Abstract
The distribution of lipids in tears is critical to their function. Lipids in human tears may retard evaporation by forming a surface barrier at the air interface. Lipids complexed with the major lipid binding protein in tears, tear lipocalin, reside in the bulk (aqueous) and may have functions unrelated to the surface. Many new lipids species have been revealed through recent mass spectrometric studies. Their association with lipid binding proteins has not been studied. Squalene, (O-acyl) omega-hydroxy fatty acids (OAHFA) and ceramides are examples. Even well known lipids such as wax and cholesteryl esters are only presumed to be unbound because extracts of protein fractions of tears were devoid of these lipids. Our purpose was to determine by direct binding assays if the aforementioned lipids can bind tear lipocalin. Lipids were screened for ability to displace DAUDA from tear lipocalin in a fluorescence displacement assay. Di- and tri-glycerides, squalene, OAHFA, wax and cholesterol esters did not displace DAUDA from tear lipocalin. However, ceramides displaced DAUDA. Apparent dissociation constants for ceramide-tear lipocalin complexes using fluorescent analogs were measured consistently in the submicromolar range with 3 methods, linear spectral summation, high speed centrifugal precipitation and standard fluorescence assays. At the relatively small concentrations in tears, all ceramides were complexed to tear lipocalin. The lack of binding of di- and tri- glycerides, squalene, OAHFA, as well as wax and cholesterol esters to tear lipocalin is consonant with residence of these lipids near the air interface.
Keywords: Tear lipocalin, Lipocalin-1, LCN1, Lipid binding, ceramide, linear spectral summation, DAUDA, squalene, tears, dry eye, wax esters, cholesterol esters, (O-acyl) omega-hydroxy fatty acids (OAHFA), di- and tri- acylglycerols
Graphical Abstract

1.0 Introduction
Numerous studies have elucidated the lipid components in human tears, Table 1.[1–23] These studies show that classes and species of lipids in tears are surprisingly consistent. The most abundant tear lipids are wax and cholesteryl esters. Phospholipids including sphingomyelin, as well as fatty acids and alcohols are present. Squalene has been recently identified in tears. [23,24] Ten species of ceramides (sphingolipids) have been identified.[18] Quantitative differences in lipid composition evident in Table 1 may be due to many factors such as variations in collection technique, samples, spectrometric methods, ion suppression, and instrument response function.[8,20,21,25]
TABLE 1.
Studies of the Lipid Composition in Human Tears
| Lipid Author | DAG | TAG | WE | Ch | CE | Foh | FA | OAHFA | PL | Squ | GL | CER |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [1] | + | + | + | + | + | + | + | |||||
| [2] | 5mM | |||||||||||
| [3] | 0.8mM | |||||||||||
| [4] | 6.9% | 7.1% | 9.7% | 18.3% | 0.9% | 55% | ||||||
| [5] | 1.45mM | |||||||||||
| [6] | 15% | <15% | 15% | |||||||||
| [7,8] | + | + | + | + | + | + | + | + | ||||
| [9,10] | + | + | + | + | + | |||||||
| [11] | + | + | + | |||||||||
| [12,13] | + | + | + | 0.5–1% | + | neg/small | + | |||||
| [14,15] | + | |||||||||||
| [16] | + | + | + | − | ||||||||
| [17] | 0.01mM | 0.02mM | 0.048mM | 0.0009mM | ||||||||
| [18,19] | 0.3% | 2.8% | 35.2% | 5.4% | 44.9% | 3% | 0.4% | 0.26% | ||||
| [20–22] | 1–2% | 18.6–45% | 8–18.6% | 35.7–54.8% | 1–4.4% | 5.4–12% | ||||||
| [23] | 10% | 45% | 38% | 7% |
% is the calculated mole percent as calculated by the various authors.
Abbreviations: DAG- diacylglycerol, TAG- triacylglycerol, WE-wax ester, Ch-cholesterol, CE-cholesteryl ester, Foh-fatty alcohol, FA-fatty acid, OAHFA-(o-acyl) omega-hydroxy fatty acid, PL- phospholipid, Squ-squalene, GL-glycolipid, CER-ceramide
The tear film has been classically modeled by components that populate each of 3 distinct layers. The components are 1. ocular surface membrane spanning mucins 2. aqueous soluble proteins including mucins and 3. lipids at the air-aqueous interface. The presumed location of lipids is based on interferometry that discern a surface layer with a different index of refraction than the bulk.[26] The mean thicknesses accounting for interference fringes as measured by a number of techniques are about 40–90 nm but with a dynamic range between ~15–160 nm.[25,27–32] Schemes for modeling the position of individual components within the layer are generally predicated on differences of polarity of classes of lipids. In such schemes non-polar lipids such as wax and cholesterol esters have been modeled at the air-interface. Polar lipids, such as fatty acids and alcohols and even phospholipids, are proffered to reside near the aqueous-lipid interface.[19,25] The assumptions for these models have been aptly challenged.[33]
Most of the tear film lipids feature long alkyl chains which are suited for retardation of evaporation.[34] However, many tear film lipids are completely insoluble in water. Molecular associations of insoluble lipids with proteins no doubt affect their position and add a dimension poorly accounted by the tear film models. For example many tear lipids, including fatty acids and alcohols, glycolipids, phospholipids, cholesterol and even diacylglycerols, have been found complexed to tear lipocalin and some have been tested with binding assays.[7,8,12,35–38] Tear lipocalin resides in the bulk. In native human tears, tear lipocalin is not completely saturated with lipid. Most lipids associated with tear lipocalin are expected to dwell in the aqueous portion of the tears not in the putative lipid layer. Other lipid binding proteins in tears are insignificant in amount compared to tear lipocalin. [39–43]
Wax and cholesterol esters are absent in extracts from native or expressed tear lipocalin as determined by thin layer chromatograms.[7,44] These lipids are presumed to reside at the air-lipid interface of the tear film based on their chemical properties. The location(s) of recently identified tear lipids, squalene, OAHFA and ceramides are unknown. These lipids were not specifically studied in earlier analysis of extracts from lipocalin.[7,12] Knowledge of their binding to tear lipocalin is critical to being able to localise these lipids to the aqueous or the lipid layer of the tear film and consequently contribute to better modelling of the lipid layer. Similarly, the binding of other lipids such as diacylglycerols (diglyceride)to tear lipocalin is controversial. Some groups presumably identified diacylglycerol in extracts of lipocalin fractions of tears [12], but not other investigators.[7] Our purpose is to fill this gap in knowledge by investigating the binding of examples of these lipid classes to tear lipocalin.
2 Materials and Methods
2.1 Reagents
C18-ceramide (ceramide- N-(octadecanoyl) sphing-4-enine, also known as N-stearoyl-D-erythro-sphingosine), was obtained from ACROS Organic (Pittsburg, PA). C6-NBD ceramide (N-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-D-erythro-sphingosine) and C12-NBD ceramide (N-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-D-erythro-sphingosine) were obtained from Avanti Polar Lipids (Alabaster, AL). Squalene and the wax ester, stearyl behenate were purchased from MP Biomedicals, LLC (Solon, OH). Behenyl stearate and behenyl oleate were purchased from Indofine Chemical Company, Inc. (Hillsborough, NJ). Cholesteryl oleate was purchased from Alfa Aesar (Tewksbury, MA). Stearic acid, cholesteryl stearate, oleoyl chloride, 16-hydroxypalmitic acid, 1,3-distearin, tristearin and NBD-Cl (4-chloro-7-nitrobenzofurazan) were obtained from Sigma (St. Louis, MO). DAUDA or 11-(((5-(dimethlyamino)-1-naphthalenyl)sulfonyl)amino) undecanoic acid was obtained from Molecular Probes (Eugene, OR). 1,2-distearoyl-sn-glycerol (1,2-distearin) was purchased from Chem-Impex Int’l Inc. (Wood Dale, IL). The structures of the key lipids in this study are shown in Figure 1 in [45]. Egg white lysozyme was obtained from ICN Biomedicals Inc. (Aurora, OH). (O-oleoyl)-16- hydroxypalmitic acid was synthesized with minor modifications from previous published methods.[46,47] The details of synthesis and mass spectrometric validation are provided (Figure 2 in [45]).
2.2 Spectroscopy
Fluorescence measurements were made at 20°C in a Jobin Yvon-SPEX Fluorolog-3 spectrofluorimeter; bandwidth for excitation was 2 nm and for emission, 4 nm. Raman and background scattering by the solvent were corrected where necessary using appropriate blank solutions.
Ultraviolet-visible absorption spectrophotometric measurements were obtained with a Shimadzu UV-2401PC instrument, (Kyoto, Japan). The concentrations of proteins and fluorescent lipids were calculated from molar extinction coefficients that have been published or derived herein (Table 2).[48–52]
TABLE 2.
Molar Extinction Coefficients in Solvents of Tested Compounds
| Chromophore | solvent | ε (M−1 cm−1) | λabs (nm) | Reference |
|---|---|---|---|---|
| DAUDA | ethanol | 4400 | 335 | [48] |
| C6-NBD ceramide | methanol | 22000 | 465 | [49] |
| C6-NBD ceramide-tear lipocalin complex | NaP, pH 7.3 | 22000 | 475 | current work |
| C12-NBD ceramide | methanol | 22000 | 465 | [49] |
| NBD-Cl | methanol | 9800 | 336 | [50] |
| Tear lipocalin | NaP, pH 7.3 | 13760 | 280 | [51] |
| Lysozyme | NaP, pH 7.3 | 37970 | 280 | [52] |
ε is the molar extinction coefficient; λabs is the peak wavelength of absorbance.
2.3 Collection of Human Tears for Protein Purification
Stimulated human tears were collected from healthy volunteers in accordance with the tenets of the Declaration of Helsinki and approved by the institutional review board. Informed consent was obtained from donors after explanation of the nature and possible consequences. Collection was performed as previously published [8,53,54] with polished glass tips and glass transfer pipettes. Tears were pooled in polytetrafluoroethylene-lined glass vials and stored under nitrogen at −80°C until use.
2.4 Purification of tear lipocalin
Fractionation of the tear proteins included: gel filtration liquid chromatography using the AKTA purifier Versatile FPLC with Superdex 75 preparative column, (GE Healthcare, Piscataway, NJ) followed by pooling of peak tear lipocalin fractions, anion exchange chromatography using DEAE-Sephadex A-25 and analysis by tricine polyacrylamide gel electrophoresis as previously published.[7,8,54] The amount tear lipocalin was determined from the molar extinction coefficient (Table 2). This purification scheme results in six isoforms that have been previously characterized by mass spectrometry and sequenced. The major isoform has 157 amino acids and a mass of 17,446.5 Da.[54,55] Even though native ligands remain complexed to lipocalin after purification, additional capacity for binding partners has been repeatedly demonstrated.[35,38,44,53,56,57] Notwithstanding slightly reduced binding, use of native tear lipocalin purified in this way avoids structural perturbations seen after partial delipidation with organic solvents.[51,58,59] An inescapable consequence is that the dissociation constants and stoichiometry derived from the use of tear lipocalin are apparent and reflect only the native protein’s additional capacity for complexing ligand.
2.5 DAUDA displacement fluorescence assay
In order to test the binding of tear lipocalin to key tear lipids, cholesteryl stearate, cholesteryl oleate, behenyl stearate, behenyl oleate, stearyl behenate, (O-oleoyl)-16- hydroxypalmitic acid, squalene, C18-ceramide, di- and tri- glycerides were screened for the displacement of DAUDA from the binding site of tear lipocalin. Displacement of DAUDA from the hydrophobic environment of the binding site results in a decrease in fluorescence (λem =490 nm, λex =345 nm). Stearic acid, which was shown previously to bind tear lipocalin, served as a positive control.[56]
In general the ligand complex resulting from a mixture of tear lipocalin and DAUDA (5 μM each) in 10 mM sodium phosphate (pH 7.3) was titrated by successive addition of 0.2μL the test lipid. Each addition increased the final concentration of test lipid by 2 μM. The final concentration was about 18 μM. The lipids in their respective solvents were: DAUDA, squalene, 1,2 distearin, 1,3 distearin, tristearin, and (O-oleoyl)-16- hydroxypalmitic acid (ethanol), cholesteryl stearate, cholesteryl oleate, behenyl stearate, stearyl behenate, and behenyl oleate (chloroform), as well as C18-ceramide (methanol). At the end of each titration experiment, the total organic solvent concentration did not exceed 2%. To ensure that the various solvents did not impact the fluorescence spectra, solvent alone was added to the solution prior to the addition of the lipid and the maximum amount of solvent used was added at the end of the experiment. The effects on the spectra were negligible.
2.6 High speed centrifugal precipitation assay for ceramide-tear lipocalin binding complexes
This binding assay is based on separating unbound lipids from those bound to tear lipocalin or other proteins by capitalizing on centrifugal precipitation of the insoluble lipid in water.[60] Long chain lipids such as ceramides are insoluble in buffer and particularly suited to this technique. C6 and C12 -NBD ceramides showed complete precipitation in 10 mM Na-phosphate pH 7.3. NBD labeled lipids have a large extinction coefficient in methanol or bound to protein (Table 2). Measurements by ultra violet- visible absorbance spectrophotometry are accurate for both unbound ceramide in methanol and protein bound ceramides provided the lipids bear an NBD label. Tear lipocalin, 10 or 20 μM, 500 μL in 10 mM sodium phosphate (pH 7.3), was incubated with successively increasing concentrations of C6 or C12 -NBD ceramides, respectively in DMSO. In experiments with C6-NBD ceramide 0.5 μL increments of 1 mM C6-NBD ceramide in DMSO were added to obtain total final concentrations of ligand from 1 to 20 μM. In experiments with C12-NBD ceramide 0.5 μL increments of 2 mM of C12-NBD ceramide were added to 500 μL 20 μM tear lipocalin in 10 mM sodium phosphate (pH 7.3) in DMSO to obtain total concentrations from 2 to 40 μM. The maximum concentration of DMSO in any sample with 20 μM of ligand was 2%. Mixtures of tear lipocalin and ligand were incubated at either 25°C for 30 min or 34°C for 16 hours, followed by centrifugation in a Sorvall Discovery M150 (Thermo Fisher Scientific, Fairlawn, NJ, USA) with an S150AT rotor at ~196,000 × g for 1 h at 25°C or 34°C. The concentrations of insoluble unbound ligand and bound ligands were determined from the absorbance spectra in methanol and buffer using appropriate extinction coefficients (Table 2). Molar extinction coefficients of ligand bound tear lipocalin were calculated from the amount of bound ligand at saturation by subtracting the free ligand from the total. Control experiments with C6 or C12 -NBD ceramides without tear lipocalin showed no absorbance in spectra of the supernatant at 400–500 nm after centrifugation. Each experiment was repeated 3 times and the means were fit to the hyperbola curve and Hill equation (Microcal Origin, Northhampton, MA, USA). To exclude non-specific binding to lipocalin (a negative control) 10 μM lysozyme was used instead of lipocalin.
2.7 Testing C6-NBD ceramide for linear spectral summation
Increasing concentrations of suspensions of C6-NBD ceramide, 1 to 20 μM, in water or buffer were obtained by addition of 1 mM C6-NBD ceramide in DMS0 to seek a range where absorbance obeys a linear relationship with concentration according to Beer’s law. Tear lipocalin, 500 μL in 10 mM sodium phosphate at pH 7.3 was titrated by successive addition of 0.5–10 μL of 1 mM C6-NBD ceramide in DMSO and the absorbance spectra were measured. Following each addition of C6-NBD ceramide, the solution was mixed and allowed to equilibrate for 5 min. At the end of the titration experiment, the DMSO concentration did not exceed 2%. Bound C6-NBD ceramide spectra were obtained from spectra of the mixture of 2 μM C6-NBD ceramide and 10 μM tear lipocalin in 10 mM sodium phosphate at pH 7.3. The molar excess of tear lipocalin ensures that essentially all C6-NBD ceramide molecules are bound to the protein. The unbound spectra were obtained from 2 μM C6-NBD ceramide (without any protein) suspended in buffer. Composite spectra were recomposed by fitting the scaled spectra of bound (2 μM) and unbound (2 μM) C6-NBD ceramide using a program created in LabView (National Instruments, Austin, TX). In addition, a baseline component was added for better quality fit. The program uses the “General linear Fit VI” subVI to fit each experimental spectrum to the linear summation of the components and the bound and unbound lipid concentrations were determined as previously described.[61] The experiment was repeated 3 times and the means were calculated.
2.8 Fluorescence Binding Assays with NBD-labeled lipids
In separate experiments C6 and C12 -NBD ceramides in DMSO were added successively to 1 μM tear lipocalin in 10 mM sodium phosphate pH 7.3. After equilibration, fluorescence spectra were obtained, λex =420 nm. The relative intensity at 523 nm was used to compute each bound label. Experiments were repeated in triplicate and the means calculated. The total solvent concentration did not exceed 0.6%.
2.9 Data Analysis
Plots were fit to a single rectangular hyperbola or the Hill equation, B/P= nL/(Kd+L)= Lx/(Lx+Kd), respectively by standard nonlinear regression techniques (Microcal Origin, Northhampton, MA, USA): where B,P and L are concentrations of bound lipid, total protein and unbound lipid, respectively to determine apparent number of binding sites (n), apparent dissociation constant Kd and Hill coefficient (x) for tear lipocalin and ligand.
2.10 Docking Analysis
Molecular Docking studies were performed in DockingServer and SwissDock. The X-ray crystallographic structure of tear lipocalin (1XKI) was retrieved from Protein Data Bank (PDB), and used as a target protein. Structures of C18 ceramide (18C), stearic acid (ZINC04978673), and sphingosine (ZINC08195650) were obtained from PubChem and ZINC databases. The docking analysis and visualization was performed with default parameters implemented by the SwissDock site.[62,63]
3 Results
3.1 DAUDA displacement of native lipids from tear lipocalin
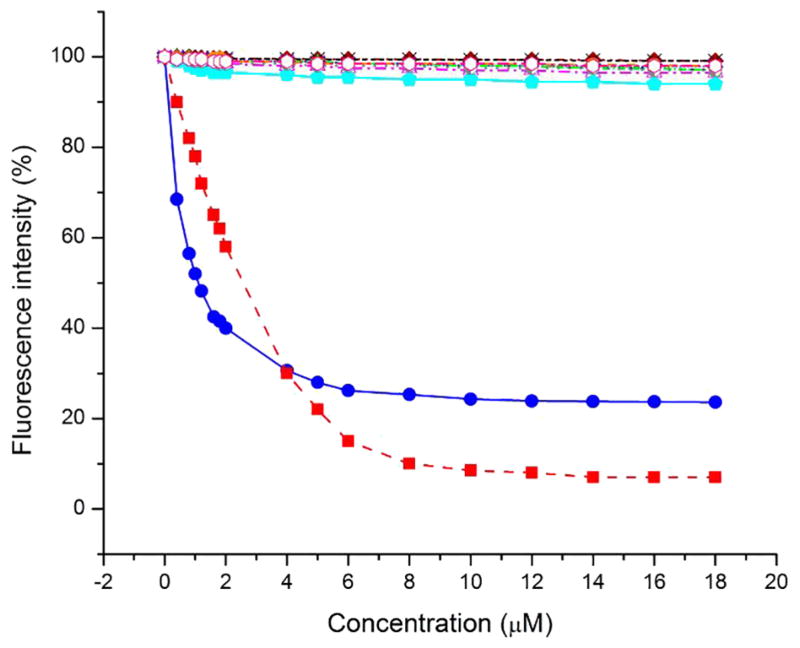
DAUDA loses fluorescence when displaced from the hydrophobic environment of the binding cavity of tear lipocalin.[56] DAUDA was displaced from the binding complex of tear lipocalin by the tear lipids, stearic acid (positive control) and C18-ceramide (Figure 1). The drop in fluorescence from displacement of DAUDA appeared at lower concentrations of ceramide than stearic acid. However, the residual fluorescence was twice as great with ceramide as that observed with stearic acid. There was negligible displacement of DAUDA by squalene, wax and cholesterol esters, (O-oleoyl)-16- hydroxypalmitic acid, as well as di- and tri-acylglycerols.
Figure 1.
DAUDA displacement assay for tear lipids. DAUDA bound to tear lipocalin displays fluorescence. Unbound DAUDA has exiguous fluorescence. Decreased fluorescence indicates displacement of DAUDA from the tear lipocalin binding site by the tested lipids. Fluorescence of tear lipocalin and DAUDA after addition of ceramide (
 ), stearic acid (
), stearic acid (
 ), cholesteryl stearate (
), cholesteryl stearate (
 ), cholesteryl oleate (
), cholesteryl oleate (
 ), stearyl behenate (
), stearyl behenate (
 ), behenyl stearate (-x-), behenyl oleate (
), behenyl stearate (-x-), behenyl oleate (
 ), squalene (
), squalene (
 ), (O-oleoyl)-16-hydroxypalmitic acid (
), (O-oleoyl)-16-hydroxypalmitic acid (
 ) 1,2-distearin (
) 1,2-distearin (
 ), 1,3-distearin (
), 1,3-distearin (
 ), tristearin (
), tristearin (
 ).
).
3.2 Testing binding of NBD fluorophores (pure label and lipids) to tear lipocalin
In order to determine apparent binding constants and explain the residual fluorescence seen with C-18 ceramide, NBD labeled lipids were tested. The results for the control NBD-CL alone are shown (Figure 3 in [45]).
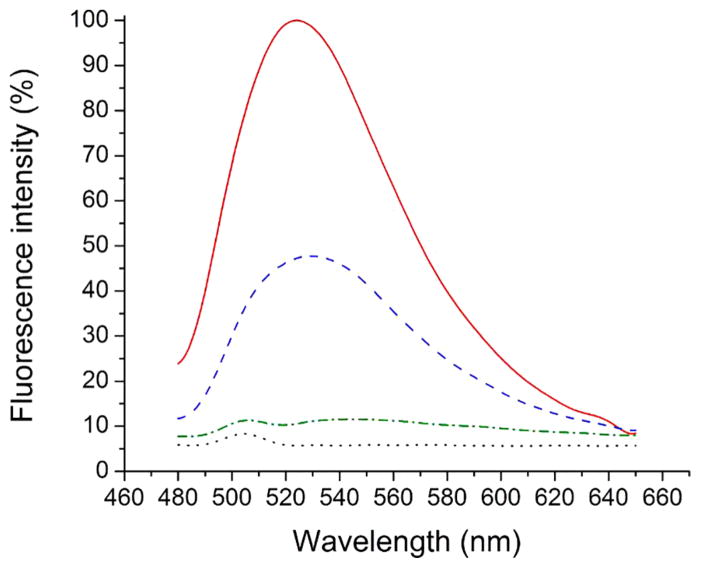
Fluorescent assays showed NBD labeled ceramides were amenable to binding experiments with tear lipocalin (Figure 2). C12 and C6 -NBD ceramides showed enhanced fluorescence in the presence of tear lipocalin compared to the unbound label. Interestingly, bound C6-NBD ceramide showed more fluorescence than C12-NBD ceramide.
Figure 2.
NBD6-Ceramide and NBD12-Ceramide show fluorescence only when bound to tear lipocalin. Fluorescence spectra of free 1 μM C6-NBD ceramide (
 ), 1 μM C12-NBD ceramide (
), 1 μM C12-NBD ceramide (
 ), 1 μM C6-NBD ceramide bound to 1 μM tear lipocalin(
), 1 μM C6-NBD ceramide bound to 1 μM tear lipocalin(
 ) and 1 μM C12-NBD ceramide 1 μM tear lipocalin (
) and 1 μM C12-NBD ceramide 1 μM tear lipocalin (
 ).
).
3.3 C6-NBD ceramide binding by linear summation, high speed centrifugal precipitation and fluorescence
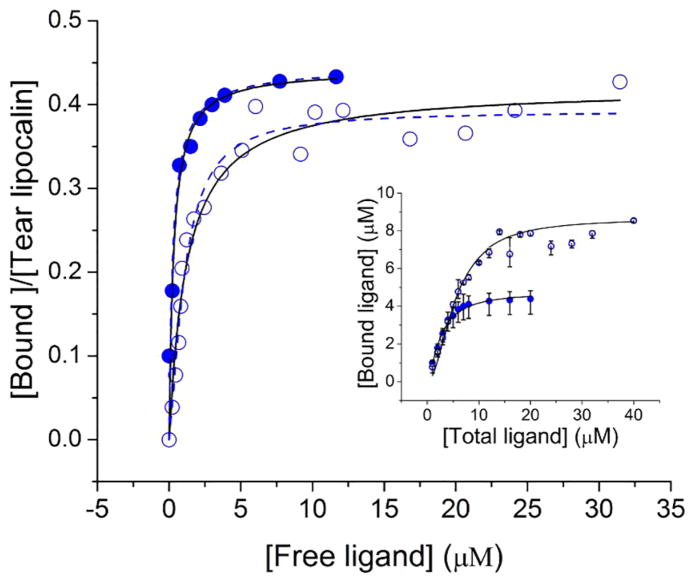
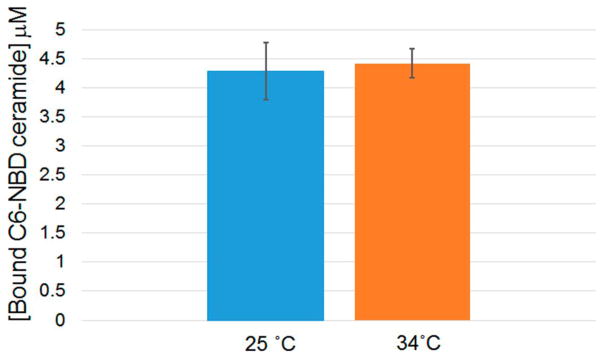
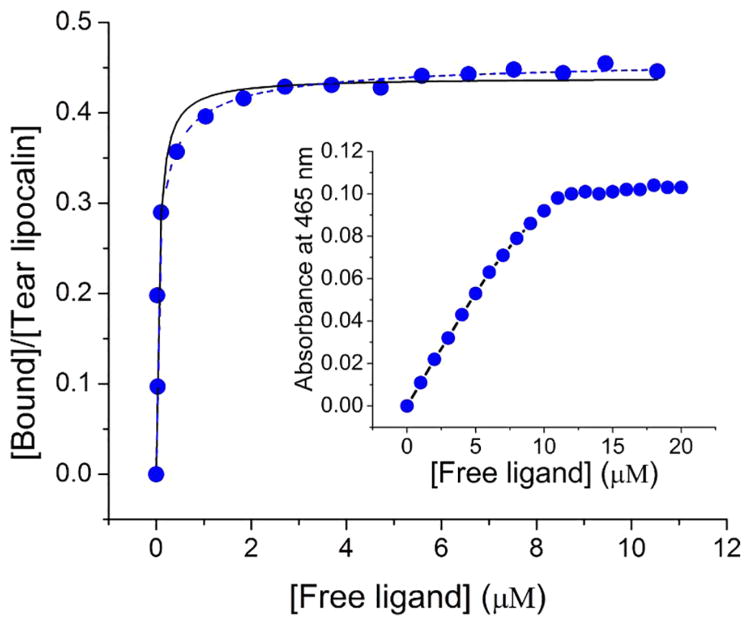
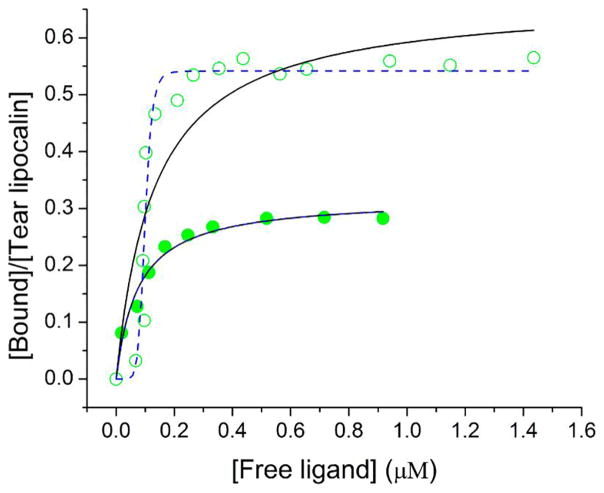
In order to determine apparent kinetic parameters for binding of the C6-NBD ceramide several techniques were investigated. The insolubility of unbound C6-NBD ceramide availed the opportunity to use centrifugal separation. The technique of centrifugal separation has the advantage of clearing any lipid that aggregates in suspension. The C6-NBD ceramide-tear lipocalin complex remains in solution and a binding curve could be generated (Figure 3 and Figure 4 in [45]). To ensure that the binding reaction equilibrated, the reactions were run at 34° C (Figure 4). The apparent Kd was noted to be sub-micromolar, n≈ 0.5 (Table 3). To rigorously verify these results, we tested the binding without centrifugal separation using the technique of linear spectral summation. This technique is predicated on an acceptable spectral separation of the bound and free ligand (Figure 4 in [45]). Previously we found that the spectral peaks of bound and free ligand must be offset by at least 7–10 nm.[61] Because unbound ceramides are suspended, this technique necessitates that the suspended fluorophore obeys Beer’s law in the range of the assay (Figure 5 inset). The apparent dissociation constant was even smaller with linear spectral summation but a similar stoichiometric relationship of the tear lipocalin-ligand complex was observed (Table 3, Figure 5). Finally, we tested the binding relationship with a standard fluorescence assay which capitalizes on the fluorescence of the bound fluorophore compared to unbound (Figure 6). The dissociation constants and stoichiometry of the fluorescence assay were similar to that of linear spectral summation (Table 3).
Figure 3.
High speed centrifugal precipitation assay to test binding of C6-NBD ceramide (
 ) and C12-NBD ceramide (
) and C12-NBD ceramide (
 ) to 10 μM or 20 μM tear lipocalin, respectively. Concentration of bound ligand-protein complex (supernatant) was determined by absorption spectra after centrifugal separation from unbound insoluble precipitant. Curve fit to a hyperbola (—) Kd= 0.32 μM, n=0.44 for C6-NBD ceramide and Kd= 1.23 μM and n=0.42 for C12-NBD ceramide; and the Hill equation (
) to 10 μM or 20 μM tear lipocalin, respectively. Concentration of bound ligand-protein complex (supernatant) was determined by absorption spectra after centrifugal separation from unbound insoluble precipitant. Curve fit to a hyperbola (—) Kd= 0.32 μM, n=0.44 for C6-NBD ceramide and Kd= 1.23 μM and n=0.42 for C12-NBD ceramide; and the Hill equation (
 ) Kd= 0.29 μM, n=0.45 for C6-NBD ceramide and Kd= 1.06 μM and n=0.39 for C12-NBD ceramide. Inset: concentration of total versus bound ligand concentration. Error bars shows the range from 3 experiments.
) Kd= 0.29 μM, n=0.45 for C6-NBD ceramide and Kd= 1.06 μM and n=0.39 for C12-NBD ceramide. Inset: concentration of total versus bound ligand concentration. Error bars shows the range from 3 experiments.
Figure 4.
Comparison of high speed centrifugal precipitation of C6- NBD ceramide at 25° C and 34° C for a single concentration. Bars show the range from 3 experiments.
TABLE 3.
Binding Parameters Calculated from Fitting of Ligand Binding to Tear Lipocalin
| Ligand | Assay used | Fitting Equation | Kd μM | n |
|---|---|---|---|---|
| C6-NBD ceramide | High speed centrifugal precipitation | Hyperbola | 0.32 | 0.44 |
| Hill | 0.29 | 0.45 | ||
| Spectral linear summation | Hyperbola | 0.06 | 0.45 | |
| Hill | 0.07 | 0.47 | ||
| Fluorescence | Hyperbola | 0.08 | 0.32 | |
| Hill | 0.08 | 0.32 | ||
| C12-NBD ceramide | High speed centrifugal precipitation | Hyperbola | 1.23 | 0.42 |
| Hill | 1.06 | 0.39 | ||
| Fluorescence | Hyperbola | 0.13 | 0.67 | |
| Hill | 0.10 | 0.21 |
Figure 5.
Binding curve of C6-NBD ceramide to tear lipocalin 10 μM by linear spectral summation (
 ). Absorbance composite spectra of mixtures were fit to the sum of varying pure free and pure bound spectra. Curve was fit to hyperbola (—) Kd= 0.06 μM, n=0.45, and to Hill equation (
). Absorbance composite spectra of mixtures were fit to the sum of varying pure free and pure bound spectra. Curve was fit to hyperbola (—) Kd= 0.06 μM, n=0.45, and to Hill equation (
 ) Kd= 0.07 μM, n=0.47. Inset, concentration dependent absorption of C6-NBD ceramide suspended in 10 mM sodium phosphate, pH 7.3.
) Kd= 0.07 μM, n=0.47. Inset, concentration dependent absorption of C6-NBD ceramide suspended in 10 mM sodium phosphate, pH 7.3.
Figure 6.
Binding curve of C6-NBD ceramide (
 ) and C12-NBD ceramide (
) and C12-NBD ceramide (
 ) to tear lipocalin by fluorescence. Curve fit to hyperbola (—) Kd= 0.08 μM and n=0.32 for C6-NBD ceramide binding and Kd= 0.13 μM and n=0.67 for C12-NBD ceramide, and to the Hill equation (
) to tear lipocalin by fluorescence. Curve fit to hyperbola (—) Kd= 0.08 μM and n=0.32 for C6-NBD ceramide binding and Kd= 0.13 μM and n=0.67 for C12-NBD ceramide, and to the Hill equation (
 ) Kd= 0.08 μM and n=0.32 for C6-NBD ceramide and Kd= 0.1 μM and n=0.21 for c12-NBD ceramide.
) Kd= 0.08 μM and n=0.32 for C6-NBD ceramide and Kd= 0.1 μM and n=0.21 for c12-NBD ceramide.
3.4 C12-NBD ceramide binding to tear lipocalin
In order to ensure that the results are not due a unique characteristic of the NBD labeled ceramide, comparison with a second ceramide, C12-NBD ceramide, was performed. Binding was tested by high speed centrifugal precipitation of the insoluble unbound precipitate from the bound soluble ligand (Figure 3). The apparent dissociation constant (Kd=1.2 μM) suggested strong binding but less affinity for this ligand compared to C6-NBD ceramide. Spectral fitting using linear summation was obviated because the peaks of the bound and unbound spectra were not well separated (Figure 4, inset in [45]). The binding curve was also performed using fluorescence of the bound ligand (Figure 6).
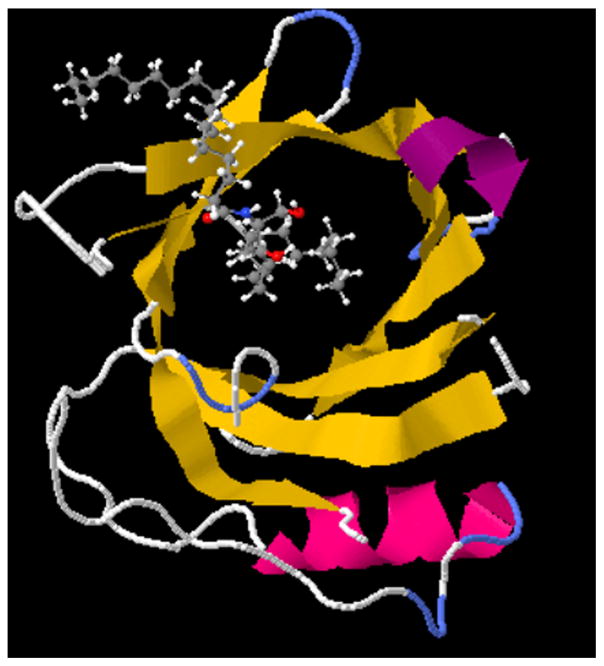
3.5 Docking studies of ceramide to tear lipocalin
The docking studies of the tear lipocalin ceramide complex showed a full fitness score −1328.48 kcal/mole with a free energy minima (ΔG =−8.38 kcal/mole ) Table 4. The predominant pose featured the alkyl chain, fatty acid side, slightly folded but fully contained in the cavity and the sphingosine portion positioned at the mouth (Figure 7). This was the case for several of the the highest rated docking poses of ceramide. The controls for the docking studies are shown in supplementary materials and include, stearic acid posed with the alkyl chain extended in the cavity (ΔG =−7.30 kcal/mole). Sphingosine alone was posed near the mouth (ΔG =−8.21 kcal/mole ) (Figures 5 and 6 in [45]).
TABLE 4.
Clustering results from the docking of Ceramide 18 into Tear Lipocalin by SwissDock
| Cluster rank for ligand poses based on fullfitness | FullFitness (kcal/mol) | Estimated ΔG (kcal/mol) |
|---|---|---|
| 1 | −1328.48 | −8.38 |
| 2 | −1324.35 | −8.86 |
| 3 | −1322.12 | −9.69 |
| 4 | −1318.96 | −8.28 |
| 5 | −1319.64 | −9.06 |
The 5 top SwissDock clusters of 30 (250 runs) are shown.
Figure 7.
Swiss Dock image of C18 ceramide complexed to tear lipocalin shows pose with the highest full fitness score (see Table 4).
4 Discussion
The key findings of this study are that 1) tear lipocalin binds ceramides 2) tear lipocalin does not appear to bind squalene, OAHFA’s, diacylglycerols, triacylglycerols, wax and cholesteryl esters 3) assays using high speed centrifugal precipitation match those using fluorescence and linear spectral summation, providing validation for these techniques and for their results and 4) docking studies suggest a predominant orientation with the alkyl chain of the fatty acid moiety of ceramide extending into the cavity and the sphingosine moiety positioned near the mouth of the cavity of tear lipocalin. Both the positive and negative results presented here have implications for the potential distribution and functions of lipids in the tear film. This study marshals strong evidence that native and fluorescent labeled ceramide analogs bind tear lipocalin. For the NBD labeled analogs the apparent binding constants are in the submicromolar to micromolar range according to 3 different binding assays. Interpretation of the precise stoichiometry and Kd are vitiated because lipocalin as purified is always complexed to other lipid products.[7,8,64] The dissociation constants may therefore be overestimated. Squalene, wax and cholesteryl esters have no discernible binding. Since tear lipocalin generally is believed to be restricted to the aqueous portion of tears, one would expect ceramides to reside in bulk tears. Squalene, wax esters and cholesteryl esters are insoluble in aqueous and therefore very likely to be integrated with lipids close to the air-surface interface. If (O-oleoyl)-16- hydroxypalmitic acid properly represents the class of OAHFA’s, one would not expect it to be soluble in the aqueous of tears without protein binding.
There are other lipid binding proteins in tears, such as lipophilin, phospholipid transfer protein and apolipoprotein D. However these proteins are estimated to be at least 10–100 times less abundant. [39–43]. Furthermore, the lipid binding profiles of these less abundant proteins do not include any of the lipids explored in this study. Therefore, it is probably safe to assume that lipids explored in this study that do not bind to tear lipocalin are absent from the aqueous.
4.1 Novel techniques for binding studies of insoluble lipids
In determining apparent binding with insoluble lipids, we have generally used a fluorescence approach. The method accrues the key advantages that often the unbound lipid shows no appreciable fluorescence and the sensitivity of fluorescence permits very low concentrations of reagents in the assays. Two approaches advanced in the current work capitalize on the absorbance of the unbound lipid in order to directly measure free and bound ligand. The results invite comparison to the results of fluorescence. In the first approach, separation by high speed centrifugation clears insoluble ligand and aggregates. We have used this technique before to study rifalazil binding to lipocalin.[60] This technique has the advantage that unbound insoluble ligand, and bound soluble ligand are both separated and measured directly by spectrophotometry in solvents appropriate for the mixtures. No assumptions are made regarding particles in suspension. Further, a fluorimeter is not required. The disadvantages include the laborious nature of the experiment, the need for higher concentrations than fluorescence, and incidental, although usually inconsequential losses when removing the supernatant. The mathematical approach for analyzing kinetics is simplified to the calculation of the solubility product (Ksp), where the concentration of free compound in solution is negligible, and the protein bound fraction and precipitated compound are easily separated and directly measurable.
Linear spectral summation was described for fluorescence previously, but altered here for absorbance spectrophotometry. This technique has 2 key prerequisites. First, the ligand, soluble or insoluble, must obey Beer’s law in the concentration range of the experiment (Figure 4, inset). Beer’s law is generally applied to solvated particles. While deviations are seen for large particles in solution, smaller particles show a linear absorbance plot that coincides with that of a condensed homogeneous film of the same material.[65] The second prerequisite is that the spectral peaks of the unbound lipid must be separated by at least 7 nm from the bound lipid.[61] That observation was supported by the experiments here. The bound and unbound peaks for C6-NBD ceramide were 27 nm apart and therefore amenable to the technique (Figure 4 in [45]). However, the spectral peak for unbound C12-NBD ceramide was broad and overlapped the sharper bound peak, obviating the technique in this case (Figure 4, inset in [45]). The advantages of linear spectra summation are rapidity, the use of routine laboratory equipment (spectrophotometer) and easily obtainable software. For C6-NBD ceramide, linear spectral summation closely matched the results for fluorescence assays for apparent Kd. Interestingly, centrifugal separation showed almost an order of magnitude larger Kd’s compared to fluorescence and linear summation giving the appearance of less binding affinity. One explanation, albeit speculative, is that during prolonged centrifugation, precipitation of unbound ligand removes suspended particles that would otherwise be accounted as solvated ligand. This reduction would convey slightly less binding for high speed centrifugal precipitation than the other techniques where the free ligand remains in suspension. The impact would be expected to be greater on the Kd than on the stoichiometry (n) since the former is determined mainly in the steeper portion of the binding curve.
4.2 Clinical Relevance of Ceramide Binding with Tear Lipocalin
Ceramides have long hydrocarbon tails and functional groups that confer both hydrophobic and polar qualities (amphiphiles). Albeit scarce in meibum of normal subjects, 0.03 mole % of total lipids,[66] ceramides are slightly more abundant in tears (Table 1). Ceramides are known to be rigid and have a high melting point. If ceramides are added in small quantities to meibum, hysteresis is dramatically elevated in Langmuir troughs experiments. Surface pressure rises with compression. Ceramides induce eventual collapse of the lipid film.[67] Ceramides comprise about 7% (average of 3 samples) of total lipids in chalazia.[68] Elevation of ceramides has been noted in moderate dry eye compared to normal subjects (although apparently not in severe dry eye).[66] Given these detrimental circumstances, sequestration of ceramides away from the lipid film in tears would appear functionally advantageous. Our data suggest that tear lipocalin binds the 3 different ceramides tested including the most abundant species in tears, C18-ceramide (Cer d18:1/18.0).[66] The low apparent dissociation constants in the submicromolar range are reflective of strong binding affinity and imply very little unbound ceramide can be expected in tears at natural concentrations. In fact, NBD ceramides have the lowest dissociation constant of any at least 20 ligands studied for tear lipocalin.[7,56,58,60,69–73]
4.3 Mechanistic features of binding
The stronger displacement of native ceramide compared to stearic acid matches the best full fitness pose provided by docking studies (Figure 7). All the poses place the alkyl chains of either the fatty acid (predominant) or sphingosine in the cavity. In each case the other chain is positioned at the mouth of the cavity. The poses vary in the degree of folding of the fatty acid or sphingosine alkyl chains in the cavity. Docking studies are known to have false positives. This study is limited because the PDB file for tear lipocalin was based on crystallographic studies that did not resolve loop structures.[74] The loop structures that overhang the cavity could limit folding of alkyl chains near the cavity mouth.[75,76] As a control, docking studies done with stearic acid show an extended fit over the full length of the cavity, which by crystallographic estimates are about 15 Å (Figure 5 in [45]).[74,77] C6 and C12- NBD ceramides molecules were not available in the proper format for docking studies. However, the NBD moiety fluoresces only in a hydrophobic environment and therefore it is safe to assume it complexes within the lipocalin cavity. NBD has an estimated maximum diameter of about 6.38 Å,[78], less than the known diameter (10 Å) of the calyx of tear lipocalin by crystallography. This is also consistent with functional studies that show the tear lipocalin cavity permits molecules up to about 6.7 Å in diameter and reject those of 9.1 Å and greater. [56]
Binding free energy differences between C6 and C12 -NBD ceramide association complexes with tear lipocalin are very small by fluorescence assays 0.4–1 kjoules/mole. At first, the minor differences initially appear incongruous to the longer alkyl chain length of C12-NBD ceramide on one arm of the molecule. However, the cavity is only 15 Å in length and therefore only a portion of the fatty acid side C12-NBD ceramide could enter the cavity. If the distance between carbon atoms (C-C distance) is assumed to be about 1.25 Å, then the cavity would be filled by NBD plus 6 carbon units reaching the limit of the hydrophobic effect. C6 and C12-NBD ceramides (Figure 1 in [45]) would be expected penetrate to similar depths with similar binding constants. As indicated in the docking studies of native ceramide the sphingosine tail could also participate in binding at the mouth of the NBD-ceramides. Alternatively, another binding mechanism involving charged interactions between the ligands and tear lipocalin cannot be completely excluded. The amphiphilic nature of ceramides might also permit a charged interaction with a trigonal cluster in tear lipocalin. Ion pairing between Lys 114 of lipocalin and the sulfonate group of ANS has been posited.[79] C12-NBD ceramide would be expected to have more exposure of the hydroxyl group at the center of the molecule than C6-NBD ceramide. A charge effect alone with tear lipocalin is unlikely to account for dissociation constants in the micromolar range.[80,81] The docking experiments do not support this mode of binding.
4.4 Squalene
Squalene, a terpenoid, is abundant in skin secretions and may arise from cells in both apocrine and sebaceous glands. Squalene is known to be an anti-oxidant in tears.[82,83] Squalene exists in meibum and ranges in mole percentage from 1–7. [84–89] Terpenoids have been identified in tears by mass spectrometry that presumably originate from the eyelid.[9,10,84] The negative results of the DAUDA displacement assay precludes significant bind of squalene to tear lipocalin. Comparison of tear lipocalin to squalene binding proteins is revealing. A protein involved in cholesterol synthesis called supernatant protein factor can bind squalene in vitro if a constraining C terminal jelly-role shaped domain is altered.[90] Supernatant protein factor has a lipid binding core domain that promotes squalene to assume a horseshoe shape in the cavity. The dimensions of tear lipocalin with overhanging loops and a restricted cavity would not permit this ligand configuration.[75] The implication is that squalene is likely to be distributed in the lipid layer of the tear film above the protein-aqueous layer. This notion is congruous with recent Langmuir trough experiments that squalene reduces the phase transition of meibum by 5 °C, and promotes disordered lipid configurations with gauche rotamers resulting in lipid expansion.[23] The lack of change achieved in maximum surface pressure by squalene was taken as evidence against its surfactant properties at the aqueous interface with meibum.[23] Our results are consistent with the findings that squalene may be active at the air interface in Langmuir-Blodgett films.
4.5 Wax and Cholesterol Esters
By most estimates the most abundant lipid components of the tear film are wax and cholesteryl esters (Table 1). Previous studies showed these components were conspicuously absent in extracts made from purified tear lipocalin despite the positive identification in tears.[7] The current study affirms these prior findings. Tear lipocalin is structurally distinct from known cholesteryl ester binding proteins. For example cholesteryl ester transfer protein, 53 kDa, is 3 times larger than tear lipocalin 17.4 kDa and sports a 60Å long hydrophobic tunnel with a highly flexible distal B barrel.[91,92] By comparison, tear lipocalin’s smaller cavity would place the long alkyl chains of cholesteryl stearate as well as wax esters in energetically unfavorable states exposed to solvent.
4.6 OAHFA
Synthetic (O-oleoyl)-16- hydroxypalmitic acid has been used as the standard to represent the class of OAHFA in tears.[46,47] By necessity experiments were limited to the synthetic compound as neither the native OAHFA’s nor their precursors that have been identified by mass spectrometry in tears are commercially available. The amphiphilic nature of these lipids have prompted some to proffer that OAHFA may be responsible for stabilizing the interface between nonpolar lipids and aqueous tears. This function was previously assigned to phospholipids.[46,93] Our data are consistent in that unlike phospholipids,[8] OAHFA is not sequestered by tear lipocalin and therefore is not likely to reside in the aqueous bulk. Therefore these lipids are key candidates to be interposed between the aqueous and surface lipids such as wax and cholesteryl esters.
4.7 Diacyl and triacylglycerides
Our prior studies failed to show di- or tri-acylglycerols in binding complexes with tear lipocalin by thin layer chromatography.[7] In contrast, a more recent work identified 1-palmitoyl-2-stearoyl-glycerol and 1,2-distearoyl-glycerol based on the fragmentation patterns of peaks with m/z values of 607 and 579 in extracts of tear lipocalin.[12]However, a contaminant, an oxidized form of Irgafos 168 from plasticware may mimic diacylglycerols, was also identified. In the current study DAUDA is not displaced by authentic class members. The conclusion is that the binding of diacyl and triacylglycerides to tear lipocalin is far less than that of DAUDA and probably functionally negligible.
5 Conclusions
The data shown here support a recurrent theme with regard to a major function of tear lipocalin. Tear lipocalin acts as a scavenger of a wide assortment of lipids.[7,35,36,53,70,94] and ceramide can now be added to that list. The protein has a relatively flexible cavity which binds a broad array of lipids including potential harmful substances such as the plasticizer, dioctyl phthalate, as well as proinflammatory mediators. The promiscuous binding properties have invited exploitation of lipocalin constructs for use pharmaceutical applications to scavenge drugs such as digitalis for use in cases of digitalis toxicity.[95] Alteration of the lipocalin loops alters binding and has lead to potential agents to even interrupt angiogenic pathways in cancer.[96,97] Native tear lipocalin has been proposed for delivery of anti-tuberculous drugs that show off-target toxicity in the unbound state.[60] The binding of ceramide in tears extends the scavenger theme to sequester potentially destabilizing lipids from the surface of the tear film.
Highlights.
Recently discovered tear lipids were tested for binding to tear lipocalin.
Novel spectroscopic applications were applied for these insoluble lipids.
Lipids bound to lipocalin are unlikely to function at the air-tear interface.
Ceramides bind to and implicate a scavenger role for tear lipocalin.
Acknowledgments
This work was supported by Public Health Service grant EY 11224 from the National Eye Institute (BG), and EY 00331 (Institute Core) and the Edith and Lew Wasserman Endowed Professorship (BG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrews JS. Human tear film lipids. I. Composition of the principal non-polar component. Exp Eye Res. 1970;10:223–7. doi: 10.1016/s0014-4835(70)80032-x. [DOI] [PubMed] [Google Scholar]
- 2.Young WH, Hill RM. Tear cholesterol levels and contact lens adaptation. Am J Optom Arch Am Acad Optom. 1973;50:12–6. doi: 10.1097/00006324-197301000-00003. [DOI] [PubMed] [Google Scholar]
- 3.van Haeringen NJ, Glasius E. Cholesterol in human tear fluid. Exp Eye Res. 1975;20:271–4. doi: 10.1016/0014-4835(75)90140-2. [DOI] [PubMed] [Google Scholar]
- 4.Stuchell, et al. Lipid composition of human tears. ARVO Abstr. 1984;25:320. [Google Scholar]
- 5.Saatçi AO, Irkeç M, Unlü N. Tear cholesterol levels in blepharitis. Ophthalmic Res. 1990;22:269–70. doi: 10.1159/000267034. [DOI] [PubMed] [Google Scholar]
- 6.Wollensak G, Mur E, Mayr A, Baier G, Göttinger W, Stöffler G. Effective methods for the investigation of human tear film proteins and lipids. Graefe’s Arch Clin Exp Ophthalmol = Albr von Graefes Arch Für Klin Und Exp Ophthalmol. 1990;228:78–82. doi: 10.1007/BF02764296. [DOI] [PubMed] [Google Scholar]
- 7.Glasgow BJ, Abduragimov AR, Farahbakhsh ZT, Faull KF, Hubbell WL. Tear lipocalins bind a broad array of lipid ligands. Curr Eye Res. 1995;14:363–72. doi: 10.3109/02713689508999934. [DOI] [PubMed] [Google Scholar]
- 8.Dean AW, Glasgow BJ. Mass spectrometric identification of phospholipids in human tears and tear lipocalin. Investig Ophthalmol Vis Sci. 2012;53:1773–82. doi: 10.1167/iovs.11-9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ham BM, Jacob JT, Keese MM, Cole RB. Identification, quantification and comparison of major non-polar lipids in normal and dry eye tear lipidomes by electrospray tandem mass spectrometry. J Mass Spectrom. 2004;39:1321–36. doi: 10.1002/jms.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ham BM, Jacob JT, Cole RB. MALDI-TOF MS of phosphorylated lipids in biological fluids using immobilized metal affinity chromatography and a solid ionic crystal matrix. Anal Chem. 2005;77:4439–47. doi: 10.1021/ac058000a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khyshiktuev BS, Tereshkov PP, Kozlov SA, Golub LA, Maksimenia MV. Fatty acid constitution of the lachrymal fluid in healthy subjects and in patients with ophthalmopathology. Klin Lab Diagn. 2005:18–9. [PubMed] [Google Scholar]
- 12.Millar TJ, Mudgil P, Butovich IA, Palaniappan CK. Adsorption of human tear lipocalin to human meibomian lipid films. Invest Ophthalmol Vis Sci. 2009;50:140–51. doi: 10.1167/iovs.08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids--a review. Curr Eye Res. 2008;33:405–20. doi: 10.1080/02713680802018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saville JT, Zhao Z, Willcox MDP, Blanksby SJ, Mitchell TW. Detection and quantification of tear phospholipids and cholesterol in contact lens deposits: the effect of contact lens material and lens care solution. Invest Ophthalmol Vis Sci. 2010;51:2843–51. doi: 10.1167/iovs.09-4609. [DOI] [PubMed] [Google Scholar]
- 15.Saville JT, Zhao Z, Willcox MDP, Ariyavidana MA, Blanksby SJ, Mitchell TW. Identification of phospholipids in human meibum by nano-electrospray ionisation tandem mass spectrometry. Exp Eye Res. 2011;92:238–40. doi: 10.1016/j.exer.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Campbell D, Griffiths G, Tighe BJ. Tear analysis and lens-tear interactions: part II. Ocular lipids-nature and fate of meibomian gland phospholipids. Cornea. 2011;30:325–32. doi: 10.1097/ICO.0b013e3181eae239. [DOI] [PubMed] [Google Scholar]
- 17.Rantamäki AH, Seppänen-Laakso T, Oresic M, Jauhiainen M, Holopainen JM. Human tear fluid lipidome: from composition to function. PLoS One. 2011;6:e19553. doi: 10.1371/journal.pone.0019553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam SM, Tong L, Duan X, Petznick A, Wenk MR, Shui G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J Lipid Res. 2014;55:289–298. doi: 10.1194/jlr.M044826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam SM, Tong L, Reux B, Duan X, Petznick A, Yong SS, Khee CBS, Lear MJ, Wenk MR, Shui G. Lipidomic analysis of human tear fluid reveals structure-specific lipid alterations in dry eye syndrome. J Lipid Res. 2014;55:299–306. doi: 10.1194/jlr.P041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohit A, Stapleton F, Brown SHJ, Mitchell TW, Willcox MDP. Comparison of tear lipid profile among basal, reflex, and flush tear samples. Optom Vis Sci. 2014;91:1391–5. doi: 10.1097/OPX.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 21.Brown SHJ, Kunnen CME, Papas EB, Lazon de la Jara P, Willcox MDP, Blanksby SJ, Mitchell TW. Intersubject and Interday Variability in Human Tear and Meibum Lipidomes: A Pilot Study. Ocul Surf. 2016;14:43–8. doi: 10.1016/j.jtos.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Brown SHJ, Kunnen CME, Duchoslav E, Dolla NK, Kelso MJ, Papas EB, Lazon de la Jara P, Willcox MDP, Blanksby SJ, Mitchell TW. A comparison of patient matched meibum and tear lipidomes. Invest Ophthalmol Vis Sci. 2013;54:7417–24. doi: 10.1167/iovs.13-12916. [DOI] [PubMed] [Google Scholar]
- 23.Ivanova S, Tonchev V, Yokoi N, Yappert MC, Borchman D, Georgiev GA. Surface Properties of Squalene/Meibum Films and NMR Confirmation of Squalene in Tears. Int J Mol Sci. 2015;16:21813–31. doi: 10.3390/ijms160921813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butovich IA. On the lipid composition of human meibum and tears: comparative analysis of nonpolar lipids. Invest Ophthalmol Vis Sci. 2008;49:3779–89. doi: 10.1167/iovs.08-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green-Church KB, Butovich I, Willcox M, Borchman D, Paulsen F, Barabino S, Glasgow BJ. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52:1979–93. doi: 10.1167/iovs.10-6997d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald JE. Surface phenomena of the tear film. Am J Ophthalmol. 1969;67:56–64. doi: 10.1016/0002-9394(69)90008-7. [DOI] [PubMed] [Google Scholar]
- 27.King-Smith PE, Hinel EA, Nichols JJ. Application of a novel interferometric method to investigate the relation between lipid layer thickness and tear film thinning. Invest Ophthalmol Vis Sci. 2010;51:2418–23. doi: 10.1167/iovs.09-4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu H, Wang MR, Wang J, Shen M. Tear film measurement by optical reflectometry technique. J Biomed Opt. 2014;19:27001. doi: 10.1117/1.JBO.19.2.027001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goto E, Dogru M, Kojima T, Tsubota K. Computer-synthesis of an interference color chart of human tear lipid layer, by a colorimetric approach. Invest Ophthalmol Vis Sci. 2003;44:4693–7. doi: 10.1167/iovs.03-0260. [DOI] [PubMed] [Google Scholar]
- 30.Korb DR, Scaffidi RC, Greiner JV, Kenyon KR, Herman JP, Blackie CA, Glonek T, Case CL, Finnemore VM, Douglass T. The effect of two novel lubricant eye drops on tear film lipid layer thickness in subjects with dry eye symptoms. Optom Vis Sci. 2005;82:594–601. doi: 10.1097/01.opx.0000171818.01353.8c. [DOI] [PubMed] [Google Scholar]
- 31.Olsen T. Reflectometry of the precorneal film. Acta Ophthalmol. 1985;63:432–8. doi: 10.1111/j.1755-3768.1985.tb01559.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Tan CLS, Tong L. Intra-observer and inter-observer repeatability of ocular surface interferometer in measuring lipid layer thickness. BMC Ophthalmol. 2015;15:53. doi: 10.1186/s12886-015-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millar TJ, Schuett BS. The real reason for having a meibomian lipid layer covering the outer surface of the tear film - A review. Exp Eye Res. 2015;137:125–38. doi: 10.1016/j.exer.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 34.La Mer VK, Healy TW. Specification of Materials for Retarding Water Evaporation-- Spreading Hexadecanol Monolayers. Science. 1964;144:564. doi: 10.1126/science.144.3618.564-a. [DOI] [PubMed] [Google Scholar]
- 35.Yeh P-T, Casey R, Glasgow BJ. A novel fluorescent lipid probe for dry eye: retrieval by tear lipocalin in humans. Invest Ophthalmol Vis Sci. 2013;54:1398–410. doi: 10.1167/iovs.12-10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gasymov OK, Abduragimov AR, Prasher P, Yusifov TN, Glasgow BJ. Tear lipocalin: evidence for a scavenging function to remove lipids from the human corneal surface. Invest Ophthalmol Vis Sci. 2005;46:3589–96. doi: 10.1167/iovs.05-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Interaction of tear lipocalin with lysozyme and lactoferrin. Biochem Biophys Res Commun. 1999;265:322–5. doi: 10.1006/bbrc.1999.1668. [DOI] [PubMed] [Google Scholar]
- 38.Glasgow BJ, Abduragimov AR, Yusifov TN, Gasymov OK. Studies of ligand binding and CD analysis with apo- and holo-tear lipocalins. Adv Exp Med Biol. 1998;438:105–12. doi: 10.1007/978-1-4615-5359-5_14. [DOI] [PubMed] [Google Scholar]
- 39.Lehrer RI, Nguyen T, Zhao C, Ha CX, Glasgow BJ. Secretory lipophilins: a tale of two species. Ann N Y Acad Sci. 2000;923:59–67. doi: 10.1111/j.1749-6632.2000.tb05519.x. [DOI] [PubMed] [Google Scholar]
- 40.Lehrer RI, Xu G, Abduragimov A, Dinh NN, Qu XD, Martin D, Glasgow BJ. Lipophilin, a novel heterodimeric protein of human tears. FEBS Lett. 1998;432:163–7. doi: 10.1016/s0014-5793(98)00852-7. [DOI] [PubMed] [Google Scholar]
- 41.Zhao C, Nguyen T, Yusifov T, Glasgow BJ, Lehrer RI. Lipophilins: human peptides homologous to rat prostatein. Biochem Biophys Res Commun. 1999;256:147–55. doi: 10.1006/bbrc.1999.0274. [DOI] [PubMed] [Google Scholar]
- 42.Jauhiainen M, Setälä NL, Ehnholm C, Metso J, Tervo TMT, Eriksson O, Holopainen JM, Setala NL, Ehnholm C, Metso J, Tervo TMT, Eriksson O, Holopainen JM. Phospholipid transfer protein is present in human tear fluid. Biochemistry. 2005;44:8111–8116. doi: 10.1021/bi050151k. [DOI] [PubMed] [Google Scholar]
- 43.Holzfeind P, Merschak P, Dieplinger H, Redl B. The human lacrimal gland synthesizes apolipoprotein D mRNA in addition to tear prealbumin mRNA, both species encoding members of the lipocalin superfamily. Exp Eye Res. 1995;61:495–500. doi: 10.1016/s0014-4835(05)80145-9. [DOI] [PubMed] [Google Scholar]
- 44.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Structural changes in human tear lipocalins associated with lipid binding. Biochim Biophys Acta. 1998;1386:145–56. doi: 10.1016/s0167-4838(98)00092-2. [DOI] [PubMed] [Google Scholar]
- 45.Glasgow BJ, Abduragimov AR. Orphan Tear Lipid Analogs, Synthesis and Binding to Tear Lipocalin. Data Br. 2017 doi: 10.1016/j.dib.2018.03.102. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butovich IA, Wojtowicz JC, Molai M. Human tear film and meibum. Very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J Lipid Res. 2009;50:2471–85. doi: 10.1194/jlr.M900252-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saville JT. Phospholipids in human tears, meibum and deposited onto contact lenses, Dr. Philos. Thesis. Sch Chem Univ Wollongong. 2013 [Google Scholar]
- 48.Haughland RP. Handbook of Fluorescent Probes and Research Chemicals. 5. Molecular Probes; Eugene, Oregon: 1992. [Google Scholar]
- 49.Raghuraman H, Shrivastava S, Chattopadhyay A. Monitoring the looping up of acyl chain labeled NBD lipids in membranes as a function of membrane phase state. Biochim Biophys Acta. 2007;1768:1258–67. doi: 10.1016/j.bbamem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Poole LB. Measurement of protein sulfenic acid content. Curr Protoc Toxicol Chapter. 2008;17:Unit17.2. doi: 10.1002/0471140856.tx1702s38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gasymov OK, Abduragimov AR, Glasgow BJ. Intracavitary ligand distribution in tear lipocalin by site-directed tryptophan fluorescence. Biochemistry. 2009;48:7219–28. doi: 10.1021/bi9005557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaari A, Fahy C, Chevillot-Biraud A, Rholam M. Insights into Kinetics of Agitation-Induced Aggregation of Hen Lysozyme under Heat and Acidic Conditions from Various Spectroscopic Methods. PLoS One. 2015;10:e0142095. doi: 10.1371/journal.pone.0142095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glasgow BJ, Gasymov OK, Abduragimov AR, Engle JJ, Casey RC. Tear lipocalin captures exogenous lipid from abnormal corneal surfaces. Invest Ophthalmol Vis Sci. 2010;51:1981–7. doi: 10.1167/iovs.09-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glasgow BJ. Tissue expression of lipocalins in human lacrimal and von Ebner’s glands: colocalization with lysozyme. Graefe’s Arch Clin Exp Ophthalmol = Albr von Graefes Arch Für Klin Und Exp Ophthalmol. 1995;233:513–22. doi: 10.1007/BF00183433. [DOI] [PubMed] [Google Scholar]
- 55.Glasgow BJ, Abduragimov AR, Yusifov TN, Gasymov OK, Horwitz J, Hubbell WL, Faull KF. A conserved disulfide motif in human tear lipocalins influences ligand binding. Biochemistry. 1998;37:2215–25. doi: 10.1021/bi9720888. [DOI] [PubMed] [Google Scholar]
- 56.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Binding studies of tear lipocalin: the role of the conserved tryptophan in maintaining structure, stability and ligand affinity. Biochim Biophys Acta. 1999;1433:307–20. doi: 10.1016/s0167-4838(99)00133-8. [DOI] [PubMed] [Google Scholar]
- 57.Abduragimov AR, Gasymov OK, Yusifov TN, Glasgow BJ. Functional cavity dimensions of tear lipocalin. Curr Eye Res. 2000;21:824–32. doi: 10.1076/ceyr.21.4.824.5551. [DOI] [PubMed] [Google Scholar]
- 58.Breustedt DA, Schönfeld DL, Skerra A. Comparative ligand-binding analysis of ten human lipocalins. Biochim Biophys Acta. 2006;1764:161–73. doi: 10.1016/j.bbapap.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Gasymov OK, Abduragimov AR, Glasgow BJ. The conserved disulfide bond of human tear lipocalin modulates conformation and lipid binding in a ligand selective manner. Biochim Biophys Acta. 2011;1814:671–83. doi: 10.1016/j.bbapap.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staudinger T, Redl B, Glasgow BJ. Antibacterial activity of rifamycins for M. smegmatis with comparison of oxidation and binding to tear lipocalin. Biochim Biophys Acta. 2014;1844:750–8. doi: 10.1016/j.bbapap.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gasymov OK, Abduragimov AR, Glasgow BJ. A simple model-free method for direct assessment of fluorescent ligand binding by linear spectral summation. J Fluoresc. 2014;24:231–8. doi: 10.1007/s10895-013-1290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grosdidier A, Zoete V, Michielin O. Fast docking using the CHARMM force field with EADock DSS. J Comput Chem. 2011;32:2149–2159. doi: 10.1002/jcc.21797. [DOI] [PubMed] [Google Scholar]
- 63.Grosdidier A, Zoete V, Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011;39:W270–W277. doi: 10.1093/nar/gkr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsukamoto S, Fujiwara K, Ikeguchi M. Fatty acids bound to recombinant tear lipocalin and their role in structural stabilization. J Biochem. 2009;146:343–50. doi: 10.1093/jb/mvp076. [DOI] [PubMed] [Google Scholar]
- 65.Stewart J. Absorption of Radiant Energy by Solid Particles in Suspension. J Res Natl Bur. 1955 [Google Scholar]
- 66.Lam SM, Tong L, Yong SS, Li B, Chaurasia SS, Shui G, Wenk MR. Meibum lipid composition in Asians with dry eye disease. PLoS One. 2011;6:e24339. doi: 10.1371/journal.pone.0024339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arciniega JC, Uchiyama E, Butovich IA. Disruption and destabilization of meibomian lipid films caused by increasing amounts of ceramides and cholesterol. Invest Ophthalmol Vis Sci. 2013;54:1352–60. doi: 10.1167/iovs.12-10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicolaides N, Flores A, Santos EC, Robin JB, Smith RE. The lipids of chalazia. Invest Ophthalmol Vis Sci. 1988;29:482–486. [PubMed] [Google Scholar]
- 69.Fluckinger M, Haas H, Merschak P, Glasgow BJ, Redl B. Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob Agents Chemother. 2004;48:3367–72. doi: 10.1128/AAC.48.9.3367-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Redl B, Holzfeind P, Lottspeich F. cDNA cloning and sequencing reveals human tear prealbumin to be a member of the lipophilic-ligand carrier protein superfamily. J Biol Chem. 1992;267:20282–7. [PubMed] [Google Scholar]
- 71.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Relaxation of beta-structure in tear lipocalin and enhancement of retinoid binding. Invest Ophthalmol Vis Sci. 2002;43:3165–73. [PubMed] [Google Scholar]
- 72.Gasymov OK, Abduragimov AR, Gasimov EO, Yusifov TN, Dooley AN, Glasgow BJ. Tear lipocalin: potential for selective delivery of rifampin. Biochim Biophys Acta. 2004;1688:102–11. doi: 10.1016/j.bbadis.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 73.Lechner M, Wojnar P, Redl B. Human tear lipocalin acts as an oxidative-stress-induced scavenger of potentially harmful lipid peroxidation products in a cell culture system. Biochem J. 2001;356:129–135. doi: 10.1042/0264-6021:3560129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breustedt DA, Korndörfer IP, Redl B, Skerra A. The 1.8-A crystal structure of human tear lipocalin reveals an extended branched cavity with capacity for multiple ligands. J Biol Chem. 2005;280:484–93. doi: 10.1074/jbc.M410466200. [DOI] [PubMed] [Google Scholar]
- 75.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Interstrand loops CD and EF act as pH-dependent gates to regulate fatty acid ligand binding in tear lipocalin. Biochemistry. 2004;43:12894–904. doi: 10.1021/bi049076o. [DOI] [PubMed] [Google Scholar]
- 76.Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Site-directed tryptophan fluorescence reveals the solution structure of tear lipocalin: evidence for features that confer promiscuity in ligand binding. Biochemistry. 2001;40:14754–62. doi: 10.1021/bi0110342. [DOI] [PubMed] [Google Scholar]
- 77.Breustedt DA, Chatwell L, Skerra A. A new crystal form of human tear lipocalin reveals high flexibility in the loop region and induced fit in the ligand cavity. Acta Crystallogr D Biol Crystallogr. 2009;65:1118–25. doi: 10.1107/S0907444909031011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mukherjee S, Chattopadhyay A, Samanta A, Soujanya T. Dipole moment change of NBD group upon excitation studied using solvatochromic and quantum chemical approaches: Implications in membrane research. J Phys Chem. 1994;98:2809–2812. doi: 10.1021/j100062a014. [DOI] [Google Scholar]
- 79.Gasymov OK, Abduragimov AR, Glasgow BJ. Ligand binding site of tear lipocalin: contribution of a trigonal cluster of charged residues probed by 8-anilino-1-naphthalenesulfonic acid. Biochemistry. 2008;47:1414–24. doi: 10.1021/bi701955e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gasymov OK, Abduragimov AR, Glasgow BJ. Evidence for internal and external binding sites on human tear lipocalin. Arch Biochem Biophys. 2007;468:15–21. doi: 10.1016/j.abb.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gasymov OK, Glasgow BJ. ANS fluorescence: potential to augment the identification of the external binding sites of proteins. Biochim Biophys Acta. 2007;1774:403–11. doi: 10.1016/j.bbapap.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelly GS. Squalene and its potential clinical uses. Altern Med Rev. 1999;4:29–36. [PubMed] [Google Scholar]
- 83.De Luca C, Valacchi G. Surface lipids as multifunctional mediators of skin responses to environmental stimuli. Mediators Inflamm. 2010;2010:321494. doi: 10.1155/2010/321494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Borchman D, Yappert MC, Milliner SE, Duran D, Cox GW, Smith RJ, Bhola R. 13C and 1H NMR ester region resonance assignments and the composition of human infant and child meibum. Exp Eye Res. 2013;112:151–9. doi: 10.1016/j.exer.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 85.Borchman D, Yappert MC, Milliner SE, Smith RJ, Bhola R. Confirmation of the presence of squalene in human eyelid lipid by heteronuclear single quantum correlation spectroscopy. Lipids. 2013;48:1269–77. doi: 10.1007/s11745-013-3844-9. [DOI] [PubMed] [Google Scholar]
- 86.Tiffany JM. Individual variations in human meibomian lipid composition. Exp Eye Res. 1978;27:289–300. doi: 10.1016/0014-4835(78)90164-1. [DOI] [PubMed] [Google Scholar]
- 87.Keith CG. Seborrhoeic blepharo-kerato-conjunctivitis. Trans Ophthalmol Soc U K. 1967;87:85–103. [PubMed] [Google Scholar]
- 88.Ehlers N. The precorneal film. Biomicroscopical, histological and chemical investigations. Acta Ophthalmol Suppl. 1965;(SUPPL 81):1–134. [PubMed] [Google Scholar]
- 89.Borchman D, Foulks GN, Yappert MC, Milliner SE. Changes in human meibum lipid composition with age using nuclear magnetic resonance spectroscopy. Invest Ophthalmol Vis Sci. 2012;53:475–82. doi: 10.1167/iovs.11-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Christen M, Marcaida MJ, Lamprakis C, Aeschimann W, Vaithilingam J, Schneider P, Hilbert M, Schneider G, Cascella M, Stocker A. Structural insights on cholesterol endosynthesis: Binding of squalene and 2,3-oxidosqualene to supernatant protein factor. J Struct Biol. 2015;190:261–270. doi: 10.1016/j.jsb.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 91.Qiu X, Mistry A, Ammirati MJ, Chrunyk BA, Clark RW, Cong Y, Culp JS, Danley DE, Freeman TB, Geoghegan KF, Griffor MC, Hawrylik SJ, Hayward CM, Hensley P, Hoth LR, Karam GA, Lira ME, Lloyd DB, McGrath KM, Stutzman-Engwall KJ, Subashi AK, Subashi TA, Thompson JF, Wang I-K, Zhao H, Seddon AP. Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat Struct Mol Biol. 2007;14:106–113. doi: 10.1038/nsmb1197. [DOI] [PubMed] [Google Scholar]
- 92.Lei D, Zhang X, Jiang S, Cai Z, Rames MJ, Zhang L, Ren G, Zhang S. Structural features of cholesteryl ester transfer protein: a molecular dynamics simulation study. Proteins. 2013;81:415–25. doi: 10.1002/prot.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schuett BS, Millar TJ. An investigation of the likely role of (O-acyl) ω-hydroxy fatty acids in meibomian lipid films using (O-oleyl) ω-hydroxy palmitic acid as a model. Exp Eye Res. 2013;115:57–64. doi: 10.1016/j.exer.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 94.Glasgow BJ, Marshall G, Gasymov OK, Abduragimov AR, Yusifov TN, Knobler CM. Tear lipocalins: potential lipid scavengers for the corneal surface. Invest Ophthalmol Vis Sci. 1999;40:3100–7. [PubMed] [Google Scholar]
- 95.Eyer F, Steimer W, Nitzsche T, Jung N, Neuberger H, Müller C, Schlapschy M, Zilker T, Skerra A. Intravenous application of an anticalin dramatically lowers plasma digoxin levels and reduces its toxic effects in rats. Toxicol Appl Pharmacol. 2012;263:352–359. doi: 10.1016/j.taap.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 96.Gille H, Hülsmeyer M, Trentmann S, Matschiner G, Christian HJ, Meyer T, Amirkhosravi A, Audoly LP, Hohlbaum AM, Skerra A. Functional characterization of a VEGF-A-targeting Anticalin, prototype of a novel therapeutic human protein class. Angiogenesis. 2016;19:79–94. doi: 10.1007/s10456-015-9490-5. [DOI] [PubMed] [Google Scholar]
- 97.Richter A, Skerra A. Anticalins directed against vascular endothelial growth factor receptor 3 (VEGFR-3) with picomolar affinities show potential for medical therapy and in vivo imaging. Biol Chem. 2016 doi: 10.1515/hsz-2016-0195. [DOI] [PubMed] [Google Scholar]