Abstract
The previous concept regarding diabetic retinopathy assigned a primary role to hyperglycemia-induced microvascular alterations, while neuronal and glial abnormalities were considered to be secondary to either ischemia or exudation. The aim of this study was to reveal the potential role of neuronal and glial cells in initial and advanced alterations of the retinopathy in human type 2 diabetes. Electron microscopy and histochemical studies were performed on 38 surgically removed human eyes (28 obtained from diabetic patients and 10 from non-diabetic patients). Morphometric analysis of basement membrane material and lipids was performed. An accumulation of metabolic by-products was found in the capillary wall with aging: this aspect was significantly more pronounced in diabetics. Müller glial cells were found to contribute to alterations of the capillary wall and to occlusion, as well as to the development of proliferative retinopathy and cystoid degeneration of the retina. Our results showed morphological evidence regarding the role of neuronal and glial cells in the pathology of diabetic retinopathy, prior and in addition to microangiopathy. These morphological findings support a neurovascular pathogenesis at the origin of diabetic retinopathy, thus the current treatment approach should be completed by neuroprotective measures.
Keywords: basement membrane, diabetic macular edema, diabetic retinopathy, glia, proliferative diabetic retinopathy
Introduction
Diabetic retinopathy (DR) is a common complication of diabetes and a leading cause of blindness in adults. The clinical signs of DR include increased vascular permeability, leading to edema, and endothelial cell proliferation. It has become increasingly clear that DR affects not only retinal vasculature but also retinal neuronal and glial cells.1,2 Normal vision depends on the normal function of retinal neurons, so visual loss in diabetes must ultimately be explained in terms of altered neuronal function. Clinically, DR has been mainly considered as a microvascular disease; however, recently, a neurodegenerative view of the disease has emerged.3 Numerous cellular and molecular studies support the hypothesis that neurodegeneration, together with functional changes in the vasculature, is an important component in DR. The principal glial cell of the retina is the Müller cell, a specialized radial glial cell spanning the entire depth of the retina.2–4 Through the extensive arborization of their processes, Müller cells constitute an anatomic and functional link between neurons and vessels. Retinal glial cells are considered channels of communication between retinal blood vessels and neurons owing to their particular spatial arrangement and regulatory functions.5 They not only provide structural support but also are involved in maintaining the complex homeostasis of the retina by regulating metabolism, the phagocytosis of neuronal debris, and the release of neurotransmitters and trophic factors. Therefore, the final role of glial cells, in particular of microglia, is not only to monitor the retinal microenvironment but also to respond to potential abnormalities maintaining the tissue homeostasis and modulating the inflammatory processes.5,6 At early stages of DR, perivascular microglial cells moderately grow in number and gradually become hypertrophic in the innermost retinal layers, leading to an increase in glial fibrillary acidic protein (GFAP) expression and a decrease in the astrocytic population. The expression of GFAP generally correlates with the functional state of the astrocytes. These changes suggest that astrocyte function is concomitant with an increased vascular permeability and with modifications in blood flow in DR.
Müller cells produce factors capable of modulating blood flow, vascular permeability, and cell survival. Moreover, their processes surround all blood vessels in the retina. The up-regulation of the above-mentioned factors contemporarily activates Müller cells and raises the production of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor, which in turn triggers pathological neovascularization and retinal fibrosis.5
In the light of the data reported in the literature, it is becoming apparent that Müller cell abnormalities are due to a direct effect of diabetes on the neural compartment of the retina and that these changes precede the vascular lesions associated with DR.
Materials and methods
Study population
In all, 38 human eyes, age 6–89 years (21 male and 17 female, mean age: 64.4 years), were selected for these studies: the patients from whom the eyes were taken were non-diabetic in 10 cases, diabetic without signs of DR in 6, with various signs of DR in 10, with proliferative diabetic retinopathy (PDR) in 10, and with diabetic macular edema (DME) in 2. All these eyes had been surgically removed several years earlier due to severe ocular trauma or malignant tumors, neither of which affected the posterior pole of the eyeball, and several thousands of images were conserved either in hard copy or in digitalized form and were re-examined for this study. The study was conducted in compliance with the Declaration of Helsinki, applicable to national and local requirements regarding the ethics committee and institutional review boards. Ethical approval was obtained from the institutional review board (Policlinico Umberto I of Rome and Department of Ophthalmology, University of Pecs, Hungary). A written informed consent was obtained before the experimental analysis of the enucleated eyeballs from each adult patient or from parents on behalf of the minors.
Electron microscopy
Small pieces of the retina were dissected at the posterior pole within 2 min of surgical removal of the eyeball, fixed at 4°C in 2% buffered glutaraldehyde for 2 h and post-fixed in 2% osmium tetroxide for another 2 h. The specimens were dehydrated, embedded in araldite, sectioned with a Reichert ultramicrotome, contrasted with lead citrate and uranyl acetate for transmission electron microscopy (TEM), and studied with a Zeiss 109 Electron Microscope.
Histochemical–morphological evaluation
After dissection, samples were washed in phosphate buffer (phosphate buffered saline (PBS), 0.1 M, pH 7.4), fixed with 10% buffered formalin, embedded in paraffin, using a standard procedure, and cut in serial sections, with a thickness of 5 µm, using a microtome. For morphological evaluation, sections were stained with hematoxylin and eosin.
Morphometry
Capillary basement membrane thickness and lipid deposits in the capillary wall were determined using NIH ImageJ program.7 Five sections from each specimen and 10 different areas of each sections were examined and the relevant areas were calculated. Morphometric analysis was performed independently by two experienced observers, and questionable cases were re-evaluated by a senior researcher.
Statistical analysis
Statistical analysis was performed using SPSS software (version 15.0; IBM Corp., Armonk, NY, USA). The Shapiro–Wilk W test was used to assess the normal distribution of the variables. During the analysis, data from non-diabetic patients, mildly diabetic patients (no visible retinopathy), and severely diabetic patients (visible retinopathy, cystoid macular edema (CME), PDR) were compared by means of one-way analysis of variance (ANOVA) tests. The effect of age and the severity of diabetes on the thickness of capillary wall and areas of lipid deposits were evaluated using multivariable regression models.
Results
Thickening of the capillary wall
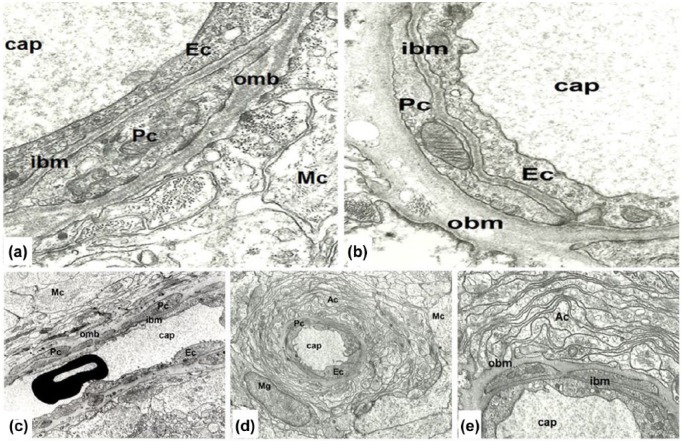
In normal young eyes, the capillary wall contains two distinct layers: the inner leaflet consisting of endothelial cells and pericytes, and the outer one containing Müller cells or astrocytes. The outer leaflet of the capillary wall changed significantly during aging: an increase in thickness was accompanied by the accumulation of vacuoles, vesicles, electron-dense granules, and collagen fibers in an amorphous basement membrane material. In comparison, the inner leaflet remained homogeneous and only slightly thickened even at an advanced age. Both endothelial cell and pericyte cytoplasm contained well-preserved organelles (Figure 1(a) and (b)).
Figure 1.
(a and b) Comparison of young and aged retinal capillary wall. (a) Retinal capillary (cap) in young age contains endothelium (Ec), pericyte (Pc), and Müller glial cells (Mc), and the basement membrane is formed by an inner leaflet (ibm) and an outer leaflet (obm). Six years male, magnification: ×24,000. (b) Aged retinal capillary (cap) contains an endothelial cell (Ec), pericyte (Pc), and significantly thickened basement membrane in which several electron-translucent, round-form vacuoles, and highly electron-dense granules are embedded. Both basement membrane thickening and lipid deposition affect the outer leaflet (obm) of the basement membrane, while the inner leaflet (ibm) is unaffected. Male 62 years, magnification ×24,000. (c–e) Retinal capillary in mild diabetes. (c) Retinal capillary (cap) in nearly longitudinal section: endothelial cells (Ec), pericytes (Pc), and their basement membrane (the inner leaflet of the capillary wall) show normal ultrastructural features. Müller cells (Mc) contain numerous, well-preserved organelles, and their basement membrane (the outer leaflet of the capillary wall) is significantly thickened; 81 years female, magnification: ×8000. (d) Capillary in the inner plexiform layer is surrounded by numerous cytoplasmic processes of astrocytes (Ac), microglia (Mg), and Müller glial cells (Mc) located around the “astrocyte-sheet.” Male 48 years, magnification ×8000. (e) Detail of the former picture in higher magnification. The inner leaflet of the basement membrane (ibm) is significantly thinner than the outer leaflet of the basement membrane (obm), which in several places extends in-between astrocytes. Endothelial cells and pericytes, as well as their basement membrane show normal appearance. Male 48 years, magnification ×20,000.
Similarly, in samples from patients with mild diabetes, that is, no visible retinal changes, the outer leaflet of the capillary wall was thickened and in several places extended into the intercellular space of Müller cells, next to the capillaries. Furthermore, in some cases, islets of basement membrane material without any apparent connection to the capillaries were observed. In addition to the thickening of the homogeneous material, electron-dense or electron-translucent vacuoles and vesicles appeared in the outer leaflet, suggesting the presence of unsaturated and saturated lipids, respectively. Müller’s cell cytoplasm contained several organelles of normal appearance and numerous intermediate filaments, microtubules, and vacuoles indicating their intense metabolic activity. The inner leaflet of the capillary basement membrane remained apparently unchanged as long as pericytes persisted. Neither endothelial cells nor pericytes in the mild DR showed a significant difference from age-related changes. The number and extension of pericytes decreased in advanced DR. In mild diabetes, the involvement of astrocytes was similar to that of Müller’s cells. Their cytoplasm contained several organelles of normal appearance, but their basement membrane was significantly thickened, protruding into the intercellular spaces (Figure 1(c)–(e)). The data concerning the thickening of capillary basement membrane are provided in detail in Table 1.
Table 1.
Thickness of capillary wall in the inner leaflet.
| Number of eyes | Media ± standard deviation | |
|---|---|---|
| Patients with PDR | 10 | 580.93 ± 10.41 nm |
| Patients with DR | 10 | 383.11 ± 4.28 nm |
| Patients without DR | 10 | 278.87 ± 7.01 nm |
| P-value | 0.00001 |
PDR: proliferative diabetic retinopathy; DR: diabetic retinopathy.
The results were considered as statistically significant when P-value <0.01.
Intravascular glial proliferation
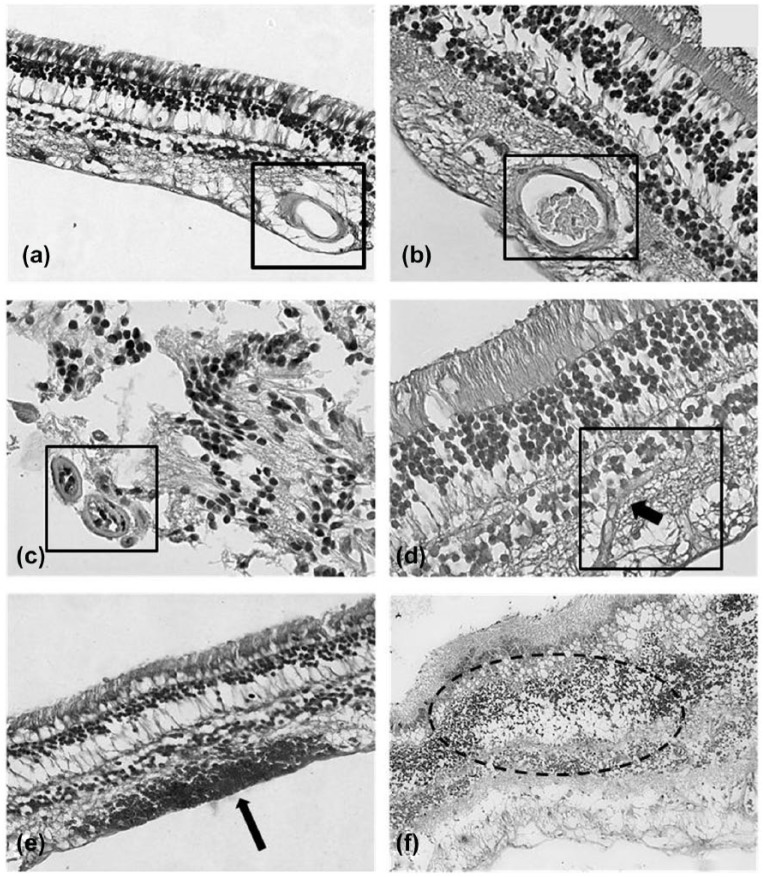
With the progression of diabetes, that is, the appearance of visible alterations in the retina (micro-aneurysms, hemorrhages, hard, and soft exudates), two types of ultrastructural changes could be observed. First, a progressive transformation of Müller cell phenotype to poorly differentiated glial cell phenotype: a decrease in organelles (mitochondria, lysosomes, endoplasmic reticulum, and Golgi’s complex) was accompanied by the accumulation of vesicles. Furthermore, the decrease in neuronal structures and the proliferation of glial cell processes in the retina were altogether considered as “reactive gliosis.” The most exciting observation was the appearance of glial cell processes in the lumen of several capillaries in DR. The intraluminal glia showed variable features: in certain cases glial processes occurred together with well-preserved endothelial cells and blood cells suggesting partial occlusion, while in others the capillary lumen was completely occluded by glial processes. In each case, intraluminal glial cells showed subcellular structures similar to those of the extra-luminal glia. Despite a thorough examination of tissue samples, we did not find evidence of a direct continuity between the intra- and extra-luminal glia through the capillary wall. However, we did observe focal vacuolization of the capillary wall, suggesting a communication between the intra- and extra-luminal glia (Figure 2(a)–(d)).
Figure 2.
(a and b) Normal human retina. H&E stain. The normal morphology of the retina appears preserved in control patients. The lumen of the capillaries is free from glial cells proliferation, magnification ×40. (c and d) Human retina of patients affected by diabetic retinopathy. H&E stain. The lumen of the capillaries is filled by intravascular glial cells proliferation. In (c), two occluded capillaries in transverse section are visible (lower left corner). In (d), a capillary (longitudinal section) is occluded by intravascular glial cells’ proliferation (arrow), magnification ×40. (e and f) Human retina of patients affected by proliferative diabetic retinopathy. H&E stain. Within the retinal tissue, some microhemorrhages (e, arrow) and cystoid degeneration of the inner layers of the retina (f) are visible, magnification ×20.
PDR
While several electron microscopic and histochemical studies are available on surgically removed intra-retinal proliferative tissue in advanced DR, we documented the earliest event of PDR, when the retinal glia breaks the altered capillaries and causes microhemorrhages (Figure 2(e) and (f)). This provides direct morphological proof that glial cells play a role in the neo-formation of vitreo-retinal fibrocellular tissue. We also demonstrated the earliest phase of neovascularization, that is, an endothelial tube without basement membrane and pericytes.
Cystoid retinal degeneration
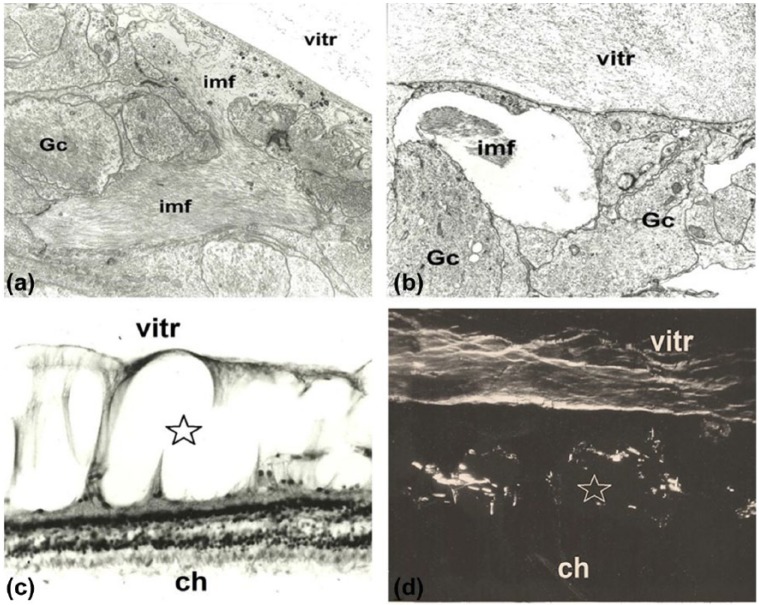
Electron microscopy showed intra-retinal accumulation of intermediate filaments mainly in the intercellular spaces or in the vitreous cavity. By means of specific histochemical reaction and polarization microscopic analysis of cystoid retinal degeneration, we identified glycoprotein-rich filamentary structures which had accumulated in both the retina and vitreous body (Figure 3).
Figure 3.
Cystoid retinal degeneration. (a) Early phase of cystoid retinal degeneration: accumulation of intermediate filaments (imf) between glial cells (Gc) is visible. Male 40 years, transmission electron microscopy. Magnification ×16,000. (b) Early cystoid degeneration of the retina with intermediate filaments (imf) in one of the empty spaces between glial cells (Gc). No neuronal elements may be identified. In the vitreous (vitr), disorganized filaments and fine granular material are visible. Female 56 years. Transmission electron microscopy. Magnification ×18,000. (c) Advanced cystoid degeneration of the retina (star). Some columns of Müller cells separate empty “cystoid” spaces of the inner retina, while outer layers are well-preserved. Note the increased thickness of the retina (ch: choroid, vitr: vitreous). Female 69 years. Light microscopy: H&E stain. Magnification ×800. 3D. Glycoprotein-rich filaments are visible in the cystoid spaces of the retina (star) and in the vitreous body (ch: choroid, vitr: vitreous). Female 69 years. Polarization microscopic picture. Magnification ×800.
Discussion
Our observations present morphological evidence of an emerging concept by demonstrating that diabetes affects the entire neurovascular unit of the retina, with early loss of neurovascular coupling, gradual neurodegeneration, and glial alteration due to neuro-inflammation occurring before appreciable vascular alterations.8 Our observations suggest the involvement of Müller’s cells and astrocytes in both early and late DR. The density of Müller’s cell increases, whereas the number of astrocytes is decreased in diabetic retinas.9 Müller’s cells contribute to inflammatory responses during the development of DR, are major sources of inflammatory mediators, and become virtually “activated” or “reactive” in response to all pathological changes in the retina.10 VEGF is rapidly released by Müller’s cells in early DR, enhancing perfusion by locally increasing the permeability of blood vessels with a concomitant decrease in the anti-angiogenic pigment epithelium-derived factor amounts.11,12 In the early stage, we observed that changes in retinal capillaries were initiated by thickening of the basement membrane adjacent to Müller’s cells. In addition, the basement membrane of glial origin protruded into the intercellular space of Müller’s cells. Similar alterations of the basement membrane were previously observed in an animal model of diabetes.13 Glial proliferation (“reactive phenotype of Müller’s cells”) is one of the most widespread alterations of DR. In streptozotocin-induced rat DR, Müller’s cells developed a complex and specific reactive phenotype, characterized by the induction of acute-phase response proteins and other inflammation-related genes,13 by a decrease in the glutamate transporter mechanism that is likely to involve oxidation14 and by an increase in the GFAP expression.15 In the thickened outer layer of the capillary wall, a various extent of lipid accumulation was observed, thus suggesting that lipid deposition is also an early event in diabetic microangiopathy.16 Similarly, thickening and compositional changes were observed in the internal limiting membrane (the basement membrane of Müller’s cells) in human DR.17 In addition to the well-known morphological changes, our study demonstrates intra-capillary proliferation and even the occlusion of capillaries due to glial cells’ proliferation observed in several electron microscopic images. Considered together, our electron microscopic findings and these histochemical observations suggest the possibility that glial cell invasion, but not endothelial cell proliferation, may occlude the vascular lumen in non-perfused areas of the retina secondary to DR. A prolonged and excessive edema thus results in neuronal cell death and disruption of Müller’s cells, typical of the final stage of the disease. Presumably this process is the result of an increased capillary leakage in which astrocytes may play a central role.
Acknowledgments
All authors have reviewed the final version of this manuscript and approved its submission for publication. J.F. and S.T. contributed equally.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The research for this paper was financially supported by Ministry of Health and Fondazione Roma.
ORCID iD: Samanta Taurone  http://orcid.org/0000-0002-0354-894X
http://orcid.org/0000-0002-0354-894X
References
- 1. Barber AJ. (2015) Diabetic retinopathy: Recent advances towards understanding neurodegeneration and vision loss. Science China Life Sciences 58(6): 541–549. [DOI] [PubMed] [Google Scholar]
- 2. Jonsson KB, Frydkjaer-Olsen U, Grauslund J. (2016) Vascular changes and neurodegeneration in the early stages of diabetic retinopathy: Which comes first? Ophthalmic Research 56(1): 1–9. [DOI] [PubMed] [Google Scholar]
- 3. Sohn EH, Van Dijk HW, Jiao C, et al. (2016) Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proceedings of the National Academy of Sciences of the United States of America 113(19): E2655–E2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lynch SK, Abràmoff MD. (2017) Diabetic retinopathy is a neurodegenerative disorder. Vision Research. Epub ahead of print 28 April DOI: 10.1016/j.visres.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coughlin BA, Feenstra DJ, Mohr S. (2017) Müller cells and diabetic retinopathy. Vision Research. Epub ahead of print 5 September DOI: 10.1016/j.visres.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sorrentino FS, Allkabes M, Salsini G, et al. (2016) The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life Sciences 162: 54–59. [DOI] [PubMed] [Google Scholar]
- 7. Abramoff MD, Magalhaes PJ, Ram SJ. (2004) Image processing with ImageJ. Biophotonics International 11(7): 36–42. [Google Scholar]
- 8. Abcouwer SF, Gardner TW. (2014) Diabetic retinopathy: Loss of neuroretinal adaptation to the diabetic metabolic environment. Annals of the New York Academy of Sciences 1311: 174–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rungger-Brandle E, Dosso AA, Leuenberger PM. (2000) Glial reactivity, an early feature of diabetic retinopathy. Investigative Ophthalmology & Visual Science 41: 1971–1980. [PubMed] [Google Scholar]
- 10. Liu X, Ye F, Xiong H, et al. (2015) IL-1beta induces IL-6 production in retinal Müller cells predominantly through the activation of p38 MAPK/NF-kappaB signaling pathway. Experimental Cell Research 331(1): 223–231. [DOI] [PubMed] [Google Scholar]
- 11. Wang J, Xu X, Elliott MH, et al. (2010) Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes 59: 2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Penn JS, Madan A, Caldwell RB, et al. (2008) Vascular endothelial growth factor in eye disease. Progress in Retinal and Eye Research 27: 331–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gerhardinger C, Costa MB, Coulombe MC, et al. (2005) Expression of acute-phase response proteins in retinal Müller cells in diabetes. Investigative Ophthalmology & Visual Science 46: 349–357. [DOI] [PubMed] [Google Scholar]
- 14. Puro DG. (2002) Diabetes-induced dysfunction of retinal Müller cells. Transactions of the American Ophthalmological Society 100: 339–352. [PMC free article] [PubMed] [Google Scholar]
- 15. Mizutani M, Gerhardinger C, Lorenzi M. (1998) Müller cell changes in human diabetic retinopathy. Diabetes 47: 445–449. [DOI] [PubMed] [Google Scholar]
- 16. Powner MB, Scott A, Zhu M, et al. (2011) Basement membrane changes in capillaries of the ageing human retina. British Journal of Ophthalmology 95: 1316–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. To M, Goz A, Camenzind L, et al. (2013) Diabetes-induced morphological, biomechanical, and compositional changes in ocular basement membranes. Experimental Eye Research 116: 298–307. [DOI] [PubMed] [Google Scholar]