Abstract
Background
Medicinal plants with immunomodulatory properties can provide good alternative therapeutics for curing visceral leishmaniasis. Bergenia ligulata (Wall.) Engl. is an interesting plant with strong antioxidant, antimicrobial, immunomodulatory and hepatoprotective properties.
Aim
The present study was planned to determine the antileishmanial activity of plant extract by modulating the immune responses of inbred BALB/c mice.
Methodology
Bergenin, the principle active component of B. ligulata, was quantitated in crude extract by performing RP-HPLC. The therapeutic potential was assessed through in vitro antileishmanial activity and in mice model through parasite load, cytokine assays, IgG antibody levels, DTH responses, histopathology and biochemical enzyme assays.
Results
B. ligulata showed the presence of glycosides, saponins, carbohydrates, tannins, flavonoids and bergenin which contributed to the antileishmanial activity of extract with IC50 of 22.70 μg/mL. Furthermore, the higher dose significantly reduced the parasite load by 95.56 %. The reduction was further associated with significant enhancement of IL-12 and IFN-γ levels in comparison to IL-10 and IL-4 cytokines. The switching towards Th1 type of immune response was also confirmed by elevated antibody levels of IgG2a isotype as compared to IgG1 as well as increased DTH responses. The histology of liver and kidney further complimented the non toxic nature of plant extract in addition to its negligible toxicity on HeLa cells.
Conclusions
The current study revealed the significant antileishmanial and immunomodulatory properties of this plant extract against murine visceral leishmaniasis. Further, the bioactive components will be explored to assess their efficacy for the development of safe and cost effective drug.
Keywords: Extract, HPLC, Parasite load, Immunological, Biochemical, Histopathological studies
Abbreviations: DMSO, Dimethyl sulphoxide; SSG, Sodium stibogluconate; BLEE, Bergenia ligulata ethanolic extract; CMI, Cell mediated immune responses; p.t.d., Post treatment days; p.i.d., Post infection days; PCT, Proximal convoluted tubules; DCT, Distal convoluted tubules; T.S, Transverse section; SRBC, Sheep red blood cells; VL, Visceral leishmaniasis
Graphical abstract
1. Introduction
Medicinal plants have been used in developing countries for the treatment of infectious diseases, cancer and parasitic diseases since ancient times. Numerous studies have been conducted on the therapeutic potential of plant extracts to evaluate their antifungal, antiprotozoal and antihelminthic properties.1 These medicinal plants contain numerous bioactive components which play an active role in the treatment of various diseases. Most of the antimalarial drugs available today are derived from plants. The ether extract of Artemisia annua was used for isolating an active component named artemisinin.2 These studies introduced a new era of using plant materials as medication for treatment of various diseases.
According to WHO, visceral leishmaniasis is one of the most neglected diseases and approximately 1.3 million new cases of leishmaniasis occur every year, of which 300,000 cases are of VL. Moreover, 90 % of VL cases occur only in Bangladesh, Brazil, Ethiopia, India, Sudan, South Sudan and Somalia. It is estimated that 20,000 to 40,000 deaths occur every year due to this disease.3 In addition, one of the major consequences of VL is suppression of protective T-helper (Th)-1 cells and induction of disease-promoting Th-2 cells.4 The treatment of visceral leishmaniasis relies primarily upon chemotherapeutic drugs and most of these drugs cause severe side effects. Resistance to first line drug i.e. antimonials (sodium stibogluconate and sodium antimony gluconate) is a serious concern.5 Alternative drugs like amphotericin B and pentamidine also have unpleasant side effects.6 Therefore, during the past decade the treatment of this disease has become a bewildering step. Unfortunately, there is no antileishmanial drug which is derived from any plant resource. Recently, a number of studies have been conducted to explore the potential of natural plant extracts and their lead compounds against different forms of leishmaniasis. However, most of the studies are restricted to in vitro effects of plant part extracts on promastigote stage of Leishmania.7 Thus, treatment of VL with natural plant products through immunomodulation is a good option. Therefore, the present study has been planned to evaluate in vitro and in vivo efficacy of traditional medicinal plant B. ligulata (Wall.) Engl. which is known to possess antiplasmodial, immunomodulatory, hepatoprotective and diuretic properties.8, 9, 10, 11
2. Methodology
2.1. Maintenance of promastigote culture
Indian strain of Leishmania donovani i.e. MHOM/IN/80/Dd8 was obtained from the London School of Hygiene and Tropical Medicine, U.K. and was used for present study. Log phase promastigotes were used for the maintenance of strain in modified NNN medium at 22 ± 1 °C. The culture was checked for any contamination and sub cultured after every 48–72 h by transferring 0.5–1.0 mL of culture suspension in Mc Cartney vials containing NNN medium and then supplemented with 3–4 mL of MEM. The pH of 7.2 of medium was maintained by adding 7.5 % NaHCO3. The promastigote culture was maintained in B.O.D. incubator at 22 ± 1 °C.12
2.2. Plant material
Rhizomes of B. ligulata (Wall.) Engl. were collected from Shimla district of Himachal Pradesh. Permission for collection of plant was taken from forest department of Himachal Pradesh, India. Specimens were authenticated by herbarium incharge of Department of Botany, Panjab University, Chandigarh. Voucher specimen was submitted in the herbarium of Department of Botany, Panjab University, Chandigarh and voucher no. 5328 was obtained.
2.3. Preparation of the extract
Rhizomes were washed thoroughly with water and dried at room temperature and then powdered. Ethanolic extract was prepared by Soxhlet extraction method. Approximately 100 g dried and powdered rhizomes were extracted with 250 mL of ethanol. Crude extract was filtered through Whatman filter paper no. 41. Filtrate was concentrated under vacuum in a rotary evaporator (Buchi, USA). Residue obtained was then lyophilized. It was equivalent to 10.5 % of the dry mass of original rhizome powder. It was stored at −20 °C till further use.
2.4. Phytochemical screening of plant extract
Phytochemical screening of the plant extract was carried out to detect alkaloids, saponins, phenols, terpenes, flavonoids, glycosides, tannins and polysterols by employing standard procedures.13, 14
2.5. Determination of bergenin in crude extract
Bergenin is the principle active component of B. ligulata, therefore reverse phase-high performance liquid chromatography was performed for this compound. RP-HPLC fingerprint profile was established for ethanolic extract using RP-HPLC instrument (Shimadzu, Kyoto, Japan). The validated analytical method was used for the quantification of bergenin in plant extract. Components were separated on a Waters C18 3.9 × 150 mm I.D. 5 μm Symmetry column. Elution was performed at a flow rate of 1 mL/min with a gradient prepared from water–phosphoric acid 99.7:0.3 (v/v) (component A) and acetonitrile–water–phosphoric acid 79.7:20:0.3 (v/v) (component B). Gradient used was: 0–5 min, 88–85 % A; 5–10 min, 85–75 % A; 10–20 min, 75–70 % A. The results were acquired and processed using Shimadzu LC-solution version 6.42 software.
Bergenin standard (total purity 98%) was purchased from TCI chemicals (India) Pvt. Ltd. Amount of active compound in plant extract was determined by plotting calibration plots established by chromatography of bergenin standards at five different concentrations. Each solution was chromatographed in triplicate. Peaks were identified by comparison of retention times and UV absorption spectra with those of standards. Linearity of detector response for the standards was assessed by linear regression analysis of each amount of standard and area of the corresponding peak on chromatogram. Linearity was also confirmed for extract. After chromatographic separation, peak areas obtained were plotted against extract concentration by linear regression analysis. Purity of peaks was checked by acquisition of spectra (λ = 200–400 nm) by use of PDA detector and multivariate analysis. Spectra were acquired at the upslope, apex, and downslope of each peak, computer normalized, and superimposed. Peaks were considered pure when there was coincidence between three spectra (match factor ≥98 %).
RP-HPLC method was validated by determination of linearity, peak purity, and limits of quantification and detection. For qualitative purposes, the method was evaluated by taking into account retention time precision, peak purity and selectivity for standards. Peak purity was studied for major peaks. Impurities or co-elution were not observed (match factors ≥95 %). Linearity and limits of detection (LOD) and quantification (LOQ) were evaluated for quantitative purposes. LOD for bergenin was 2.10 and LOQ was 6.37 respectively, implying the method was suitable for quantification of this compound. R2 values for the compound was >0.99, confirming the linearity of the method.
2.6. Estimation of parasite viability by flowcytometry
L. donovani promastigotes at a concentration of 2 × 106/mL were dispensed into 24-well culture plates. Each well was then supplemented with specific concentration (10–100 μg/mL) of plant extract. Test was performed in duplicate series. Negative control cultures were supplemented with an equal volume of 1 % DMSO. In addition, positive control cultures were incubated with 10–100 μg/mL sodium stibogluconate (SSG). Plates were incubated at 22 ± 1 °C for 48 h. After 48 h, promastigotes of L. donovani were stained with propidium iodide.15 The percentage viability was calculated by probit analysis using SPSS 18.0 software.
2.7. Cytotoxic effect on HeLa cells
Growth of HeLa cells was quantitated by studying the ability of living cells to reduce yellow dye 3-(4, 5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) to a blue formazan product. 6 × 103 cells of HeLa cell line were subsequently supplemented with different concentrations (10-1000 μg/mL) of plant extracts and incubated for 48 h. Then medium in each well was replaced by MTT solution (1 mg/mL) and incubated for 3–4 h. Formazan crystals produced by viable cells were dissolved in 100 μL of DMSO. Absorbance was then measured in ELISA plate reader at 560 nm. The effect of extracts was expressed by CC50 values (the drug concentration which reduces the absorbance of treated cells by 50% with respect to untreated cells).
2.8. Acute toxicity
Acute toxicity of the extract was determined by Limit test of Lorke.16 For this, inbred BALB/c mice were administered with different concentration of extract by oral route. The plant extract was dissolved in standard suspension vehicle (5 g of carboxy methyl cellulose, 5 mL of benzyl alcohol and 4 mL of Tween 80 in 1000 mL of 0.9% aqueous sodium chloride). The upper dose limit of 5 g/kg body weight of extracts was administered orally to 5 normal mice fasted for 4 h. All mice were observed for 14 days after the administration of different concentrations of extracts for adverse side effects in the form of mortality. Histopathological studies of organs i.e. liver and kidney were carried out at the end of study. LD50 was obtained by plotting linear dose-response curve of extract used and percentage of mortality obtained.
2.9. Groups of animals
The inbred BALB/c mice of both sexes, 5–-6 weeks old having 20–25 g weight were procured from Institute of Microbial Technology and reared in the Central Animal House of Panjab University, Chandigarh for present study. All the mice were kept in appropriate cages and fed with water and food ad libitum throughout the study period. Various groups included normal mice, infected mice, mice infected and treated with 40 mg/kg b.wt. of SSG for 5 days, mice infected and treated orally with 500 and 1000 mg/kg b.wt. of Bergenia ligulata ethanolic extract (BLEE) for 15 days. Each infected and treated group was divided into four subgroups. Each group comprised of 24 mice which further divided into four subgroups consisting of 6 mice in each subgroup. Subgroups were then sacrificed on 1,7,14 and 21 post treatment days (p.t.d.). The ethical clearance was obtained from Institutional Animal Ethics Committee (PU/IAEC/S/14/154) and all the experiments were carried out according to the guidelines of CPCSEA. Mice of different groups were infected intracardially with 107 promastigotes which were harvested from 2 to 3 days old culture.17
2.10. Assessment of parasite load
Parasite load was assessed in Giemsa stained liver imprints and expressed as Leishman Donovan Units (LDU) by the following formula18:
2.11. Determination of immune responses by
2.11.1. Enzyme linked immunosorbent assay (ELISA) for parasite-specific IgG1 and IgG2a isotypes
Serum concentrations of immunoglobulin G (IgG) isotype antibody responses were measured by conventional ELISA.17
2.11.2. Delayed type hypersensitivity (DTH) responses
DTH responses have been used to assess CMI in vivo. All groups of mice were challenged in the right foot pad with a subcutaneous injection of leishmanin. After 48 h, thickness of the right and left foot pads were measured using a pair of vernier callipers. The percentage increase in the thickness of the right foot pad as compared to the left was calculated.17
2.11.3. Cytokine responses
IL-12, IL-4, IL-10 and IFN-γ cytokine levels were assayed by ELISA in serum samples of mice using Diaclone kits.
2.12. Biochemical parameters
2.12.1. Liver and kidney function tests
Serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), alkaline phosphatase (ALP), acid phosphatase (ACP), lactate dehydrogenase (LDH), urea, creatinine and uric acid were estimated in serum samples of mice by using commercially available kits of Thermo Fisher Scientific Pvt. Ltd.
2.13. Histopathological parameters
Kidney and liver tissues from each subgroup were stained with hematoxylin and eosin (HE) to study histology.17
2.14. Statistical analysis
Statistical analysis of differences between mean values obtained from different experimental groups was done by means of two way ANOVA using Tukey post hoc test. P values less than 0.05 were considered as significant.
3. Results
3.1. Phytochemical constituents
The various phytochemical constituents found in BLEE are listed in Table 1.
Table 1.
Phytochemical constituents of B. ligulata ethanolic extract.
| Alkaloids | Flavonoids | Saponins | Triterpenes | Polysterols | Phenols | Tannins | Glycosides |
|---|---|---|---|---|---|---|---|
| +++ | ++ | + | +++ | ++ | + | + | ++ |
3.2. RP-HPLC analysis of bergenin in crude extract
The quantitative analysis of bergenin by RP-HPLC showed that the crude extract of B. ligulata contained 15.87 μg/mL of bergenin as obtained by regression equation (Fig. 1).
Fig. 1.
A, B. Chromatograms obtained from bergenin standard and crude extract of B. ligulata at 214 nm.
3.3. Antileishmanial activity
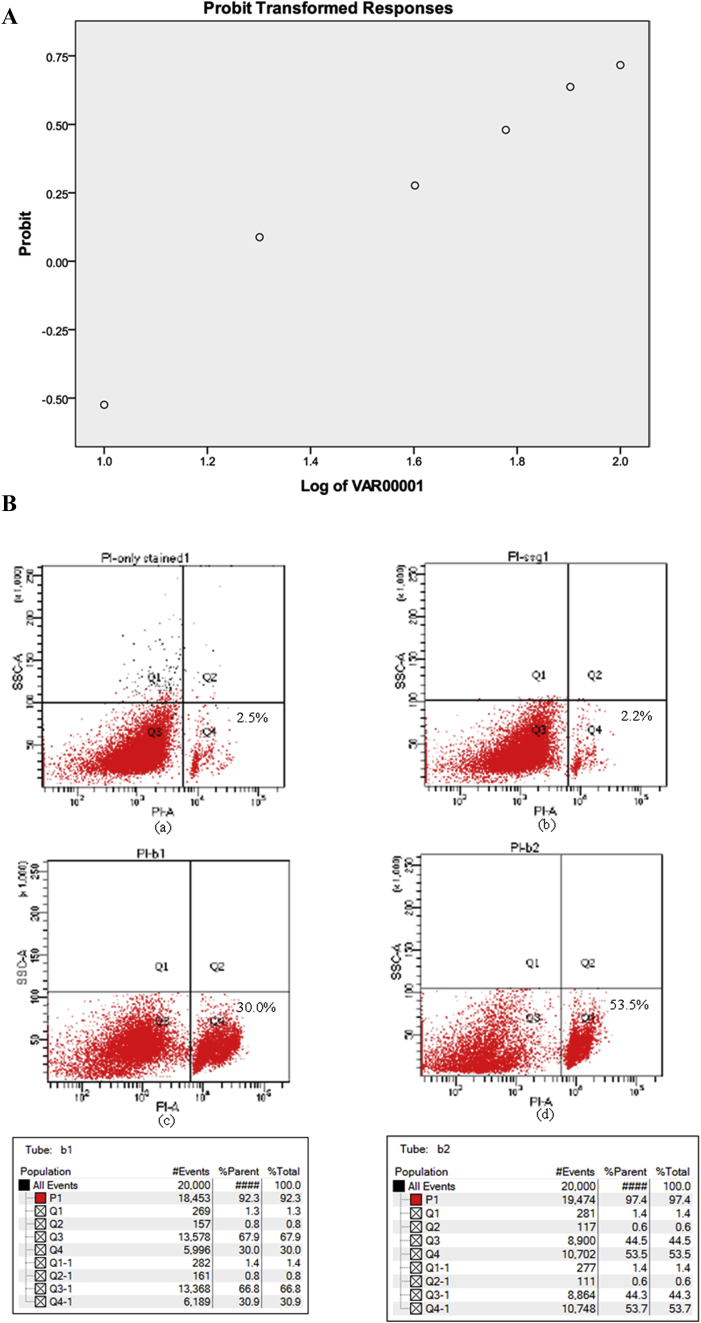
Promastigotes treated with different concentrations of BLEE extract showed significant growth inhibition after 48 h of treatment. Percentage growth inhibition was observed to be in a dose dependent manner. IC50 of BLEE was found to be 22.70 μg/mL by plotting graph using SPSS software (Fig. 2A,B).
Fig. 2.
A. Percentage growth inhibition of L. donovani promastigotes with B. ligulata ethanolic extract. B. Flow cytometry scatter plot of (a) only stained untreated promastigotes (b) promastigotes treated with 10 μg/mL of SSG (c) promastigotes treated with 10 μg/mL of BLEE (d) promastigotes treated with 20 μg/mL of BLEE.
3.4. Cytotoxicity of plant extract
HeLa cells were incubated for 48 h with the plant extract and their CC50 was calculated. The CC50 was revealed to be 6499 ± 195.47 μg/mL for BLEE and for SSG drug CC50 was found to be more than 1000 μg/mL which revealed that extract is non-toxic to HeLa cell line.
3.5. Acute toxicity
The acute toxicity of BLEE was found to be more than 5 g/kg b.wt. All the mice tolerated this concentration and no mortality was observed during two weeks observation period. No changes were observed in behaviour of mice within 2 h of dose administration. Histopathological examination of liver and spleen after 2 weeks did not reveal hepatomegaly/splenomegaly and were well comparable to normal tissues. All these results pointed towards the safety of BLEE.
3.6. Parasite load
Parasite load was observed in Leishman Donovan Units in the macrophages of liver smears. A significant reduction in parasite load was observed in animals treated with both the concentrations of plant extract. Maximum reduction in the parasite load (p < 0.05) was observed in animals treated with B. ligulata (BLEE) 1000 mg/kg b.wt. and the parasite load reduced by 91.10 % (466.14 ± 26.72) and 95.63 % (185.33 ± 26.1) respectively on 14 and 21 p.t.d. as compared to infected controls, where the parasite load was 5227.8 ± 136.0 on 60 post infection days (p.i.d.) and 4268.80 ± 124.00 on 67 p.i.d. Similarly, infection was suppressed to 76.87 % (1211.39 ± 42.88) and 79.88 % (853.0 ± 26.22) on 14 and 21 p.t.d. when infected animals were treated with BLEE 500 mg/kg b.wt. However, mice treated with standard drug SSG showed suppression of parasite load by 89.5 % (548.6 ± 25.4) and 92.04 % (339.6 ± 11.08) on 14 and 21 p.t.d. (Fig. 3).
Fig. 3.
Parasite load in different groups of animals. P value: Infected vs infected + SSG 40 mg/kg b.wt./infected + BLEE 500 mg/kg b.wt./infected + BLEE 1000 mg/kg b.wt. p* < 0.05.
3.7. IgG1 and IgG2a isotypes
IgG2a antibody response is an indicator of protective Th1 type of immune response. Therefore, treated animals produced significantly enhanced levels of this antibody when compared with the infected controls. Maximum amount of IgG2a antibody was produced by treatment of animals with 1000 mg/kg b.wt. of ethanolic extracts of B. ligulata (Fig. 4A). IgG1 antibody response points toward Th2 type (non protective type) of immune response. Therefore, it was found to be more in infected animals as compared to treated ones. Levels were found to be significantly reduced in treated animals as compared to infected controls. Minimum antibody levels were observed in animals treated with BLEE 1000 mg/kg b.wt. (Fig. 4B).
Fig. 4.
A, B: Levels of IgG2a and IG1 antibodies in different groups of animals. P value: Infected vs infected + SSG/infected + BLEE 500 mg/kg b.wt./infected + BLEE 1000 mg/kg b.wt. *p < 0.05.
3.8. Th1/Th2 type immune responses
The Th1 type of immune response was assessed through the levels of IFN-γ and IL-12 cytokines. Levels of IL-10 and IL-4 were used as indicators of Th2 type immune response. Animals treated with 1000 mg/kg b.wt. of BLEE produced maximum levels of IFN-γ (2188.61 ± 75.64) on 14 p.t.d. as compared to infected mice where significant and gradual reduction in IFN-γ levels from 501.01 ± 6.05 to 175.21 ± 12.5 pg/mL was observed on 46, 53, 60 and 67 p.i.d. Similarly in animals treated with BLEE 500 mg/kg b.wt., concentration was found to be significantly (p < 0.05) upregulated to 1598.29 ± 11.2 pg/mL when compared with infected mice (Fig. 5A). IL-12 is another cytokine produced by T cells which plays a critical role in prevention of disease progression. Level of this cytokine was estimated to be minimum in infected animals. But when mice were treated with both the concentrations of BLEE the levels were found to be significantly (p < 0.05) enhanced (Fig. 5B). However, cytokines IL-10 and IL-4 produced by spleen cells stimulate the progression of disease and their levels were found to be maximum in infected mice, whereas their concentration reduced significantly (p < 0.05) in treated groups of animals (Fig. 5C,D).
Fig. 5.
A, B, C, D: Concentration of cytokines IFNγ, IL-12, IL-4, IL-10 in serum samples of different groups of animal. P value: Infected vs infected + SSG/infected + BLEE 500 mg/kg b.wt./infected + BLEE 1000 mg/kg b.wt. *p < 0.05.
3.9. Delayed type hypersensitivity response
DTH response predicts the peripheral blood T cell immunity to VL. A slight increase (10.25 ± 0.62 to 12.88 ± 0.59%) in footpad thickness was observed in infected mice as compared to normal mice. But a significant (p < 0.05) rise in DTH response was observed when the mice were treated with both the concentrations of plant extract in all post treatment days (Fig. 6).
Fig. 6.
Delayed type hypersensitivity response in animals of different groups. P value: Infected vs infected + SSG/infected + BLEE 500 mg/kg b.wt./infected + BLEE 1000 mg/kg b.wt. *p < 0.05.
3.10. Liver function tests
SGOT, SGPT, ALP, ACP and LDH concentrations were estimated in various groups of mice after post infection and post treatment days in order to evaluate the normal functioning of liver. SGOT and SGPT enzymes were found to be produced at significantly (p < 0.05) higher level in serums of mice infected with L. donovani promastigotes as compared to normal control. The concentration of SGOT was 48.39 ± 1.04, 50.82 ± 2.01, 57.22 ± 4.57and 64.47 ± 3.04 IU/L in infected mice on 46, 53, 60 and 67 post infection days respectively. However, significant (p < 0.05) decrease of SGOT was observed in infected groups treated with 500 and 1000 mg/kg b.wt. of BLEE to normal levels with increase in post treatment days. Similarly, enzyme activity of SGPT increased significantly (p < 0.05) in infected mice from 150.19 ± 1.46 to 164.76 ± 4.35 IU/L on 46 to 67 p.i.d. as compared to normal mice. But normal levels were attained in all the infected mice treated with both concentrations of plant extract. SGPT activity in SSG treated mice was comparatively lower than infected animals but significantly higher than normal mice. However, ALP, ACP and LDH enzyme levels were in normal range in all groups of mice (Table 2).
Table 2.
Acitivities of liver enzymes in different groups of animals.
| Enzymes (normal range) | PT days | Normal control | Infected control | SSG (40mg/kg b.wt.) | Infected+ SSG (40mg/kg b.wt.) | BLEE (500 mg/kg b.wt.) | Infected+ BLEE (500 mg/kg b.wt.) | BLEE (10000mg/kg b.wt.) | Infected+ BLEE (1000 mg/kg b.wt.) |
|---|---|---|---|---|---|---|---|---|---|
|
SGOT (5–40IU/L) |
1 | 25.66 ± 1.19 | 48.39 ± 1.04 | 42.89 ± 2.17 | 60.47 ± 1.79∗ | 27.78 ± 2.17 | 30.35 ± 2.34∗ | 28.84 ± 2.48 | 23.34 ± 1.36∗ |
| 7 | 27.28 ± 1.24 | 50.82 ± 2.01 | 30.67 ± 1.68 | 34.56 ± 2.48 | 27.15 ± 1.38 | 30.17 ± 1.36∗ | 27.79 ± 2.71 | 22.02 ± 0.24∗ | |
| 14 | 28.15 ± 3.53 | 50.82 ± 4.57 | 28.91 ± 2.2 | 34.47 ± 1.48 | 26.16 ± 3.16 | 24.74 ± 2.0∗ | 27.2 ± 1.01 | 25.67 ± 3.41∗ | |
| 21 | 23.66 ± 3.13 | 64.47 ± 3.04 | 29.19 ± 1.29 | 27.09 ± 1.27∗ | 24.06 ± 1.01 | 22.88 ± 2.74∗ | 26.82 ± 0.98 | 24.07 ± 4.09∗ | |
|
SGPT (5–35IU/L) |
1 | 23.26 ± 1.45 | 70.19 ± 5.46 | 60.64 ± 6.89 | 97.56 ± 8.26∗ | 25.75 ± 2.36 | 30.35 ± 2.34∗ | 25.62 ± 1.35 | 30.34 ± 1.36∗ |
| 7 | 24.48 ± 1.74 | 81.82 ± 7.15 | 37.87 ± 4.16 | 45.34 ± 6.18 | 24.87 ± 3.45 | 31.17 ± 3.36∗ | 25.87 ± 1.62 | 32.02 ± 1.24∗ | |
| 14 | 24.52 ± 3.85 | 85.39 ± 4.88 | 33.28 ± 8.87 | 39.02 ± 7.13 | 25.54 ± 2.48 | 35.51 ± 4.7∗ | 26.66 ± 2.25 | 32.48 ± 1.33∗ | |
| 21 | 25.97 ± 2.66 | 92.76 ± 6.35 | 30.49 ± 5.46# | 35.89 ± 5.05 | 26.52 ± 3.21 | 33.52 ± 1.84∗ | 25.55 ± 1.8 | 30.78 ± 2.47∗ |
P value: Infected vs infected + SSG, infected + BLEE 500 mg/kg b.wt., infected + BLEE1000 mg/kg b.wt. *p < 0.05.
3.11. Kidney function tests
The effect of visceral leishmaniasis on kidney function of mice treated with BLEE was investigated. Abnormally high levels of urea pointed towards the renal dysfunction caused by visceral leishmaniasis. The serum samples from infected mice contained significantly (p < 0.05) higher levels of urea as compared to normal mice. Concentration of urea was 50.18 ± 2.41 on 1 p.t.d. and 51.96 ± 2.18 mg/dL on 21 p.t.d. when compared with levels of urea in mice treated with 500 and 1000 mg/kg b.wt. of BLEE which was found in normal range. However, levels of creatinine and uric acid were in normal range of 0.85–1.35 mg/dL and 3–6.7 mg respectively in all groups of animals (Table 3).
Table 3.
Serum levels of urea in various groups of animals.
| Enzymes (normal range) | PT days | Normal control | Infected control | SSG (40 mg/kg b.wt.) | Infected + SSG (40 mg/kg b.wt.) | BLEE (500 mg/kg b.wt.) | Infected + BLEE (500 mg/kg b.wt.) | BLEE (10000 mg/kg b.wt.) | Infected + BLEE (1000 mg/kg b.wt.) |
|---|---|---|---|---|---|---|---|---|---|
|
UREA (10–45 mg/dl) |
1 | 24.36 ± 1.81 | 43.18 ± 2.41 | 35.15 ± 2.23 | 45.26 ± 2.34∗ | 24.16 ± 1.38 | 25.6 ± 1.54∗ | 24.5 ± 1.41 | 25.62 ± 1.04∗ |
| 7 | 25.26 ± 1.28 | 45.04 ± 1.89 | 32.47 ± 2.17 | 37.39 ± 2.26∗ | 24.17 ± 1.48 | 25.24 ± 1.27∗ | 24.38 ± 1.32 | 25.71 ± 1.18∗ | |
| 14 | 23.72 ± 2.27 | 43.21 ± 3.99 | 29.36 ± 0.69 | 39.28 ± 1.54∗ | 24.04 ± 1.88 | 24.32 ± 3.21∗ | 23.99 ± 1.32 | 25.66 ± 3.58∗ | |
| 21 | 23 ± 2.54 | 42.96 ± 2.18 | 31.43 ± 0.84 | 39.61 ± 0.68∗ | 24.22 ± 1.91 | 25.67 ± 3.15∗ | 23.87 ± 1.87 | 25.29 ± 2.55∗ |
P value: Infected vs infected + SSG, infected + BLEE 500 mg/kg b.wt., infected + BLEE1000 mg/kg b.wt. *p < 0.05.
3.12. Histopathological studies
3.12.1. Kidney
The results of histopathology supported the biochemical parameters used to study the normal functioning of liver and kidney. Kidney tissue of infected mice revealed the presence of some foci of lymphocytic infilteration in the interstitium which showed focal interstitial nephritis. However, in SSG treated animals, the kidney architecture was normal except at some places where swelling and congestion in dilated glomeruli was observed. When mice were treated with BLEE, the glomeruli, PCT, DCT and Bowman's capsule appeared to be normal (Fig. 7).
Fig. 7.
T.S. of kidney of BALB/c mice stained with haematoxylin and eosin stain (A) Normal control (10X) (B) Infected Control (10X) (C) Infected mice treated with SSG (10X) (D) Mice treated with 1000 mg/kg b.wt. of BLEE (40X).
3.12.2. Liver
The hematoxylin/eosin stained transverse sections of livers of infected mice showed vacuolated hepatocytes, lymphocytic aggregates along with kupffer cell hyperplasia. In addition, there were several intralobular granuloma formations which caused hepatocyte atrophy. In contrast, light microscopic observations of liver tissue of mice treated with both concentrations of BLEE showed normal large hepatic lobules with polygonal cells having hepatic sinusoids arranged in between the hepatic cords with fine arrangement of kupffer cells (Fig. 8).
Fig. 8.
T.S. of liver of BALB/c mice stained with haematoxylin and eosin stain (A) Normal control (10X) (B) Infected Control (40X) (C) Infected mice treated with SSG (10X) (D) Mice treated with 1000mg/kg b.wt. of BLEE (10X).
4. Disscussion
Bergenia ligulata, commonly known as Pashanbhed, belongs to family Saxifragaceae and is a well known Indian traditional medicinal plant which has been extensively used in the past for various ailments. Rhizomes of this plant are referred by Ayurvedic system for the treatment of kidney stones since decades. The active component bergenin isolated from rhizomes of B. ligulata has been known to ameliorate the renal dysfunction in hyperoxaluric rats by inhibiting the growth of calcium oxalate crystals.11 It is also known to have antiviral, antihepatotoxic, antiulcerogenic, anti-HIV, antifungal, antiplasmodial, hepatoprotective, antiarrhythmic, neuroprotective, anti-inflammatory, immunomodulatory and burn wound healing properties.19 Moreover, ethanolic extract of rhizomes of B. ligulata and bergenin have also been acclaimed for their hepatoprotective, diuretic and antipyretic properties.10 These properties of B. ligulata encouraged us to evaluate its antileishmanial efficacy during L. donovani infection in BALB/c mice.
In the present study, BLEE exhibited significant inhibitory activity against promastigotes of L. donovani with IC50 of 22.70 μg/mL and is comparable to methanol extract of Achillea biebersteinii (26.9 ± 2.9 μg/mL) used against L. amazonensis.20 It was also found to have negligible cytotoxicity against HeLa cells, therefore, the selectivity index of >100 i.e. 144 inspired us to further examine the efficacy of BLEE in murine animal model. A significant reduction of ∼95% of amastigotes in liver macrophages of mice treated with higher dosage of BLEE can be accredited to its active phytochemical compounds. The major phytochemicals present in this extract were found to be alkaloids, flavonoids, terpenes, polysterols, tannins, anthroquinones and cardiac glycosides. Previous studies also reported the presence of various active biochemical constituents like bergenin, pashaanolactone, β-sitosterol, stigmesterol, tannic acid, gallic acid, parasorbic acid, isovaleric acid, 1,8-cineole which may be responsible for its medicinal properties.21 Supportive evidence for the confirmation of above mentioned statement was obtained from various studies as bergenin and its natural derivative 11-O-galloylbergenin isolated from B. ligulata showed antiplasmodial activities with IC50 value less than 2.5 μM against Plasmodium falciparum.8 Bergenin also showed an inhibitory effect on the growth of Trypanosoma brucei with an IC50 value of 1 mM.22 Ethanolic extract of Bergenia ciliata also exhibited significant schizonticidal activity at a dosage of 1000 mg/kg b.wt. against Plasmodium berghei in Swiss albino mice.23
The suppression or progression of visceral leishmaniasis is mainly characterized by cell mediated immune responses which include DTH responses and Th1/Th2 cytokine responses. The delayed type hypersensitivity response is an important type of defense mechanism against VL and is an indication of efficacy of immune system to establish a long lasting antigen specific T cell responses.24 Therefore, cellular response to Leishmania infection was evaluated in vivo by DTH skin test using leishmanin as antigen. Our study also demonstrated the strong immunogenic property of both the doses of BLEE with significant increase in foot pad thickness. It was also evident from a previous study that significant increase in delayed type hypersensitivity response occurred in mice treated with 2 mg/mL of pectic polysaccharide bergenan for three weeks isolated from Bergenia crassifolia against ovalbumin. Bergenan (100 mg/mL) was also found to enhance the uptake capacity of human neutrophils and was shown to stimulate the generation of oxygen radicals by mouse peritoneal macrophages.25 In addition, VL also results in marked suppression of Th1 type of immune responses as evidenced by weak proliferation of T cells and production of IL-12 and IFN-γ. In contrast, predominant Th2 type of immune response is characterized by higher levels of IL-10 and IL-4.26 IL-12 is secreted by antigen-presenting cells i.e. macrophages which further stimulate the proliferation of CD4 + Th1 cells. These cells secrete IL-2 and interferon gamma cytokines, thus eliciting the protective immune response against VL.27
Our results also reported the enhanced levels of IL-10 and IL-4 cytokines as compared to IL-12 and IFN-γ in infected mice. A relatively higher concentration of Th1 cytokines i.e. IL-12 and IFN-γ was observed in mice treated with both the doses of BLEE as compared to IL-10 and IL-4 cytokines. The triterpenoids fractions from the rhizomes of Astilbe chinensis of same family saxifragaceae also remarkably increased proliferation of splenocytes, NK cell activity, level of IL-2 secreted by splenocytes in tumor bearing mice, promote the DTH reaction and enhanced anti-SRBC antibody levels in naive mice.28 Bergenin and non bergenin isolates from B. stracheyi also showed the potential Th1/Th2 cytokine balancing activity against adjuvant induced arthritis.29 In our laboratory studies, Withania somnifera and Tinospora cordifolia in combination with cisplatin significanty upregulated Th1 type of immune responses by enhancing the levels of IL-2 and IFN-γ over IL-10 and IL-4 against visceral leishmaniasis.30, 31 Although the cell mediated immune response plays a pivotal role in progression or suppression of disease, but the role of humoral immune response cannot be ignored since high levels of Leishmania specific antibodies are seen in serum samples immediately after infection and prior to immunological abnormalities.32 Likewise, high levels of IgG2a were observed in serum samples of infected mice treated with both concentrations of plant extracts as compared to IgG1 antibody.
Treatment of mice with both doses of BLEE helped in restoration of normal levels of liver enzymes. These results are consistent with another study where the hepatotoxicity caused by carbon tetrachloride was cured by alcoholic extract of 500 mg/kg b.wt. of rhizomes of B. ligulata by significantly reducing the levels of SGOT, SGPT, ALP and total bilirubin.21 Increase in the levels of liver enzymes is also related to damage to liver caused by visceral leishmaniasis. It has been shown in various studies that the infection with L. chagasi caused intense reaction of the kupffer cells, capsule and portal inflammation and presence of intralobular granulomas in the different clinical groups of symptomatic and asymptomatic dogs.33 Similarly, in present study the light microscopic examination revealed an increase in kupffer cells and lymphocytic infiltration in liver tissues of infected mice which restored to normal after treatment of mice with both the doses of plant extract.
The involvement of visceral leishmaniasis with nephropathy is only partially understood. However, the most frequent pathologies found are proliferative glomerulonephritis and interstitial nephritis.34 However, in the present study, the levels of urea, creatinine and uric acid were found to be in normal range in all the groups of animals.
Visceral leishmaniasis continues to constitute a severe public health threat,35 causing considerable mortality and major disability in the Indian subcontinent36 due to its challenging behaviour as a re-emerging disease at a fast deteriorating rate with urban epidemics and burden is upsurging year after year.37 In the current scenario of non availability of any safe, non toxic, effective and affordable drug, the crude plant extract has been explored to have antileishmanial activity. The present study illustrates considerable antileishmanial activity of BLEE against L. donovani infection in both in vitro and in vivo studies by switching on the Th1 type of protective immune responses along with their safety as depicted by normal hepatic and renal function tests. Therefore, this extract proves to be good alternative for the treatment of visceral leishmaniasis. Further studies will be performed to isolate active phytoconstituents from this extract which might be responsible for its antileishmanial and immunomodulatory activities. The studies will also be carried on higher animal models for a better understanding of the immune responses before it is to be tested in leishmaniasis patients.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgement
The authors are thankful to University Grants Commission- Basic Science Research (UGC-BSR) and CAS-II for their financial assistance. The authors are also grateful to DST-FIST for flowcytometer.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Gellis A., Dume tre A., Lanzada G. Preparation and antiprotozoal evaluation of promising b-carboline alkaloids. Biomed Pharmacother. 2011;66:339–347. doi: 10.1016/j.biopha.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Wright C.W. Traditional antimalarials and the development of novel antimalarial drugs. J. Ethnopharmacol. 2005;100:67–71. doi: 10.1016/j.jep.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 3.WHO Leishmaniasis in high burden countries: an epidemiological update based on data reported in 2014. Wkly Epidemiol. 2016;91:285–296. Rec No. 22. [PubMed] [Google Scholar]
- 4.Kaur S., Chauhan K., Sachdeva H. Protection against experimental visceral leishmaniasis by immunostimulation with herbal drugs derived from Withania somnifera and Asparagus racemosus. J Med Microbiol. 2014;63:1328–1338. doi: 10.1099/jmm.0.072694-0. [DOI] [PubMed] [Google Scholar]
- 5.Dias L.C., de Lima G.M., Pinheiro C.B. Design, structural and spectroscopic elucidation of new nitroaromatic carboxylic acids and semicarbazones for the in vitro screening of anti-leishmanial activity. J Mol Struct. 2015;1079:298–306. [Google Scholar]
- 6.Kieffer C., Cohen A., Verhaeghe P. Looking for new antileishmanial derivatives in 8-nitroquinolin-2(1H)-one series. Eur J Med Chem. 2015;92:282–294. doi: 10.1016/j.ejmech.2014.12.056. [DOI] [PubMed] [Google Scholar]
- 7.de Carvalho P.B., Ferreira E.I. Leishmaniasis phytotherapy. Nature's leadership against an ancient disease. Fitoterapia. 2001;72:599–618. doi: 10.1016/s0367-326x(01)00301-x. [DOI] [PubMed] [Google Scholar]
- 8.Uddin G., Sadat A., Siddiqui B.S. Comparative antioxidant and antiplasmodial activities of 11-O-galloylbergenin and bergenin isolated from Bergenia ligulata. Trop Biomed. 2014;31(1):143–148. [PubMed] [Google Scholar]
- 9.Bajracharya G.B. Diversity, pharmacology and synthesis of bergenin and its derivatives: potential materials for therapeutic usages. Fitoterapia. 2015;101:133–152. doi: 10.1016/j.fitote.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Singh N., Juyal V., Gupta A.K., Gahlot M. Evaluation of ethanolic extract of root of Bergenia ligulata for hepatoprotective, diuretic and antipyretic activities. J Pharm Res. 2009;2(5):958–960. [Google Scholar]
- 11.Aggarwal D., Kaushal R., Kaur T., Bijarnia R.K., Puri S., Singla S.K. The most potent antilithiatic agent ameliorating renal dysfunction and oxidative stress from Bergenia ligulata rhizome. J. Ethnopharm. 2014;158:85–93. doi: 10.1016/j.jep.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Rao R.R., Mahajan R.C., Ganguly N.K. Modified media for in vitro cultivation of Leishmania promastigotes. a comparative study. Bull P.G.I. 1984;18:125–128. [Google Scholar]
- 13.Trease G.E., Evans W.C. In: Trease and Evans Pharmocognosy. 13th edition. Tindall B., editor. W.B. Saunders Company; London: 1989. p. 672. [Google Scholar]
- 14.Tiwari P., Bimlesh K., Kaur M., Kaur G., Kaur H. Phytochemical screening and extraction: a review. Int Pharma Sci. 2011;1:98–106. [Google Scholar]
- 15.Riccardy C., Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Proctoc. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- 16.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 17.Sharma M., Sehgal R., Kaur S. Evaluation of nephroprotective and immunomodulatory activities of antioxidants in combination with cisplatin against murine visceral leishmaniasis. PLoS Negl Trop Dis. 2012;6(5):e1629. doi: 10.1371/journal.pntd.0001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley D.J., Kirkley J. Regulation of Leishmania population within host 1. The variable course of L. donovani infections in mice. Clin Exp Immunol. 1977;30:119–129. [PMC free article] [PubMed] [Google Scholar]
- 19.Patel D.K., Patel K., Kumar R., Gadewar M., Tahilyani V. Pharmacological and analytical aspects of bergenin: a concise report. Asian Pac J Trop Dis. 2012;2:163–167. doi: 10.1016/S2221-1691(12)60239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Sokari S.S., Ali N.A.A., Monzote L., Al-Fatimi M.A. Evaluation of antileishmanial activity of Albaha medicinal plants against Leishmania amazonensis. Biomed Res Int. 2015;2015:938747. doi: 10.1155/2015/938747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruby K.M., Dwivedi J., Chouhan R. Pashanbheda a golden herb of Himalaya: a review. Int J Pharm Rev Res. 2012;2(2):97–105. [Google Scholar]
- 22.Nyasse B., Nono J., Sonke B., Denier C., Fontaine C. Trypanocidal activity of bergenin, the major constituent of Flueggea virosa, on Trypanosoma brucei. Pharmazie. 2004;59:492–494. [PubMed] [Google Scholar]
- 23.Walter N.S., Bagai U., Kalia S. Antimalarial activity of Bergenia ciliata (Haw.) Sternb. against Plasmodium berghei. Parasitol Res. 2013;112(9):3123–3128. doi: 10.1007/s00436-013-3487-z. [DOI] [PubMed] [Google Scholar]
- 24.Lechner A., Ritter U., Varona R., Marquez G., Bogdan C., Körner H. Protective immunity and delayed type hypersensitivity reaction are uncoupled in experimental Leishmania major infection of CCR6-negative mice. Microbe Infect. 2007;9:291–299. doi: 10.1016/j.micinf.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Popov S.V., Popova G.Y., Nikolaeva S.Y., Golovchenko V.V., Ovodova R.G. Immunostimulating activity of pectic polysaccharide from Bergenia crassifolia(L) Fritsch. Phytother. Res. 2005;19:1052–1056. doi: 10.1002/ptr.1789. [DOI] [PubMed] [Google Scholar]
- 26.Mutiso J.M., Macharia J.C., Taracha E., Wafula K., Rikoi H., Gicheru M.M. Safety and skin delayed-type hypersensitivity response in vervet monkeys immunized with Leishmania donovani sonicate antigen delivered with adjuvants. Rev Inst Med Trop. 2012;54(1):37–41. doi: 10.1590/s0036-46652012000100007. [DOI] [PubMed] [Google Scholar]
- 27.Sharma U., Sharma S. Immunobiology to leishmaniasis. Indian J Exp Biol. 2009;47:412–423. [PubMed] [Google Scholar]
- 28.Tu J., Sun H.X., Ye Y.P. Immunomodulatory and antitumor activity of triterpenoid fractions from the rhizomes of Astilbe chinensis. J Ethnopharmacol. 2008;119(2):266–271. doi: 10.1016/j.jep.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Nazir N., Koul S., Qurishi M.A. Immunomodulatory effect of bergenin and norbergenin against adjuvant-induced arthritis-a flow cytometric study. J Ethnopharmacol. 2007;112(2):401–405. doi: 10.1016/j.jep.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Sachdeva H., Sehgal R., Kaur S. Studies on the protective and immunomodulatory efficacy of Withania somnifera along with cisplatin against experimental visceral leishmaniasis. Parasitol Res. 2013;112:2269–2280. doi: 10.1007/s00436-013-3387-2. [DOI] [PubMed] [Google Scholar]
- 31.Sachdeva H., Sehgal R., Kaur S. Tinospora cordifolia as a protective and immunomodulatory agent in combination with cisplatin against murine visceral leishmaniasis. Exp Parasitol. 2014;137:53–65. doi: 10.1016/j.exppara.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Saha S., Mondal S., Banerjee A., Ghose J., Bhowmick S., Ali N. Immune responses in kala-azar. Indian J Med Res. 2006;123(3):245–266. [PubMed] [Google Scholar]
- 33.Giunchetti R.C., Mayrink W., Carneiro C.M. Histopathological and immunohistochemical investigations of the hepatic compartment associated with parasitism and serum biochemical changes in canine visceral leishmaniasis. Res Vet Sci. 2008;84(2):269–277. doi: 10.1016/j.rvsc.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Daher E.F., Sampaio A.M., Martiniano L.V.M., Vieira A.P.F., Silva Junior G.B. Acute kidney injury in visceral leishmaniasis: a cohort of 10 patients admitted to a specialized intensive care unit in northeast of Brazil. Asian Pac J Trop Dis. 2013;3:41–46. [Google Scholar]
- 35.Bhunia G.S., Kesari S., Chatterjee N., Kumar V., Das P. The burden of visceral leishmaniasis in India: challenges in using remote sensing and GIS to understand and control. ISRN Infect Dis. 2013;2013:1–14. [Google Scholar]
- 36.Singh O.P., Singh B., Chakravarty J., Sundar S. Current challenges in treatment options for visceral leishmaniasis in India: a public health perspective. Infect Dis Poverty. 2016;5:1–15. doi: 10.1186/s40249-016-0112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leta S., Dao T.H.T., Mesele F., Alemayehu G. Visceral leishmaniasis in Ethiopia: an evolving disease. PLoS Negl Trop Dis. 2014;8:e3131. doi: 10.1371/journal.pntd.0003131. [DOI] [PMC free article] [PubMed] [Google Scholar]