Abstract
Gastrointestinal stromal tumors are the most common primary mesenchymal tumors of the gastrointestinal tract accounting for 0.1%–3.0% of all gastrointestinal malignancies. The stomach is the most common site (60%) followed by the small bowel (30%–35%) particularly jejunum and ileum, colorectum (5%) and rarely affect esophagus and appendix. Most gastrointestinal stromal tumors arise sporadically, however, less commonly they develop in association with various clinical syndromes like Carney triad, Carney–Stratakis syndrome, familial gastrointestinal stromal tumor syndrome and neurofibromatosis type1 (NF1). We report a 65-year-old male patient presented with gastric mass (7.5 × 4.5 × 3.5 cm) arising from the posterior gastric wall. Histologic examination revealed neoplastic proliferation of spindled and epithelioid cells with focal plexiform pattern and low mitotic activity 3/50 HPF. No evidence of cytological atypia, abnormal mitosis or necrosis. Interestingly enough, there were focal areas of mature bone formation/osseous differentiation associated with calcification. The tumor cells were strongly positive for CD117, DOG1 with focal immunoreactivity against CD34. The morphologic features and the immunoprofile were diagnostic of gastrointestinal stromal tumor. Herein, we present a rare case of gastric gastrointestinal stromal tumor with mature bone formation, osseous metaplasia and calcification. To the best of our knowledge, this is the second case report of gastric gastrointestinal stromal tumor with osseous differentiation and mature bone formation.
Keywords: Gastrointestinal stromal tumor, stomach, metaplasia, calcification, osseous differentiation
Introduction
Gastrointestinal stromal tumors (GISTs) are mesenchymal-derived neoplasms of the gastrointestinal tract that has gained significant clinicopathologic and therapeutic interest over the last decades. GISTs originate from the interstitial cells of Cajal, located in the gastrointestinal wall, which regulate the autonomic motor activity in the gastrointestinal tract.1 GISTs exhibit slow-growing clinical behavior that can lead to late signs and symptoms. Most commonly reported symptoms were vague abdominal pain, nausea, vomiting and abdominal discomfort. However, acute abdomen and intestinal obstruction has been reported as the first clinical presenting symptoms. Radiologically, GISTs appear as a well-circumscribed mass occasionally with a peripheral rim of enhancement due to necrosis. Determining the risk assessment of the disease recurrence is very helpful tool for those patients whom likely to benefit from adjuvant therapy rather than surgery alone. The three histopathologic most reliable factors are tumor size, site and mitotic activity.2 A wide range of morphological phenomenon in GISTs have been reported in the literature. Focal calcification is not unusual event in large tumors; Nevetheless, extensive calcification in GISTs is a rare occasion. However, a mature bone formation is an extremely rare event in GIST and has been only reported once in the literature.3 Molecular genetic testing of GISTs demonstrate that 80%–85% of tumors harbor KIT (tyrosine-protein kinase kit)-activating mutation (Exon 11 most commonly), and they show dramatic response to imatinib therapy. Nevertheless, platelet-derived growth factor receptor alpha (PDGFRA) mutated GISTs (Exon 8 mostly) account for 5%–10% and are resistant to imatinib therapy. Wild-type GISTs account for 10%–15% in which the tumors lack KIT and PDGFRA mutations. It has been reported that mutations in succinate dehydrogenase (SDH) subunits (SDHA, SDHB, SDHC and SDHD), neurofibromatosis 1 (NF1) gene, retrovirus-associated DNA sequences (RAS) family (HRAS, NRAS, KRAS) and BRAF may play a role in the pathogenesis of wild-type GISTs.
Case report
A 65-year-old male patient who is a known case of benign prostatic hyperplasia, hypertension and dyslipidemia on medication, presented to our hospital as a case of gastric mass for further evaluation. Apart from mild abdominal tenderness, physical examination was unremarkable. Abdominal computed tomography (CT) scan with contrast (Figure 1) showed an ill-defined mass below the gastro duodenal junction, mural-based heterogeneous with multiple calcified foci, not associated with liver lesions, peritoneal spread or metastatic lymph nodes. The resection specimen demonstrated a fairly well-circumscribed, multilobular mass measuring 7.5 × 4.5 × 3.5 cm (Figure 2), arising from the posterior gastric wall. The mass appeared lobulated with white, firm, cut surfaces and foci of calcification. No areas of necrosis seen. Histopathological examination (Figure 3) revealed diffuse sheets of mixed pattern spindle and epithelioid cells with focal multinodular/plexiform growth pattern. Mitosis count is less than 3/50 HPF. Multiple areas of osseous differentiation and mature bone formation were identified. All submitted margins were negative for tumor. By immunohistochemistry, the tumor cells were positive for DOG1 (Figure 4(a)) and CD117 (Figure 4(b)), with focal immunoreactivity against CD34. Histopathologic appearance and immunohistochemical studies of the tumor were diagnostic of a GIST, low grade with mature bone formation, osseous metaplasia and calcification. Follow-up for 13 months after surgery revealed that the patient was doing well, and no tumor recurrence and/or metastasis was detected.
Figure 1.

Abdominal CT scan with contrast shows an ill-defined mass below the gastro duodenal junction.
Figure 2.

Gross photo of a fairly well-circumscribed gastric mass.
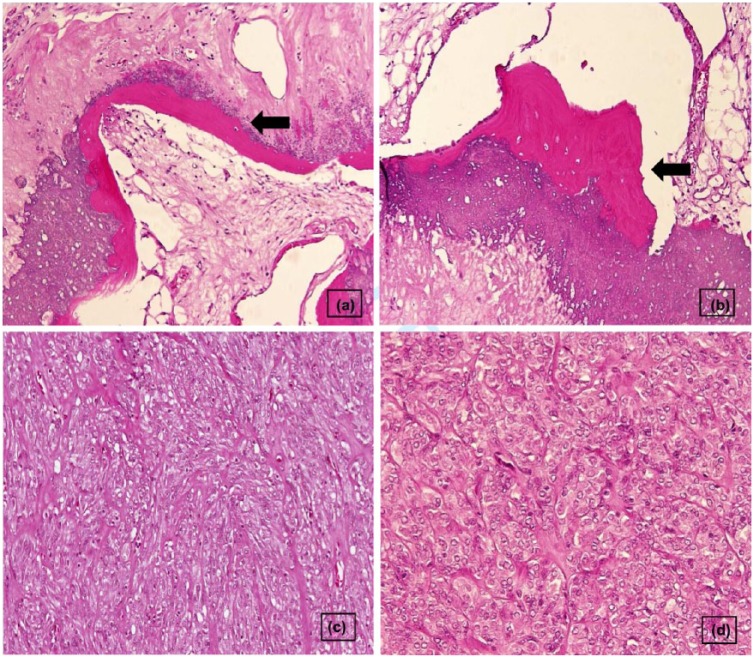
Figure 3.
Histopathology of a gastric gastrointestinal stromal tumor (hematoxylin and eosin stain): (a and b) (arrow head), multiple areas of calcification with osseous differentiation and mature bone formation (H and E stain; magnification ×10), (c) mixed growth patterns of both epithelioid and spindle cells (H and E stain; magnification ×20) and (d) low mitotic count, less than 3/50 HPF (H and E stain; magnification ×40).
Figure 4.
Immunohistochemistry of gastric gastrointestinal stromal tumor: (a) tumor cells are showing diffuse positivity for DOG1 (immunohistochemistry stain; magnification ×20) and (b) tumor cells are showing positive staining for CD117 (immunohistochemistry stain; magnification ×20).
Discussion
GISTs are the most common primary mesenchymal neoplasms of the gastrointestinal tract and known to arise from a cell of Cajal.4 It peaks at the age of 60 with female predominance in SDH-deficient GIST. Somatic mutations in KIT or PDGFRA form the majority of GIST mutation, which are dependent to receptor tyrosine kinases (RTK). This somatic mutation will lead to downstream signaling pathways and activation of RTK through Ras/Raf/mitogen-activated protein kinases (MAPK), signal transducer and activator of transcription 3 (STAT3), and phosphoinositide 3-kinase (PI3K)/Akt pathways. GIST can be treated by tyrosine kinase inhibitors (TKI). TKI therapy such as imatinib can block the activation of KIT and PDGFRA, which shows a dramatic response in patients with high risk, advanced stage and overall 2-year survival rate 70%–80%. Sadly, some GIST patients treated with TKI show resistance to therapy and left with no further standardized treatment. New searches are focusing nowadays to overcome the TKI resistance by new approaches. These include new KIT/PDGFRA inhibitors, KIT expression dysregulation and degradation, compounds targeting dysregulated signaling pathways, inhibitory effect against multiple RTK, cell cycle inhibitors and immunotherapeutics. Morphologically, GISTs exhibit variable growth patterns including spindle, epithelioid and mixed patterns. Spindle cell GIST is the most common type (70%), followed by epithelioid GIST (20%). The latter, usually harbors PDGFRA mutation. Histologically, SDH-deficient GIST shows a characteristic multinodular and plexiform growth pattern with predominant epithelioid cell morphology.5 Peculiar histological patterns ranging from stromal hyalinization, sclerosis, collagen and myxoid changes have been described in GISTs. Stromal lymphocytic infiltrate may be seen occasionally. Some GISTs might display an inconspicuous background vascular network while occasionally may show large, ectatic “staghorn-like” vessels. Large aggressive tumors might show perivascular tumor cell preservation in a background of geographic necrosis. Paraganglioma-like organoid pattern is an uncommon morphological pattern.6 Hypo- or paucicellularity, stromal fibrosis, hyalinization, myxoid changes, rhabdomyoblastic differentiation and or calcification are likely to be seen in treated GIST. Although some cases were reported in GISTs with extensive calcification prior to therapy,2,7 only one case has been reported in the literature that shows osseous differentiation.3 The exact mechanism of calcification and chondro-osseous or osseous differentiation is not clearly understood. Dystrophic calcification usually indicates the slow progressive nature of the underlying neoplastic growth, may also be seen in association with large tumors that underwent areas of hemorrhage, cystic degeneration and necrosis. Some studies have suggested that the pathogenesis is related to underlying inflammation, ischemia and necrosis in close vicinity to the tumor.8–10 Osseous differentiation and mature bone formation in GIST is an extremely rare finding. Multiple inflammatory cell mediators, including PDGFRA which is involved in the pathogenesis of GIST, are thought to play an important role in inflammation and osseous metaplasia. Moreover, bone morphogenetic protein (BMP) is well-known protein in osteogenic activity and bone formation.11,12 It has been demonstrated that BMP-2, BMP-4, BMP-5 and BMP6 are secreted by the tumor cells into the surrounding stromal environment causing stimulation to the stromal cells and leading to heterotopic osseous metaplasia. One hypothesis associates these changes, especially in treated GISTs, to imatinib suppressive effect which allows the osseous differentiation. Although, the clinical predictive value of GIST calcification is not well-established due to limited cases in the medical literature, calcified GISTs tend to behave clinically in a less aggressive manner.2
Conclusion
In conclusion, we present an interesting case of low-grade GIST of the stomach with focal calcification and extremely rare manifestation of osseous differentiation and mature bone formation. The exact mechanism of bone and osseous differentiation remains unclear due to rarity-reported GISTs with such phenomenon in the medical literature. We believe upon getting a reasonable number of GISTs with osseous metaplasia, exploring the underlying molecular changes would tremendously add to our current knowledge and help to better understand their clinical significance and expected behavior.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Ethical approval to report this case was obtained from King Faisal Specialist Hospital & Research Centre, Jeddah, Saudi Arabia (IRB-CR-2017-10).
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Verbal and written informed consents were obtained from the patient for their anonymized information to be published in this article.
References
- 1. Liegl-Atzwanger B, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Archiv 2010; 456(2): 111–127. [DOI] [PubMed] [Google Scholar]
- 2. Salati M, Orsi G, Bonetti LR, et al. Heavily calcified gastrointestinal stromal tumors: pathophysiology and implications of a rare clinicopathologic entity. World J Gastrointest Oncol 2017; 9(3): 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giorlandino A, Caltabiano R, Gurrera A, et al. Gastrointestinal stromal tumour of the stomach with osseous differentiation: a case report. Pathologica 2014; 106(4): 345–347. [PubMed] [Google Scholar]
- 4. Greenson JK. Gastrointestinal stromal tumors and other mesenchymal lesions of the gut. Modern Pathol 2003; 16(4): 366–375. [DOI] [PubMed] [Google Scholar]
- 5. Miettinen M, Lasota J. Succinate dehydrogenase deficient gastrointestinal stromal tumors (GISTs)—a review. Int J Biochem Cell Biol 2014; 53: 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miettinen M, Makhlouf H, Sobin LH, et al. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol 2006; 30(4): 477–489. [DOI] [PubMed] [Google Scholar]
- 7. Kim HS, Sung JY, Park WS, et al. Gastrointestinal stromal tumors of the stomach with extensive calcification: report of two cases. Intern Med 2012; 51(18): 2555–2558. [DOI] [PubMed] [Google Scholar]
- 8. Izawa N, Sawada T, Abiko R, et al. Gastrointestinal stromal tumor presenting with prominent calcification. World J Gastroenterol 2012; 18(39): 5645–5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshida H, Mamada Y, Taniai N, et al. Spurt bleeding from a calcificated gastrointestinal stromal tumor in the stomach. J Nippon Med Sch 2005; 72(5): 304–307. [DOI] [PubMed] [Google Scholar]
- 10. Ichiishi E, Kogawa T, Takeda S, et al. Eight cases of gastric tumors with calcification. Intern Med 1995; 34(10): 1038–1042. [DOI] [PubMed] [Google Scholar]
- 11. Huang RS, Brown RE, Buryanek J. Heterotopic ossification in metastatic colorectal carcinoma: case report with morphoproteomic insights into the histogenesis. Ann Clin Lab Sci 2014; 44(1): 99–103. [PubMed] [Google Scholar]
- 12. Komai Y, Morimoto S, Saito K, et al. Possible involvement of bone morphogenetic protein 2 in heterotopic ossification in metastatic lesion from urothelial carcinoma of bladder. Int J Urol 2006; 13(8): 1126–1128. [DOI] [PubMed] [Google Scholar]