Abstract
Mono(2-ethylhexyl) phthalate (MEHP) is the bioactive metabolite of di(2-ethylhexyl) phthalate, a plasticizing agent and persistent environmental contaminant associated with obesity, developmental abnormalities, and oxidative stress. Nrf2 (Nfe2l2) is a transcription factor that regulates cytoprotective genes as part of the adaptive antioxidant response. We previously identified the pancreas as a sensitive target of oxidative stress during embryonic development. The goals of this study were to 1) characterize the effects of MEHP exposure on pancreatic development, and 2) determine whether oxidative stress contributes to MEHP embryotoxicity. Zebrafish (Danio rerio) embryos from AB wildtype and Tg(ins:GFP;nrf2afh318/fh318) were exposed to 0 or 200 µg/L MEHP at 3 hours post fertilization (hpf) through 168 hpf to assess pancreatic organogenesis. MEHP exposure significantly decreased ß-cell area at all timepoints (48, 72, 96, 168 hpf), but Nrf2a did not significantly protect against islet hypomorphism. Tg(gcga:GFP) embryos exposed to MEHP showed a decrease in α-cell area in the islet across the same timepoints. Tg(ptf1a:GFP) embryos were assessed at 80 and 168 hpf for exocrine pancreas length. MEHP exposure decreased growth of the exocrine pancreas. Expression of pancreas genes insa, sst2 and ptf1a was significantly reduced by MEHP exposure compared to controls. Glutathione (GSH) concentrations and redox potentials were quantified at 72 hpf by HPLC, but no significant changes were observed. However, expression of the GSH-related genes gstp1 and gsr were significantly altered by MEHP exposure. These data indicate that the developing pancreas is a sensitive target tissue of embryonic exposure to MEHP.
Keywords: Islet, endocrine, exocrine, toxicology, redox, Nfe2l2
1. Introduction
Phthalates are a class of ubiquitous environmental toxicants, several of which are associated with metabolic and reproductive health disorders (Tickner et al. 2001, Corbasson et al. 2016). Added to polyvinylchloride (PVC)-based plastics to increase flexibility, phthalates are not covalently bound and can leach from plastics when exposed to extreme conditions such as heat or UV light (Tickner et al. 2001). Phthalates have been detected in human biological samples, including blood, pancreatic and intestinal epithelial tissues (Lee et al. 2006). In addition, they have been detected in amniotic fluid and fetal cord blood samples, which indicate that phthalates can cross the placental barrier and pose a potential risk to the exposed fetus (Sathyanarayana et al. 2008).
One of the most widely studied phthalates is di(2-ethylhexyl) phthalate (DEHP), found in consumer products including food packaging, nail polish, medical tubing and toys. Human exposure to DEHP typically occurs through ingestion or via medical equipment (Lin et al. 2011). Once it enters the body, DEHP is rapidly hydrolyzed to mono(2-ethylhexyl) phthalate (MEHP), a highly reactive and toxic metabolite (Frederiksen, Skakkebaek and Andersson 2007). One of the enzymes that hydrolyzes DEHP to MEHP is pancreatic lipase (Daniel and Bratt 1974). In mouse toxicokinetic studies, MEHP has been detected at high levels in pancreatic tissue, more so than in liver, kidney, blood, or placenta (Tomita et al. 1986). These findings suggest the pancreas is an important target tissue of phthalate exposures.
Both DEHP and MEHP are capable of inducing oxidative stress by reducing available glutathione (GSH) and disrupting GSH-related gene expression (Sant et al. 2016a, Wang et al. 2012), and both have been associated with metabolic dysfunction, insulin resistance and type 2 diabetes (Corbasson et al. 2016, Lin et al. 2011). Exposure to MEHP during early developmental windows has been shown to negatively impact development due to induction of oxidative stress, implicated by increased frequencies of neural tube defects and altered male and female genital morphology following MEHP exposure in rodent models (Sant et al. 2016a, Wang et al. 2012, Gray et al. 2000). We have recently shown that developmental exposure to prooxidants negatively impacts pancreatic development and produces structural islet variant morphologies and truncation of the exocrine pancreas, similar to those produced by embryonic MEHP exposure (Sant et al. 2016b). Thus, we hypothesize that disruptions in redox signaling may be a potential mechanism by which MEHP alters pancreatic morphology.
The zebrafish model is widely integrated in developmental biology and toxicology research (Truong et al. 2014, Tiso, Moro and Argenton 2009, Yang et al. 2009). The availability of pancreas-specific transgenics make it a particularly insightful model in which to investigate toxicological impacts on pancreas development, as the development of both the endocrine and exocrine pancreas can be easily visualized in real-time throughout development in live embryos. In zebrafish, the first pancreatic bud forms dorsally from the endoderm at the 16-somite stage during organogenesis, governed by expression of the pancreatic and duodenal homeobox gene (pdx1). Between 24 and 48 hpf, the dorsal bud rotates along with the gut and gives rise to the primary endocrine islet, a core of ß-cells surrounded by α-cells and δ-cells. A second bud arises anteroventrally during the pharyngula stage around 40 hpf (reviewed in Tiso et al. 2009), and elongates to form the bulk of exocrine tissue between 48 and 72 hpf. The anteroventral bud differentiates to form the pancreatic ducts, governed by dual expression of pdx1 and the pancreas-specific transcription factor alpha (ptf1a) (Tiso et al. 2009). Importantly, these developmental signaling pathways and processes of pancreatic organogenesis are highly conserved among vertebrates, including zebrafish (Lele and Krone 1996). Additional advantages of the zebrafish model include large clutches of transparent embryos that develop rapidly, externally to the mother. These attributes make the zebrafish embryo one of the best-suited models for investigating pancreatic development in vivo.
We recently reported that prooxidant compounds and MEHP have the ability to produce similar structural pancreatic anomalies, including hypomorphic beta cell clusters in primary islets of zebrafish embryos (Sant et al. 2016b). Here, we aim to characterize the observed effects of embryonic exposure to MEHP on endocrine and exocrine pancreatic organogenesis, identify the pancreas as a sensitive target tissue of developmental exposure to MEHP, and provide the groundwork to investigate the functional toxicological impacts of MEHP on developing pancreas in the zebrafish embryo model.
2. Methods
2.1. Chemicals
All chemicals used in these experiments were of the highest purity grade available. Mono(2-ethylhexyl)phthalate (MEHP) was purchased from AccuStandard (New Haven, CT, USA). Dimethylsulfoxide (DMSO) was purchased from Fisher Scientific (Pittsburg, PA, USA). A stock solution of 2 mg/mL was prepared by diluting neat MEHP in DMSO. Solutions were stored in amber vials at −20 °C and vortexed before each use. All procedures involving the handling of MEHP were performed utilizing proper PPE and standard laboratory safety precautions. All other chemicals used in this study were purchased from Fisher Scientific.
2.2. Animals
Adult zebrafish were maintained in accordance with the guidelines laid out in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health, and with authorization from the University of Massachusetts Amherst Institutional Animal Care and Use Committee (Animal Welfare Assurance Number A3551-01). Fish were maintained in a recirculating zebrafish system (Aquaneering, San Diego, CA, USA) at 28.5 °C on a daily 14 h light:10 h dark cycle, and fed once daily with the recommended amount of Gemma Micro 300 Skretting; Westbrook, ME, USA). Breeding fish populations were maintained in tanks containing an approximate 1:2 ratio of males to females. Zebrafish embryos were collected daily from tanks approximately 0–1 hours post fertilization (hpf), washed thoroughly, and placed in 100 mm polystyrene petri dishes containing 0.3× Danieau’s solution (Westerfield 2007).
Wildtype AB zebrafish were obtained from Boston Children’s Hospital (Boston, MA) and were used in the redox and gene expression experiments. Transgenic zebrafish strains (on AB background) were obtained from Dr. Philip diIorio at the University of Massachusetts Medical School (Worcester, MA, USA), and Dr. Jennifer Moss at Duke University. The Tg(ins:GFP) and Tg(gcga:GFP) strains, which co-express green fluorescent protein (GFP) with insulin and glucagon expression respectively, were used to visualize pancreatic islet development (diIorio et al. 2002, Pauls et al. 2007). A Tg(ptf1a:GFP) strain was used to visualize the development of the exocrine pancreas (Lin et al. 2004).
Wild type (nrf2a+/+) and nrf2afh318/fh318 (loss of function) zebrafish embryos on an AB strain background were used to investigate whether the detoxification of MEHP is governed by the Nrf2 pathway. Zebrafish heterozygous for the Nrf2afh318 mutation were generated by the TILLING mutagenesis project (R01HD076585), and obtained from Dr. Mark Hahn as embryos from the Moens Laboratory at the Fred Hutchinson Cancer Research Center (Seattle, WA, USA). Adults were bred in house to achieve homozygosity for wild type (nrf2a+/+) and mutant (nrf2afh318/fh318) animals. Adult homozygous wild type and mutants were then crossed with Tg(ins:GFP) zebrafish to obtain wild type and mutant transgenics. Adult heterozygotes were then crossed and bred to homozygosity for the wild type and mutant genotype. All breeding adults were genotyped as previously described to confirm homozygosity (Rousseau et al. 2015).
2.3. Exposures
Zebrafish embryos were exposed to MEHP beginning at the mid-blastula transition (3 hpf). Embryos were transferred to 20 mL glass scintillation vials containing 10 mL dosing solutions (0.01% DMSO control, 200 µg/L MEHP in 0.3× Danieau’s medium), with 5 embryos per vial. Solutions were refreshed daily through 168 hpf (7 days post fertilization) by completely changing media of both control and exposed groups. Embryos were manually dechorionated at 24 hpf using watchmaker’s forceps under a dissection microscope (Leica Microsystems, IL, USA). The concentration of MEHP was chosen based on other zebrafish studies in the literature which showed 200 µg/L to produce sub-lethal effects(e.g. (Zhai et al. 2014). Our preliminary exposure studies confirmed that this concentration does not produce gross malformations in developing zebrafish.
2.4. Microscopy
To observe larval growth and the development of the primary pancreatic islet, Tg(ins:GFP) eleutheroembryos and larvae were imaged at 48, 72, 96, and 168 hpf, using an inverted fluorescence microscope with a light cube GFP filter (EVOS FL Auto, Life Technologies, Pittsburgh, PA, USA). Images were captured with 4×, 10× and 20× objectives under transmitted light and GFP filters to assess gross morphology and islet development. To observe the extension of the exocrine pancreas, Tg(ptf1a:GFP) larvae were imaged at 80 and 168 hpf, previously identified as sensitive timepoints in exocrine pancreas development (Sant et al. 2017b). Larvae were examined using an upright Olympus compound fluorescence microscope equipped with a Zeiss Axiocam 503 camera and Zen analysis software (Zeiss, USA). Images were captured with 4× and 10× objectives under transmitted light and GFP filters to assess total fish length and exocrine pancreas length.
Prior to imaging, eleutheroembryos and larvae were washed in petri dishes containing 30–40 mL 0.3× Danieau’s, briefly anesthetized in 0.3× Danieau’s containing MS-222 (Westerfield 2007), and staged in 3% methylcellulose. The zebrafish pancreas is located on the right side of the body, and fish were oriented laterally to optimize pancreas visualization on either the right or left side depending on whether the inverted or upright microscope was used in the acquisition. After imaging, the fish were washed thoroughly in fresh Danieau’s medium.
2.5. Image analysis and morphometrics
Prior to analysis, images were blinded, and then analyzed using EVOS or Zen (Zeiss) analysis software. To account for acquisition artifact, all images captured on the inverted EVOS were mirror-flipped prior to analysis using Image J software (downloaded from NIH.gov). Gross morphology and image quality was assessed first to determine whether the images met quality control parameters, including correct staging, exposure, and image acquisition. Gross morphology was assessed by measuring total fish length, yolk sac area, and pericardial sac area. To determine total fish length, images acquired with a 2× objective were used to draw a straight line from the fish’s tail to jaw, and length was calculated by the software. Yolk sac and pericardial sac areas were measured by tracing the perimeter of each structure. To determine the area of the endocrine islets (either β-cells or α-cells), images acquired with a 10× objective captured under a GFP filter were used to trace the perimeter of the cell cluster, and areas were calculated by the software. Exocrine pancreas length was measured after imaging with a 5× objective in the same fashion, by drawing a straight line from the posterior tail of the pancreas to the posterior end of the endocrine islet, visualized as a dark indent in the fluorescent exocrine tissue. Relative exocrine pancreas length was determined by calculating a ratio of exocrine pancreas length to total fish length.
2.6. Gene Expression
At 96 hpf, RNA was collected from AB eleutheroembryos to assess gene expression of pancreas glucoregulatory hormones and digestive enzymes, and genes related to glutathione. A total of 6 samples from each exposure group were collected with pools of 7 fish per sample, and stored in RNA Later (Fisher Scientific) at −80 °C until RNA isolation. RNA was isolated using the GeneJET RNA Purification Kit purchased from Fisher Scientific (Waltham, MA, USA), following the included isolation protocol for whole tissue purification. RNA concentrations and sample purity were determined using a µLITE spectrophotometer purchased from BioDrop (Cambridge, UK). Immediately following RNA quantification, 500 ng RNA were converted to cDNA by reverse transcription using the iScript cDNA Synthesis Kit made by Bio-Rad, and all samples were diluted to 0.25 ng/µL cDNA in nuclease-free water, and stored at −20 °C until use.
Using a Bio-Rad CFX Connect Real-Time PCR Detection System, Quantitative Real-Time PCR (qRT-PCR) was performed to assess the expression of the pancreas hormone genes and glutathione-related genes. A 20 µL reaction mixture was prepared containing 5 µL nuclease-free water, 5 pM of each primer, and 10 µL 2× iQ SYBR Green Supermix (BioRad), and 4 µL cDNA template. Previously designed and optimized primers for ß-actin (actb), ß-2-microglobulin (b2m) and preproinsulin a (insa) have been described in published literature (Timme-Laragy et al., 2015). Additional pancreas genes, glucagon (gcga), ghrelin (ghrl), somatostatin 2 (sst2), pdx1, ptf1a, trypsin (try), chymotrypsinogen B1 (crtb1), and α-amylase 2A (amy2a) were investigated using PrimePCR primers purchased from Bio-Rad. Primers for the genes encoding glutathione-cysteine ligase catalytic subunit (gclc), glutathione-S-transferase A1 (gsta1), glutathione-S-transferase P (gstp), glutathione-disulfide reductase (gsr), and gamma-glutamyltransferase 1b (ggt1b), have been previously described in published literature (Sant et al. 2017a). Gene expression data were analyzed using Bio-Rad CFX software, and the ΔΔCT method was used to calculate fold-changes (Livak and Schmittgen, 2001). All fold changes were standardized relative to the biweight mean center of ß-actin and b2m expression (Dombi 2010).
2.7. Redox Analysis
Quantification of glutathione and cysteine redox pairs was performed using high-performance liquid chromatography, as previously described in (Jones 2002, Harris and Hansen 2012) and previously performed in (Sant et al. 2017a, Timme-Laragy et al. 2013). Briefly, five pools of 15–20 embryos were preserved in buffer containing perchlorate, boric acid, and γ-glutamylglutamate and stored at −80°C until use. S amples were derivatized using dansyl chloride, and analyzed using a Waters 2475 fluorescence detector coupled to a Waters 2695 separations module fitted with a Supelcosil LC-NH2 column. Reduced and oxidized glutathione (GSH, GSSG) and cysteine (Cys, CySS) was quantified using Waters Empower software, using excitation and emission wavelengths of 335 and 518 nm, respectively. Flow rate was 1 mL/min, using a gradient method switching between two mobile phases described in (Harris and Hansen 2012). Redox potentials were calculated using the Nernst equation (pH 7.4): Eh = E0 + (RT/nF) * log([GSSG]/[GSH]2), where E0 = −264 mV and (RT/nF) = 30.
2.8. Statistics
All microscopy experiments were repeated in triplicate. Statistical significance was determined by Students unpaired t-tests, chi-squared tests, or ANOVA with a Fishers post hoc test. Gene expression was analyzed using the ΔΔCT method (Livak K. J. 2001). Fold change calculations were normalized to the biweight mean center of two housekeeping genes, bactin and b2m expression, and the DMSO controls. Data are presented as the mean plus or minus the standard error of the mean (SEM).
3. Results
3.1. Gross Morphometrics
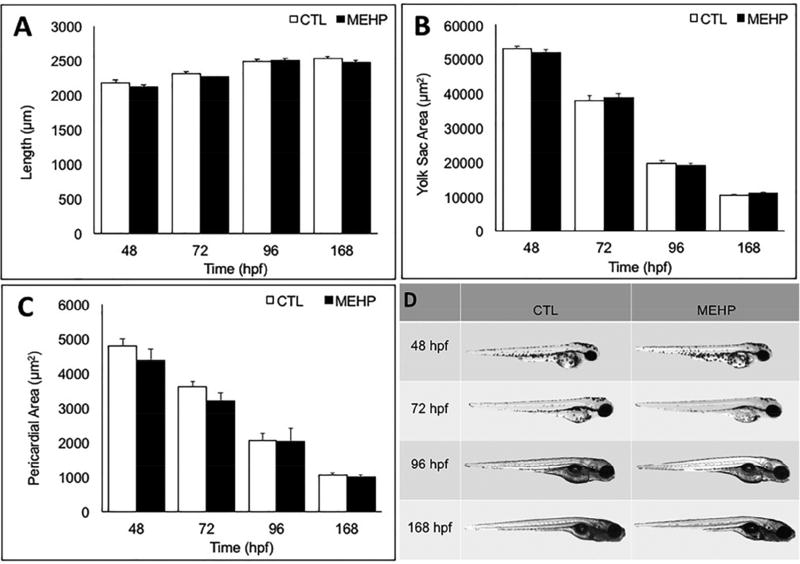
Developmental exposures to toxicants including MEHP have the ability to alter gross morphology. However, we selected a dosing concentration that is both environmentally relevant, and has been shown to be below embryotoxicity thresholds in previous zebrafish studies (Swan 2008, Sant et al. 2016a, Zhai et al. 2014). To determine if any pancreas variants seen were associated with gross malformations in the fish, total fish length, yolk sac area, and pericardial sac area were measured to assess MEHP effects on growth, yolk utilization, or cardiovascular health. No significant changes in length, yolk sac area, or pericardial sac area were observed due to MEHP exposure at this concentration (200 µg/L) (Fig. 1A–1D).
Figure 1.
Embryonic exposure to 200 µg/L MEHP does not alter gross morphology from 48–168 hpf. A) Length (rostral to caudal), B) yolk sac area, and C) pericardial sac area were measured at 48, 72, 96, and 168 hpf using Zen analysis software with a 2× objective. No significant differences in length, yolk sac area, pericardial area, or gross deformities were detected between exposed or control eleutheroembryos and larvae at any time point examined (n=30 fish; p>0.05).
3.2. Endocrine Islet Morphometrics
The Islets of Langerhans are essential to maintain glucose homeostasis, as they contain the only cells capable of secreting insulin and glucagon. It has been well-established that a decrease or loss of endocrine islets, especially the β-cells, is a common characteristic of both type 1 and 2 diabetes (Akirav, Kushner and Herold 2008, Matveyenko and Butler 2008). To assess whether early embryonic MEHP exposure alters the growth of the endocrine islet during development, Tg(ins:GFP) and Tg(gcga:GFP) zebrafish were used to visualize the area of the insulin-producing ß-cells and glucagon-producing α-cells, respectively.
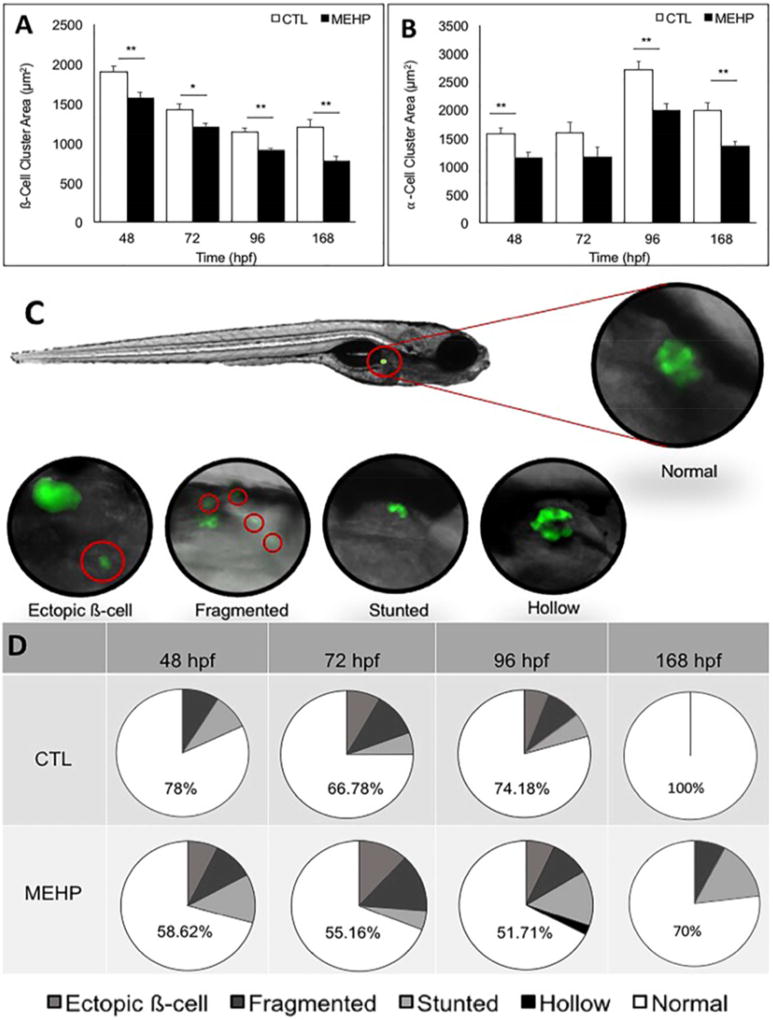
Following subchronic MEHP exposure, ß-cell cluster area was quantified by fluorescence microscopy using Tg(ins:GFP) embryos, eleutheroembryos, and larvae at 48, 72, 96, and 168 hpf. We found that ß-cell cluster area was significantly decreased in exposed fish compared to controls at all timepoints, most significantly at 48 hpf (p<0.001) where there was a 17.6% reduction in area (Fig. 2A). Similarly, α-cell area was assessed using Tg(gcga:GFP) embryos and eleutheroembryos at 48, 72, 96, and 168 hpf, using fluorescence microscopy. At each timepoint observed, α-cell area was also decreased in MEHP exposed groups compared to controls, most significantly at 96 and 168 hpf, with 27% and 32.38% reductions in α-cell area, respectively (p<0.001) (Fig. 2B). The β-cell cluster is surrounded by the glucagon-producing α-cells; together, these data demonstrate that developmental exposure to MEHP results in hypomorphic islets.
Figure 2.
Embryonic MEHP exposure significantly decreases pancreatic islet area compared to controls at 48, 72, 96, and 168 hpf, and significantly increases the incidence of pancreatic islet variants. A) Pancreatic ß-cell cluster area was visualized using Tg(ins:GFP) zebrafish eleutheroembryos and larvae following treatment with MEHP or DMSO, and islet area was measured using Zen analysis software. B) Pancreatic α-cell cluster area was visualized in Tg(gcga:GFP) zebrafish eleutheroembryos and larvae following treatment with MEHP or DMSO and measured using Zen analysis software. Islet architechture was assessed using Tg(ins:GFP) zebrafish eleutheroembryos and larvae. Morphologies that deviated from the normal spherical islet shape were categorized as fragmented, stunted, hollow, or having ectopic ß-cells. Variants were quantified across three study replicates. C) The position of the endocrine islet is shown on a 96 hpf eleutheroembryo, as well as representative images of the islet variants observed. D) Pie charts show the distribution of each variant, and numbers shown are the percentage of normal islets at each timepoint (n=40 for 48, 72, 96; n=20 for 168 hpf; *p<0.05; **p<0.005).
In addition to measuring islet area, Tg(ins:GFP) and Tg(gcga:GFP) zebrafish were used to assess islet morphology. While the islet normally develops in a compact, spherical cluster of endocrine cells, several variant morphologies have been previously described following toxicant exposures using the Tg(ins:GFP) strain, including fragmented, stunted, and hollow islets, as well as ectopic ß-cells (Sant et al. 2016b, Sant et al. 2017b). To observe if MEHP exposure results in any of these variants in addition to the overall decrease in islet area, islet morphology was assessed at 48, 72, 96, and 168 hpf. At all timepoints observed, MEHP exposure increased the frequency of pancreatic islet morphology variants compared to controls when ß-cell area was assessed (Fig. 2D). No islet variants were observed when α-cell area was assessed. While these variants were occasionally observed among controls, a significantly greater frequency of total variant islet morphologies was seen in MEHP-exposed groups (p<0.05).
3.3. Endocrine Hormone Gene Expression
To address whether the MEHP-induced reduction in islet area resulted in changes to gene expression, expression of several endocrine hormone genes was quantified at 96 hpf by qPCR and analyzed using the ΔΔCT method, normalized to two housekeeping genes, ß-actin and beta-2-microglobulin (b2m). Responsible for stimulating cellular uptake of glucose, Insulin (encoded by preproinsulin a; insa) is secreted by the ß-cells of the endocrine islet. In contrast to insulin action, the α-cells produce Glucagon (encoded by glucagon a; gcga), which acts on the liver to break down glycogen and release free glucose into the bloodstream. Secreted by the δ-cells, Somatostatin (encoded by somatostatin 2; sst2) has many signaling responsibilities, including the inhibition of insulin receptor signaling. Ghrelin (encoded by ghrelin; ghrl), thought of as the “hunger hormone”, is produced by the ε-cells of the islet to stimulate food-seeking behavior, and works inversely to Leptin, which is produced by adipocytes. In addition to these endocrine hormones, we examined the expression of Pdx1 (encoded by pdx1), which mediates the expression of the aforementioned glucoregulatory genes (Tiso et al. 2009). In combination, these genes are responsible for maintaining glucose homeostasis, and disruption of their expression could have significant metabolic consequences.
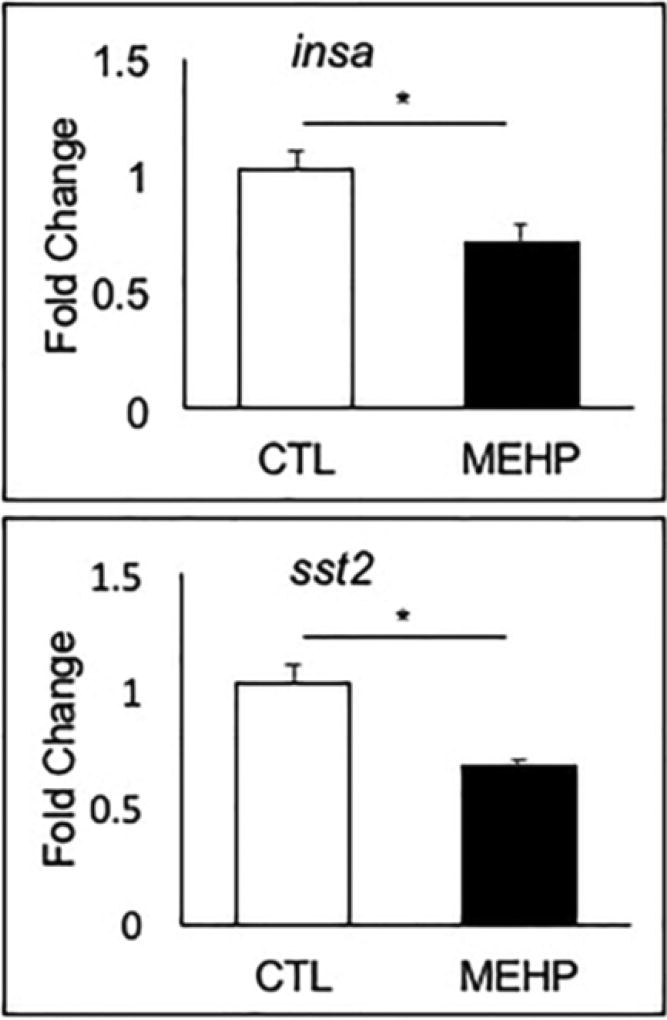
We found that embryonic MEHP exposure resulted in either a statistically significant downregulation, or trend towards downregulation, in expression of each of the endocrine hormone genes examined (Fig. 3A, 3B; Supp. Fig. 1). Expression of insa and sst2 were reduced by 31.5% and 35.5% compared to controls (p=0.0069 and p=0.0018), respectively (Fig. 3). Expression of gcga, ghrl and pdx1 trended towards reduction by 13%, 40.6% and 17.2% (p=0.541, p=0.0617, and p=0.392), respectively (Supp. Fig. 1).
Figure 3.
Embryonic MEHP exposure reduced expression of pancreatic hormone genes insa and sst2. AB zebrafish were exposed to MEHP from 3–96 hpf, and gene expression was assessed at 96 hpf. Data are presented as mean fold changes normalized to the biweight mean center of bactin and b2m expression ± SEM (n=6; *p<0.05; **p<0.005).
3.4. Exocrine Pancreas Morphometrics
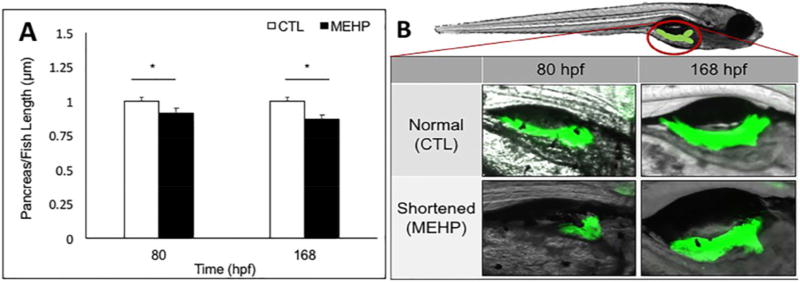
While the endocrine islets produce the majority of the glucoregulatory hormones, the body of the pancreas is composed primarily of exocrine tissue, which produces digestive peptides. The exocrine pancreas extends posteriorly during the 48 to 96 hpf window of zebrafish development. The primary islets appear between 24 and 48 hpf, and are located in the region proximal to the gut, while the secondary islets begin to develop in the distal body and tail of the exocrine pancreas, around 168 hpf, once extension is complete (Tiso et al. 2009). Congenital shortening of the pancreas has been associated with diabetic phenotypes in human studies (Elayat, el-Naggar and Tahir 1995, Agabi and Akhigbe 2016). Toxicological perturbations have been proposed as a potential mechanism for exocrine pancreas insufficiency, and for this reason we examined whether MEHP exposure may impact the development and extension of the exocrine pancreas (Sant et al. 2016b). Exocrine pancreas extension was visualized at 80 and 168 hpf following subchronic MEHP exposure using the Tg(ptf1a:GFP) transgenic line, and pancreas length was measured from the center of the primary endocrine islet to the posterior pancreatic tail using Zen analysis software. Total fish length was also measured, and pancreas length is reported as a proportion of total length to account for size variability. We found a significant overall decrease in the ratio of exocrine pancreas to total fish length in MEHP exposed individuals compared to controls at both timepoints (p<0.05), which suggests that MEHP exposure may contribute to a shortened pancreas phenotype (Fig. 4A, 4B).
Figure 4.
Embryonic MEHP exposure shortens the ratio of exocrine pancreas to total fish length at 80 and 168 hpf. Exocrine pancreas morphology was assessed using Tg(ptf1a:GFP) zebrafish eleutheroembryos and larvae. A) The position of the exocrine pancreas is shown, as well as representative images of shortened pancreata observed. B) Pancreas length was measured using Zen analysis software, from the center of the primary endocrine islet to the tail of the pancreas. Exocrine pancreas length was normalized to total fish length. Data presented are mean ratios of pancreas to fish length ± SEM (n=30; *p<0.05).
3.5. Exocrine Hormone Gene Expression
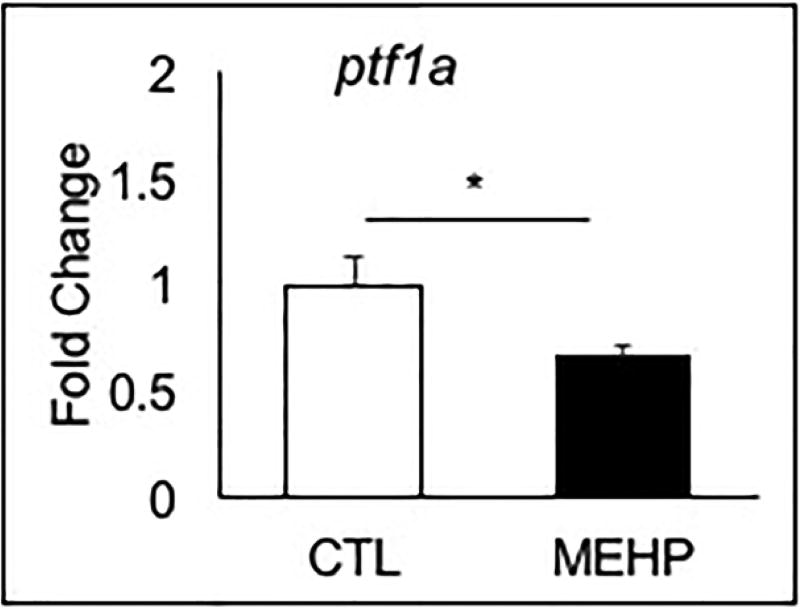
While the endocrine pancreas is responsible for secreting glucoregulatory hormones, the exocrine pancreas also plays a role in maintaining glucose homeostasis by producing digestive peptides. We measured expression of four critical exocrine genes: pancreas-specific transcription factor 1a (ptf1a), the proteases trypsin (try) and chymotrypsinogen B1 (crtb1), and pancreatic amylase (amy2a). We observed altered expression of each exocrine gene in the exposed group compared to the controls. Consistent with the shortened pancreas phenotype observed in the Tg(ptf1a:GFP) line, expression of ptf1a in the MEHP exposed group was significantly reduced compared to controls by 33.9% (p=0.023) (Fig. 5). We also observed a trend towards a decreased expression of crtb1, and increased expression of try and amy2a but this was not statistically significant (Supp. Fig. 2).
Figure 5.
Embryonic MEHP exposure significantly reduces expression of ptf1a at 96 hpf. AB zebrafish were exposed to MEHP from 3–96 hpf, and gene expression was assessed at 96 hpf. Data are presented as mean fold change normalized to the biweight mean center of bactin and b2m expression ± SEM (n=6; *p<0.05).
3.6 Redox Analysis and GSH Gene Expression
MEHP has been reported to cause oxidative stress in numerous studies. Here, we used biochemical, molecular, and genetic approaches to determine whether MEHP caused oxidative stress in embryos, and whether this contributed to the aberrant β-cell development. First, we measured the content of the redox couples GSH:GSSG and Cys:CySS to identify disrupted redox homeostasis in whole embryos. Quantification of these redox couples revealed no significant changes in GSH, Cys, GSSG, CySS, or redox potential (Eh) of GSH or Cys (Supp. Fig. 3). Eh (GSSG/GSH) remained constant between exposed and control samples, at −230.656 mV and 229.583 mV, respectively (p=0.634). Eh CySS/Cys also remained constant between exposed and control samples at −154.68 mV and 155.543 mV, respectively (p=0.741). Total GSH remained unchanged between exposed and control samples, at 3459.544 µM and 3447.519 µM, respectively (p=0.964), while total Cys increased slightly from 1526.006 µM in control samples to 1646.364 µM in exposed samples (p=0.634) (Supp. Fig. 3A–C). While the means of these measures were not significantly different, the variation within the MEHP-treated embryos was much less than the controls (Supp. Fig. 3C).
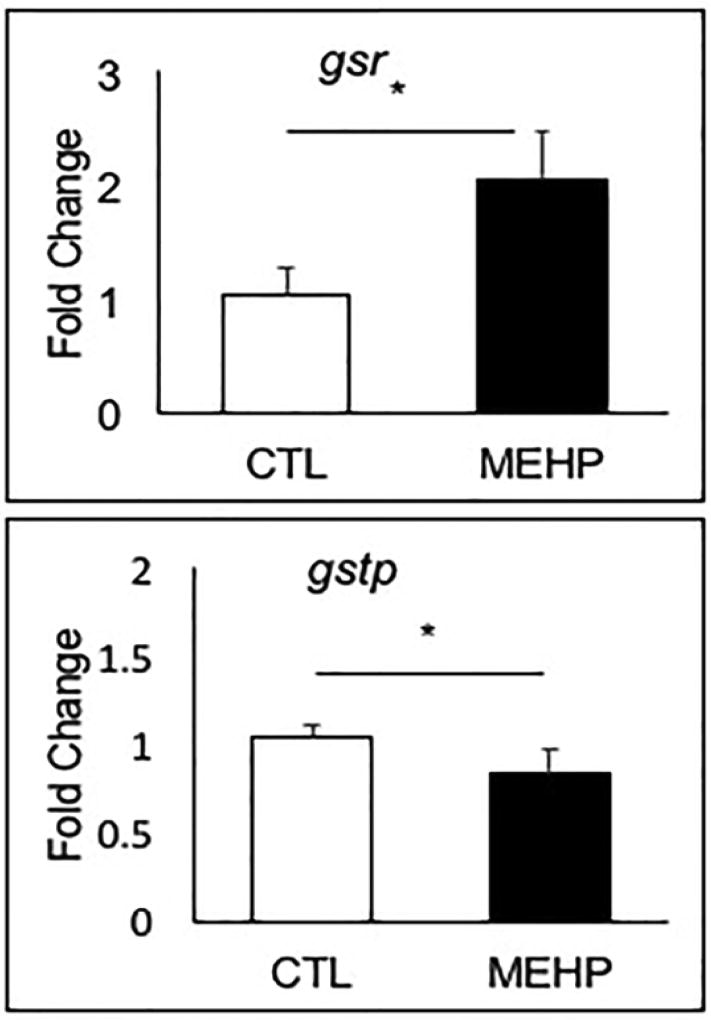
To expand our investigation into how MEHP exposure may influence the redox environment in the developing embryo, we assessed the expression of selected genes encoding enzymes and subunits involved in GSH synthesis, recycling, and utilization. During oxidative stress conditions, S-glutathionylation of proteins is catalyzed by glutathione S-transferase pi 1 (gstp1) and glutathione S-transferase alpha 1 (gsta1) to stabilize protein structure and aid in cellular detoxification. Availability of GSH is increased by glutathione-disulfide reductase (gsr), which recycles oxidized glutathione-disulfide (GSSG) into reduced GSH. Additional enzymes encoded in part by gamma-glutamyltransferase 1b (ggt1b) and glutamate-cysteine ligase catalytic subunit (gclc) increase cellular cysteine availability and catalyze glutathione synthesis respectively (Sant et al. 2017a). We observed no change in expression of gsta1, ggt1b, and gclc in response to MEHP exposure (Supp. Fig. 3). However, we did find significant alterations in expression of gstp1 and gsr. Gstp1 expression decreased significantly by 20 % (p=0.042), while gsr expression doubled (p=0.0073) (Fig. 6).
Figure 6.
Embryonic MEHP exposure significantly altered the expression of gstp1 and gsr. AB zebrafish were exposed to MEHP from 3–96 hpf, and gene expression was assessed at 96 hpf. Data are presented as mean fold changes normalized to the biweight mean center of bactin and b2m expression ± SEM (n=6; *p<0.05).
3.7 Nrf2afh318/fh318 Mutant Endocrine Islet Morphometrics
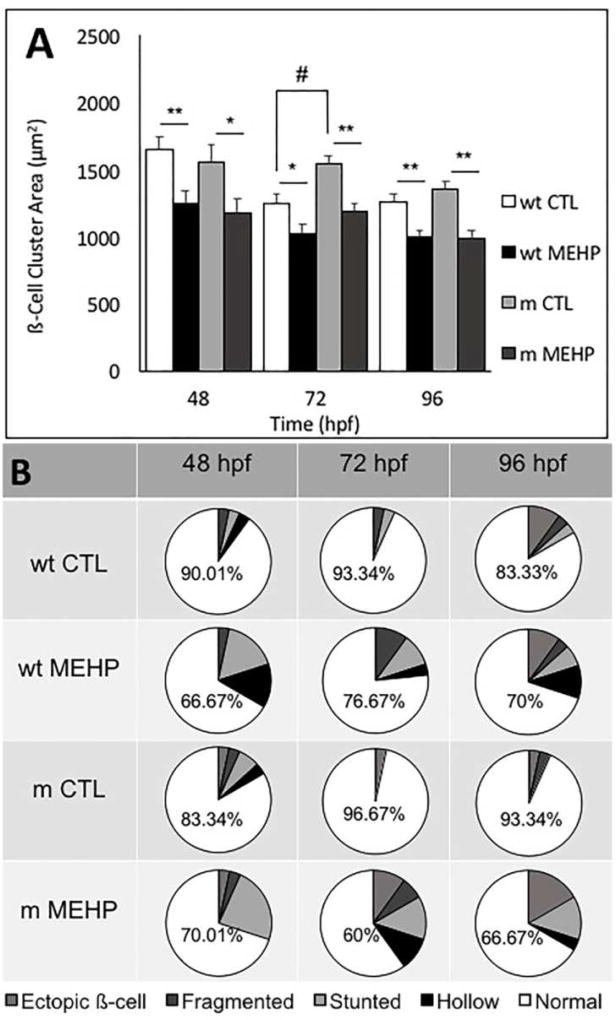
To determine whether the observed disruptions to pancreatic organogenesis may be due in part to MEHP-induced oxidative stress, we used a Nrf2a(fh318/fh318) mutant zebrafish line, characterized by an impaired Nrf2 response to oxidative conditions due to a point mutation in the DNA binding domain. These mutant fish have been previously characterized to be more sensitive to oxidative stress, and more susceptible to adverse effects of toxicant exposures that involve oxidative stress (Rousseau et al. 2015, Mukaigasa et al. 2012). No significant changes in gross morphology (length, yolk sac area, pericardial area) were observed in wild type or Nrf2afh318/fh318 mutants. Islet morphometrics were additionally reexamined as above (section 3.2) using wild type and mutant (Nrf2afh318/fh318) Tg(ins:GFP) transgenic lines. We observed reductions in ß-cell cluster area across genotype, consistent with findings from the previous islet morphometrics experiment, and an increase in the variant β-cell morphologies greater than that found in wild type MEHP-exposed embryos was observed in the MEHP-exposed Nrf2a mutant embryos at 72 hpf (Fig. 7A, 7B). The distribution of variant morphologies also differed between the Nrf2a mutant and wildtype embryos exposed to MEHP. For example, at 48 hpf, MEHP exposure resulted in mainly stunted and hollow islet morphologies in the wildtype embryos, but mainly stunted islets in the Nrf2a mutants (Fig. 7B).
Figure 7.
Embryonic MEHP exposure alters pancreatic structure in wild type (wt) and Nrf2afh318/fh318 mutant (m) zebrafish embryos. results in A) significantly reduced ß-cell cluster area, and B) significantly increased incidence of pancreatic islet variants at all time points observed. Data are presented as the mean ± SEM. Asterisks (*) indicate statistical significance between control and exposed within genotype; hash marks (#) indicate significant differences between controls across genotype (n=30; *p<0.05; **p<0.005; #p<0.05).
4. Discussion
Human exposure to MEHP is widespread and poses a health risk to the developing embryo. Epidemiology studies have detected MEHP in nearly 100% of biological samples taken from the U.S. population, and MEHP exposure has been associated with increased risk for cancer, obesity, neurological disorders and reproductive dysfunction in both men and women (Sun et al. 2014, Corbasson et al. 2016, Benjamin et al. 2017). Experimental evidence in humans and animals have demonstrated that MEHP is capable of crossing the placental barrier and exposing the developing embryo (Sathyanarayana et al. 2008).
Previous studies in zebrafish have demonstrated that developmental MEHP exposure results in embryotoxicity and endocrine disruption. At elevated concentrations (250–500 µg/L), embryonic MEHP exposures result in lethality and overt embryo toxicity (Lammer et al. 2009, Kroese et al. 2015). While both terrestrial and aquatic species are rarely exposed to such concentrations, low-dose effects of MEHP exposure in zebrafish have also been characterized. These include thyroid endocrine disruption, exhibited by significantly reduced levels of whole body triiodothyronine (T3) and thyroxine (T4), occurring at concentrations of 200 µg/L (Zhai et al. 2014). Additionally, chronic MEHP exposure induced reproductive dysfunction in both male and female zebrafish by reducing gamete production and quality at concentrations as low as 4 µg/L (Zhu et al. 2016).
In the present study, we show that early embryonic MEHP exposure is capable of disrupting the development of both the endocrine and exocrine pancreas, demonstrated by alterations to pancreatic growth, structure, and gene expression. While non-lethal pancreas defects often go undiagnosed, the presence of several congenital variants has been associated with increased risk for adult-onset diabetes and pancreatitis. The endocrine pancreas is responsible for the production of essential endocrine hormones that maintain glucose homeostasis, while the enzymes secreted by the exocrine pancreas are critical for digestion and facilitate the breakdown of complex carbohydrates. While uncommon, structural alterations of the endocrine pancreas, such as ectopic endocrine tissue, and exocrine pancreas malformations such as pancreatic agenesis (a shortened pancreas), are associated with type 1 and 2 diabetes (Bento, Baptista and Oliveira 2013)
We observed a significantly elevated frequency of hypomorphic and variant endocrine islet morphologies in response to MEHP exposure. Using Tg(ins:GFP) and Tg(gcga:GFP) transgenic lines to visualize ß- and α-cell development, respectively, we observed decreased islet areas which persisted from 48 to 168 hpf. This finding is consistent with rodent studies which have shown that developmental exposure to the parent compound of MEHP, DEHP, disrupts mitochondrial function in ß-cells, resulting in reduced ß-cell cluster area and architectural variation of the islet, effects which persist into adulthood and disrupted glucose metabolism throughout the life course (Lin et al. 2011). Additionally, we show an elevated frequency of variants in islet morphology. Several of these islet variants are similar to those found in humans, such as ectopic pancreatic tissue, which occurs in approximately 10% of the human population (Bento et al. 2013). In addition to ectopic tissue, we have shown an increased frequency of “hollow” ß-cell clusters, which appear as a circular ring of fluorescent cells. This “hollow islet” morphology phenocopies islet morphology identified in pdx1 zebrafish morphants (knockdown by antisense morpholino oligonucleotides), which suggests that MEHP may be disrupting pdx1 function to produce this phenotype (Kimmel et al. 2011). We recently reported that developmental exposures to prooxidants produce highly similar pancreatic structural anomalies. Embryonic exposure to tert-butylhydroperoxide (tBOOH) resulted in hypomorphic islet area and elevated frequencies of islet variants in Tg(ins:GFP) zebrafish eleutheroembryos and larvae (Sant et al. 2016b).
We also observed shortened ratios of exocrine pancreas length to total fish length, suggesting that embryonic MEHP exposure reduces exocrine mass, a phenotype that has been commonly associated with diabetes in human studies, known as dorsal pancreatic agenesis. Dorsal pancreatic agenesis is characterized by a shortened pancreatic tail, which leads to functional metabolic abnormalities such as hyperglycemia. Such functional abnormalities are primarily due to decreased endocrine tissue mass, as the majority of secondary islets are clustered within the distal body and exocrine tail, and thus a shortened length may impede their development (Bento et al. 2013). Reduced ratios of exocrine pancreas to total body length were similarly produced by developmental exposure to tBOOH in previous studies, indicating that oxidative stress may play a role in the development of this phenotype (Sant et al. 2016b).
Congruent with the hypomorphic pancreatic islets and shortened pancreas length observed in our microscopy experiments, expressions of the pancreas genes insa, sst2, and ptf1a were significantly decreased at 96 hpf in MEHP-exposed fish compared to controls. Several trends emerged in gene expression following MEHP exposure. Expression of each endocrine hormone gene investigated was downregulated by MEHP exposure, consistent with reduced islet mass. However, while expression of ptf1a and crtb1 were also reduced, try and amy2a were upregulated, perhaps due to a compensatory or adaptive response to the toxicological insult.
We have demonstrated that exposure to MEHP during organogenesis clearly alters the structure of the pancreas, as well as expression of pancreas-related genes. However, the mechanism by which this toxicant is disrupting pancreatic development remains unknown. Previous work has shown that MEHP causes neurulation defects associated with altered GSH redox potential in mouse whole embryo cultures during organogenesis (Sant et al. 2016a). Based on this finding and other reports of MEHP-induced oxidative stress (e.g. (Meruvu, Zhang and Choudhury 2016, Tetz et al. 2013, Wang et al. 2012, Yang et al. 2012)), we hypothesized that oxidative stress is involved in the disruption of pancreas organogenesis by MEHP. We observed contrasting trends that indicate a more reduced potential for GSH, and a more oxidized potential for cysteine (Cys). Because GSH synthesis requires Cys, it is possible that the increased GSH and decreased Cys are due to shunting of Cys. However, total glutathione and total cysteine concentrations were both increased, suggesting that intracellular cysteine is mostly accumulating in its oxidized state. Likewise, ggt1 (cysteine import), gclc (glutathione synthesis) and gsta1 expression were all unchanged due to treatment. Because gene expression and redox potentials were quantified 24 h following the most recently exposure timepoint, it is likely that embryos were able to mostly recover within this timeframe, as observed in mouse embryos (Sant et al. 2016a). Upon investigation of GSH-related gene expression, we found that MEHP altered expression of gsr and gstp1. We found that gsr was significantly upregulated, indicating that more cellular GSSG is present after MEHP exposure, which must be converted into GSH by gsr before it can be used. Additionally, gstp1 expression was significantly reduced by MEHP, which suggests that limited cellular detoxification is occurring due to lack of this enzyme. These results suggest that MEHP exposure is disrupting the cellular redox environment. Disruption of the sensitive redox balance can result in altered cell fate decisions, such as premature differentiation, which may have lasting consequences on both morphology and function (Rovira M. 2011). MEHP has been shown to produce the same structural changes in the endocrine and exocrine pancreas as classic prooxidants such as tBOOH, strengthening the evidence that MEHP is disrupting the cellular redox environment and resulting in altered pancreatic morphology (Sant et al. 2016b).
Previous studies have identified an association between MEHP-induced oxidative stress and Nrf2 induction. In rat INS-1 cells, treatment with DEHP resulted in significantly increased ROS production, dysregulation of the Nrf2-mediated antioxidant response, and inhibition of glucose-stimulated insulin secretion (Sun et al. 2015). The results of our microscopy assay using wild type and Nrf2afh318/fh318 mutants showed a similar decrease in ß-cell cluster area that was largely due to exposure rather than genotype, with the exception being a transient increase in aberrant β-cell cluster morphology in the Nrf2a mutant fish exposed to MEHP assessed at the 72 hpf time point. Similar trends in the wild type and mutant strains indicate that embryonic MEHP exposure may affect organogenesis of the endocrine pancreas through a mechanism independent of Nrf2a, or perhaps the other Nrf-related proteins are able to compensate for deficient Nrf2a, as has been shown for the regulation of the glutathione system during zebrafish development (Sant et al. 2017a).
Numerous studies have associated chronic phthalate exposure with symptoms of metabolic dysfunction such as obesity, insulin resistance and hyperglycemia in humans. However, much remains to be learned about the biological mechanisms by which phthalate exposure may contribute to altered disease susceptibility (Sun et al. 2014). Here, we have shown that developmental exposures to the phthalate metabolite MEHP is capable of disrupting pancreatic organogenesis in zebrafish, reducing the size of the endocrine and exocrine pancreas, producing variant pancreatic morphologies, altering gene expression, and modifying the redox environment in the developing zebrafish embryo.
Supplementary Material
Highlights.
The pancreas is a sensitive target tissue of developmental exposure to MEHP.
MEHP exposure decreases islet area and exocrine pancreas length.
MEHP exposure results in aberrant morphology of the primary β-cell cluster.
MEHP exposure alters expression of pancreas-specific and GSH-related genes.
Acknowledgments
We are grateful to the members of the Timme-Laragy Lab for providing excellent fish care and to Dr. Craig Harris (University of Michigan) for use of his redox analysis facility and equipment. This work was supported by grants from the NIH (R01ES025748 and R01ES028201 to AT-L), F32 (F32ES028085 to KES), and (P20GM103423 to LMW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing interests to declare.
References
- Agabi JO, Akhigbe AO. Comparative sonographic evaluation of the anteroposterior dimensions of the pancreas in diabetics and nondiabetics. Niger J Clin Pract. 2016;19:175–81. doi: 10.4103/1119-3077.175969. [DOI] [PubMed] [Google Scholar]
- Akirav E, Kushner JA, Herold KC. Beta-cell mass and type 1 diabetes: going, going, gone? Diabetes. 2008;57:2883–8. doi: 10.2337/db07-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin S, Masai E, Kamimura N, Takahashi K, Anderson RC, Faisal PA. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J Hazard Mater. 2017;340:360–383. doi: 10.1016/j.jhazmat.2017.06.036. [DOI] [PubMed] [Google Scholar]
- Bento A, Baptista H, Oliveira F. Congenital pancreas malformations: a clinical case report. Rev Assoc Med Bras (1992) 2013;59:35–9. [PubMed] [Google Scholar]
- Corbasson I, Hankinson SE, Stanek EJ, Reeves KW., 3 Urinary bisphenol-A, phthalate metabolites and body composition in US adults, NHANES 1999–2006. Int J Environ Health Res. 2016;26:606–17. doi: 10.1080/09603123.2016.1233524. [DOI] [PubMed] [Google Scholar]
- Daniel JW, Bratt H. The absorption, metabolism and tissue distribution of di(2-ethylhexyl)phthalate in rats. Toxicology. 1974;2:51–65. doi: 10.1016/0300-483x(74)90042-0. [DOI] [PubMed] [Google Scholar]
- diIorio PJ, Moss JB, Sbrogna JL, Karlstrom RO, Moss LG. Sonic hedgehog is required early in pancreatic islet development. Developmental Biology. 2002;244:75–84. doi: 10.1006/dbio.2002.0573. [DOI] [PubMed] [Google Scholar]
- Dombi GW. Using biweights for handling outliers. SAS Institute; 2010. [Google Scholar]
- Elayat AA, el-Naggar MM, Tahir M. An immunocytochemical and morphometric study of the rat pancreatic islets. J Anat. 1995;186(Pt 3):629–37. [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Mol Nutr Food Res. 2007;51:899–911. doi: 10.1002/mnfr.200600243. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–65. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Harris C, Hansen J. Oxidative Stress, Thiols, and Redox Profiles. In: Harris C, Hansen JM, editors. Developmental Toxicology. Humana Press; 2012. pp. 325–346. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redox potential of GSH/GSSG couple: Assay and biological significance. In: Helmut S, Lester P, editors. Methods in Enzymology. Academic Press; 2002. pp. 93–112. [DOI] [PubMed] [Google Scholar]
- Kimmel RA, Onder L, Wilfinger A, Ellertsdottir E, Meyer D. Requirement for Pdx1 in specification of latent endocrine progenitors in zebrafish. BMC biology. 2011;9:75. doi: 10.1186/1741-7007-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese ED, Bosgra S, Buist HE, Lewin G, van der Linden SC, Man HY, Uibel F. Evaluation of an alternative in vitro test battery for detecting reproductive toxicants in a grouping context. Reproductive Toxicology. 2015;55:11–19. doi: 10.1016/j.reprotox.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Lammer E, Carr GJ, Wendler K, Rawlings JM, Belanger SE, Braunbeck T. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2009;149:196–209. doi: 10.1016/j.cbpc.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, Jacobs DR. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes. Diabetes careDiabetes care. 2006;29:1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- Lele Z, Krone PH. The zebrafish as a model system in developmental, toxicological and transgenic research. Biotechnology advances. 1996;14:57–72. doi: 10.1016/0734-9750(96)00004-3. [DOI] [PubMed] [Google Scholar]
- Lin JW, Biankin AV, Horb ME, Ghosh B, Prasad NB, Yee NS, Leach SD. Differential requirement for ptf1a in endocrine and exocrine lineages of developing zebrafish pancreas. Developmental biologyDevelopmental biology. 2004;27:491–503. doi: 10.1016/j.ydbio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Lin Y, Wei J, Li Y, Chen J, Zhou Z, Song L, Wei Z, Lv Z, Chen X, Xia W, Xu S. Developmental exposure to di(2-ethylhexyl) phthalate impairs endocrine pancreas and leads to long-term adverse effects on glucose homeostasis in the rat. Am J Physiol Endocrinol Metab. 2011;301:E527–38. doi: 10.1152/ajpendo.00233.2011. [DOI] [PubMed] [Google Scholar]
- Livak KJ, D ST. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Matveyenko AV, Butler PC. Relationship between beta-cell mass and diabetes onset. Diabetes Obes Metab. 2008;10(Suppl 4):23–31. doi: 10.1111/j.1463-1326.2008.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruvu S, Zhang J, Choudhury M. Mono-(2-ethylhexyl) Phthalate Increases Oxidative Stress Responsive miRNAs in First Trimester Placental Cell Line HTR8/SVneo. Chem Res Toxicol. 2016;29:430–5. doi: 10.1021/acs.chemrestox.6b00038. [DOI] [PubMed] [Google Scholar]
- Mukaigasa K, Nguyen LT, Li L, Nakajima H, Yamamoto M, Kobayashi M. Genetic evidence of an evolutionarily conserved role for Nrf2 in the protection against oxidative stress. Mol Cell Biol. 2012;32:4455–61. doi: 10.1128/MCB.00481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls S, Zecchin E, Tiso N, Bortolussi M, Argenton F. Function and regulation of zebrafish nkx2. 2a during development of pancreatic islet and ducts. Developmental biology. 2007;304:875–890. doi: 10.1016/j.ydbio.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Rousseau ME, Sant KE, Borden LR, Franks DG, Hahn ME, Timme-Laragy AR. Regulation of Ahr signaling by Nrf2 during development: Effects of Nrf2a deficiency on PCB126 embryotoxicity in zebrafish (Danio rerio) Aquat Toxicol. 2015;167:157–71. doi: 10.1016/j.aquatox.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira M, H W, Yusuff S, Shim JS, Ferrante AA, Liu JO, Parsons M. Chemical screen identifies FDA-approved drugs and target pathways that induce precocious pancreatic endocrine differentiation. PNAS. 2011;108:19264–19269. doi: 10.1073/pnas.1113081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant KE, Dolinoy DC, Jilek JL, Sartor MA, Harris C. Mono-2-ethylhexyl phthalate disrupts neurulation and modifies the embryonic redox environment and gene expression. Reproductive Toxicology. 2016a;63:32–48. doi: 10.1016/j.reprotox.2016.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant KE, Hansen JM, Williams LM, Tran NL, Goldstone JV, Stegeman JJ, Hahn ME, Timme-Laragy A. The role of Nrf1 and Nrf2 in the regulation of glutathione and redox dynamics in the developing zebrafish embryo. Redox Biol. 2017a;13:207–218. doi: 10.1016/j.redox.2017.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant KE, Jacobs HM, Borofski KA, Moss JB, Timme-Laragy AR. Embryonic exposures to perfluorooctanesulfonic acid (PFOS) disrupt pancreatic organogenesis in the zebrafish, Danio rerio. Environ Pollut. 2017b;220:807–817. doi: 10.1016/j.envpol.2016.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant KE, Jacobs HM, Xu J, Borofski KA, Moss LG, Moss JB, Timme-Laragy AR. Assessment of Toxicological Perturbations and Variants of Pancreatic Islet Development in the Zebrafish Model. Toxics. 2016b;4:20. doi: 10.3390/toxics4030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Calafat AM, Liu F, Swan SH. Maternal and infant urinary phthalate metabolite concentrations: are they related? Environ Res. 2008;108:413–8. doi: 10.1016/j.envres.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Cornelis MC, Townsend MK, Tobias DK, Eliassen AH, Franke AA, Hauser R, Hu FB. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses' Health Study (NHS) and NHSII cohorts. Environ Health Perspect. 2014;122:616–23. doi: 10.1289/ehp.1307201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Lin Y, Huang Q, Shi J, Qiu L, Kang M, Dong S. Di(2-ethylhexyl) phthalate-induced apoptosis in rat INS-1 cells is dependent on activation of endoplasmic reticulum stress and suppression of antioxidant protection. Journal of Cellular and Molecular Medicine. 2015;19:581–594. doi: 10.1111/jcmm.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environmental research. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetz LM, Cheng AA, Korte CS, Giese RW, Wang P, Harris C, Meeker JD, Loch-Caruso R. Mono-2-ethylhexyl phthalate induces oxidative stress responses in human placental cells in vitro. Toxicol Appl Pharmacol. 2013;268:47–54. doi: 10.1016/j.taap.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickner JA, Schettler T, Guidotti T, McCally M, Rossi M. Health risks posed by use of di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: A critical review. American Journal of Industrial Medicine. 2001;39:100–111. doi: 10.1002/1097-0274(200101)39:1<100::aid-ajim10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Timme-Laragy AR, Goldstone JV, Imhoff BR, Stegeman JJ, Hahn ME, Hansen JM. Glutathione redox dynamics and expression of glutathione-related genes in the developing embryo. Free Radical Biology and Medicine. 2013;65:89–101. doi: 10.1016/j.freeradbiomed.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiso N, Moro E, Argenton F. Zebrafish pancreas development. Mol Cell Endocrinol. 2009;312:24–30. doi: 10.1016/j.mce.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Tomita I, Nakamura Y, Yagi Y, Tutikawa K. Fetotoxic effects of mono-2-ethylhexyl phthalate (MEHP) in mice. Environmental health perspectives. 1986;65:249. doi: 10.1289/ehp.8665249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong L, Reif DM, St Mary L, Geier MC, Truong HD, Tanguay RL. Multidimensional in vivo hazard assessment using zebrafish. Toxicological Sciences. 2014;137:212–233. doi: 10.1093/toxsci/kft235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Craig ZR, Basavarajappa MS, Hafner KS, Flaws JA. Mono-(2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol Reprod. 2012;87:152. doi: 10.1095/biolreprod.112.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. A guide for the laboratory use of zebrafish (Danio rerio) Eugene, OR: University of Oregon Press; 2007. [Google Scholar]
- Yang G, Zhou X, Wang J, Zhang W, Zheng H, Lu W, Yuan J. MEHP-induced oxidative DNA damage and apoptosis in HepG2 cells correlates with p53-mediated mitochondria-dependent signaling pathway. Food Chem Toxicol. 2012;50:2424–31. doi: 10.1016/j.fct.2012.04.023. [DOI] [PubMed] [Google Scholar]
- Yang L, Ho NY, Alshut R, Legradi J, Weiss C, Reischl M, Strähle U. Zebrafish embryos as models for embryotoxic and teratological effects of chemicals. Reproductive Toxicology. 2009;28:245–253. doi: 10.1016/j.reprotox.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Zhai W, Huang Z, Chen L, Feng C, Li B, Li T. Thyroid endocrine disruption in zebrafish larvae after exposure to mono-(2-ethylhexyl) phthalate (MEHP) PLoS One. 2014;9:e92465. doi: 10.1371/journal.pone.0092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hua R, Zhou Y, Li H, Quan S, Yu Y. Chronic exposure to mono - (2 - ethylhexyl) - phthalate causes endocrine disruption and reproductive dysfunction in zebrafish. Environmental toxicology and chemistry. 2016;35:2117–2124. doi: 10.1002/etc.3369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.