmation of HBsAg negativity to the time of HBsAg positivity), survival rate (the percentage of subjects who survived from the first administration of Hepabulin to the end of the study), survival time (the period from the first administration of the drug to the death), and changes in serological markers for hepatitis B [HBsAg, hepatitis B surface antibody (HBsAb), hepatitis B e antigen (HBeAg), hepatitis B e antibody (HBeAb), HBV DNA]. Serological markers were measured at each visit, and HBsAg, HBsAb, HBeAg, and HBeAb were analyzed by electrochemiluminescence immunoassay (ECLIA). HBV DNA was analyzed by real-time polymerase chain reaction (PCR) and mutation tests were performed using PCR and direct sequencing.
All of the adverse events (AEs) that occurred during the entire study period were included in stability assessment, and the stability of the test drug was assessed based on vital signs, physical examination, laboratory tests, chest X-ray, and electrocardiogram at each visit. Laboratory tests included complete blood cell count (CBC), blood chemistry including glucose, blood urea nitrogen (BUN), creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, blood coagulation test including prothrombin time (PT), partial thromboplastin time (PTT), urinalysis, and pregnancy test. A total of 87 patients who had received at least 1 dose of the clinical trial drug were selected for the safety test. The incidence rates of AEs following administration of the drug for clinical trials are presented. Serious AE refers to any of the following AEs or adverse drug reactions occurring at any dose of a drug used in clinical trials: death, admission to hospital, need to increase the admission period of a patient who had already been admitted, the occurrence of persistent or meaningful disability or malfunction, and other medically important situations.
The changes in serial hepatitis B markers, including HBsAg, HBeAg, and HBV DNA, were evaluated. After Hepabulin administration, changes in serum levels of HBsAb were examined and the factors affecting the concentrations of HBsAb after 1 week and 12 months were investigated.
Statistical analysis
The clinical trial results were evaluated by safety analysis, FAS analysis, and PP analysis. Safety analysis included all data from subjects who received Hepabulin at least once. The FAS method included subjects assessed for recurrence by measuring HBsAg 1 or more times after Hepabulin administration among subjects who received Hepabulin at least once. The PP method was used for the analysis of data from subjects included in FAS analysis who completed treatment according to the protocol without any significant violations. In cases with a missing value in the main outcome variable, the previous observation was carried forward to replace the most recent missing value based on the time of occurrence. The FAS method was used as the primary analysis method in principle, and PP analysis was additionally performed. The safety data were principally based on safety analysis.
The primary endpoint was serum HBsAg recurrence rates for subjects who relapsed from 4 to 52 weeks from negative to positive HBsAg, and the Wald chi-square test was performed on binomial ratios. The significance level was 0.025 for 1 side and 97.5% for the confidence interval. Among the secondary endpoints, the survival rate was calculated using the frequency and percentage of surviving subjects from the first administration of Hepabulin to the end of the study. The recurrence rate of HBsAg and the survival rate were analyzed using the Kaplan-Meier method. Serological parameters were analyzed using the paired t test to determine whether there was any change in intragroup variation. In all analyses, P<0.05 was taken to indicate statistical significance.
Results
Patient characteristics
Of the 87 subjects enrolled in the study, 71 (81.6%) were men and the mean age was 52.3±6.5 years. The mean body mass index (BMI) was 23.8±2.7, and 54 (62.1%) subjects were overweight (BMI >23). Fifty subjects (57.5%) were classified as Child-Pugh class A, 24 (27.6%) as class B, and 11 (12.6%) as class C. The mean model for end-stage liver disease (MELD) score was 12.31±5.62. There were 65 HCC patients (74.7%). All HCC patients were within the Milan criteria. The average duration of hepatitis B infection was 229±125 months (range, 4–517 months) and 81 (93.1%) of the 87 subjects had used antiviral agents. Among the 87 subjects, 27 (31.0%) were HBeAg-positive and 40 (45.9%) were HBV DNA-positive. Fifty-eight (66.7%) subjects had HBsAg titer >6000 IU/mL and 18 (20.7%) had HBV DNA titer > 500 IU/mL. Among the 40 HBV DNA-positive cases, the HBV mutation test was performed in 19 patients, with the exclusion of 21 subjects who could not be tested because of low HBV DNA copy numbers. The mutation was detected in 16 of 19 patients (84.2%); precore mutation was found in 10 (62.5%), core mutation in 16 (100%), surface mutation in 8 (50%), and drug resistance mutation in 5 (31.3%) (Table 1).
Table 1.
Patient characteristics.
| Characteristics | Data (n=87) |
|---|---|
| Age | 52.3±6.5 |
| Male, n (%) | 71 (81.6%) |
| BMI | 23.8±2.7 |
| Child score, n (%) | |
| Class A | 50 (58.8%) |
| Class B | 24 (28.2%) |
| Class C | 11 (13.0%) |
| HCC, n (%) | 65 (74.7%) |
| Hepatitis B (duration, months) | 229±125 (4–517) |
| Pre-transplant antiviral agent, n (%) | 81 (93.1%) |
| Duration (months) | 36±41 (1–33) |
| Pre-transplant HBV viral markers | |
| HBsAg (+), n (%), mean ±SD (IU/L) | 87 (100.0%), 6629.03±3013.99 |
| HBsAb (+), n (%), mean ±SD (IU/L) | 4 (4.6%), 30.74±14.86 |
| HBeAg (+), n (%), mean ±SD (Index) | 27 (31.0%). 17.59±49.28 |
| HBeAb (+), n (%), mean ±SD (Index) | 63 (72.4%), 0.23±0.32 |
| HBV DNA (+), n (%), mean ±SD (IU/mL) | 40 (45.9%), 285,123±923,308 |
| HBV mutation | 16 (18.4%) |
| HBV precore mutation | 10 (62.5%) |
| HBV core mutation | 16 (100%) |
| HBV surface mutation | 8 (50%) |
| HBV drug resistance mutation | 5 (31.3%) |
BMI – body mass index; HBV – hepatitis B virus; HBsAg – hepatitis B surface antigen; HBsAb – hepatitis B surface antibody; HBeAg – hepatitis B e antigen; HBeAb – hepatitis B e antibody.
Effects of Hepabulin
As the primary endpoint, none of the subjects showed relapse from HBsAg-negative to HBsAg-positive after liver transplantation from 4 to 52 weeks after administration of the trial drug. In addition, the serum HBsAg recurrence rate was 0% in the FAS and PP analysis groups. Of the 80 subjects in the FAS group, 1 died between first administration of the drug and the end of the clinical trial, and the survival rate (the secondary endpoint) was 98.75%. This patient died from sepsis due to acute cholangitis, which was not related to the clinical trial drug (Table 2).
Table 2.
Efficacy assessment: HBsAg recurrence rate and survival.
| Endpoint | Data (n=80) | |
|---|---|---|
| Primary endpoint | HBsAg recurrence rate | 0% |
| Secondary endpoint | Survival rate | |
| Survival | 79 (98.7%) | |
| Mortality | 1 (1.3%) | |
Adverse events of hepabulin
A total of 876 AEs occurred in 83 (95.40%) of the total population of 87 patients. The most common AEs were gastrointestinal disorders (57 patients, 65.5%), such as diarrhea, constipation, and abdominal pain. Thirteen patients (14.9%) showed abnormal LFT, but no acute cellular rejection was confirmed in biopsy. Serious AEs occurred in 44 (50.6%) of 87 patients, and 119 (13.58%) of 876 AEs were serious. Two deaths occurred (2.29%), one due to acute septic cholangitis during the clinical trial and the other due to hepatocellular carcinoma (HCC) recurrence after being dropped in the clinical trial. No changes were made to the dose of clinical trial drug in 113 cases of serious AE, while the drug was discontinued in the remaining 6 cases. Drug administration was discontinued due to tumor recurrence in 5 cases, while the sixth patient died because of septic cholangitis. Hepatobiliary disorder was the most common disease among the major AEs, and occurred in 18 patients (20.7%). The most common hepatobiliary disorder was biliary stricture. None of the AEs were related to the clinical trial drug.
Changes in HBV markers following hepabulin administration
We analyzed 80 patients in the FAS group. The mean preoperative HBsAg titer was 6668±3018 IU/mL, but it was 182±670 IU/mL at 1 day after surgery and 28 patients (35%) were positive for the antigen. On the 7th postoperative day, the mean HBsAg titer was 0.63±0.11 IU/mL and all patients were negative for HBsAg. After the administration of Hepabulin, HBeAg titer was negative in all patients at 1 week after surgery. Of the 37 patients (46.25%) positive for HBV DNA, the average preoperative HBV DNA titer was 142,351±664 IU/mL, which was 17±49 IU/mL on postoperative day 1, and 13 patients (16.25%) were HBV DNA-positive. On postoperative day 7, mean HBV DNA titer was 37±147 IU/mL and 13 patients (16.25%) were positive. We analyzed the changes of DNA titer in HBV DNA-positive patients (n=13) at 1 week postoperatively (Figure 3). At 2 weeks postoperatively, HBV DNA titer was 7±30 IU/mL, and 6 patients (7.5%) were HBV DNA-positive. DNA positivity was observed in 3 patients (3.75%) at 3 weeks postoperatively, in 1 patient (1.25%) at 4 weeks postoperatively, and in 1 patient (1.25%) at 8 weeks postoperatively. However, all were negative at 3 months. The preoperative factors affecting HBV DNA positivity (n=13) at 1 week postoperatively were evaluated (Table 3). The preoperative HBsAg titer (P=0.015) and ALT level (P=0.003) were higher in the HBV DNA-positive group than in the HBV DNA-negative group. In addition, there were more preoperative mutation-positive patients in the HBV DNA-positive group (P=0.004). Preoperative HBV DNA level and HBeAg positivity were not significantly related, but the preoperative HBV DNA titer and incidence of HBeAg positivity tended to be higher in the HBV DNA-positive group at 1 week postoperatively (P=0.076 and P=0.055).
Figure 3.
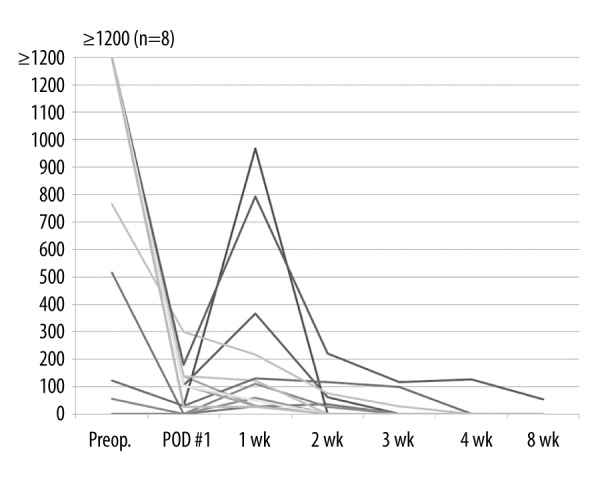
Changes in HBV DNA titer after Hepabulin administration in HBV DNA-positive patients (n=13) at 1 week after surgery.
Table 3.
Preoperative factors affecting HBV DNA positivity at 1 week postoperatively.
| HBV DNA(+) (n=13) | HBV DNA(−) (n=66) | P-value | |
|---|---|---|---|
| ALT (U/L) | 63.08±37.39 | 33.72±29.73 | 0.003 |
| Total bilirubin (mg/dL) | 5.64±9.46 | 2.56±4.40 | 0.272 |
| Creatinine (mg/dL) | 0.86±0.25 | 0.87±0.23 | 0.911 |
| Albumin (g/dL) | 3.28±0.71 | 3.46±0.67 | 0.384 |
| HBsAg (IU/L) | 8343±1630 | 6283±3109 | 0.015 |
| HBeAg positivity | 7 (53.85%) | 17 (25.76%) | 0.055 |
| HBV DNA (IU/mL) | 823,143±1,506,950 | 10,412±81,586 | 0.076 |
| Mutation positivity | 8 (61.54%) | 8 (12.12%) | 0.004 |
HBsAg – hepatitis B surface antigen; HBV – hepatitis B virus; DNA – deoxyribonucleic acid; ALT – alanine aminotransferase; HBeAg – hepatitis B e antigen.
Changes in HBsAb and factors affecting the levels of HBsAb by HBIG administration
Preoperative HBsAb titer was 3.51±6.95 IU/L, and increased to 1426±2107 IU/L on the first day after Hepabulin administration. The highest value of 8114±3037 IU/L was recorded at 7 days postoperatively after daily administration. After weekly administration, HBsAb titer was 5965±4000 IU/L at 1 month after surgery, and was 1587±646 IU/L at 1 year after surgery after monthly administration (Figure 4). When HBIG was administered monthly, the mean titers of HBsAb ranged from 1569 to 2806 IU/L. Factors affecting antibody levels at 1 week (visit 3) and 1 year (visit 18) after surgery were examined (Table 4). The factors affecting HBsAb titer at 1 week postoperatively were Child score (P=0.017), MELD score (P=0.001), total bilirubin level (P=0.001), albumin level (P=0.001), HBsAg titer (P=0.019), HBV DNA status (P=0.021), and mutation status (P=0.022). The factors affecting HBsAb titer at 52 weeks after surgery were sex (P=0.011) and ALT level (P=0.002).
Figure 4.
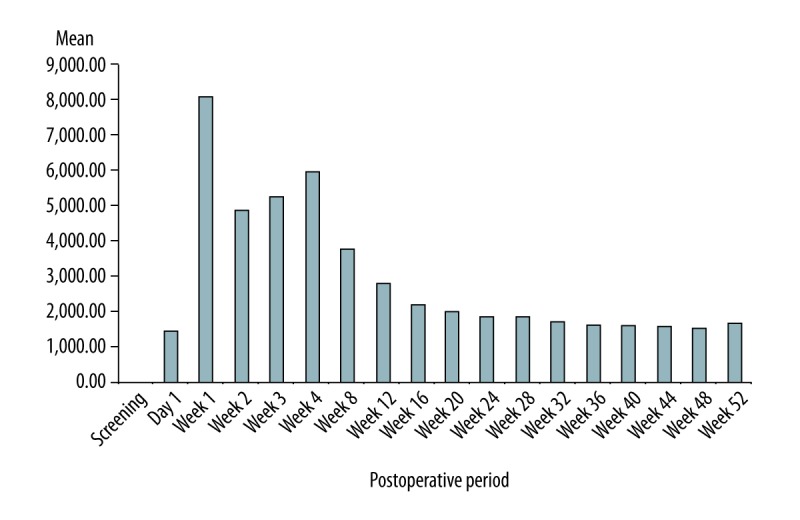
Changes in mean HBsAb titer after administration of Hepabulin.
Table 4.
Clinical factors influencing HBsAb titer after Hepabulin administration at 1 and 52 weeks postoperatively.
| HBsAb (IU/L – 1 week) | HBsAb (IU/L – 52 weeks) | ||||||
|---|---|---|---|---|---|---|---|
| n | Mean ±SD | P-value | n | Mean ±SD | P-value | ||
| Recipient age | <50 | 25 | 8349.04±2452.64 | 0.626 | 22 | 1602.40±689.74 | 0.876 |
| ≥50 | 54 | 8001.03±3139.87 | 48 | 1576.16±633.15 | |||
| Sex | Male | 65 | 7839.23±3048.41 | 0.075 | 59 | 1500.63±589.98 | 0.011 |
| Female | 14 | 9373.68±1903.33 | 11 | 2033.75±775.78 | |||
| BMI | <25 | 54 | 8432.04±3099.26 | 0.154 | 45 | 1655.06±707.43 | 0.222 |
| ≥25 | 25 | 7418.07±2434.98 | 25 | 1457.22±507.97 | |||
| Child score | <10 | 67 | 8449.20±2863.35 | 0.017 | 58 | 1612.76±624.19 | 0.586 |
| ≥10 | 11 | 6180.64±2778.31 | 11 | 1495.81±777.93 | |||
| MELD score | <15 | 55 | 8687.87±2819.40 | 0.001 | 50 | 1626.34±639.62 | 0.216 |
| ≥15 | 16 | 5911.75±2378.87 | 14 | 1373.95±763.26 | |||
| Total bilirubin (mg/dL) | <3.0 | 58 | 8606.02±2788.55 | 0.001 | 68 | 1607.91±636.19 | 0.076 |
| ≥3.0 | 14 | 5892.64±2552.87 | 2 | 785.30±640.21 | |||
| ALT (U/L) | <50 | 56 | 8235.70±3034.42 | 0.342 | 59 | 1686.80±618.52 | 0.002 |
| ≥50 | 15 | 7414.78±2608.46 | 11 | 1035.18±521.16 | |||
| Creatinine (mg/dL) | <1.0 | 53 | 7944.99±2766.78 | 0.569 | 31 | 1516.35±661.16 | 0.436 |
| ≥1.0 | 18 | 8407.58±3503.91 | 39 | 1638.50±638.06 | |||
| Albumin (g/dL) | <3.0 | 24 | 6539.67±2703.63 | 0.001 | 1 | 332.60 | 0.050 |
| ≥3.0 | 47 | 8839.77±2787.71 | 69 | 1602.55±633.06 | |||
| HBsAg (IU/L) | <6668 | 32 | 9038.62±3228.68 | 0.019 | 29 | 1522.59±696.41 | 0.505 |
| ≥6668 | 47 | 7479.70±2551.79 | 41 | 1628.13±613.87 | |||
| HBeAg | Negative | 55 | 8258.80±2860.74 | 0.501 | 47 | 1536.15±655.28 | 0.376 |
| Positive | 24 | 7772.83±3114.38 | 23 | 1683.01±630.91 | |||
| HBV DNA | Negative | 42 | 8817.95±2768.52 | 0.021 | 36 | 1475.41±595.21 | 0.148 |
| Positive | 37 | 7308.86±2934.03 | 34 | 1699.81±686.71 | |||
| Mutation | Negative | 63 | 8489.13±2652.38 | 0.022 | 54 | 1547.48±631.50 | 0.384 |
| Positive | 16 | 6622.93±3543.47 | 16 | 1709.03±701.60 | |||
Discussion
This was a 52-week, multicenter, open-label, single-arm, phase III trial to determine the efficacy and safety of the new HBIG, Hepabulin (SK Plasma), administered as a single high dose without concomitant antiviral agent. At present, combination therapy using low-dose HBIG and nucleos(t)ide analogs (NAs) is considered the most efficacious and cost-effective prophylaxis for post-LT HBV reinfection [6]. The use of dual therapy significantly reduces HBV recurrence rate and improves liver transplantation outcome in HBV patients. Some authors have argued that HBIG should be abandoned due to good results with use of antiviral agents alone [7]. However, most of these reports were based on small case series with a short follow-up period and a low viral load, so care is required when making conclusions in endemic areas where viral load is high. As shown in a recent systematic review, the timing of HBIG withdrawal is unclear and more adequately-powered studies are required [8]. In this study, we performed HBIG monotherapy to examine the pure effect of HBIG (Hepabulin) as prophylaxis. We used high-dose HBIG monotherapy, and evaluated the efficacy of Hepabulin after 1 year, when treatment was changed to combination therapy using low-dose HBIG and NAs. At present, the European Association for the Study of the Liver (EASL) guideline recommends combination of HBIG and a potent NA is recommended after liver transplantation for the prevention of HBV recurrence [9]. In endemic areas with high viral load, HBIG still plays an important role in the prevention of HBV recurrence. The disadvantage of HBIG is its high cost, which also causes non-compliance. If Hepabulin competes with existing HBIG drugs, it will help lower drug prices. Various routes of administration (e.g., intramuscular versus intravenous) and dosing regimens of HBIG have been studied [10]. However, intravenous treatment is still the most commonly used route. We chose to evaluate a regularly scheduled regimen of intravenous high-dose HBIG based on previous reports [11,12].
HBsAg recurrence was the primary endpoint in the present study, and the recurrence rate was zero in both FAS and PP analyses. In patients with hepatitis B, the recurrence rate was >70% after 2 years without hepatitis prevention. Considering the high recurrence rate of HBIG treatment at 1 year in clinical trials of other HBIG preparations [11,13,14], the results in this study were excellent. In particular, this result is much lower than the recurrence rate of 7.1% for HepaGam B (Aptevo Bio Therapeutics, Seattle, WA), the version of the drug approved overseas, which was set as a reference for this study protocol. These observations confirmed that Hepabulin has excellent efficacy in preventing the recurrence of hepatitis B infection.
Safety, an important element of this trial, was evaluated through physical examination and laboratory tests. All of the 87 subjects who received Hepabulin even once in the study were included in the safety analysis group. A total of 119 (13.58%) of 876 AEs were serious. Mortality occurred in 2 cases (2.29%), neither of which was related to the trial drug (causes of death were sepsis and HCC recurrence). The incidence rates of AEs and serious AEs were high because of the close observation over a period of 52 weeks in critical liver transplant recipients. Compared to the total of 177 AEs reported in the first 14 patients treated with HBIG [12], the AE rate was not particularly high in this study. The study population in this trial was living donor liver transplantation (LDLT) recipients. LDLT is characterized by a higher rate of biliary complications than deceased donor liver transplantation. In this study, biliary stricture was the most common serious AE. All of the AEs were judged to be unrelated to Hepabulin, and most were mild.
HBIG is known to bind to HBsAg or to the surface of HBV particles and neutralize HBsAg or protect hepatocytes from HBV infection [15,16]. In this study, both HBsAg and HBeAg titers decreased rapidly on the first day after LT, and only 28 (35%) and 4 (5%) patients were positive for HBsAg and HBeAg, respectively, and all were negative at 2 weeks after LT. HBV DNA level decreased rapidly after surgery, but remained at an average of 37 IU/mL at 7 days postoperatively and the number of positive cases remained at 13 (16.25%). Only 1 patient remained HBV DNA-positive until 8 weeks postoperatively, but all were negative by 3 months. HBV DNA remains long after surgery, which is associated with preoperative viral load, such as HBsAg titer, HBeAg, and HBV DNA level.
HBsAb titer was well-correlated with Hepabulin dose after Hepabulin administration. Circulating HBsAg and other factors determine the changing half-life of HBsAb in the initial postoperative period [17]. In our study, the factors affecting HBsAb titer at 1 week postoperatively were general condition (Child score, MELD score, total bilirubin level, and albumin level) and HBV viral load (HBsAg titer, HBV DNA status, and mutation status). However, within several weeks to months, the half-life lengthened and stabilized in these patients, although there seem to be patient-to-patient variations [18]. In our study, the factors affecting HBsAb titer at 52 weeks after surgery were sex and serum ALT level.
Conclusions
Prevention of recurrence of HBV after liver transplantation is very important to improve the prognosis of liver transplantation. Hepabulin is a hepatitis B immunoglobulin substance with good safety and efficacy in preventing recurrence of HBV after transplantation. This study was conducted with HBIG monotherapy without antiviral agent to demonstrate the efficacy and safety of new HBIG, Hepabulin. The new HBV treatment guidelines, such as time interval and proper titer, require further study.
Footnotes
Source of support: This study was funded by SK Plasma, Seoul, South Korea
References
- 1.Schreibman IR, Schiff ER. Prevention and treatment of recurrent Hepatitis B after liver transplantation: The current role of nucleoside and nucleotide analogues. Ann Clin Microbiol Antimicrob. 2006;5:8. doi: 10.1186/1476-0711-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korea Centers for Disease Control and Prevention. The Third Korea National Health and Nutrition Examination Survey (KNHANES III), 2005. Seoul: Korea Centers for Disease Control and Prevention; 2006. p. 68. [Google Scholar]
- 3.Samuel D, Muller R, Alexander G, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993;329:1842–47. doi: 10.1056/NEJM199312163292503. [DOI] [PubMed] [Google Scholar]
- 4.Kim WR, Poterucha JJ, Kremers WK, et al. Outcome of liver transplantation for hepatitis B in the United States. Liver Transpl. 2004;10:968–74. doi: 10.1002/lt.20217. [DOI] [PubMed] [Google Scholar]
- 5.Kasraianfard A, Watt KD, Lindberg L, et al. HBIG remains significant in the era of new potent nucleoside analogues for prophylaxis against hepatitis B recurrence after liver transplantation. Int Rev Immunol. 2016;35:312–24. doi: 10.3109/08830185.2014.921160. [DOI] [PubMed] [Google Scholar]
- 6.Onoe T, Tahara H, Tanaka Y, Ohdan H. Prophylactic managements of hepatitis B viral infection in liver transplantation. World J Gastroenterol. 2016;22:165–75. doi: 10.3748/wjg.v22.i1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung J, Chan SC, Cheung C, et al. Oral nucleoside/nucleotide analogs without hepatitis B immune globulin after liver transplantation for hepatitis B. Am J Gastroenterol. 2013;108:942–48. doi: 10.1038/ajg.2013.111. [DOI] [PubMed] [Google Scholar]
- 8.Cholongitas E, Papatheodoridis GV. High genetic barrier nucleos(t)ide analogue(s) for prophylaxis from hepatitis B virus recurrence after liver transplantation: A systematic review. Am J Transplant. 2013;13:353–62. doi: 10.1111/j.1600-6143.2012.04315.x. [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Vierling JM. Management of HBV infection in liver transplantation patients. Int J Med Sci. 2005;2:41–49. doi: 10.7150/ijms.2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terrault NA, Kilic M, Karademir S, et al. HepaGam B after liver transplant in patients with Hepatitis B virus. US Gastroenterol Rev. 2007;2:39–45. [Google Scholar]
- 12.Terrault NA, Zhou S, Combs C, et al. Prophylaxis in liver transplant recipients using a fixed dosing schedule of hepatitis B immunoglobulin. Hepatology. 1996;24:1327–33. doi: 10.1002/hep.510240601. [DOI] [PubMed] [Google Scholar]
- 13.Nymann T, Shokouh-Amiri MH, Vera SR, et al. Prevention of hepatitis B recurrence with indefinite hepatitis B immune globulin (HBIG) prophylaxis after liver transplantation. Clin Transplant. 1996;10:663–67. [PubMed] [Google Scholar]
- 14.Olivera-Martinez MA, Gallegos-Orozco JF. Recurrent viral liver disease (hepatitis B and C) after liver transplantation. Arch Med Res. 2007;38:691–701. doi: 10.1016/j.arcmed.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Krugman S, Giles JP, Hammond J. Viral hepatitis, type B (MS-2 strain) prevention with specific hepatitis B immune serum globulin. JAMA. 1971;218:1665–70. [PubMed] [Google Scholar]
- 16.Szmuness W, Prince AM, Goodman M, et al. Hepatitis B immune serum globulin in prevention of nonparenterally transmitted hepatitis B. N Engl J Med. 1974;290:701–6. doi: 10.1056/NEJM197403282901302. [DOI] [PubMed] [Google Scholar]
- 17.Adler R, Safadi R, Caraco Y, et al. Comparison of immune reactivity and pharmacokinetics of two hepatitis B immune globulins in patients after liver transplantation. Hepatology. 1999;29:1299–305. doi: 10.1002/hep.510290446. [DOI] [PubMed] [Google Scholar]
- 18.McGory RW, Ishitani MB, Oliveira WM, et al. Improved outcome of orthotopic liver transplantation for chronic hepatitis B cirrhosis with aggressive passive immunization. Transplantation. 1996;61:1358–64. doi: 10.1097/00007890-199605150-00013. [DOI] [PubMed] [Google Scholar]


