Abstract
The present study was proposed to elucidate the effect of Commiphora mukul gum resin elthanolic extract treatment on alterations in carbohydrate and lipid metabolisms in rats fed with high-fructose diet. Male Wistar rats were divided into four groups: two of these groups (group C and C+CM) were fed with standard pellet diet and the other two groups (group F and F+CM) were fed with high fructose (66 %) diet. C. mukul suspension in 5% Tween-80 in distilled water (200 mg/kg body weight/day) was administered orally to group C+CM and group F+CM. At the end of 60-day experimental period, biochemical parameters related to carbohydrate and lipid metabolisms were assayed. C. mukul treatment completely prevented the fructose-induced increased body weight, hyperglycemia, and hypertriglyceridemia. Hyperinsulinemia and insulin resistance observed in group F decreased significantly with C. mukul treatment in group F+CM. The alterations observed in the activities of enzymes of carbohydrate and lipid metabolisms and contents of hepatic tissue lipids in group F rats were significantly restored to near normal values by C. mukul treatment in group F+CM. In conclusion, our study demonstrated that C. mukul treatment is effective in preventing fructose-induced insulin resistance and hypertriglyceridemia while attenuating the fructose induced alterations in carbohydrate and lipid metabolisms by the extract which was further supported by histopathological results from liver samples which showed regeneration of the hepatocytes. This study suggests that the plant can be used as an adjuvant for the prevention and/or management of insulin resistance and disorders related to it.
Keywords: Commiphora mukul, Glycolytic enzymes, High fructose diet, Insulin resistance, Lipid metabolic enzymes
Graphical abstract
1. Introduction
The last 25 years have witnessed a marked increase in total per capita fructose intake as a sweetener in the food industry, primarily in the form of sucrose (a disaccharide consisting of 50% fructose) and high-fructose corn syrup (HFCS; 55–90% fructose content).1 Processed-food manufacturers often prefer HFCS to sucrose because it is inexpensive, sweeter and mixes well in many foods. The increase in HFCS consumption far exceeds the increases in intake of any other food or food group. The disturbing fact is fructose consumption (excluding that which occurs naturally in fruits and vegetables) increased from less than 0.5 g/day in 1970 to more than 40 g/day in 1997 (more than 80-fold increase).2
In humans, high fructose in diets reduces insulin sensitivity3 and elevates plasma triglycerides in both fed and fasting conditions.4 Diets high in fructose cause multiple symptoms of metabolic syndrome such as insulin resistance5 impaired glucose tolerance,6 hyperinsulinemia, hypertension,7 and hypertriglyceridemia.6 Thus, high consumption of dietary fructose, primarily from sucrose and high-fructose corn syrup, has been implicated as a contributing factor to the development of obesity and accompanying metabolic abnormalities such as insulin resistance and hypertriglyceridemia. The prevalence of metabolic syndrome has dramatically increased worldwide due to modern lifestyle and increase of consumption of high-sugar diets especially fructose.8 Animal studies revealed that high fructose diet-fed rats display hepatic oxidative damage and altered lipid metabolism due to hepatic stress as a result of the burden of fructose metabolism.9
Liver is an insulin dependent tissue, which plays a pivotal role in glucose and lipid homeostasis and is severely affected during Diabetes mellitus. It has been shown to be decreasing the activities of enzymes in the glycolytic and pentose phosphate pathways, while increasing the activities of gluconeogenic and glycogenolytic pathways.10 Several plant extracts are known to have antidiabetic properties and a large number of compounds from plant extracts have been reported to have beneficial effects for the treatment of diabetes mellitus.11 The WHO Expert Committee recommended the importance of investigating the hypoglycemic agents from plant origin, which were used in traditional medicine for the treatment of diabetes mellitus.12 The antihyperglycemic agents were focused on plants used for the traditional medicine because they may be a better treatment than currently used synthetic drugs.13
Insulin sensitizers and antioxidants are found to be effective in preventing not all but at least a majority of the abnormalities induced by fructose.14, 15 Current treatments with thiazolidinediones and particularly metformin does not adequately address the issue of IR.16 Hence, there is a need to search for new agents with better efficacy and minimal side effects. Plants and herbs are mines of a large number of bioactive phytochemicals that might serve as leads for the development of effective, safe, cheap novel drugs.
Commiphora mukul (family Burseraceae) commonly called as gum guggulu is highly valued in Ayurveda, an Indian system of medicine practised in India, Bangladesh and Pakistan. The gum resin extract of C. mukul tree has been used in Ayurvedic medicine for more than 2000 years to treat a variety of ailments like obesity, lipid disorders, rheumatoid arthritis,17 bone fracture, cardiovascular disorder disease18 and antihyperglycemic and antioxidant activities.19
The oleoresin secreted by this plant, known as guggul, is a yellowish substance with balsamic odor. Traditional (India) uses of C. mukul (CM) are anti-inflammatory, antispasmodic, carminative, emmenagogue, hypoglycemic, alternative, antiseptic, and astringent, a thyroid stimulant, anthelminitic and antihyperlipidemia properties. It is an important herb used in the treatment of several degenerative disorders in modern medicine too and established as a hypolipidemic drug.20 Ayurvedic medicines containing gum guggul often contain a guggul in their names, such as in Shunthi-guggul, and Yogaraja guggul. All the formations used for traditional Ayurvedic treatments for obesity contained gum guggul among its herbal ingredients. Guggulipid, an ethyl acetate extract of the resin of plant C. mukul is an established hypolilpidemic agent. Various studies have been conducted to understand and illustrate the mechanism of action and potential of guggulsterone as a therapeutic agent using synthetic E and Z isomers.21
Guggulsterones inhibited cholesterol synthesis in the liver via antagonism of the forsenoid X receptor and the bile acid receptor.22 A number of clinical trials have been conducted to evaluate the hypolipidemic effect of guggulipid. Most of these studies were carried out in India and one in the United States. Consistent with the preclinical data, most of these studies demonstrated hypolipidemic activity of guggul or gugulipid with an average of 10.30% and 10.20% decrease in total cholesterol and triglycerides, respectively. With proven hypolipidemic efficacy in rats, guggulsterone was used as a positive control to assess the hypolipidemic activity of other chemical compounds.23 C. mukul and guggulsterone were effective antioxidants against LDL oxidation.24 Hepatic microsomal lipid peroxidation was also significantly reduced by guggulipid. In addition, guggulipid significantly reversed the cardiac damage and biochemical changes induced by isoproterenol.25 Till date, several chemical components such as diterpenes, sterols, steroids, esters and higher alcohols were identified in C. mukul.24 No significant side effects were observed on renal and liver functions, hematological parameters, and electrolytes.26 Such a safety profile is consistent with its long history of use in Ayurvedic medicine and practice. Our previous work suggested that C. mukul gum resin ethanolic extract has antidiabetic and antioxidant activities in – streptozotocin (STZ)-induced insulin-dependent diabetes mellitus and fructose-fed induced insulin resistance in animals.19, 27 High-fructose diets that induce hypertriglyceridemia, insulin resistance, and hypertension in Wistar rats are the established models of fructose-induced insulin resistance and hypertriglyceridemia.28 We are currently experimenting with the fructose-enriched diet (FED) rat model, and aim of the present work is characterizing its efficacy against fructose-induced alterations in carbohydrate and lipid metabolisms by assessing the activities of key regulatory enzymes in liver and adipose tissues.
2. Material and methods
2.1. Chemicals
Acetyl Co-A, malonyl Co-A, pyruvate kinase, lactate dehydrogenase, and glucose-6-phosphate dehydrogenase were obtained from the Sigma Chemical Co., St. Louis, MO, USA. All other chemicals and solvents were of analytical grade and procured from Sisco Research Laboratories (P) Ltd., Mumbai, India.
2.2. Plant material
Ethanolic extract of C. mukul gum resin (CM; brown, dry powder with Lot No. L5111031) was obtained from the manufacturers and exporters of herbal extracts, Ms Plantex Pvt. Ltd., Vijayawada, Andhra Pradesh, India. Procedure followed by the firm for the preparation of extract is as follows: the plant was identified by Dr. K. Narasimha Reddy, Taxonomist, Lailaimpex R & D Center, Vijayawada. The collected plant sample (resin) was washed thoroughly with tap water, dried at room temperature away from sun light, cut into small pieces, and then powdered. Ethanolic extract was prepared by cold maceration of gum resin powder in ethanol for 7 days. The extract was filtered, concentrated under reduced pressure and finally dried in vacuum desiccators. Herb-to-product ratio was 8:1. A voucher specimen has been deposited in the Department of Biochemistry, Sri Krishnadevaraya University, Anantapur, under number DSK-CM-09. The extract was stored at 0–4 °C and dissolved in water just before use.
2.3. Animals
Male Wistar rats weighing 140–160 g were procured from Sri Venkateswara Enterprises (Bangalore, India), acclimatized for 7 days in our animal house (Regd. no. 470/01/a/CPCSEA) before dietary manipulation. Animals were housed two per cage in an air-conditioned room (22 ± 2 °C) with 12 h light/dark cycle and had free access to standard pellet diet and water. All the procedures were performed in accordance with the Institutional Animal Ethics Committee.
2.4. Control and high fructose diet
The control diet for the rats contained 66% starch, 15% protein, 8% fat, 4% cellulose, 3.5% AIN 93 mineral mix, 1% AIN 93 vitamin mix, and 0.3% l-cystine. The fructose diet contained 66% of fructose instead of starch and the remaining composition was the same as that of control diet. Both of these diets were obtained from the National Centre for Laboratory Animal Sciences, National Institute of Nutrition (Hyderabad, India).
2.5. Animal model and experimental design
All the animals were of 7 weeks age, weighing around 185 g at the time of dietary manipulation. They were randomly assigned into four groups of eight each: Control (C); Control rats treated with C. mukul gum resin ethanol extract (C + CM); high fructose fed rats (F); and fructose fed rats treated with C. mukul gum resin ethanol extract (F + CM). C and C + CM group animals received standard pellet diet whereas F and F + CM groups received high fructose diet. F + CM and C + CM animals received C. mukul gum resin ethanol extract suspension (200 mg/kg body weight) in 2 ml of 5% Tween 80 daily for 60 days through orogastric tube, whereas, 2 ml of 5% tween 80 was administered to C and F group rats. The dose of C. mukul gum resin ethanolic extract in the current study is based on the preliminary experiments on dose-dependent antihyperglycemic effect in STZ-induced diabetic rats.19 At the end of experimental period of 60 days, 12-h fasted animals were sacrificed by cervical decapitation. Liver, Heart, Muscle and visceral adipose tissues were collected.
2.6. Biochemical analysis
At the time of sacrifice, blood was collected by cardiac puncture from all the rats in each group. Plasma glucose was estimated by the glucose oxidase-peroxidase method by using the Span diagnostic kit (Span Diagnostics Ltd., Surat, India) Plasma triglycerides (TG) were estimated by enzymatic colorimetric end-point methods using the Span diagnostic reagent kit. Plasma insulin was estimated by using the radioimmunoassay kit (RIA K-1) from Bhabha Atomic Research Center (Mumbai, India) according to the method of Yallow and Berson.29 Homeostasis model assessment (HOMA), used as an index to measure the degree of IR, was calculated by the formula: insulin (μU/ml) X glucose (mmol/l)/22.5.30
Liver, kidney heart and visceral adipose samples were taken from all the animals. The extraction of lipids from liver and kidney was carried out according to the procedure of Folch et al, 1957.31 Total cholesterol (TC), triglycerides (TG) (Span diagnostic kit, Surat, India), free fatty acids (FFA)32 (Duncombe, 1963) and phospholipids33 (Connerty et al), were analyzed. Hepatic glycogen content was determined by using anthrone reagent as adapted by Carrol et al.34
2.7. Assay of carbohydrate and lipid metabolic enzymes
Liver and kidney homogenates (10%) were prepared in 0.1 M Tris–HCl buffer, pH7.4 and centrifuged at 12,000 rpm for 45 min. The supernatant was collected and protein content in the supernatant was estimated by the method of Lowry et al35 and the same supernatant was used for the assay of hexokinase (Brandstrup et al),36 Phosphofructokinase (Sadava et al),37 pyruvate kinase37 (Sadava et al), glucose-6-phosphotase,38 King, fructose-1,6-bisphosphotase37 (Sadava et al), glycogen phosphorylase39 (Sutherland, 1995), glucose-6-phosphate dehydrogenase (Beutler, 1975),40 fructokinase (Adelman et al),41 and malic enzymes (Geer et al)42 were also assayed. For the fatty acid synthetase, the tissue was homogenized at 4 °C in 0.1 M potassium phosphate buffer, pH8.0. The homogenate was centrifuged at 30,000 rpm at 4 °C for 30 min and the clear supernatant collected was used for the assay of fatty acid synthetase (Gibson and Hubbard).43 Assay of lipoprotein lipase (LPL) in adipose tissue was done following the method of Shirai and Jackson.44
2.8. Histological studies
Histopathological parameters were studied at the Department of Pathology, S.V. Veterinary University, Tirupati, A.P, India. The tissues were washed, dehydrated with alcohol, cleared with xylene and paraffin blocks were made. Serial sections of 5 um thickness was cut by using a rotary microtone. The sections were then deparaffinised with xylene and hydrated in descending grades of alcohol. The slides were then transferred to haematoxylin for 10 min, followed by rinsing with water, differentiated in 1% acid alcohol, rinsed in water, bleuing in running tap water or 1% lithium carbonate. Later they were counter stained with eosin, rinsed with water, dehydrated with ascending grades of alcohol, cleared with xylene and mounted on glass slides. These slides were examined under 10X and 40X using microscope.
The histopathological studies of the liver tissue was conducted to know the alterations under fructose fed conditions and to study the protection/harmful effects of C. mukul administration in metabolic syndrome rat model and normal rats.
2.9. Statistical analysis
Data were expressed as the mean ± SE for the number, n = 8 of animals in the group as indicated in (Table 1, Table 2, Table 3, Table 4). The data were subjected to statistical analysis by Duncan's Multiple Range (DMR) test (Duncan, 1955).45 Values of p < 0.05 were considered statistically significant.
Table 1.
Body weight and levels of fasting plasma glucose, triglycerides, insulin and insulin sensitivity index of rats at the end of 60 days.
| Parameter | C | C + CM | F | F + CM |
|---|---|---|---|---|
| Body weight (g) | 282.84 ± 1.82a | 279.88 ± 1.63a | 306.48 ± 2.32b | 284.84 ± 2.46a |
| Blood glucose (mg/dl) | 78.48 ± 1.24a | 80.28 ± 1.46a | 118.84 ± 2.12b | 81.54 ± 0.84a |
| Insulin (μU/ml) | 42.85 ± 1.82a | 40.88 ± 1.35a | 102.66 ± 2.24b | 62.82 ± 0.86c |
| Insulin sensitivity index (HOMA-IR) | 9.42 ± 0.406a | 8.86 ± 0.420a | 28.68 ± 1.234b | 11.48 ± 0.248c |
| Triglycerides (mg/dl) | 76.84 ± 0.98a | 72.46 ± 1.63a | 148.24 ± 2.86b | 78.96 ± 1.84c |
C: Control diet, C + CM: Control diet plus CM treatment, F; high fructose diet, F + CM: high-fructose diet plus CM treatment.
Values not sharing a common superscript letter differ significantly at p < 0.01 (DMRT).
Table 2.
Effect of high fructose diet and C. mukul gum resin treatment on glycogen, glycolytic enzymes of liver and muscle of rats.
| Parameter | Tissue | C | C + CM | F | F + CM |
|---|---|---|---|---|---|
| Hexokinase (μmol of G6P formed/mi/mg protein) | Liver | 4.31 ± 0.069a | 4.44 ± 0.067a | 2.88 ± 0.09b | 4.1 ± 0.059a |
| Muscle | 4.79 ± 0.095a | 4.9 ± 0.12a | 3.10 ± 0.03b | 4.85 ± 0.10a | |
| Phosphofructokinase (μmol of F16Bis phosphate formed/mi/mg protein) | Liver | 3.81 ± 0.06a | 3.73 ± 0.036a | 2.75 ± 0.06c | 3.31 ± 0.23a |
| Muscle | 4.25 ± 0.07a | 4.23 ± 0.069a | 2.74 ± 0.10b | 4.32 ± 0.06a | |
| Pyruvate kinase (μmol of NADH oxidized/min/mg protein) | Liver | 2.64 ± 0.07a | 2.7 ± 0.05a | 3.72 ± 0.18c | 2.8 ± 0.04a |
| Muscle | 2.56 ± 0.04a | 2.63 ± 0.07a | 3.88 ± 0.09c | 2.94 ± 0.01a | |
| F1, 6BPase (nmol of F6P formed/min/mg protein) | Liver | 0.41 ± 0.005a | 0.44 ± 0.0329a | 0.56 ± 0.026c | 0.41 ± 0.006a |
| kidney | 0.13 ± 0.005a | 0.14 ± 0.01a | 0.17 ± 0.005b | 0.13 ± 0.014a | |
| G6Pase (nmol of Pi formed/mi/mg protein) | Liver | 21.83 ± 0.58a | 21.93 ± 0.424a | 34.45 ± 1.00b | 22.66 ± 0.29a |
| kidney | 18.16 ± 0.86a | 18.9 ± 0.56a | 21.50 ± 0.70b | 19.28 ± 0.21a | |
| Glycogen phosphorylase (μmol of Pi formed/min/mg protein) | Liver | 0.13 ± 0.0045a | 0.14 ± 0.0036a | 0.08 ± 0.002b | 0.116 ± 0.0063c |
C: Control diet, C + CM: Control diet plus CM treatment, F; high fructose diet, F + CM: high-fructose diet plus CM treatment.
Values not sharing a common superscript letter differ significantly at p < 0.01 (DMRT).
Table 3.
Effect of high fructose diet and C. mukul gum resin treatment on liver and adipose tissues enzymes of rats.
| Parameter | Tissue | CON | CON + CMEE | FRU | FRU + CMEE |
|---|---|---|---|---|---|
| Malic enzyme (μmol of NADP reduced/min/mg protein) | Liver | 6.16 ± 0.33a | 6.26 ± 0.17a | 7.9 ± 0.14b | 6.7 ± 0.09c |
| Fatty acid synthase (μmol of NADPH utilized/min/mg protein) | Liver | 0.49 ± 0.02a | 0.48 ± 0.02a | 0.69 ± 0.01b | 0.52 ± 0.01a |
| Lipoprotein lipase (μmol of PNP/min/mg protein) | Adipose | 8.54 ± 0.03a | 8.46 ± 0.11a | 7.16 ± 0.17 b | 9.24 ± 0.03c |
C: Control diet, C + CM: Control diet plus CM treatment, F; high fructose diet, F + CM: high-fructose diet plus CM treatment.
Values not sharing a common superscript letter differ significantly at p < 0.01 (DMRT).
Table 4.
Effect of high fructose diet and C. mukul gum resin treatment on hepatic and cardiac tissue lipids of rats.
| Parameter | Tissue | C | C + CM | F | F + CM |
|---|---|---|---|---|---|
| TC mg/100 g tissue | Liver | 150.88 ± 2.04a | 136.53 ± 3.05b | 186.66 ± 2.31c | 152.93 ± 3.69a |
| Heart | 64.67 ± 1.89a | 58.03 ± 1.52b | 89.45 ± 1.97c | 66.56 ± 1.53a | |
| TG mg/100 g tissue | Liver | 154.66 ± 1.74a | 146.20 ± 1.31b | 182.20 ± 2.29c | 154.51 ± 2.25a |
| Heart | 69.88 ± 0.97a | 62.58 ± 1.02b | 84.94 ± 1.38c | 71.81 ± 1.46a | |
| PLP μg/100 g tissue | Liver | 889.3 ± 12.01a | 843.83 ± 13.43b | 1036.5 ± 15.41c | 896.0 ± 10.90a |
| Heart | 449.0 ± 11.01a | 418.8 ± 10.07a | 736.0 ± 6.59c | 436.5 ± 10.93a | |
| FFA mg/100 g tissue | Liver | 210.20 ± 3.64a | 201.65 ± 3.05a | 290.75 ± 4.57c | 212.23 ± 4.16a |
| Heart | 124.86 ± 2.39a | 109.85 ± 1.98b | 192.11 ± 3.06c | 129.60 ± 4.35a |
C: Control diet, C + CM: Control diet plus CM treatment, F; high fructose diet, F + CM: high-fructose diet plus CM treatment.
Values not sharing a common superscript letter differ significantly at p < 0.01 (DMRT).
3. Results
The following general observations were made in the study: In the present experiments, none of the animals chronically treated with C. mukul died or no visible side effects (weight loss and edema) and variations in animal behavior (respiratory distress, abnormal locomotion, and catalepsy) were observed in group F from one week onwards till the experimental period compared to group C.
Table 1 presents the data on body weight, plasma glucose, triglycerides, insulin and insulin sensitivity index (HOMA). Body weight, plasma glucose, insulin, and HOMA values at different intervals of time (day 0, 15, 30, 45 and 60) for these experimental groups were reported previously.27 At the end of experimental period, body weight, plasma glucose, insulin, HOMA and triglyceride levels were significantly higher in the fructose-fed rats as compared to those in the control rats. IR in the fructose-fed rats was indicated by higher HOMA values as compared to the control rats. C. mukul treatment completely prevented the fructose-induced increased body weight, hyperglycemia, and hypertriglyceridemia. Hyperinsulinemia and insulin resistance observed in group F were significantly decreased with C. mukul treatment in group F + CM.
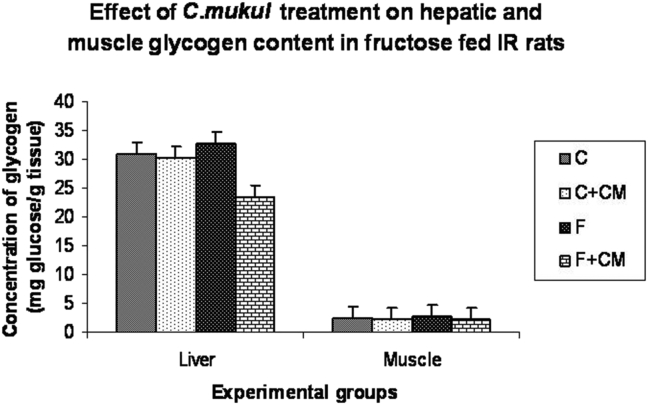
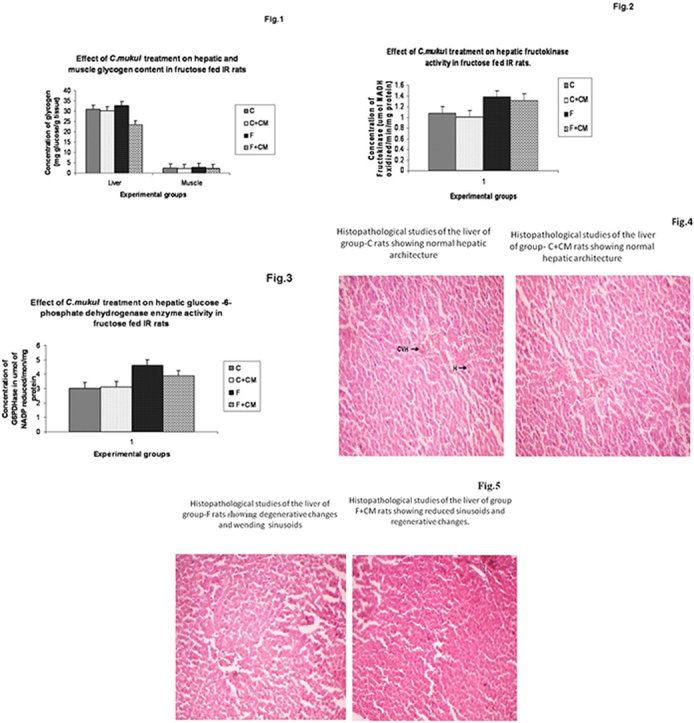
Fig. 1 summarizes the liver and muscle glycogen levels in different experimental groups. Group F showed significantly enhanced glycogen content in liver and muscle (12.8, 13.0%) when compared to group C. C. mukul administration for 60 days to the fructose-fed rats (group F + CM) resulted in a significant decrease in a glycogen content of liver and muscle (28.4, 16.0%) when compared to group F.
Fig. 1.
Change in glycogen content of C, C + CM, F and F + CM groups during the 60 days experimental period. Values are ± S.E., (n = 8 animals).
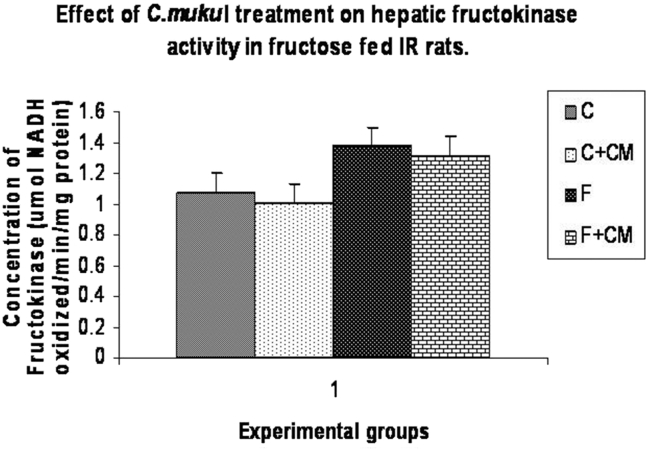
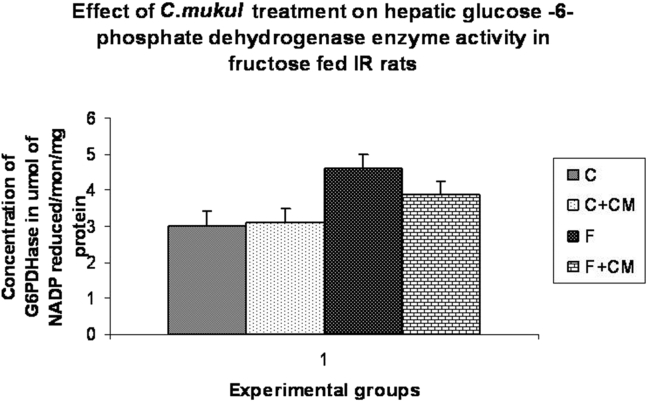
Table 2, Fig. 2, Fig. 3 show activities of enzymes of carbohydrate metabolism in liver, muscle and kidney. Group F showed significantly lower activities of hepatic and muscle hexokinase (33.6%, 35.2%) phosphofructokinase (27.8%,35.5%), hepatic glycogen phosphorylase (38.4%) and higher activities of liver and muscle pyruvate kinase (40.9%,51.5%), hepatic fructokinase (27.7%), hepatic glucose-6-phosphate dehydrogenase (53.0%), liver and kidney glucose-6-phosphatase (57.8%,19.4%) and fructose 1,6 bis-phosphatase (36.5%), (30.7%) as compared to group C.
Fig. 2.
Change in fructokinase activity of C, C + CM, F and F + CM groups during the 60 days experimental period. Values are ± S.E., (n = 8 animals).
Fig. 3.
Change in glucose-6, phosphate dehydrogenase activity of C, C + CM, F and F + CM groups during the 60 days experimental period. Values are ± S.E., (n = 8 animals).
C. mukul treatment to the fructose fed rats (group F + CM) limited the fall in activities of hepatic and muscle hexokinase to 5.0%, 1.5%, hepatic glycogen phosphorylase to 10.8% and glucose 6 phosphate dehydrogenase to 29.0%, liver and kidney glucose-6-phosphatase to 4.0%, 6.5% fructose-1, 6-bis phosphatase activities near to normal value as compared with group C. Fructokinase activity in group F + CM was significantly lower than in group F, but still significantly higher than the group C.
The data presented in Table 3 pertain to activity of hepatic malic enzyme and FAS of four experimental groups. F-group showed a significantly enhanced activity of hepatic malilc enzyme (28.2%) and FAS (40.8%) when compared to C-group. C. mukul administration to fructose fed rats (F + CM group) resulted in significant decrease in hepatic malic enzyme (15.1%) and FAS (24.6%) activities when compared to F-group. A significantly decreased activity (16.1%) of LPL of adipose tissue was observed in fructose fed rats (F-group) when compared to C-group. The F + CM group showed significantly enhanced adipose LPL activity when compared to F and C-group.
The data presented in the Table 4 reveal the hepatic and cardiac tissue TC, TG, PLP and FFA of the four experimental groups. The data revealed that liver tissue contains higher proportions of lipid fractions than heart. All fractions of tissue lipids are significantly enhanced in F group compared to C-group. When compared to C-group, the per cent increase in hepatic and cardiac TC, TG, PLP and FFA are 23.7, 18.2, 16.5, 38.4% and 38.3, 21.6, 63.9, 54.8% respectively in F-group. C. mukul administration for 60 days restored the tissue lipid fractions to the normal value in insulin resistance rats (F + CM).
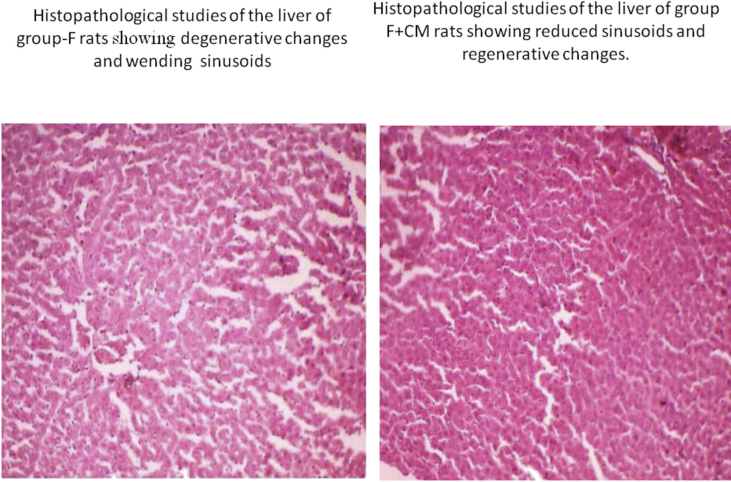
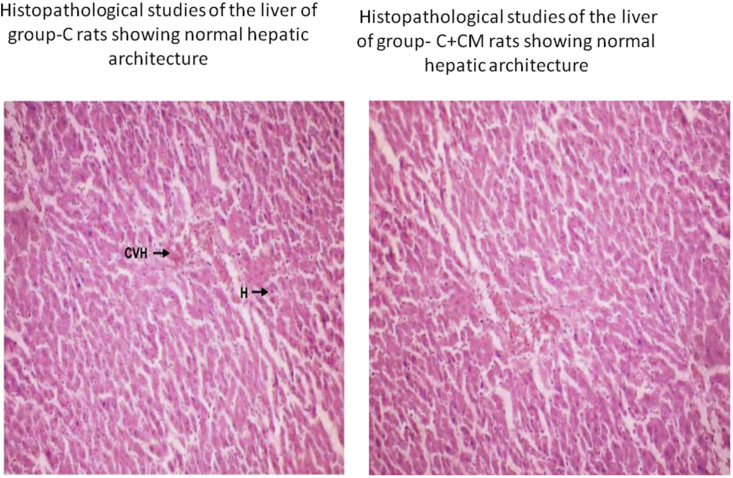
Sections of liver tissue from C. mukul treated rats showed a cytoprotective effect by these phytochemicals. A marginal reduction in the size of pancreatic β-cells in high fructose diet fed rats was observed (Fig. 5) which was normalized in rats treated with C. mukul (Fig. 5) almost to the level of control rats Fig. 4 showed marks of severe steatosis i.e., accumulation of lipid droplets which was evident in the form of vacuoles (Fig. 5) as compared to the control animal liver (Fig. 4). However, treatment with C. mukul not only reversed this deformity but at the same time did not show any signs of toxicological effects as compared to the control group (Fig. 4).
Fig. 5.
Histopathological studies of the liver of group-F rats showing degenerative changes and wending sinusoids. Histopathological studies of the liver of group-F + CM rats showing reduced sinusoids and regenerative changes.
Fig. 4.
Histopathological studies of the liver of group-C and C + CM rats showing normal hepatic architecture.
4. Discussion
Insulin resistance as a widespread feature of atherogenic diseased predisposes the affected individuals to various diseases including weight gain, hypertension, obesity, cardiovascular disease, hypertriglyceridemia and type 2 diabetes mellitus.46 Lowering endogenous insulin levels is a key step to successful therapy directed at insulin resistance related diseases.47
Fructose feed administration to human also causes features of metabolic syndrome.48 The development of insulin resistance in fructose fed rats is well documented in the literature49 and was also established in our laboratory.27 Results of the current study showed that high fructose feeding to rats for 60 days leads to fasting hyperglycemia, hypertriglyceridemia, hyperinsulinemaia, glucose intolerance and impaired antioxidant potential leading to the development of insulin resistance. Further, fructose-induced insulin resistant animal model has been recommended for assessing the therapeutic efficacy of insulin sensitizers and drugs that are likely to be have an effect on insulin sensitivity. Hence this model was selected to study the efficacy of C. mukul gum resin ethanol extract in preventing the insulin resistance.
In the present study, the fructose-fed animals showed significantly enhanced plasma glucose, insulin, and IR as reflected by HOMA values. It has been demonstrated that fructose does not directly promote insulin secretion from pancreatic cells, which is necessary for glucose metabolism. However, glucose produced as a result of fructose metabolism stimulates insulin release, but the fructose-induced insulin resistance prevents the insulin from effectively metabolizing glucose resulting in hyperglycemia. In addition, insulin resistance also led to compensatory hyperinsulinemia, where the body attempts to balance the reduced effect of insulin by producing and releasing more insulin which leads to hyperinsulinemia.50 Increased plasma triglyceride levels in the fructose-fed rats have been already reported by many including this study. Increased triglyceride with fructose is due to increased hepatic lipogenesis, overproduction of very low-density lipoprotein (VLDL), and impairment in their peripheral catabolism.51 Administration with C. mukul prevented fructose-induced hyperglycemia, hyperinsulinemia, hypertriglyceridemia, and insulin resistance. In the present experiments, none of the animals chronically treated with C. mukul showed behavioral changes. In fact, these animals showed a general tendency to be healthier with unaltered parameters of carbohydrate and lipid metabolisms when compared with group C.
The data presented in the Table 2 and Fig. 2, Fig. 3 clearly indicate the beneficial effects of C. mukul in normalizing the decreased glycolytic enzyme activities both in liver and muscle and in rectifying the decreased utilization of glucose for energy production under diabetic condition. Studies of Weber and Convery,52 indicated that insulin administration restored the decreased glycolytic enzyme activities of diabetic animals. Insulin upregulates the transcription of glucokinase, phosphofructokinase, and pyruvate kinase genes.53
The development of insulin resistance in fructose fed rats as also reported in the present study is well documented in the literature. Defects in post-receptor events in insulin signaling54 and in enzymes involved in glucose metabolism55 were reported. The hyperglycemia prevailing in fructose fed conditions may lead to increased uptake of glucose into hepatocytes through GLUT-2, an insulin independent glucose transporter. In addition, decreased operation of glycolysis is also responsible for the enhancement of the cellular glucose concentration in the liver. High intracellular glucose exerts toxic effects on structure and function of organs and induces insulin resistance, a phenomenon referred to as glucose toxicity as reported in skeletal muscle of diabetic rats.56 In the span of glycolytic reactions from glyceraldehyde-3-phosphate to pyruvate and lactate, the rate-controlling step is catalyzed by pyruvate kinase.
C. mukul administration to fructose fed rats prevented the fructose feed induced decrease in HK and PFK activity and increase in PK activity in muscle and liver. The current results of glycolytic enzymes activities represent the beneficial effects of C. mukul treatment in rectifying the insulin resistance of hepatic level and correcting the impairment in the insulin mediated glucose transport observed in the muscle tissue of fructose fed rats.
Insulin plays a direct role in the control of liver glycogen metabolism by the regulation of the glycogenesis which controls this process by regulating the conversion of a glucose-6-phosphate-dependent form into a glucose-6-phosphate-independent form of glycogen synthase.57, 58 Basal amounts of insulin inhibit glycogenolysis by about 60%. Glycogen levels in various tissues especially in skeletal muscle is a direct reflection of insulin activity as insulin promotes intracellular glycogen deposition by stimulating glycogen synthase and inhibiting glycogen phosphorylase.59 Our results of enhanced glycogen content in fructose fed rats are in accordance with the earlier reports.60 However, a few contradictory observations of decreased hepatic glycogen content in fructose fed rats also appeared in literature.61 When compared with glucose, fructose is most predominantly utilized by skeletal muscle. Fructose appears to be more readily incorporated into glycogen. After an intravenous infusion, fructose has been demonstrated to disappear from the blood stream more rapidly than glucose and leads to a greater production of lactate.62 In man, skeletal muscle glycogen content has been reported to increase in response to fructose infusion.63
In agreement with our studies guggulsterone administration has rectified the decreased hepatic and skeletal muscle glycogen content high fat diet induced type II diabetic rats.64 C. mukul treatment for 60 days prevented the fructose feeding induced enhancement in the glycogen content of liver and muscle as observed in F + CM-group. In the present study, the enhanced hepatic glycogen stores in fructose fed rats is due to the the observed decrease in the activity of glycogen phosphorylase, which can be explained by enhanced production of fructose-1-phosphate and increased activity of fructokinase observed in these animals.
Previous investigations using magnetic-resonance spectroscopy confirmed the accumulation of fructose-1-phosphate in the human liver after intravenous administration of fructose.65 Although most of these effects were obtained by using physiological concentrations of fructose, experiments with isolated hepatocytes using graded concentrations of fructose demonstrated that significant elevations of fructose-1-phosphate occurred at concentrations of 0.5 and 1.0 mmol fructose/L which are available in the portal vein in vivo after a fructose meal.66 Further, inhibition of glycogen phoshorylase and activation of glycogen synthase by increased concentration of glucose-6-phosphate may also be possible because of enhanced operation of gluconeogenesis in fructose fed animals. Thus conversion of fructose to liver glycogen is increased because of enzyme adaptation. Correction of enhanced glycogen content by fructose feeding in C. mukul treated fructose fed rats can be explained by the observed significant enhancement in glycogen phosphorylase activity of F + CM-group compared to F-group.
The increased activities of hepatic enzymes related to lipogenesis viz., fatty acid synthetase, malic enzyme, glucose-6-phosphate dehydrogenase, and decreased activities of these enzymes in adipose tissue suggest that a diet containing high percentage of fructose causes a shift in the site of lipid synthesis from adipose tissue to liver.67 The activity of malic enzyme, particularly high in lipogenic tissues, is elevated in conditions favoring fatty acid synthesis. Further, increased concentrations of hepatic total lipids, total cholesterol, triglycerides, and free fatty acids in group F as compared to group C clearly indicate the state of increased lipid synthesis in the liver of group F.
Administration of C. mukul at a dose of 200 mg/kg/day was found to be effective in preventing the adverse effects of high fructose feeding. Hyperglycemia, hypertriglyceridemia, hyperinsulinemia, and IR observed in group F were completely prevented in group F + CM. Further, the treatment also prevented alterations in the activities of carbohydrate and lipid metabolizing enzymes when administered along with high fructose feed for 60 days. Hepatic total lipids, cholesterol, triglycerides, and free fatty acids were significantly lower as compared to group F. The activity of hepatic fructokinase of group F + CM was significantly lower than that of group F, but still significantly higher than group C. The reason for this increased activity compared to group C might be due to high intake of fructose. The decreased activity of fructokinase as compared to group F may be due to decrease in intake of diet or influence on activity of the enzyme or both by the plant extract. Preservation of insulin action in group F + CM could be responsible for the regulation of lipid metabolism. However, studies showed that C. mukul might also have a direct role in lipid metabolism. For example, antihyperglycemic and antioxidant action of this plant in STZ induced diabetic rats.19
The presence of wending sinusoid spaces and degenerative changes were observed in hepatocytes of fructose fed rats in the present study reflecting the oxidative stress caused by fructose fed diet. Increased oxidative stress can initiate lipid peroxidation which inturn stimulates glycation of protein, inactivation of enzymes and alterations in the structure and function of collagen and other basement membranes and play a role in cellular destruction.68 In our present study, hyperlipidemia was observed in the liver of group-F animals which reflects an elevated lipid peroxidation and excessive formation of ROS. The observed elevation of transaminase activities in liver of group-F animals is an indication of increased damage of hepatocytes. C. mukul treatment caused the major reversal of degenerative changes in hepatocytes and reduction in wending sinusoid spaces which are induced by high fructose diet. The hepatoprotective activity of C. mukul clearly indicates that it attenuates the excessive formation of ROS. Thus though the exact mechanism of hepatoprotective action of C. mukul is not yet known, its antioxidant activity as revealed in some earlier studies seems to be the most important mode of its hepatoprotective action. In addition hepatoprotective activity of C. mukul was also reported in STZ induced diabetic rats.19
5. Conclusion
C. mukul gum resin ethanolic extract is capable of averting high fructose diet induced insulin resistance and alterations in carbohydrate and lipid metabolisms. In conclusion, C. mukul gum resin ethanolic extract would seem useful as an adjuvant for the prevention and/or management of prediabetic state of insulin resistance and for subjects who need to increase insulin sensitivity. Further comprehensive biochemical and pharmacological investigations are needed to elucidate active principles and identify their molecular mechanism of action.
Conflict of interest
The authors declare that have no conflicts of interest.
Acknowledgement
I wish to express my special thanks to Prof. P.B.B.N Charyulu, Department of Microbiology, Sri Krishnadevaraya University for proofreading of the manuscript. We appreciate the National Centre for Laboratory Animal Sciences, Hyderabad (ICMR) for the kind supply of fructose feed and starch feed for experimental animals.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Bray G.A., Nielsen S.J., Popkin B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 2.Gaby A.R. Adverse effects of dietary fructose. Altern Med Rev. 2005;10:294–306. [PubMed] [Google Scholar]
- 3.Beck-Nielsen H., Pedersen O., Lindskov H.O. Impaired cellular insulin binding and insulin sensitivity induced by high-fructose feeding in normal subjects. Am J Clin Nutr. 1980;33:273–278. doi: 10.1093/ajcn/33.2.273. [DOI] [PubMed] [Google Scholar]
- 4.Bantle J.P., Raatz S.K., Thomas W. Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr. 2000;72:1128–1134. doi: 10.1093/ajcn/72.5.1128. [DOI] [PubMed] [Google Scholar]
- 5.Thorburn A.W., Storlien L.H., Jenkins A.B. Fructose induced in vivo insulin resistance and elevated plasma triglyceride levels in rats. Am J Clin Nutr. 1989;49:1155–1163. doi: 10.1093/ajcn/49.6.1155. [DOI] [PubMed] [Google Scholar]
- 6.Kasim-Karakas S.E., Vriend H., Almario R. Effects of dietary carbohydrates on glucose and lipid metabolism in golden Syrian hamsters. J Lab Lin Med. 1986;128:208–213. doi: 10.1016/s0022-2143(96)90013-x. [DOI] [PubMed] [Google Scholar]
- 7.Hwang I.S., Ho H., Hoffman B.B. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–516. doi: 10.1161/01.hyp.10.5.512. [DOI] [PubMed] [Google Scholar]
- 8.Misra A., Khurana L.J. Obesity and the metabolic syndrome in developing countries. Clin Endocrinol Metab. 2009;93:S9–S30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 9.Kelley G.L., Allan G., Azhar S. High dietary fructose induces a hepatic stress response resulting in cholesterol and lipid dysregulation. Endocrinology. 2004;145:548–555. doi: 10.1210/en.2003-1167. [DOI] [PubMed] [Google Scholar]
- 10.McAnuff M.A., Omoruyi F.O., Morrison S.T. Changes in some liver enzymes in streptozotocin-induced diabetic rats fed sapogenin extract from Bitter yam (Dioscorea polygonoides) or Commercial Diosgenin. West Indian Med J. 2005;54:97–101. doi: 10.1590/s0043-31442005000200002. [DOI] [PubMed] [Google Scholar]
- 11.Anhauser M. Pharmacists seek the solution of a shaman. Drug Discov Today. 2003;88:68–69. doi: 10.1016/s1359-6446(03)02856-3. [DOI] [PubMed] [Google Scholar]
- 12.Alarcon-Aguilera F.J., Roman-Ramos R., Perez-Gutierrez S.J. Study of the antihyperglycemic effect of plants used as antidiabetics. Ethnopharmacology. 1998;6:1101–1110. doi: 10.1016/s0378-8741(98)00020-8. [DOI] [PubMed] [Google Scholar]
- 13.Hu X., Sato J., Oshida Y. Effect of Gosha-jinkigan(Chinese herbal medicine: Niu- e- en-qi-wan) on insulin re-sistance in streptozotocin induced diabetic rats. Diabetes Res Clin Pract. 2003;59:103–111. doi: 10.1016/s0168-8227(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 14.Faure P., Rossini E., Wiernsperger N., Richard M.J., Favier A., Halimi S. An insulin sensitizer improves the free radical defense system potential and insulin sensitivity in high fructose-fed rats. Diabetes. 1999;48:353–357. doi: 10.2337/diabetes.48.2.353. [DOI] [PubMed] [Google Scholar]
- 15.Verma S., Bhanot H., McNeill J.H.J. Antihypertensive effects of metformin in fructose- fed hyperinsulinemic, hypertensive rats. Pharmacol Exp Ther. 1994;1994(27):11334–11347. [PubMed] [Google Scholar]
- 16.Hollenberg N.K. Considerations for management of fluid dynamic issues associated with thiazolidinediones. Am J Med. 2003;115:S111–S115. doi: 10.1016/j.amjmed.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Dev S. A modern look at an age-old Ayurvedic drug Guggul. Sci Agenda. 1987;5:13. [Google Scholar]
- 18.Chander R., Rizvi F., Khanna A.K. Cardioprotective activity of synthetic guggulsterone (E and Z – Isomers) in isoproternol induced myocardial Ischemia in rats: a comparative study. Indian J Clin Biochem. 2003;18:71–79. doi: 10.1007/BF02867370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramesh B., Karuna R., Sreenivasa Reddy S. Antihyperglycemic and antioxidant activities of alcoholic extract of Commiphora mukul gum resin in STZ induced diabetic rats. J Pathophysiol. 2011;2011(18):255–261. doi: 10.1016/j.pathophys.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Ulbricht C., Basch E., Szapary P. Guggul for hypolipidemia: a review by the natural standard research collaboration. Complement Ther Med. 2005;13:279. doi: 10.1016/j.ctim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Ichikawa H., Aggarwal B.B. Guggulsterone inhibits osteoclastogenesis induced by receptor activator of nuclear factor-kB ligand and by tumor cells by suppressing nuclear factor-kB activation. Clin Cancer Res. 2006;12:662–668. doi: 10.1158/1078-0432.CCR-05-1749. [DOI] [PubMed] [Google Scholar]
- 22.Nagarajan M., Waszkuc T.W., Sun J. Simultaneous determination of E- and Zguggulsterones in dietary supplements containing Commiphora mukul extract (guggulipid) by liquid chromatography. JAOAC Int. 2001;84:24–28. [PubMed] [Google Scholar]
- 23.Kumari K., Augusti K.T. Lipid lowering effect of S-methyl cysteine sulfoxide from Allium cepa Linn in high cholesterol diet fed rats. J Ethnopharmacol. 2007;2007(109):367–371. doi: 10.1016/j.jep.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 24.Wang X., Greilberger J., Ledinski G. The hypolipidemic natural product Commiphora mukul and its component guggulsterone inhibit oxidative modification of LDL. Atherosclerosis. 2004;172:239–246. doi: 10.1016/j.atherosclerosis.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Batra S., Srivastava S., Singh K. Syntheses and biological evaluation of 3-substituted amino-1-aryl-6-hydroxy-hex-2-ene-1-ones as antioxidant and hypolipidemic agents. Bioorg Med Chem. 2000;8:2195–2209. doi: 10.1016/s0968-0896(00)00159-0. [DOI] [PubMed] [Google Scholar]
- 26.Szapary P.O., Wolfe M.L., Bloedon L.T. Guggulipid for the treatment of hypercholesterolemia: a randomized controlled trial. JAMA. 2003;290:765–772. doi: 10.1001/jama.290.6.765. [DOI] [PubMed] [Google Scholar]
- 27.Ramesh B., Saralakumari D. Antidiabetic and antioxidant property of Ethanolic extract of Commiphora mukul gum resin on fructose induced insulin resistant rats. J Physiol Biochem. 2012;68:573–582. doi: 10.1007/s13105-012-0175-x. [DOI] [PubMed] [Google Scholar]
- 28.Reddy S.S., Ramatholisamma P., Ramesh B. Beneficiary effect of Tinospora cordifolia against high fructose diet induced abnormali-ties in carbohydrate and lipid metabolism in Wistar rats. 2009;41:741–746. doi: 10.1055/s-0029-1220922. [DOI] [PubMed] [Google Scholar]
- 29.Yalow R.S., Berson S.A. Immunoassay of plasma insulin in man. Diabetes. 1961;10:339. doi: 10.2337/diab.10.5.339. [DOI] [PubMed] [Google Scholar]
- 30.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetoligia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 31.Folch J., Lees M., Solane Stanley G.H. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–597. [PubMed] [Google Scholar]
- 32.Duncombe W.G. The calorimetric micro-determination of long-chain fatty acids. Biochem J. 1963:887–910. doi: 10.1042/bj0880007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Connerty H.V., Briggs A.R., Eaton E.H. Simplified determination of the lipid components of blood serum. J Clin Chem. 1961;73:7–53. [PubMed] [Google Scholar]
- 34.Carrol N.V., Longley R.W., Roe J.H. The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem. 1956;220:583. [PubMed] [Google Scholar]
- 35.Lowry O.H., Rosenbrough N.J., Farr A.L., Randall R.J.J. Protein measurement with the Folins-phenol reagent. Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 36.Brandstrup N., Kirk J.E., Bruni C. The hexokinase and phosphoglucoisomerase activities of aortic and pulmonary artery tissue in individuals of various ages. J Gerontol. 1957;121:66–171. doi: 10.1093/geronj/12.2.166. [DOI] [PubMed] [Google Scholar]
- 37.Sadava D., Alonso D., Hong H., Barrett D. Effect of methadone addiction on glucose metabolism in rats. Gen Pharmacol. 1997;28:27–29. doi: 10.1016/s0306-3623(96)00165-6. [DOI] [PubMed] [Google Scholar]
- 38.King J. Van Nostrand Co.; London: 1956. Practical Clinical Enzymology; pp. 85–234. [Google Scholar]
- 39.Sutherland E.W. Polysaccharide phosphorylase, vol. 2: liver. In: Colowick S.P., Kaplan N.O., editors. Methods in Enzymology. Academic; New York: 1995. pp. 215–218. [Google Scholar]
- 40.Beutler E. 2nd ed. Grune & Stratton; New York: 1975. Red Cell Metabolism, a Manual of Biochemical Methods; p. 66. [Google Scholar]
- 41.Adelman R.A., Spoiler P., Dweinhouse S. Dietary and hormonal regulation of enzymes of fructose metabolism in rat liver. J Biol Chem. 1966;241:54–67. [PubMed] [Google Scholar]
- 42.Geer B.W., Krochko D., Oliver M.J., Walker V.K., Williamson J.H. A comparative study of the NADP-malic enzymes from Drosophila and chick liver. Comp Biochem Physiol. 1980;65:25–34. [Google Scholar]
- 43.Gibson D.M., Hubbard D.D. Incorporation of malonyl Co-A into fatty acids by liver in starvation and alloxandiabetes. Biochem Biophys Res Commun. 1960;3:531. doi: 10.1016/0006-291x(60)90169-8. [DOI] [PubMed] [Google Scholar]
- 44.Shirai K., Jackson R.L. Lipoprotein lipasecatalyzed hydrolysis of p-nitrophenyl butyrate. Interfacial activation by phospholipid vesicles. J Biol Chem. 1982;257:1253–1258. [PubMed] [Google Scholar]
- 45.Duncan D.B. Multiple range and multiple F tests. Biometrics. 1955;42:1–42. [Google Scholar]
- 46.Zheng S., Qian Z., Tang F., Sheng L. Suppression of vascular cell adhesion molecule-1 expression by crocetin contributes to attenuation of atherosclerosis in hypercholesterolemic rabbits. Biochem Pharmacol. 2005;70:1192–1199. doi: 10.1016/j.bcp.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 47.Goldstein B.J. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol. 2002:903G–910G. doi: 10.1016/s0002-9149(02)02553-5. [DOI] [PubMed] [Google Scholar]
- 48.Segal M.S., Gollub E.S., Johnson R. Is the Fructose Index more relevant with regards to cardiovascular disease than the glycemic index? Eur J Nutr. 2007;46:406–417. doi: 10.1007/s00394-007-0680-9. [DOI] [PubMed] [Google Scholar]
- 49.Yagi N., Takasu N., Higa S., Ishikawa K., Murakami K., Mimura G. Effect of troglitazone, a new oral antidiabetic agent, on fructose-induced insulin resistance. Horm Metab Res. 1995;27:439–441. doi: 10.1055/s-2007-979997. [DOI] [PubMed] [Google Scholar]
- 50.Teff K.L., Elliott S.S., Tschop M. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 51.Busserolles J., Gueux E., Rock E., Mazur A., Rayssiguier Y. Substituting honey for refined carbohydrates protects rats from hypertriglyceridemic and prooxidative effects of fructose. J Nutr. 2002;132:3379–3382. doi: 10.1093/jn/132.11.3379. [DOI] [PubMed] [Google Scholar]
- 52.Weber G., Convery H.J.H. Insulin; inducer of glucose-6-phosphate-dehydrogenase. Life Sci. 1966;5:1136–1146. doi: 10.1016/0024-3205(66)90098-1. [DOI] [PubMed] [Google Scholar]
- 53.Howard C.T. Metabolic regulation of gene transcription in mammals. J Biol Chem. 1995;270:23235–23238. doi: 10.1074/jbc.270.40.23235. [DOI] [PubMed] [Google Scholar]
- 54.Bezerra R.M., Ueno M., Silva M.S., Tavares D.Q., Carvalho C.R.O., Saad M.J.A. A high fructose diet affects the early steps of insulin action in muscle and liver of rats. J Nutr. 2000;130:1531–1535. doi: 10.1093/jn/130.6.1531. [DOI] [PubMed] [Google Scholar]
- 55.Blakely S.R.B., Hallfrisch J., Reiser S., Prather E.S. Long term effects of moderate fructose feeding on glucose tolerance parameters in rats. J Nutr. 1981;111:307–314. doi: 10.1093/jn/111.2.307. [DOI] [PubMed] [Google Scholar]
- 56.Kahn B.B., Rossetti L., Lodish H.F., Charron M.J. Decreased in vivo glucose uptake but normal expression of GLUT1and GLUT4 in skeletal muscle diabetic rats. J Clin Investig. 1991;87:2197–2206. doi: 10.1172/JCI115254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan A.W.H., Nuttal F.Q. Regulation of synthase phosphatase and phosphorylase phosphatase in rat liver. Biochim Biophys Acta. 1976;445:118–130. doi: 10.1016/0005-2744(76)90165-0. [DOI] [PubMed] [Google Scholar]
- 58.Khandelwal R.L., Zinman S.M., Zebrowski E.J. The effect of streptozotocin induced diabetes and of insulin supplementation on glycogen metabolism in rat liver. J Biochem. 1977;168:541–548. doi: 10.1042/bj1680541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ortmeyer H.K., Bodkin N.L., Hansen B.C. Insulin regulates liver glycogen synthase and glycogen phosphorylase activity reciprocally in rhesus monkeys. Am J Physiol Endocrinol Metab. 1997;272:133–138. doi: 10.1152/ajpendo.1997.272.1.E133. [DOI] [PubMed] [Google Scholar]
- 60.Murakami T., Shimomura Y., Fujitsuka N., Sokabe M., Okamura K., Sakamoto S. Enlargement of glycogen store inn rat liver and muscle by fructose-diet intake and exercise training. J Appl Physiol. 1997;82:772–775. doi: 10.1152/jappl.1997.82.3.772. [DOI] [PubMed] [Google Scholar]
- 61.Rajasekar P., Anuradha C.V. Effectof L-Carnitine on skeletal muscle lipids and oxidative stress in rats fed high-fructose diet. Exp Diabetes Res. 2007:1–8. doi: 10.1155/2007/72741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zierath J.R., Nolte L.A., Wahlstrom E. Carrier mediated fructose uptake significantly contributes tocarbohydrate metabolism in human skeletal muscle. Biochem J. 1995;311:517–521. doi: 10.1042/bj3110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nillson L.H., Hultman E. Liver and muscle glycogen in man after glucose and fructose infusion. Scand J Clin Lab Investig. 1974:335–410. doi: 10.3109/00365517409114190. [DOI] [PubMed] [Google Scholar]
- 64.Sharma B., Salunki R., Srivastava S., Majumder Roy C. Effects of guggulsterone isolated from Commiphora mukul in high at diet induced diabetic rats. Food Chem Toxicol. 2009;47:2631–2639. doi: 10.1016/j.fct.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 65.Segebarth C., Gnivegnee A.R., Longo R., Luyten P.R., den Hollander J.A. In vivo monitoring of fructose metabolism in the human liver by means of magnetic resonance spectroscopy. Biochimie. 1990;73:105–108. doi: 10.1016/0300-9084(91)90082-c. [DOI] [PubMed] [Google Scholar]
- 66.Vanden Berghe G. Fructose: metabolism and short-term effects on carbohydrate and purine metabolic pathways. Prog Biochem Pharmacol. 1986;21:1–32. In: Macdonald I, Vrana A, eds. Metabolic effects of dietary carbohydrates. [PubMed] [Google Scholar]
- 67.Chevalier M.M., Wiley J.H., Leveille G.A. Effect of dietary fructose on fatty acid synthesis in adipose tissue and liver of the rat. J Nutr. 1972;102:337–342. doi: 10.1093/jn/102.3.337. [DOI] [PubMed] [Google Scholar]
- 68.Baynes J., Thorpe S.R. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999:481–489. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]