Abstract
Bovine ephemeral fever virus (BEFV), identified as the causative pathogen of bovine ephemeral fever (BEF), is responsible for increasing numbers of epidemics/outbreaks and has a significant harmful effect on the livestock industry. Therefore, a rapid detection assay is imperative for BEFV diagnosis. In this study, we described the development of lateral-flow dipstick isothermal recombinase polymerase amplification (LFD-RPA) assays for detection of BEFV. RPA primers and LF probes were designed by targeting the specific G gene, and the amplification product can be visualized on a simple lateral flow dipstick with the naked eyes. The amplification reaction was performed at 38 °C for 20 min and LFD incubation time within 5 min. The detection limit of this assay was 8 copies per reaction, and there was no cross-reactivity with other bovine infectious viruses such as bovine viral diarrhea virus, infectious bovine rhinotracheitis virus, bovine respiratory syncytial virus, bovine coronavirus, bovine parainfluenza virus type 3, bovine vesicular stomatitis virus. In addition, the assay was performed with total 128 clinical specimens and the diagnostic results were compared with conventional RT-PCR, real-time quantative(q) PCR. The result showed that the coincidence rate of BEFV LFD-RPA and real-time qPCR was 96.09% (123/128), which was higher than conventional RT-PCR. The RPA combined with LFD assay probably provides a rapid and sensitive alternative for diagnosis of BEFV infections outbreak.
Keywords: Bovine ephemeral fever virus (BEFV), Recombinase polymerase amplification (RPA), Lateral flow dipstick
Highlights
-
•
RPA combined with LFD assay was developed first time to detect BEFV.
-
•
The detection from cDNA could be completed within 30 min and be easily visualized with the naked eyes.
-
•
The RPA combined with LFD assay probably provides a alternative for diagnosis of BEFV.
1. Introduction
Bovine ephemeral fever (BEF) caused by bovine ephemeral fever virus (BEFV) is an insect-borne viral disease of cattle. BEFV infection is characterized by variable clinical signs, such as acute febrile reaction, lameness, stiffness, reduced-milk production, lowered-male fertility, and also death in cattle [[1], [2], [3]]. The disease has been reported in tropical and subtropical regions of Asia, Australia and Africa. In addition, in recent years there have been several major outbreaks in China and much more widespread, and it has led to significant economic losses to dairy industry [[4], [5], [6], [7], [8], [9], [10], [11]]. Diagnosis and vaccination now are of great importance in the management of BEFV. However, vaccines at presently in use are less than ideal due to the poor heat-stability that might be incapable of providing reliable and durable protection following a single dose [3], and further research is required to develop a rapid diagnostic assay that will provide reliable tools for the prevention and control of BEFV.
BEFV belongs to a typical species of the genus Ephemerovirus within the family Rhabdoviridae. It is a single stranded RNA virus that is comprised of 12 genes arranged in the order 3′-l-N-P/C-M-G-GNS-α1/α2/α3-β-γ-L-t-5′. Among those genes and encoded proteins, most research to date has focused on developing diagnostic assay and recombinant vaccine targeting on the G gene [[12], [13], [14], [15], [16], [17], [18], [19]].
Cell culture and indirect immunofluorescent detection have long been used for the detection of BEFV [17,[20], [21], [22]]. In addition, BEFV can be routinely detected by the conventional RT-PCR, real-time quantative(q) PCR, and enzyme-linked immunosorbent assay (ELISA) as well as the virus neutralization test [[23], [24], [25], [26]]. However, those common detection methods are laboratory-based and require sophisticated instruments operated by special trained personnel.
Recombinase polymerase amplification (RPA) is a proliferation of next-generation molecular diagnostics relative to traditional PCR [27]. It is performed at a low and constant temperature (optimal temperatures 37°C-42 °C) with the simple set-up and no thermal or chemical melting of DNA is required. The technology takes advantage of three major proteins including a recombinase enzyme to separate the DNA duplex, single-stranded DNA-binding proteins to stabilize the open complex, and polymerase to begin DNA synthesis using two opposing primers and probe chemistries. Much like PCR, if target sequence is indeed present, an exponential DNA amplification reaction is initiated. It can specifically amplify nucleic acid sequences in an isothermal format ranging from trace levels to detectable amounts of product with a rapid turnaround time. Commonly, the RPA amplification products can be detected by gel electrophoresis, probe-based fluorescence monitoring, lateral flow dipsticks depending on the specific oligonucleotide primers and/or a probe. Recently, RPA amplification system combined with enzyme-linked immunosorbent assay [28], aptamer based bio-barcode (ABC) [29], and hybridization in microarray format [30] have emerged. Lateral flow dipsticks detection based systems requires an additional oligonucleotide which is typically labeled on the 5′ end of FAM and conjoined with the opposing amplification primer typically labeled with biotin on the 5′ end. Consequently, this amplicons can be detected in a ‘sandwich’ assay. Although RPA technology has been widely used for detection of various pathogens since its initial development [[31], [32], [33], [34]], there is no RPA used for BEFV detection, so far.
In this study, we aimed to develop the lateral flow dipsticks recombinase polymerase amplification (LFD-RPA) assays for rapid detection of BEFV. RPA primers and LF probes were designed by targeting the BEFV G gene, and the analytical sensitivity and specificity of the method were evaluated for etiology that are likely to cause bovine infectious viruses. Finally, the performance of the RPA assay was also evaluated on clinical samples for diagnosis of BEFV.
2. Materials and methods
2.1. Viruses, cell and clinical specimens
Bovine viral diarrhea virus (BVDV)/NADL strain, infectious bovine rhinotracheitis virus (IBRV)/BarthaNu/67 strain, bovine parainfluenza virus type 3(BPIV-3)/BN-1 strain were preserved in our laboratory, and the viruses were cultured in the Madin-Darby Bovine Kidney (MDBK) cell using the Dulbecco's Modified Eagle Medium (DMEM, HyClone, USA), containing antibiotics (1000 UI/L penicillin, 100 mg/L streptomycin) with 8% horse serum (HyClone, USA). Other bovine virus strains used in this study were provided by full-length cDNA clone sequence: entire genome sequence of bovine respiratory syncytial virus (BRSV)/A51908 strain, bovine ephemeral fever virus (BEFV)/(Gene Bank No: NC_002526.1), bovine coronavirus (BcoV)/ENT strain (Gene Bank No:NC_003045.1), respectively. The cDNA of bovine vesicular stomatitis virus was kindly provided by Wenqi He (Jilin University, China). A total of 128 clinical specimens were collected from cattle farms suspected to be infected with BEFV in eastern China.
2.2. Isolation of DNA/RNA, cDNA synthesis
IBRV DNA was extracted from 200 μL of infected cell culture supernatant using the UNIQ-10 viral DNA extraction kit (Sangon Biotech, Shanghai, China). RNA of BVDV, BPIV-3 was prepared from 200 μL of infected cell culture supernatant with the QIAamp viral RNA minikit (Qiagen, Germany) according to manufacturer's instructions, respectively. The DNA/RNA was eluted in 50 μL of nuclease-free water. The extracted RNA was used as template for cDNA synthesis using reverse transcription with random primers according to the instructions of the RevertAid First Strand cDNA Synthesis Kit (Fermentas, Canada). All templates were stored at −70 °C until further needed.
2.3. Generation of DNA/RNA molecular standard
The BEFV G gene (1872 bp) cloned into pMD18-T vector was previously described by our laboratory [35], and designated as G-T3-RPA. The G-T3-RPA standard DNA was measured using a Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Germany). The DNA copy number was calculated by the equation: DNA cope number=(M × 6.02 × 1023 × 10−9)/(n × 660), M: molecular weight, n: plasmid concentration measured at 260 nm. DNA standard was stored at −20 °C until further used.
RNA molecular standard were prepared as described previously [36] with some modifications. In brief, the BEFV G gene (1872 bp) was cloned into pGEM-T Easy vector (Promega, USA). The recombinant plasmid was linearized by NdeI (Thermo Fisher Scientific, USA), and then purified using the Monarch PCR & DNA Cleanup Kit (New England Biolabs, USA). The RNA was transcribed by using the RiboMAX Large Scale RNA Production System-T7 (Promega, USA) and measured with the Quant-iTTM RiboGreen RNA Assay Kit (Thermo Fisher Scientific, Germany) in accordance with the manufacturer's instructions. The copy number of RNA molecules was calculated by the following formula: Amount (copies/μL) = [RNA concentration (g/μL)/(transcript length in nucleotides × 340)] × 6.02 × 1023.
2.4. Design of RPA primers and LF probe
By alignment analysis of the G gene with the data from NCBI/GenBank (JX234571.1; M94266.1; KP403941.1; KJ729108.1; KM276084.1; KC470313.1; JX564640.1; JX564639.1; JX564638.1; JX564637.1; NC_002526.1), a series of primers and LF probes were designed according to RPA operating instructions of TwistDx (TwistDx, Ltd., Cambridge, United Kingdom), and synthesized by Sangon Biotech (Shanghai, China). In summary, the length of 30 bp to 35 bp for RPA Primers is recommended. TwistAmp LF Probe oligonucleotide backbone includes a 5′-antigenic label FAM group, an internal abasic nucleotide analogue ‘dSpacer’ and a 3′-polymerase extension blocking group C3-spacer. One amplification primer opposing TwistAmp LF Probe is labeled with Biotin at its 5′ end, thus dual-labeled amplicons can be detected simultaneously on the LFD. Oligonucleotide sequences of RPA primers and LF probes used in the study were listed in Table 1 .
Table 1.
The sequences of primers and probes designed for screening in the study.
| Primer/probe sets |
Primers Name | Oligonucleotide sequences (5′-3′) | Product sizes (bp) |
|---|---|---|---|
| 1 | F1-1 | 5′-agagcttggtgtgaatacagaccttttgttgac-3′ | 251 |
| R1-1 | 5'-【Biotin】tcgaatttgatcaatttttgataatcctctatc-3′ | ||
| 1LF | 5'-【FAM】ttggaaacagcgccggcaggaggaagctc【dSpacer】ggaaataggctgaatgta【C3-spacer】-3′ | ||
| 2 | F1-2 | 5′-attgaatggacataacatgtctgggaatcatg-3′ | 130 |
| R1-2 | 5'-【Biotin】caaatataatttagttggttccacgaagatcattc-3′ | ||
| 1LF | 5'-【FAM】ttggaaacagcgccggcaggaggaagctc【dSpacer】ggaaataggctgaatgta【C3-spacer】-3′ | ||
| 3 | F1-3 | 5′-agagcttggtgtgaatacagaccttttgttgac-3′ | 169 |
| R1-3 | 5'-【Biotin】ttgataatcctctatcccctcatatagagatt-3′ | ||
| 1LF | 5'-【FAM】ttggaaacagcgccggcaggaggaagctc【dSpacer】ggaaataggctgaatgta【C3-spacer】-3′ | ||
| 4 | F1-4 | 5′-agagcttggtgtgaatacagaccttttgttgac-3′ | 217 |
| R1-4 | 5'-【Biotin】gttctcttccacattatcatactccattacctcg-3′ | ||
| 1LF | 5'-【FAM】ttggaaacagcgccggcaggaggaagctc【dSpacer】ggaaataggctgaatgta【C3-spacer】-3′ | ||
| 5 | 2F-1 | 5′-cggaaataggctgaatgtaactctaaacggaatg-3′ | 178 |
| 2R-1 | 5'-【Biotin】tttctcatcctcctcatacctaatcaagttctc-3′ | ||
| 2LF | 5'-【FAM】ctctatatgaggggatagaggattatcaaa【dSpacer】attgatcaaattcgagg【C3-spacer】-3′ | ||
| 6 | 2F-2 | 5′-cggaaataggctgaatgtaactctaaacggaatg-3′ | 189 |
| 2R-2 | 5’-【Biotin】gtgcttgtaaaccaacctacaacagcagataaaac-3′ | ||
| 2LF | 5'-【FAM】ctctatatgaggggatagaggattatcaaa【dSpacer】attgatcaaattcgagg【C3-spacer】-3′ | ||
| 7 | 3F | 5′- aggatggagaatggtggagtatagaaaacc -3′ | 235 |
| 3R | 5'-【Biotin】ctcgtgggagccaagtaagacatatctaatgt -3′ | ||
| 3LF | 5'-【FAM】aggatgcacacagatagaacagagtttgaa【dSpacer】aattagatattaaggc【C3-spacer】-3′ | ||
| 8 | 4F | 5′-ctaaacggaatgatcttcgtggaaccaacta-3′ | 279 |
| 4R | 5'-【Biotin】cgtgcttgtaaaccaacctacaacagcagata-3′ | ||
| 4LF | 5'-【FAM】aatggagtatgataatgtggaagagaactt【dSpacer】attaggtatgaggagg【C3-spacer】-3′ |
Note: F: forward primer; R: reverse primer; LF: probe; FAM: Carboxyfluorescein; dSpacer: a tetrahydrofuran residue; C3-spacer: 3′-block.
2.5. BEFV RPA assays
TwistAmp™ nfo kits were supplied as the dry enzyme pellet in eight strips vacuum-sealed pouches (TwistDx, Ltd., Cambridge, United Kingdom). The BEFV RPA was performed in a 50 μL final reaction volume according to the instructions outlined in the Twist Amp nfo kit manual. In brief, the rehydration solution contained 2 μL DNA template, 2.1 μL (10 μM) forward primers and reverse primers, respectively, 0.6 μL (10 μM) TwistAmp LF Probe, and 29.5 μL rehydration buffer. Resuspend the reaction pellet with 47.5 μL of the rehydration solution, and then the reaction was initiated by adding 2.5 μL of magnesium acetate (280 mM). The tubes were then incubated at 38 °C in an incubator block for 4 min. As recommended, the samples were blended top down and bottom up 6–8 times after 4 min' incubation, and an additional incubation was continued for 21 min. In addition, in each run, a positive template control supplied with the Twist Amp nfo kit (primers/probe and template) and a negative template control (nuclease-free) water were included.
The amplicon of TwistAmp LF Probe system was visualized using a simple ‘sandwich’ LFD assay and 2% agarose gel. Milenia's Genline Hybridetect-1/Hybridetect-2 lateral flow strips (Germany) duplexes labeled with anti-FAM gold conjugates and anti-Biotin antibodies from Milenia GmbH were used in this study for detection of LFD-RPA amplified nucleic acids. 2 μL of hybridization products were mixed with 98 μL of PBST (1 × phosphate buffered saline with 0.1% Tween20) running buffer. The LFD were then placed into the PBST dilution in a 96-well plate. The positive amplification product was indicated by both test line and control line on the strip visualized simultaneously within 5 min, the negative control (no template) should generate a separate control line found further up test line on the strip. The absence of a control line on the LF strip indicates the strip could not work correctly.
The outcome of TwistAmp LF Probe systems were also analyzed by agarose gel-electrophoresis (AGE). In brief, the amplification product was purified by commercial PCR purification kits. The comparable size of required amount of the amplification products were visualized by electrophoresis on a 2% agarose-gel.
To determine the optimal reaction condition, different reaction temperature (25 °C, 30 °C, 35 °C, 38 °C, 42 °C and 50 °C) and different incubation time (10 min, 15 min, 20 min, 25 min and 30 min) were optimized for this methodology.
2.6. Analytical of sensitivity and specificity of the assay
To determine the detection limit of BEFV genomic copies, ten-fold serial dilutions standard G-T3-RPA plasmid by ranging from 8 × 107 to 8 × 10−1 copies per reaction were detected within the same sample run.
The analytical specificity of the assay was assessed among other viral pathogens of cattle. DNA of IBRV, RNA of BVDV and BPIV-3 were prepared from cell culture supernatant, and cDNA of BVDV, BPIV-3 were prepared as above mentioned. Clone of full-length genome of BRSV and BcoV were supplied as templates in the LFD-RPA reaction. Additionally, 8 × 105 copies per reaction of standard G-T3-RPA plasmid and BEFV-free samples were used as a positive control and a negative control respectively.
2.7. Clinical specimen preparation
BEFV-free blood and lung tissue specimens were obtained from healthy calves and 104 clinical blood specimens and 24 tissue samples including 16 lung tissue specimens, 8 lymph gland specimens were collected from suspected dairy cattle cases of BEFV infections in eastern China. The details of clinical specimen were listed in Table 2. Tissue specimens were homogenized in 10 mM phosphate buffered saline (PBS) with a dilution of 1:10 (W/V), centrifuged at 10,000 × g at 4 °C for 15 min, 200 μL sample suspension or blood sample suspension was separated for RNA extraction with the QIAamp viral RNA minikit (Qiagen, Germany) according to manufacturer's instructions. The RNA was eluted in 50 μL of nuclease-free water. The extracted RNA was used as template for cDNA synthesis using reverse transcription with random primers according to the instructions of the RevertAid First Strand cDNA Synthesis Kit (Fermentas, Canada). The volume of 2 μL of cDNA extracted from each specimen was used as a template in the RPA reactions.
Table 2.
Comparative performances of RT-PCR, LFD-RPA and Real-time qPCR assays for detection of suspected BEFV infectious clinical specimens.
| Samples type | RT-PCR |
LFD-RPA |
Real-time qPCR |
|||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |
| blood | 68 | 36 | 76 | 28 | 75 | 29 |
| lymph node | 6 | 2 | 7 | 1 | 7 | 1 |
| lung | 9 | 7 | 13 | 3 | 13 | 3 |
| Total | 83 | 45 | 96 | 32 | 95 | 33 |
2.8. Real-time qPCR and RT-PCR for amplification of BEFV
The RT-qPCR was conducted with the primers and probe targeting glycoprotein gene described by Yehuda Stram [25]. In briefly, the primer pairs for real-time qPCR used for amplification of BEFV were Bef70f: GAGATCAAATGTCCACAACGTTTAA, Bef117R: AATGTTCATCCTTTGCAAGATTATGA, and MGB probe: AATTATCACTTCAAGCCC labeled with FAM. The reaction was performed in an ABI Prism 7000 apparatus (ABI, USA) in a 20 μL volume, and the reaction conditions were: 50 °C for 2 min, 95 °C for 10 min, and then 40 cycles of 95 °C for 15 s and 60 °C for 1 min. In diagnostic real-time RT-PCR assay, it was customary to regard results between cycle threshold (Ct) 35 and 40 as equivocal and above Ct 40 as negative. The clinical sample assessment was also carried out by reverse transcription PCR (RT-PCR) assay with gel electrophoresis detection previously published by Niwa et al. [24]. Two methods were operated with the same amount of template.
3. Results
3.1. Design and screening of BEFV RPA primer and probe sets
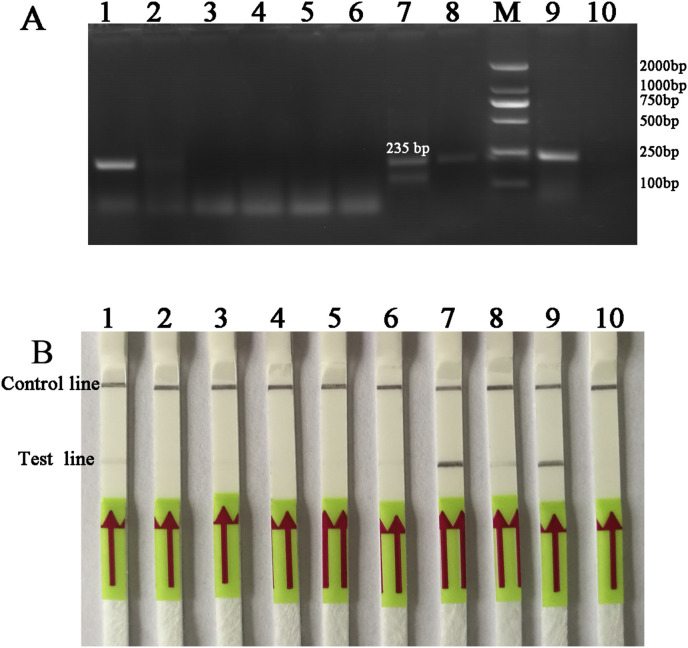
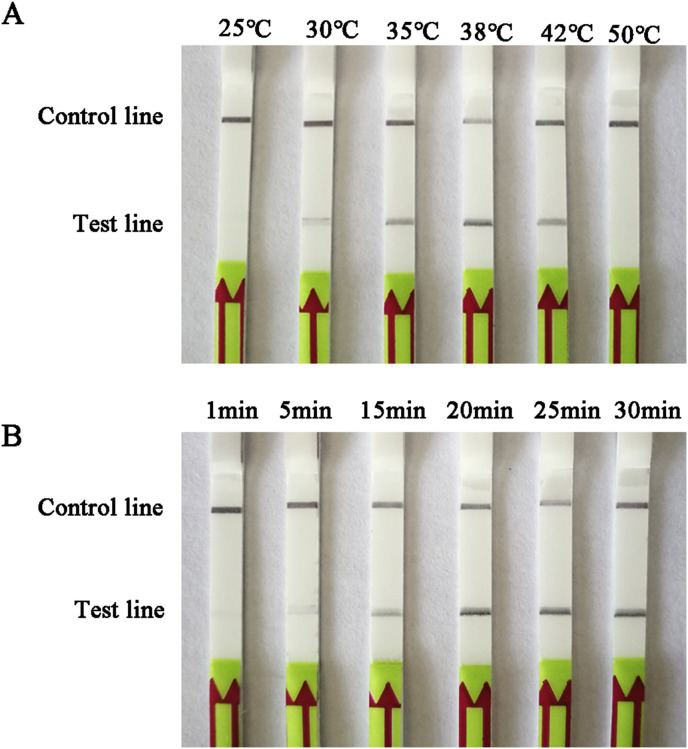
16 forward and reverse and four LF probe combinations of candidate primers/probes targeting G gene were designed and screened for the detection of BEFV (Table 1). The candidate primers/probe for the LFD-RPA assay was screened by performing with TwistAmp nfo reactions and preliminarily analyzed its amplified specificity on 2% agarose gel with labeled amplicons. Of these, 3F/3R/3LF sets were identified as capable of specific amplification efficiency with the expected size of 235 bp (Fig. 1 A), and LFD-RPA test line showed faster than other sets within 5 min (Fig. 1B). Therefore, 3F/3R/3LF primer set was chosen for subsequent evaluation (Supplementary Fig. 1). Besides that, different reaction temperature and different incubation time were optimized for this methodology. The results showed that the best amplified product were observed in reactions incubated at 38 °C (Fig. 2 A) and could be detected 15 min or longer (Fig. 2B). Thus, the process of amplification reaction of the BEFV LFD-RPA assay was performed at 38 °C for 20 min.
Fig. 1.
Screening of the designed primers/probe of BEFV LFD-RPA assay.
(A) Agarose gel electrophoresis of RPA products generated using designed primers/probes. Lane M: molecular weight standard (DNA Marker 2000). Lane1 to 8 was designed primer and probe sets: F1-1/R1-1/1LF, F1-2/R1-2/1LF, F1-3/R1-3/1LF, F1-4/R1-4/1LF, 2F-1/2R-1/2LF, 2F-2/2R-2/2LF, 3F/3R/3LF and 4F/4R/4LF, respectively. Lane 9: positive control (supplied by Twist Amp nfo kit); Lane 10: negative control (DNase-free water); Specifically, Lane 7 was primers/probe set 3F/3R/3LF, and the expected size of the product was 235bp.
(B) Lateral-flow strip end-point analysis of RPA products generated by using designed primers/probe set 3F/3R/3LF. Lane 1 to 8: BEFV amplicons performed with RPA primer pair F1-1/R1-1/1LF, F1-2/R1-2/1LF, F1-3/R1-3/1LF, F1-4/R1-4/1LF, 2F-1/2R-1/2LF, 2F-2/2R-2/2LF, 3F/3R/3LF and 4F/4R/4LF, respectively. Lane 9: positive control (supplied by Twist Amp nfo kit); Lane 10: negative control (DNase-free water).
Fig. 2.
Determination of reaction temperature and time.
(A) The LFD-RPA works effectively in a broad range of constant reaction temperatures.
(B) The LFD-RPA amplification can be visible on the LFD at 15min or longer.
3.2. Sensitivity and specificity of the BEFV RPA reaction
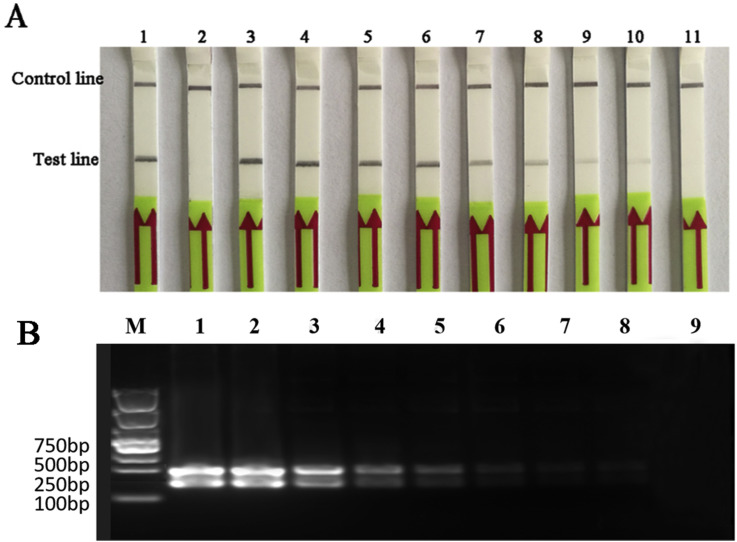
To determine the sensitivity of RPA assay, we carried out the BEFV LFD-RPA assay with the quantity of template ranging from 8 × 107 to 8 × 10−1 copies per reaction. The result showed that the minimum virus detection limits of RPA were as low as 8 copies per reaction (Fig. 3 A&3B). The copy number of RNA molecules transcribed from standard G-T3-RPA calculated by RNA molecular standard was 24 RNA molecules.
Fig. 3.
Analytical sensitivity of the BEFV LFD-RPA assay.
(A) Sensitivity of the LFD-RPA assay. Molecular sensitivity test results of RPA using 10-fold serially diluted template of standard plasmid. Lane 1: positive control (supplied by Twist Amp nfo kit); Lane 2:negative control (DNase-free water); BEFV templates of lane 3 to 11 in these reactions ranged from 8 × 107 to 8 × 10−1 copies per reaction, respectively. Samples were tested in triplicate with one reaction displayed in figure for each triplicate.
(B) BEFV RPA reaction products were detected on a 2% agarose gel. BEFV templates of Lane 1 to Lane 9 in these reactions ranged from 8 × 107 to 8 × 10−1 copies per reaction, respectively.
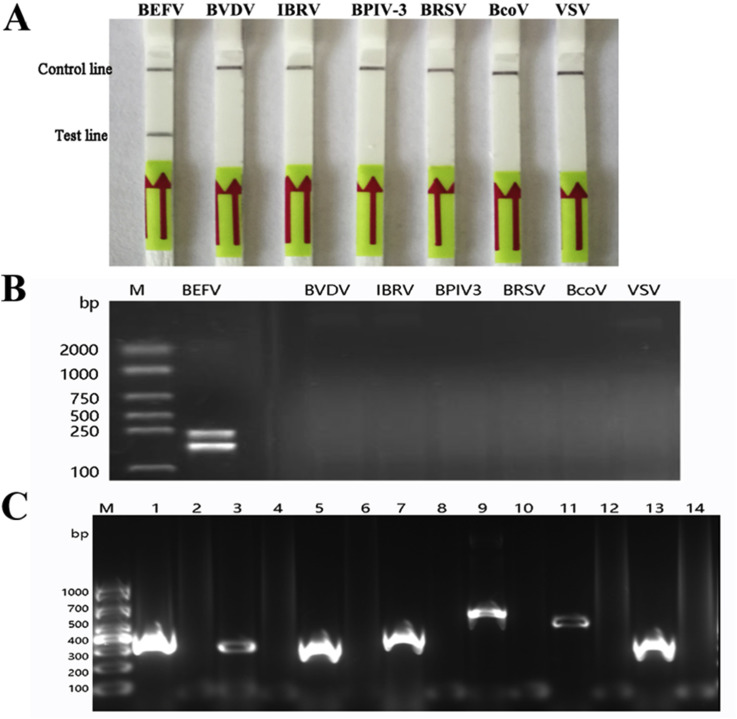
The specificity of the assay was assessed among other viral pathogens of cattle with similar clinical signs including IBRV, BVDV, BPIV-3, BRSV and BcoV as well as similar etiologies such as VSV cDNA and clone of full-length genome of other viral pathogens were supplied in the LFD-RPA reaction with an excess of templates. The results indicated that no cross-reactions of the IBRV, BVDV, BPIV-3, BRSV, BcoV and VSV were observed on the dipstick within 5 min (Fig. 4 A&4B). To check the quality of RNA/DAN preparations used for the specificity of the assay, we undertook PCR reaction firstly with positive primer of corresponding template respectively on 2% agarose gel (Fig. 4C). The viral specific primers used in this study were listed in additional Table 1.
Fig. 4.
Analytical specificity of the BEFV LFD-RPA assay.
(A) Specificity of the LFD-RPA assay. The specificity of the assay was assessed for other bovine viral pathogens with similar clinic and etiologies. Lane 1: positive control of BEFV; Lanes 2 to 7: BVDV, IBRV, BPIV-3, BRSV, BcoV and VSV, respectively. Samples were tested in triplicate with one reaction displayed in figure for each triplicate.
(B) The results of amplification products of the LFD-RPA on 2% agarose gel. Lane 1: positive control of BEFV; Lanes 2 to 7: BVDV, IBRV, BPIV-3, BRSV, BcoV and VSV, respectively.
(C) The quality detection of RNA/DNA of BVDV, IBRV, BPIV-3, BRSV, BcoV and VSV. The RNA/DNA of BEFV, BVDV, IBRV, BPIV-3, BRSV, BcoV and VSV prepared for specificity detection were undertook PCR reaction with viral specific primers (Supplementary Table 1). The positive amplification results were shown in Lane 1, Lane 3, Lane 5, Lane 7, Lane 9, Lane 11, Lane 13, respectively. Lane 2, Lane 4, Lane 6, Lane 8, Lane 10, Lane 12, Lane 14 were negative controls with DNase-free water as template.
3.3. Performance of BEFV LFD RPA assay on clinical samples
To evaluate the BEFV LFD RPA assay, we performed this method on clinical samples detection. In addition, suspected of BEFV infectious clinical blood specimens and tissue samples were obtained from submitted for inspection to our laboratory. A volume of 2 μL of cDNA from each blood and tissue specimen extraction was used as a template in conventional RT-PCR, LFD RPA, and real-time qPCR assay, respectively. The results of those assays showed that a total of 83 clinical specimens were tested positive by conventional RT-PCR, while the similar performance that 96 specimens were detected positive by BEFV RPA nucleic acid amplification assays on LFD within 5 min, and 95 specimens were positive with the Ct values below 35 using the real-time qPCR assay. Therefore, the positive rate detected by conventional RT-PCR, RPA, and real-time qPCR was 64.84% (83/128), 75.00% (96/128), 74.22% (95/128), respectively (Table 2 ). This particular RPA therefore was highly sensitive compared with the conventional RT-PCR, and the result indicated that the sensitivity, specificity and coincidence rate of BEFV LFD-RPA and real-time qPCR was 97.89% (93/95), 90.91% (30/33), 96.09% (123/128), respectively (Table 3 ). Moreover, there was very good correlation between the results from LFD RPA and real-time qPCR of the same sample.
Table 3.
Sensitivity, specificity and coincidence rate of BEFV LFD-RPA and Real-time qPCR.
| Real-time qPCR |
Sensitivity | Specificity | CR | ||||
|---|---|---|---|---|---|---|---|
| Pos | Neg | Total | |||||
| LFD-RPA | Pos | 93 | 3 | 96 | |||
| Neg | 2 | 30 | 32 | 97.89% | 90.91% | 96.09% | |
| Total | 95 | 33 | 128 | ||||
Note: Pos: Positive; Neg: Negative; CR: Coincidence rate. Coincidence rate of BEFV LFD-RPA and real-time qPCR was calculated by the equation: Coincidence rate = [(93 + 30)/128]*100%.
4. Discussion
In this study, a rapid LFD RPA assay that was an alternative method for the confirmation of BEFV infection in cattle was developed. Moreover, this is the first report about the development of a LFD-RPA for detection of BEFV. Consistent with previous various nucleic acid tests for detection BEFV, the primers were designed for the amplification of G gene among isolates obtained from Mainland China, as well as Taiwan, Japan, Australia, Turkey and Israel, and this method might also be useful for the detection of BEFV strains isolated from other countries and regions [37].
As far as RPA system is concerned, unlike PCR-based DNA amplification assays, there is no design support software program available for RPA. In this study, several primer and probe combinations were designed and screened for specific amplification efficiency for the RPA assay (Table 1). It is worth mentioning that, design and determine of ideal BEFV primers and probes are much more stringent as it requires longer primers (30–35 bases) and probes (46–52 bases) leading to the formation of secondary structures. Furthermore, the amplicons of RPA combined with LF Probe system were visualized using both a simple ‘sandwich’ LFD assay and 2% agarose gel. In this study, we firstly performed primers/probe screening on 2% agarose gel to exclude non-specific amplification (Fig. 1A), and analyzed its amplification efficiency with labeled amplicons on the LFD (Fig. 1B).
The aim of this study was to develop a sensitive, specific, simple, and rapid diagnostic assay that is applied to detection of BEFV infections. According to previous published reports, an extensive performance on detecting BEFV was carried out including reliable assay protocol for identification of diseases (RAPID)-bioactive amplification with probing (BAP) assay, RT-LAMP, real time qPCR and conventional RT-PCR [12,23,25,38]. With respect to analytical sensitivity, the limit of detection of the assay was 8 copies per reaction determined by testing replicates of various concentrations of G-T3-RPA plasmid (Fig. 3A). It seems to be similar to other techniques reported previously such as RT-LAMP (20 copies/tube of RNA), real-time-RT-PCR (lies between 10 and 100 copies), and higher than RT-PCR (200 copies/tube of RNA), but lower than that the detection limit of RAPID-BAP assay was 1 copy/μL. To some extent, BEFV LFD-RPA outperforms RT-LAMP and real time qPCR: on the one hand, RPA reaction only utilizes two primers and one probe and three binding sites, in contrast, the RT-LAMP assay needs at least four primers and six binding sites, a higher reactive temperature (60–65 °C) [38]. On the other hand, RPA reagents provided in a lyophilized are stable even when stored for 3 weeks at 45 °C, which allow independence from the cooling chain [39]. Moreover, it only requires a simple heat block to run (38 °C) (Fig. 2) and the results can also be visualized by LFD with naked eyes inspection while the method of real-time-qPCR requires sophisticated instruments operated by specially trained personnel. What's more important, the study of analytical specificity is not only typically evaluated for etiologies that are likely to cause clinical presentations similar to the target etiology, but also evaluating those more closely related to BEFV that considered as potential sources of false-positives (Fig. 3B). It is worth mentioning that the high sensitivity and specificity of this lateral flow based detection systems assay gave the credit to good use of gold-labeled anti-FAM and anti-Biotin antibodies to capture RPA product simultaneously [40]. Although some biochemical methods including the flow type quartz crystal microbalance immunosensor have been published that are able to detect of BEFV [41], the procedure is somewhat complicated and requires the BEFV monoclonal antibodies on the gold surface of a quartzcrystal microbalance (QCM), and the whole process of this method needs to be operated by a professional personnel.
Increasing numbers of reports showed that PCR-based detection of BEFV is now widely used for rapid laboratory diagnosis [23,25,42]. As the applications of LFD-RPA, conventional RT-PCR and real time qPCR methods for detection BEFV genomes from clinical samples (Table 2), the results clearly indicated the potential benefits of the developed assay over PCR-based methods. Furthermore, it is much rapid than other nucleic acid tests at present and the reaction is performed at an ambient constant temperature instead of an expensive and sophisticated thermo cycler. However, the typical benefit of lateral-flow assays is their ability to be used as pen-side tests, this does not appear to be possible in this situation due to the requirement the prepared of BEFV genomes to make cDNA prior to performing the test. Therefore, it is possible that the performance of the BEFV RPA assay could be improved from that observed in this study.
5. Conclusions
The LFD RPA assay are successfully developed for the rapid, sensitive and specific detection of BEFV. In addition, this methodology of BEFV LFD-RPA may aid in identification of BEFV infected individuals in beef and dairy farms. The results are gratifying but the method also requires a larger number of samples for further optimization and validation. Furthermore, the assay has potential to be a promising alternative to other isothermal methods for rapid detection of BEFV.
Ethics approval and consent to participate
Experimental protocols for obtaining cattle blood and tissue samples used in this study were carried out in strict accordance with China law on use of animal on research and all procedures were approved by the Shandong Normal University Animal Care and Use Committee.
Competing interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
The study was conceived, designed and critically revised by HHB and WHM. Data collection, study execution and the drafted manuscript were done by HPL. Clinical samples detection and laboratory data analysis was performed by HCQ, ZGM and HYJ. All authorship gave final approval and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work.
Funding
This work was partially supported by grants from National Natural Science Fund of China (31502064, 31672556), Taishan Scholar and Distinguished Experts (TSTP2016) (H. H.), Shandong Major Agricultural Application Technology Innovation Project (2015WHM) (H·W), Primary Research & Developement Plan of Shandong Province (2015GNC113006, 2016GNC113006).
Acknowledgements
Not applicable.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.mcp.2017.12.003.
Contributor Information
Peili Hou, Email: apeilihou@163.com.
Guimin Zhao, Email: zgmnefu@163.com.
Hongmei Wang, Email: hongmeiwang@sdnu.edu.cn.
Chengqiang He, Email: hchqiang@sdnu.edu.cn.
Yanjun Huan, Email: 201601011@qau.edu.cn.
Hongbin He, Email: hongbinhe@sdnu.edu.cn.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
S.Fig. 1.
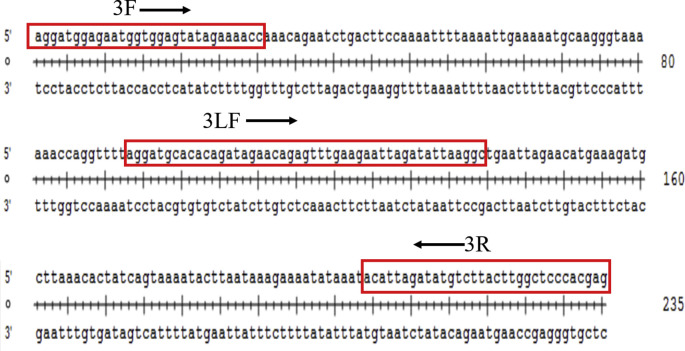
Schematic drawing of the nucleotide size and the amplified gene region of 3F/3R/3LF sets. The points linked to gene sequence amplification locus and positions of the forward (3F) and reversed (3R) primers and LF probe (3LF) used in this study.
References
- 1.Hsieh Y.C., Chen S.H., Chou C.C., Ting L.J., Itakura C., Wang F.I. Bovine ephemeral fever in Taiwan (2001-2002) J. Vet. Med. Sci. 2005;67(4):411–416. doi: 10.1292/jvms.67.411. [DOI] [PubMed] [Google Scholar]
- 2.Nandi S., Negi B.S. Bovine ephemeral fever: a review. Comp. Immunol. Microbiol. Infect. Dis. 1999;22(2):81–91. doi: 10.1016/s0147-9571(98)00027-7. [DOI] [PubMed] [Google Scholar]
- 3.Walker P.J. Bovine ephemeral fever in Australia and the world. Curr. Top. Microbiol. Immunol. 2005;292:57–80. doi: 10.1007/3-540-27485-5_4. [DOI] [PubMed] [Google Scholar]
- 4.Bai W.B., Jiang C.L., Davis S.S. Preliminary observations on the epidemiology of bovine ephemeral fever in China. Trop. Anim. Health Prod. 1991;23(1):22–26. doi: 10.1007/BF02361265. [DOI] [PubMed] [Google Scholar]
- 5.Li Z., Zheng F., Gao S., Wang S., Wang J., Liu Z., Du J., Yin H. Large-scale serological survey of bovine ephemeral fever in China. Vet. Microbiol. 2015;176(1–2):155–160. doi: 10.1016/j.vetmic.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Shirakawa H., Ishibashi K., Ogawa T. A comparison of the epidemiology of bovine ephemeral fever in South Korea and south-western Japan. Aust. Vet. J. 1994;71(2):50–52. doi: 10.1111/j.1751-0813.1994.tb06153.x. [DOI] [PubMed] [Google Scholar]
- 7.Tonbak S., Berber E., Yoruk M.D., Azkur A.K., Pestil Z., Bulut H. A large-scale outbreak of bovine ephemeral fever in Turkey, 2012. J. Vet. Med. Sci. 2013;75(11):1511–1514. doi: 10.1292/jvms.13-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker P.J., Cybinski D.H. Bovine ephemeral fever and rhabdoviruses endemic to Australia. Aust. Vet. J. 1989;66(12):398–400. doi: 10.1111/j.1751-0813.1989.tb13557.x. [DOI] [PubMed] [Google Scholar]
- 9.Walker P.J., Klement E. Epidemiology and control of bovine ephemeral fever. Vet. Res. 2015;46:124. doi: 10.1186/s13567-015-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeruham I., Sharir B., Yadin H., Tiomkin D., Chai D. Bovine ephemeral fever in beef cattle herds in the Jordan Valley, Israel. Vet. Rec. 2003;152(3):86–88. doi: 10.1136/vr.152.3.86. [DOI] [PubMed] [Google Scholar]
- 11.Yeruham I., Van Ham M., Stram Y., Friedgut O., Yadin H., Mumcuoglu K.Y., Braverman Y. Epidemiological investigation of bovine ephemeral Fever outbreaks in Israel. Vet. Med. Int. 2010;2010 doi: 10.4061/2010/290541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh Y.C., Chen S.H., Chou C.S., Hsiao H.W., Chen S.Z., Lee Y.F., Liu H.J. Development of a reliable assay protocol for identification of diseases (RAPID)-bioactive amplification with probing (BAP) for detection of bovine ephemeral fever virus. J. Virol Meth. 2005;129(1):75–82. doi: 10.1016/j.jviromet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Johal J., Gresty K., Kongsuwan K., Walker P.J. Antigenic characterization of bovine ephemeral fever rhabdovirus G and GNS glycoproteins expressed from recombinant baculoviruses. Arch. Virol. 2008;153(9):1657–1665. doi: 10.1007/s00705-008-0164-0. [DOI] [PubMed] [Google Scholar]
- 14.Kato T., Aizawa M., Takayoshi K., Kokuba T., Yanase T., Shirafuji H., Tsuda T., Yamakawa M. Phylogenetic relationships of the G gene sequence of bovine ephemeral fever virus isolated in Japan, Taiwan and Australia. Vet. Microbiol. 2009;137(3–4):217–223. doi: 10.1016/j.vetmic.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Zheng F., Qiu C. Phylogenetic relationships of the glycoprotein gene of bovine ephemeral fever virus isolated from mainland China, Taiwan, Japan, Turkey, Israel and Australia. Virol. J. 2012;9:268. doi: 10.1186/1743-422X-9-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng F.Y., Lin G.Z., Qiu C.Q., Zhou J.Z., Cao X.A., Gong X.W. Development and application of G1-ELISA for detection of antibodies against bovine ephemeral fever virus. Res. Vet. Sci. 2009;87(2):211–212. doi: 10.1016/j.rvsc.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Bakhshesh M., Abdollahi D. Bovine ephemeral fever in Iran: diagnosis, isolation and molecular characterization. J. arthropod-borne Dis. 2015;9(2):195–203. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M., Ge J., Wen Z., Chen W., Wang X., Liu R., Bu Z. Characterization of a recombinant Newcastle disease virus expressing the glycoprotein of bovine ephemeral fever virus. Arch. Virol. 2017;162(2):359–367. doi: 10.1007/s00705-016-3078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yazdani F., Bakhshesh M., Esmaelizad M., Sadigh Z.A. Expression of G1- epitope of bovine ephemeral fever virus in E. coli : a novel candidate to develop ELISA kit. Vet. Res. Forum Int. Q J. 2017;8(3):209–213. [PMC free article] [PubMed] [Google Scholar]
- 20.Snowdon W.A. Bovine ephemeral fever: the reaction of cattle to different strains of ephemeral fever virus and the antigenic comparison of two strains of virus. Aust. Vet. J. 1970;46(6):258–266. doi: 10.1111/j.1751-0813.1970.tb15773.x. [DOI] [PubMed] [Google Scholar]
- 21.Tzipori S. The isolation of bovine ephemeral fever virus in cell cultures and evidence for autointerference. Aust. J. Exp. Biol. Med. Sci. 1975;53(4):273–279. doi: 10.1038/icb.1975.30. [DOI] [PubMed] [Google Scholar]
- 22.Fenglan Tian C.W., Bai Wenbin, Jiang Chunling, Zhang Zigang. Application of indirect immunofluorescent assay for the detection of bovine ephemeral fever virus[in Chinese] Anim. Infect. Dis. 1985;25(4):7–9. [Google Scholar]
- 23.Blasdell K.R., Adams M.M., Davis S.S., Walsh S.J., Aziz-Boaron O., Klement E., Tesh R.B., Walker P.J. A reverse-transcription PCR method for detecting all known ephemeroviruses in clinical samples. J. Virol Meth. 2013;191(2):128–135. doi: 10.1016/j.jviromet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Niwa T., Shirafuji H., Ikemiyagi K., Nitta Y., Suzuki M., Kato T., Yanase T. Occurrence of bovine ephemeral fever in Okinawa Prefecture, Japan, in 2012 and development of a reverse-transcription polymerase chain reaction assay to detect bovine ephemeral fever virus gene. J. Vet. Med. Sci. 2015;77(4):455–460. doi: 10.1292/jvms.14-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stram Y., Kuznetzova L., Levin A., Yadin H., Rubinstein-Giuni M. A real-time RT-quantative(q)PCR for the detection of bovine ephemeral fever virus. J. Virol Meth. 2005;130(1–2):1–6. doi: 10.1016/j.jviromet.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Zakrzewski H., Cybinski D.H., Walker P.J. A blocking ELISA for the detection of specific antibodies to bovine ephemeral fever virus. J. Immunol. Meth. 1992;151(1–2):289–297. doi: 10.1016/0022-1759(92)90129-h. [DOI] [PubMed] [Google Scholar]
- 27.Piepenburg O., Williams C.H., Stemple D.L., Armes N.A. DNA detection using recombination proteins. PLoS Biol. 2006;4(7) doi: 10.1371/journal.pbio.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santiago-Felipe S., Tortajada-Genaro L.A., Puchades R., Maquieira A. Recombinase polymerase and enzyme-linked immunosorbent assay as a DNA amplification-detection strategy for food analysis. Anal. Chim. Acta. 2014;811:81–87. doi: 10.1016/j.aca.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Loo J.F., Lau P.M., Ho H.P., Kong S.K. An aptamer-based bio-barcode assay with isothermal recombinase polymerase amplification for cytochrome-c detection and anti-cancer drug screening. Talanta. 2013;115:159–165. doi: 10.1016/j.talanta.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 30.Kersting S., Rausch V., Bier F.F., von Nickisch-Rosenegk M. Multiplex isothermal solid-phase recombinase polymerase amplification for the specific and fast DNA-based detection of three bacterial pathogens. Mikrochim. Acta. 2014;181(13–14):1715–1723. doi: 10.1007/s00604-014-1198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Euler M., Wang Y., Nentwich O., Piepenburg O., Hufert F.T., Weidmann M. Recombinase polymerase amplification assay for rapid detection of Rift Valley fever virus. J. Clin. Virol. Offic. Publ. Pan Am. Soc. Clin. Virol. 2012;54(4):308–312. doi: 10.1016/j.jcv.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Kersting S., Rausch V., Bier F.F., von Nickisch-Rosenegk M. Rapid detection of Plasmodium falciparum with isothermal recombinase polymerase amplification and lateral flow analysis. Malar. J. 2014;13:99. doi: 10.1186/1475-2875-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosser A., Rollinson D., Forrest M., Webster B.L. Isothermal Recombinase Polymerase amplification (RPA) of Schistosoma haematobium DNA and oligochromatographic lateral flow detection. Parasites Vectors. 2015;8:446. doi: 10.1186/s13071-015-1055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y.D., Zhou D.H., Zhang L.X., Zheng W.B., Ma J.G., Wang M., Zhu X.Q., Xu M.J. Recombinase polymerase amplification (RPA) combined with lateral flow (LF) strip for equipment-free detection of Cryptosporidium spp. oocysts in dairy cattle feces. Parasitol. Res. 2016;115(9):3551–3555. doi: 10.1007/s00436-016-5120-4. [DOI] [PubMed] [Google Scholar]
- 35.He C.Q., Liu Y.X., Wang H.M., Hou P.L., He H.B., Ding N.Z. New genetic mechanism, origin and population dynamic of bovine ephemeral fever virus. Vet. Microbiol. 2016;182:50–56. doi: 10.1016/j.vetmic.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 36.Wang J.C., Yuan W.Z., Han Q.A., Wang J.F., Liu L.B. Reverse transcription recombinase polymerase amplification assay for the rapid detection of type 2 porcine reproductive and respiratory syndrome virus. J. Virol Meth. 2017;243:55–60. doi: 10.1016/j.jviromet.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Oguzoglu T.C., Erturk A., Cizmeci S.G., Koc B.T., Akca Y. A report on bovine ephemeral fever virus in Turkey: antigenic variations of different strains of EFV in the 1985 and 2012 outbreaks using partial glycoprotein gene sequences. Transboundary Emerg. Dis. 2015;62(5):e66–70. doi: 10.1111/tbed.12187. [DOI] [PubMed] [Google Scholar]
- 38.Zheng F., Lin G., Zhou J., Wang G., Cao X., Gong X., Qiu C. A reverse-transcription, loop-mediated isothermal amplification assay for detection of bovine ephemeral fever virus in the blood of infected cattle. J. Virol Meth. 2011;171(1):306–309. doi: 10.1016/j.jviromet.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 39.Lillis L., Siverson J., Lee A., Cantera J., Parker M., Piepenburg O., Lehman D.A., Boyle D.S. Factors influencing Recombinase polymerase amplification (RPA) assay outcomes at point of care. Mol. Cell. Probes. 2016;30(2):74–78. doi: 10.1016/j.mcp.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou P., Wang H., Zhao G., He C., He H. Rapid detection of infectious bovine rhinotracheitis virus using recombinase polymerase amplification assays. BMC Vet. Res. 2017;13(1):386. doi: 10.1186/s12917-017-1284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Y.G., Chang K.S. Application of a flow type quartz crystal microbalance immunosensor for real time determination of cattle bovine ephemeral fever virus in liquid. Talanta. 2005;65(5):1335–1342. doi: 10.1016/j.talanta.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Finlaison D.S., Read A.J., Zhang J., Paskin R., Kirkland P.D. Application of a real-time polymerase chain reaction assay to the diagnosis of bovine ephemeral fever during an outbreak in New South Wales and northern Victoria in 2009-10. Aust. Vet. J. 2014;92(1–2):24–27. doi: 10.1111/avj.12139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.