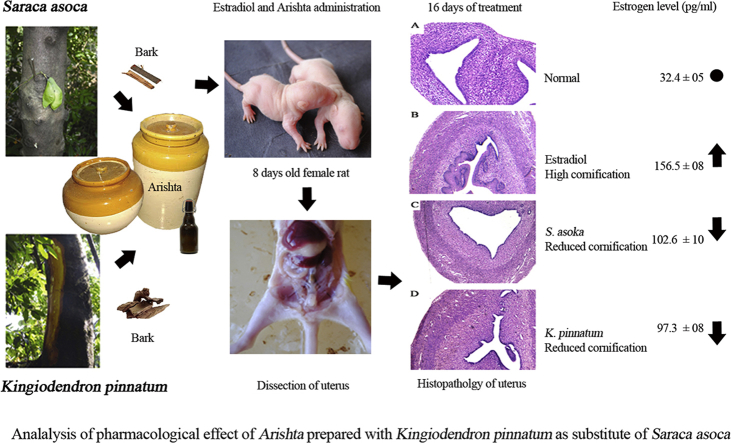
Abstract
Saraca asoca (Fabaceae) is a prime ingredient in Asokarishta, a well-known Ayurvedic preparation for gynecological ailments. Due to scarcity, adulteration or substitution of related raw drugs is a common practice in its preparation. The bark of Kingiodendron pinnatum (Roxb. ex DC.) Harms, morphologically similar to S. asoca (Asoka) is a widely used substitute. The present study aimed to evaluate the pharmacological effectiveness of K. pinnatum as an alternative for S. asoca in Asokarishta by determining the inhibitory effect of estrogen induced uterus endometrial thickening in immature female rats. Arishta was prepared using S. asoca and with the substitute, K. pinnatum as per Ayurvedic Pharmacopeia. Uterus endometrial thickening was induced by the administration of estradiol (20 μg/kg b. wt, i.p) to 8-day-old rats for 5 alternate days. On day 16, following estradiol administration, the serum estrogen level was found elevated to 156.5 ± 8 pg/ml from the normal value 32.4 ± 5 pg/ml and consequently increased the thickness of uterus endometrium from 16.7 ± 1.4 to 75.2 ± 15.3 μm. Upon oral administration of 400 μl/kg b. wt Asokarishta (ASA) and Arishta made with K. pinnatum (AKP), the thickening was reduced to 42.5 ± 12.7 and 47.1 ± 10.5 μm and the estrogen level diminished to 102.6 ± 10 and 97.3 ± 8 pg/ml, respectively. Arishta also reduced the chronic/acute inflammations in mice and improved the antioxidant status of rats. No toxic symptom was observed in the animals by the treatment of Arishta. The study supports the use of K. pinnatum as an alternative to S. asoca in Asokarishta and gives a scientific validation for Asokarishta in gynecological ailments.
Keywords: Asokarishta, Substitutes, Anti-estrogenic, Anti-inflammatory, Uterus endometrium, Cornification, Metaplasia
Graphical abstract
1. Introduction
Ayurveda, the most ancient and widely practiced traditional system of medicine in India, is a holistic approach to prevent the incidence or cure of diseases by maintaining homeostasis among the three body control systems, Vata (air), Pitta (fire) and Kapha (water). A balance of these systems is achieved by practising the basic principles of nature and the use of herbal decoctions or medicinal preparations.1 Because of the pronounced adverse effects of synthetic products, a greater emphasis has recently been received by the herbal systems in disease management. Unfortunately, India has not been able to capitalize the Ayurveda by promoting its use due to various reasons such as loss of ancient knowledge to select the pertinent medicinal plants, non-standardized forms of drug preparations, substitution or adulteration, deforestation, improper harvesting and processing, lack of quality control measures and research and development on herbal drugs etc.2, 3 The unavailability of genuine raw materials is a serious concern in Ayurvedic drug preparations. In most of the Indian systems of medicine, the botanical source of a raw drug is often attributed to one species and the continuous extraction of a particular species lead to its rarity or loss. Thus, many treasured medicinal plants are over-exploited and have become depleted or even lost from their natural habitats.4 Because of the scarcity of the actual plant materials, the usage of related species as substitutes in many medicinal preparations without proper scientific evaluation is continuing.5, 6 The adulteration in medicinal preparations may reduce the reputation of Ayurvedic system of medicine largely, which is a burning problem facing Ayurvedic industry.7, 8 However, the substitution of the herbs is a need of the hour due to the fact that more than 300 valuable medicinal plants are being red listed in India. Hence, the identification of chemical constituents as well as the pharmacological properties of plants is to be evaluated before using as a substitute. These measures can bring the herbal drugs to the standards of WHO9 and may promote the practice of Ayurveda globally.10, 11
Among the threatened medicinal plants, Saraca asoca (Roxb) de Wilde belonging to the family Fabaceae is used in large quantities in Ayurvedic industry as Asoka12 and one of the foremost plants utilized from antiquity till to date. Asoka is a sacred tree of India and renowned for its use in treating gynecological disorders especially menorrhagia.13, 14 The bark of Saraca asoca is an important raw drug in Asokarishta, a fermented formulation and in several other medicinal preparations. The tannins contained in bark are thought to provide the astringent action for halting excessive menstrual bleeding, bleeding hemorrhoids, bleeding ulcers and hemorrhagic dysentery.15, 16 The annual consumption of Asoka in Ayurvedic drug industry in India is estimated to be nearly 850 tonnes/year.17 Thus, over the years, due to over exploitation of the plant, the size of the population has been largely dwindling or almost depleting from its natural habitat. International Union for Conservation of Nature and Natural Resources (IUCN) has listed this species under the threat category, Globally Vulnerable.18 Thus the scarcity has led to the substitution with the bark of related trees of the same family including largely Kingiodendron pinnatum (Roxb. ex DC.) Harms. K. pinnatum is a large tree sparsely distributed in the evergreen forests of Western Ghats of India. Even though the population is less in wild, the species is widely used as substitute due to its giant size and the possibility of getting large volume bark compared to an Asoka tree. Traditionally, an oleo-gum-resin extracted from K. pinnatum is being used by tribes for gonorrhoea, catarrhal conditions of genito-urinary and respiratory tracts and in curing sores in elephants. There are no reports on the pharmacological properties of K. pinnatum except antioxidant19 and antibacterial effects. Some biologically active components like phenols, flavonoids, glycosides and diterpenes were also reported in the plant.20, 21 On this perspective, the focus of the study was to identify the suitability of Kingiodendron pinnatum as alternative for Asoka in Arishta preparation.
2. Materials and methods
2.1. Preparation of Arishta
The bark of Saraca asoca was collected from medicinal garden of Kerala Forest Research Institute (KFRI), Thrissur, Kerala and Kingiodendron pinnatum from Wayanad forest, Kerala. The voucher specimens of Saraca asoca (No. KFRI 4849) and Kingiodendron pinnatum (No. KFRI 4725) are deposited in Herbarium of KFRI. Arishta with S. asoca (ASA) and with K. pinnatum (AKP) were prepared as per Indian Ayurvedic Pharmacopeia. Briefly, the chopped bark of the principal component and other 14 plants as minor ingredients were mixed together and boiled in water until the volume got reduced to quarter. The sediments were removed and the filtrate kept for 30 days in an airtight china clay jar with the flowers of Woodfordia fruticosa and sugar candy for fermentation. After 30 days, the Arishta was filtered and used for the study.
2.2. Animals
Female young Wistar rats (8 day old) and mature Swiss albino mice (8–10 week old, 25–30 g) were purchased from Small Animal Breeding Station, College of Veterinary, Kerala Veterinary and Animal Sciences University (KVASU), Thrissur, Kerala. The animals were maintained under standardized environmental conditions (22–28 °C, 60–70% relative humidity, 12 h dark/light cycle) and fed with standard rat feed (Lipton, India) and water ad libitum. All the animal experiments were carried out in Amala Cancer Research Centre with the prior permission of Institutional Animal Ethics Committee (IAEC).
2.3. Experimental design for anti-keratinization study
Eight-day old female Wistar rats were used for the study. Total of 210 animals were distributed in to 7 groups comprising 30 animals each. Keratinization in uterus of all the rats was induced by the i. p. injection of 20 μg/kg b. wt. estradiol in 0.05 ml of propanediol for 5 alternative days22 except first and second groups of animals, which was kept as normal and vehicle control receiving propanediol, respectively. Among these, the animals of third group were kept as control without drug treatment. Fourth and fifth groups received Asokarishta (ASA) at 200 and 400 μl/kg b. wt and sixth and seventh groups received Arishta prepared with K. pinnatum (AKP) at 200 and 400 μl/kg b. wt orally for 5 alternative days along with the estradiol injection. During the experiment, 6 animals from each group were sacrificed at 8, 16, 24, 32 and 40 days after birth. The uterus was dissected and kept in formalin. The sections of uterus were prepared and stained using hematoxyline-eosin. The pattern of keratinization on endometrium of uterus was analysed microscopically using Leica Application Suit software. On 16th day of treatment (24 days after birth), the serum estrogen level was estimated by radioimmuno assay (RIA).
2.4. Free radical scavenging property analysis
The scavenging activity of Arishta on various free radicals was analysed. Superoxide anion radical was determined by light induced superoxide generation with riboflavin and subsequent reduction of nitro blue tetrazolium (NBT).23 Hydroxyl radical (OH−) generated by the Fe3+/ascorbate/H2O2 system (Fenton reaction) was measured by the thiobarbituric acid reacting substances (TBARS).24 Lipid peroxidation induced in rat liver homogenate25 was estimated by thiobarbituric acid reactive substances.26 ABTS (2,2-azobis-3-ethylbenthiozoline-6-sulfonic acid) and DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity and ferric reducing power (FRAP) of the Arishta were also determined. The scavenging of free radicals and inhibition of lipid peroxidation by ASA and AKP was calculated using the formula, % of inhibition = (OD of Control–OD of treated/OD of Control) × 100.
2.5. In vivo antioxidant property analysis
Male Swiss albino mice were used for the study. After the treatment of ASA and AKP orally for 5 alternative days, the animals were sacrificed, liver excised and blood was collected into the heparinized tubes by cardiac puncture for hematological and biochemical analysis. Blood samples were centrifuged at 1000×g for 15 min and the upper portion of the centrifuged samples was removed and the packed erythrocytes at the bottom were washed three times with phosphate buffer saline (pH 7.4). A known volume of red blood cells were lysed with hypotonic phosphate buffer. After removing the red blood cell debris by centrifugation of the mixtures (3000×g for 15 min), the lysates were recovered. Superoxide dismutase (SOD)23 and catalase27 activities and the level of reduced glutathione (GSH)28 in blood and liver were determined.
2.6. Anti-inflammatory activity on formalin and carrageenan induced paw oedema
Swiss albino mice were used for the study. The animals were divided in to 6 groups comprising six animals each and the acute inflammation was induced by the sub-plantar injection of 0.02 ml freshly prepared 1% suspension of carrageenan in 0.1% carboxymethyl cellulose (CMC) on right hind paw in all the groups of animals.29 Group 1 served as control by receiving carrageenan alone and group 2 was given standard drug diclophenac (10 mg/kg b. wt.), intraperitoneally which served as reference standard. Group 3 (200 μl/kg b. wt.) and 4 (400 μl/kg b. wt.) were treated with ASA orally and group 5 (200 μl/kg b. wt.) and 6 (400 μl/kg b. wt.) with AKP, respectively for five consecutive days prior to the carrageenan injection. The footpad thickness was measured using Vernier calipers for every hour for 5 h following carrageenan injection. The percentage of inhibition in paw swelling was calculated according to the following formula [(Vt − Vo) control-(Vt − Vo) treated group/(Vt − Vo) control] × 100, where Vt is the paw oedema at various time intervals and Vo is the initial paw oedema.
Chronic inflammation was induced by sub-plantar injection of freshly prepared 0.02 ml of 2% formalin on the right hind paw in all groups of animals. The drug was administered to the animals as described previously. The paw thickening was measured for every day for 5 days and the percentage inhibition in paw thickness was calculated.
2.7. Statistical analysis
The values are presented as mean ± SD. Differences between group's means were estimated using one way analysis of variance followed by Dunnett's test. The results were considered statistically significant when P < 0.05.
3. Results
3.1. Inhibitory effect of Arishta on estradiol induced keratinization in rat uterus
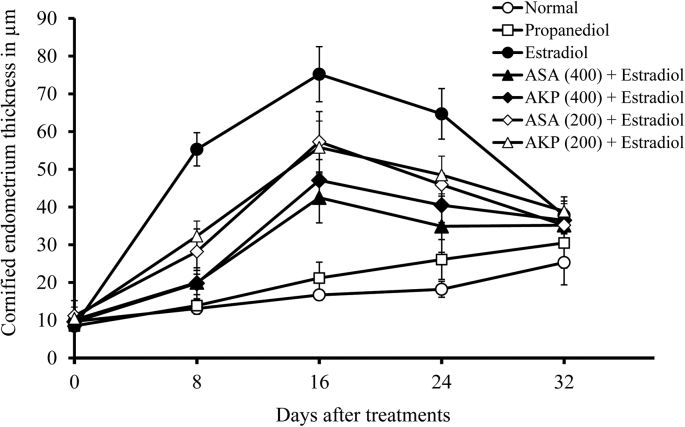
Upon histopathological examination, a massive proliferative thickened endometrium in immature rat uterus was observed by the administration of estradiol. The increase in proliferation was noticed on 8th day of estradiol administration and the maximum thickening was observed on 16th day (24th day of age). During this period of time, metaplasia was observed in some animal uterus with five to seven stratified keratinized epithelial layers with 75.2 ± 15.3 μm thickness. In animals treated with both Arishta, the thickening was found to be reduced considerably. On 16th day, the thickness of the 400 μl/kg b. wt ASA treated animals was 42.5 ± 12.7 μm which was gradually decreased to 35.2 ± 7.2 μm on 32nd day of treatment. In the case of AKP, the extent of thickness was 47.1 ± 10.5 μm on 16th day. Comparative reduction in cornification was also noticed with the dose of 200 μl/kg b. wt Arishta. A gradual decrease in the cornified keratinized layers in all the groups was observed as shown in Fig. 1, Fig. 2.
Fig. 1.
Inhibitory effect of Arishta on estradiol induced keratinization in rat uterus.
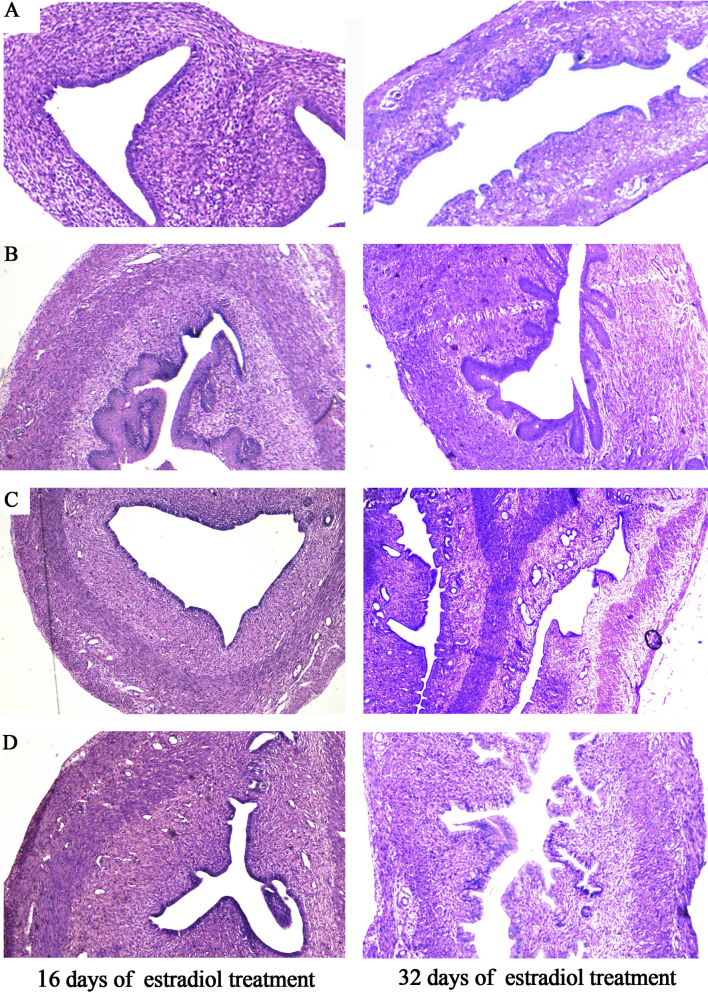
Fig. 2.
Photomicrograph (40×) of a portion of the uterus of rat stained with hematoxylin and eosin for the detection of the keratinized epithelium after 16 and 32 days of estradiol treatment. The paraffin wax embedded microtome sections were used. (A) Normal-without any treatment, (B) Estradiol, (C) Estradiol and ASA (400 μl), (D) Estradiol and AKP (400 μl).
3.2. Anti-estrogenic property of Arishta
On 16th day, the concentration of estrogen in serum was increased to 156.5 ± 8 pg/ml by the administration of estradiol from its normal value 32.4 ± 5 pg/ml. The increased level of estrogen was found considerably reduced by the administration of Arishta. The concentration of estrogen estimated was 102.6 ± 10 and 97.3 ± 8 in animals treated with ASA and AKP at the dose of 400 μl/kg b. wt (Table 1).
Table 1.
Effect of Arishta on blood estrogen level in immature rat.
| Groups | Treatment group | Estrogen level (pg/ml) |
|---|---|---|
| 1 | Normal (without any treatment) | 32.4 ± 5 |
| 2 | Vehicle control (Propanediol) | 35 ± 2 |
| 3 | Estradiol (20 μg/kg b. wt) | 156.5 ± 8 |
| 4 | ASA (400 μg/kg b. wt) + Estradiol | 102.6 ± 10** |
| 5 | ASA (200 μg/kg b. wt) + Estradiol | 128 ± 4** |
| 6 | AKP (400 μg/kg b. wt) + Estradiol | 97.3 ± 8** |
| 7 | AKP (200 μg/kg b. wt) + Estradiol | 135 ± 4** |
Each value represents the mean ± SD (n = 6). *P < 0.05 and **P < 0.01.
3.3. Free radical scavenging effect of Arishta
Arishta shows significant activity on scavenging various free radicals in in vitro assay systems. The concentration of ASA and AKP required for preventing 50% hydroxyl radical generation was estimated to be 5 and 4.5 μl/ml and for scavenging superoxide is 6.8 and 10.21. In the case of ABTS radical, the IC50 values were 0.3 and 0.15 μl/ml for ASA and AKP, respectively. Both the Arishta were also found to be effective in reducing the stable radical DPPH by donating hydrogen equivalent to DPPH-H. The IC50 values for ASA and AKP were estimated to be 7.5 and 1.35 μl/ml. Fe3+–ascorbate system induced a significant amount of lipid peroxidation in rat liver homogenate as shown by the elevated level of TBARS. Additions of Arishta were effective in reducing the lipid peroxidation (Table 2). For ASA, the concentration needed for 50% inhibition was 7.6 μl/ml, while the IC50 value of AKP was calculated to be 20 μl/ml. In FRAP assay, the ferric reducing power of 5 μl/ml ASA was equal to the reducing power of 0.24 μmol/ml FeSO4·7H2O and AKP was equal to 0.36 μmol/ml of FeSO4.7H2O.
Table 2.
Scavenging effect of Arishta on various in vitro free radicals.
| ASA (IC50 in μl/ml) | AKP (IC50 in μl/ml) | |
|---|---|---|
| Superoxide radicals | 6.8 | 10.21 |
| Hydroxyl radicals | 5.0 | 4.5 |
| Lipid peroxidation | 7.6 | 20.0 |
| DPPH radicals | 7.5 | 1.35 |
| ABTS | 0.3 | 0.15 |
3.4. Antioxidant enzyme status of mice treated with Arishta
Effect of Arishta on antioxidant molecules in blood and liver of mice for a period of 30 days after drug administration is shown in Table 3, Table 4. The activity of superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase and the level of glutathione (GSH) were found to be increasing considerably. The SOD activity in blood increased by 16.89 and 31.33% in animals treated with 200 and 400 μl/ml of ASA and 24.43 and 39.05% with AKP. The percentage increase of GPx in liver tissue estimated was 19.67 and 22.52% with the administration of 200 μl/ml ASA and AKP, respectively. At the concentration of 400 μl/ml, the percentage increase was estimated to be 28.61 and 27.12 (Table 3). In blood, 19.97 and 33.17% increase in catalase activity was noticed with 200 and 400 μl/ml ASA treatment while the hike was 10.09 and 36.87% in the case of AKP. The level of GSH was found increased to 25.98 and 51.69% in blood of the animals treated with respective concentrations of ASA and 19.21 and 43.76% for AKP. Respective increase in activity of catalase and level of GSH was observed in liver tissue as shown in Table 4.
Table 3.
Effect of Arishta on antioxidant enzyme activities and GSH level in liver tissue.
| Treatment | Catalase (K/g Hb) | Glutathione Peroxidase (U/g Hb) | Glutathione (nmols/ml) |
|---|---|---|---|
| Normal | 11.2 ± 0.46 | 16.82 ± 2.6 | 6.7 ± 2.34 |
| ASA (200 μl/kg b. wt) | 13.41 ± 3.35 (16.5%) | 20.94 ± 3.85 (19.67%) | 7.1 ± 1.78 (5.7%) |
| ASA (400 μl/kg b. wt) | 14.01 ± 2.07 (20%) | 23.56 ± 3.26* (28.61%) | 9.9 ± 1.43* (32.4%) |
| AKP (200 μl/kg b. wt) | 11.75 ± 2.02 (4.7%) | 21.71 ± 4.93 (22.52%) | 6.9 ± 1.3 (3%) |
| AKP (200 μl/kg b. wt) | 13.46 ± 2.18 (16.8%) | 23.08 ± 4.85* (27.12%) | 7.5 ± 1.77 (10.7%) |
| Vitamin C (15 mg/kg b. wt) | 14.31 ± 1.35 (21.8%) | 22.45 ± 3.21 (25%) | 8.2 ± 1.16 (18.3%) |
K = the measure of catalase activity (the difference in extinction at 240 nm per 15 s). The percentage increase is showed in bracket. Each value represents the mean ± SD (n = 6). *P < 0.05 and **P < 0.01.
Table 4.
Effect of Arishta on antioxidant enzyme activities and GSH level in blood.
| Treatment | Catalase (K/g Hb) | Superoxide dismutase (U/g Hb) | Glutathione (nmols/ml) |
|---|---|---|---|
| Normal | 80.75 ± 16.73 | 910.52 ± 52.2 | 23.13 ± 2.25 |
| ASA (200 μl/kg b. wt) | 100.9 ± 15.41 (19.97%) | 1095.55 ± 82.61** (16.89%) | 31.25 ± 2.63* (25.98%) |
| ASA (400 μl/kg b. wt) | 120.83 ± 15.76** (33.17%) | 1326.02 ± 58.09** (31.33%) | 47.88 ± 7.04** (51.69%) |
| AKP (200 μl/kg b. wt) | 89.81 ± 28.72 (10.09%) | 1204.93 ± 3.64** (24.43%) | 28.63 ± 4.75 (19.21%) |
| AKP (400 μl/kg b. wt) | 127.91 ± 26.58** (36.87%) | 1493.78 ± 55.74** (39.05%) | 41.13 ± 5.54** (43.76%) |
| Vitamin C (15 mg/kg b. wt) | 110.3 ± 10.13 (26.8%) | 994.33 ± 49.4 (8.43%) | 49.35 ± 3.65** (53.14%) |
K = the measure of catalase activity (the difference in extinction at 240 nm per 15 seconds). The percentage increase is showed in bracket. Each value represents the mean ± SD (n = 6). ∗P < 0.05 and ∗∗P < 0.01.
3.5. Anti-inflammatory effect of Arishta
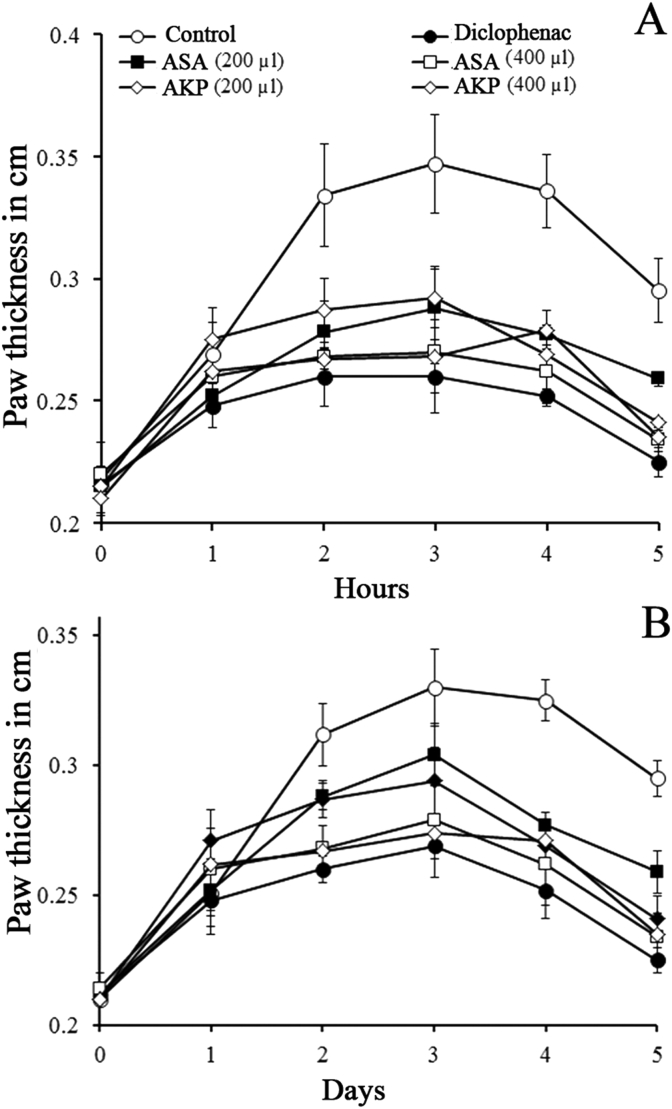
The paw oedema induced by carrageenan and formalin in the hind limb of mice was increased gradually and attained the maximum size at 3rd h and 3rd day, respectively. The administration of Arishta to the mice reduced oedema considerably. The reduction in acute oedema was estimated to be 65.80 and 60% for 400 μl/kg b. wt. of ASA and AKP, respectively. The results were comparable to that of the reference drug, diclophenac (Fig. 3A). In formalin induced model, reduced paw oedema was observed with a percentage reduction of 52.13 and 52.59% by ASA and AKP, respectively. Diclophenac exhibited a decrease of 58.59% against the formalin induced chronic oedema (Fig. 3B).
Fig. 3.
Anti-inflammatory effect of Arishta. (A) Carrageenan induced acute inflammation. (B). Formalin induced chronic inflammation on mice.
4. Discussion
Dysmenorrhea and Dysfunctional Uterine Bleeding (DUB), the major gynecological problems of women have similar physiological manifestations resulting from acute inflammatory processes. Local factors including prostaglandin, inflammatory cytokines, vascular endothelial growth factors and several matrix metalloproteinases (MMPs) are supposed to be involved in pathophysiology.30 For the management of the pain and crumb, the hormonal contraceptives preferring the estrogenic supplementation in dysmenorrhea and anti-estrogenic or progesterone for DUB have been recommended. However, the clinical evidence supporting their effectiveness is reported to be limited. Hence, the non-steroidal anti-inflammatory drugs (NSAIDs) are still the first-line of treatment.31 Asokarishta is effectively used to manage all types of disorders related to female reproductive system including dysmenorrhea and DUB in Ayurvedic system of medicine.13 According to a recent study, Asokarishta is an effective drug in psychological as well as somatic problems related with menopausal syndrome and is also a safe alternative to the modern drugs.14 But, the mechanism of action is still not explained fully. In the present study, Asokarishta prepared using S. asoca (ASA) as well as K. pinnatum (AKP) has been found to considerably reduce the estrogenic level in blood and uterine endometrial thickening in young rats. Moreover, both the Arishta inhibit acute as well as chronic inflammation in mice significantly.
Estrogen and progesterone level in the body regulates the menstrual cycle in females. Progesterone prepares uterus endometrium for implantation, failure of it leads to the reduction in progesterone level and up regulation of prostaglandin level. The high prostaglandin has been reported in the pathophysiological processes of dysmenorrhea where excessive bleeding, spasm and pain prevails.31 Asokarishta has clinically proved to reduce the spasm and pain associated with dysmenorrhea. It is supposed that the possible role of Asokarishta is to act as an inhibitor of prostaglandin synthesis or an antagonist to progesterone action. Saraca asoca bark, the main ingredient of Asokarishta has been reported to inhibit cyclooxygenase activity22 and prostaglandin synthesis13 and to have anti-inflammatory property.32 In the present study, Asokarishta shows anti-inflammatory activity in animal models. This information suggests that the inhibition of cyclooxygenase and prostaglandin might be the reason for Asokarishta's clinical efficacy. The anti-inflammatory activity of K. pinnatum containing Arishta is the same as that of Asokarishta. Further, estrogen has reported to have antagonistic action to progesterone. Even though various reports support the phytoestrogenic activity of the plant S. asoca and its formulation Asokarishta, some reports suggest vice versa.33, 22 It is not sure at present whether these phytoestrogens possess estrogen like activity to prevent the action of progesterone which needs further studies.
On the other hand, DUB in anovulatory or ovulatory condition, the normal cyclical secretion of progesterone does not occur and the subsequent excessive estrogen stimulates the proliferation of endometrium unopposed and eventually outgrowing its blood supply.34 It is then fleshed out incompletely and bleeds irregularly or sometimes profusely for a long time.35 When this abnormal process occurs repeatedly, the endometrium can become hyperplastic, sometimes with atypical or cancerous.36, 37 In this condition also, Asokarishta has found clinically effective. Here, an anti-estrogenic activity of Asokarishta is indicated. Saraca asoca has been found to reduce serum estrogen level in young rats and also in animals received estradiol. These animals have been found to reduce estradiol induced endometrial thickening and uterine metaplasia. In the present study, ASA and AKP equally reduced the serum estrogen concentration as well as estradiol induced thickening of uterine endometrium considerably in rats. It is assumed that the Arishta might have cleared the excess estrogen from the blood or the phytoestrogenic components might have mimicked or inhibited the estrogenic receptors. This study does not provide clear evidence whether Asokarishta prevents the synthesis of estrogen itself. More studies are required to unravel the anti-estrogenic action of Asokarishta.
Further, the present study also demonstrates that the oral administration of both the Arishta promote the antioxidant enzymes activities and molecules. In corroboration with this observation, the extent of lipid peroxidation has been found to reduce in treated animals. This indicates the ability of the Arishta to act as exogenous antioxidant supplement. The antioxidant effect of S. asoca extract has been previously reported by several workers.38, 39 Antioxidant activity of K. pinnatum has been documented.19 Therefore, it is expected that the anti-oxidant and anti-inflammatory activities of the Arishta are provided by its major ingredients, S. asoca and K. pinnatum and may support its clinical success. Moreover, no toxic symptom was observed to mice by the administration of Arishta up to the concentration of 1 ml/kg b. wt (data not shown).
5. Conclusion
Altogether, the present study reveals the mechanistic basis of the Asokarishta in gynecological disorders. Arishta prepared with K. pinnatum also possess similar pharmacological property as that of Asokarishta. The study thus provides a scientific validation for the use of Kingiodendron pinnatum in Arishta preparations. This may reduce the production cost as substitutes are comparatively cheaper.
Conflict of interest
No conflict of interest.
Acknowledgement
The authors are thankful to National Medicinal Plants Board (NMPB), Ministry of AYUSH, Government of India, New Delhi for the financial support (No. Z 18017/187/Pr.GO/KE-05/2007-08-NMPB).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Mishra L., Singh B.B., Dagenais S. Ayurveda: a historical perspective and principles of the traditional healthcare system in India. Altern Ther Health Med. 2001;7(2):36–42. [PubMed] [Google Scholar]
- 2.Patwardhan B. Ayurveda: the designer medicine. Indian Drugs. 2000;37:213–227. [Google Scholar]
- 3.Mukherjee P.K., Harwansh R.K., Bahadur S. Development of Ayurveda – tradition to trend. J Ethnopharmacol. 2016:30782–30786. doi: 10.1016/j.jep.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Vaidya A.D.B., Devasagayam T.P.A. Current status of herbal drugs in India: an overview. J Clin Biochem Nutr. 2007;41(1):1–11. doi: 10.3164/jcbn.2007001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubey N.K., Rajesh K., Pramila T. Global promotion of herbal medicine: India's opportunity. Curr Sci. 2004;86:37–41. [Google Scholar]
- 6.Dubey R.B., Savant B.S. Current scenario of adulterants and substitutes of medicinal plants: a review. J Pharm Sci Innov. 2015;4(5):247–250. [Google Scholar]
- 7.Subrat N., Iyer M., Prasad R. Ecotech Services (India) Pvt. Ltd; New Delhi: 2002. The Ayurvedic Medicine Industry: Current Status and Sustainability. Edition. [Google Scholar]
- 8.Poornima B. Adulteration and substitution in herbal drugs a critical analysis. Int J Res Ayurveda Pharm. 2010;1(1):8–12. [Google Scholar]
- 9.WHO . World Health Organization; Geneva: 2000. General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine. [Google Scholar]
- 10.Joshi P.R., Patel B.R., Shukla V.J. An overview of the causes of current practices in Pratinidhi Dravyas (substitution of drugs) in Ayurveda including newer techniques for their evaluation. Ayu. 2012;33(4):481–485. doi: 10.4103/0974-8520.110518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pushpendra Kumar KNS., Priyadarshini Holla BS., Ravishankar B., Yashovarma B. Quality standards for Hutabhugadi curṇa (Ayurvedic formulary of India) J Tradit Complement Med. 2016;6(1):78–88. doi: 10.1016/j.jtcme.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warrier P.K., Nambier V.P.K., Ganpathy P.M. International Development Research Centre; New Delhi: 2000. Some Important Medicinal Plants of the Western Ghats, India: A Profile; pp. 343–360. [Google Scholar]
- 13.Middelkoop T.B., Labadie R.P. Evaluation of Asokarishta, an indigenous medicine in Sri Lanka. J Ethnopharmacol. 1983;8(3):313–320. doi: 10.1016/0378-8741(83)90068-5. [DOI] [PubMed] [Google Scholar]
- 14.Modi M.B., Donga S.B., Dei L. Clinical evaluation of Ashokarishta, Ashwagandha Churna and Praval Pishti in the management of menopausal syndrome. Ayu. 2012;33(4):511–516. doi: 10.4103/0974-8520.110529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradhan P., Joseph L., Gupta V. Saraca asoca (Ashoka): a review. J Cheml Pharm Res. 2009;1(1):62–71. [Google Scholar]
- 16.Shukla R., Chakravarty M., Gautam M.P. Indigenous medicine used for treatment of gynecological disorders by tribal of Chhattisgarh, India. J Med Plant Res. 2008;2(12):356–360. [Google Scholar]
- 17.Tewari D.N. Govt. of India; 1999. Report of the Task Force on Conservation and Sustainable Use of Medicinal Plants. [Google Scholar]
- 18.Anjan B.N., Hombe G.H.C., Vasudeva R. A note on air layering in Saraca asoca (Roxb). De Wilde. J Non-Timber For Prod. 2004;11:34–35. [Google Scholar]
- 19.Kumar J.K., Prasasd A.G.D., Richard S.A. Biochemical activity of endangered medicinal plant K. Pinnatum. Asian J Plant Sci Res. 2011;1(4):70–75. [Google Scholar]
- 20.Javarappa K.K., Prasad A.G.D., Prasad A.J.M., Mane C. Bioactivity of diterpens from the ethyl acetate extract of Kingiodendron pinnatum Rox. Hams Pharmacogn Res. 2016;8(4):287–291. doi: 10.4103/0974-8490.188871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheik S., Chandrashekar K.R. Antimicrobial and antioxidant activities of Kingiodendron pinnatum (DC.) Harms and Humboldtia brunonis Wallich: Endemic plants of the Western Ghats of India. J Natn Sci Found Sri Lanka. 2014;42(4):307–313. [Google Scholar]
- 22.Shahid A.P., Salini S., Sasidharan N., Padikkala J., Raghavamenon A.C., Babu T.D. Effect of Saraca asoca (Asoka) on estradiol induced keratinizing metaplasia in rat uterus. J Basic Clin Physiol Pharmacol. 2015;26(5):509–515. doi: 10.1515/jbcpp-2014-0124. [DOI] [PubMed] [Google Scholar]
- 23.McCord J.M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 24.Elizabeth K., Rao M.N.A. Oxygen radical scavenging activity of Curcumin. Int J Pharm. 1990;58:237–240. [Google Scholar]
- 25.Bishayee S., Balasubramanian A.S. Lipid peroxide formation in rat brain. J Neurochem. 1971;18:909–920. doi: 10.1111/j.1471-4159.1971.tb12020.x. [DOI] [PubMed] [Google Scholar]
- 26.Ohkawa H., Ohishi W., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Biochemistry. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 27.Catalase Aebi H. In: Bergmeyer H.V., editor. vol. 2. Academic Press; New York: 1974. pp. 674–684. (Methods in Enzymatic Analysis). [Google Scholar]
- 28.Moron M.A., Depierre J.W., Manner, Mannervick B. Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat liver. Biochimica et Biophysica Acta. 1979;582:67–68. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 29.Winter C.A., Risley E.A., Nuss G.W. Carrageenan induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biolmed. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 30.Wallace S., Keightley A., Gie C. Review dysmenorrhea. TOG. 2010;12(3):149–154. [Google Scholar]
- 31.Marjoribanks J., Proctor M., Farquhar C., Derks R.S. Non-steroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2010;1:CD001751. doi: 10.1002/14651858.CD001751.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Ahmad F., Misra L., Tewari R., Gupta P., Mishra P., Shukla R. Anti-inflammatory flavanol glycosides from Saraca asoca bark. Nat Prod Res. 2016;30(4):489–492. doi: 10.1080/14786419.2015.1023728. [DOI] [PubMed] [Google Scholar]
- 33.Kamat S.K., Barde P.J., Raut S.B. Evaluation of the estrogenic activity of Indian medicinal plants in immature rats. Anc Sci Life. 2015;35(2):90–95. doi: 10.4103/0257-7941.171669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polaneczky M.M., Slap G.B. Menstrual disorders in the adolescent: dysmenorrhea and dysfunctional uterine bleeding. Pediatr Rev. 1992;13(3):83–87. doi: 10.1542/pir.13-3-83. [DOI] [PubMed] [Google Scholar]
- 35.Lobo R.A. Abnormal uterine bleeding: ovulatory and anovulatory dysfunctional uterine bleeding, management of acute and chronic excessive bleeding. In: Katz V.L., Lentz G.M., Lobo R.A., Gershenson D.M., editors. Comprehensive Gynecology. 5th ed. Mosby Elsevier; Philadelphia, Pa: 2007. [Chapter 37] [Google Scholar]
- 36.Firmin-Gomes-Teixeira Z., Anthonioz P. Estrogens induce malpighian metaplasia of the endo- cervical junction in female rats immunohistochemistry study. Biol Struct Morphog. 1989;2(2):45–53. [PubMed] [Google Scholar]
- 37.Zhang Q., Shen Q., Celestino J. Enhanced estrogen-induced proliferation in obese rat endometrium. Am J Obstet Gynecol. 2009;200(2):186. doi: 10.1016/j.ajog.2008.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panchawat S., Sisodia S.S. In vitro antioxidant activity of Saraca asoca roxb. de Wilde stem bark extracts from various extraction processes. Asian J Pharm Clin Res. 2010;3(3):231–233. [Google Scholar]
- 39.Sadhu S.K., Khatun A., Phattanawasin P., Ohtsuki T., Ishibashi M. Lignan glycosides and flavonoids from Saraca asoca with antioxidant activity. J Nat Med. 2007;61:480–482. [Google Scholar]