Abstract
Elastin is expressed in most tissues that require elastic recoil. The protein first appeared coincident with the closed circulatory system, and was critical for the evolutionary success of the vertebrate lineage. Elastin is expressed by multiple cell types in the lung, including mesothelial cells in the pleura, smooth muscle cells in airways and blood vessels, endothelial cells, and interstitial fibroblasts. This highly crosslinked protein associates with fibrillin-containing microfibrils to form the elastic fiber, which is the physiological structure that functions in the extracellular matrix. Elastic fibers can be woven into many different shapes depending on the mechanical needs of the tissue. In large pulmonary vessels, for example, elastin forms continuous sheets, or lamellae, that separate smooth muscle layers. Outside of the vasculature, elastic fibers form an extensive fiber network that originates in the central bronchi and inserts into the distal airspaces and visceral pleura. The fibrous cables form a looping system that encircle the alveolar ducts and terminal air spaces and ensures that applied force is transmitted equally to all parts of the lung. Normal lung function depends on proper secretion and assembly of elastin, and either inhibition of elastin fiber assembly or degradation of existing elastin results in lung dysfunction and disease.
Keywords: elastin, elastic fiber, lung development, fibrillin, microfibril, emphysema
1. INTRODUCTION
As animals moved from an aquatic to a terrestrial habitat, the functional design of the respiratory system changed to accommodate appropriate ventilation and oxygen exchange. Invertebrates evolved versatile organs, including gills, skin, book lungs, and tracheae systems, for air breathing [1]. In contrast, the method of ventilation used in respiration by many lower land-dwelling vertebrates is via a pulse pump. Air movement in the pulse pump is driven by muscle within the respiratory structure itself or by movement of the structures in the mouth in what is called buccal pumping. This method of ventilation is inefficient, but is nonetheless used by all air-breathing amphibians and, to a varying extent, by reptile species [2].
In mammals, we see remarkable changes in lung design and function. To meet the higher metabolic demands of terrestrial mammals, the lung is subdivided into long narrow airways and progressively smaller air spaces, rendering the pulse pump inefficient. In its place evolved the aspiration pump, where the lungs are located within the pump (the chest cavity) so air is sucked in, or aspirated, by low pressure created around the lungs [2]. The two important evolutionary innovations that made aspiration ventilation possible were the muscular diaphragm and the extracellular matrix (ECM) protein elastin. Elastin imparts elasticity to tissues and its presence allows the lung to function as an elastic bag. In aspiration pumping, the potential energy created by contraction of the diaphragm during inhalation is stored in the elastic tissues of the lung, and is released when the lung recoils during exhalation. It is therefore no surprise that damage to lung elastic fibers can be detrimental to lung mechanics and overall respiratory efficiency.
Three elastic fiber systems are present and develop independently in the lung. They include: (a) the pleura intersegmental and interlobular connective tissue; (b) the blood vascular system; and (c) the bronchi and respiratory units [3]. All three continue to develop postnatally up to the young adult period when elastin production diminishes [4, 5]. This review will discuss these three elastin-containing networks and focus on the importance of elastin to lung architecture and function. How mutations in the elastin gene and degradation of elastin protein lead to pulmonary disease will also be addressed.
2. ELASTIN
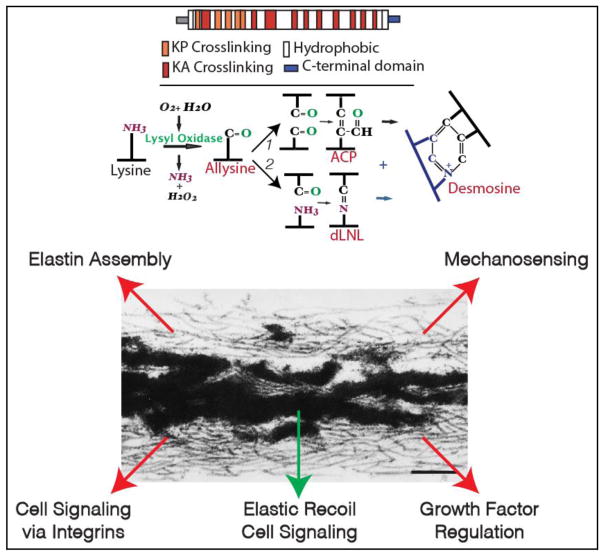
Unlike many matrix proteins that are phylogenetically ancient, elastin appeared relatively late in evolution and is present in all species from sharks to humans, but absent in lower chordates and invertebrates [6–9]. Its arrival coincides with the appearance of the closed circulatory system and the elastic lung, where elastic recoil is critical for appropriate tissue function. Elastin is encoded by a single gene whose transcript undergoes extensive alternative splicing [10–13]. It is secreted as a 70 kDa protein, called tropoelastin, that crosslinks with other tropoelastin molecules to form an insoluble, polymeric protein (Figure 1). It is this highly cross-linked form of elastin that functions as an elastomer [14, 15]. Lysyl oxidase, a copper-dependent enzyme, initiates cross-link formation by catalyzing the oxidative deamination of specific lysyl residues on the elastin precursor molecule to from α-aminoadipic δ-semialdehyde (trivial name allysine) [16]. There are two major bifunctional cross-links in elastin: dehydrolysinonorleucine, formed through the condensation of one residue of allysine and one of lysine, and allysine aldol formed through the association of two allysine residues [17–19]. These two cross-links can condense with each other, or with other intermediates, to form the tetrafunctional cross-links desmosine or isodesmosine (Figure 1) [20, 21]. Other cross-links that have been identi ed include a trifunctional cross-link, dehydromerodesmosine [22, 23], a cyclopentenosine trifunctional cross-link formed through the condensation of three allysine residues [24], and desmopyridine and isodesmopyridine found in trace amounts that form through the condensation of ammonia and allysine [25]. There is also evidence that desmosine/isodesmosine cross-links can be oxidized by reactive oxygen species resulting in dihydrooxopyridine forms [26].
Figure 1.
Elastin protein has a repeating domain structure consisting of lysine-containing sequences that populate crosslinking sites and hydrophobic sequences that contribute to elastic recoil (top). The ε-amino group of lysine residues in alanine-rich (KA) or proline-rich (KP) sequences are oxidized by the enzyme lysyl oxidase resulting in bifunctional and tetrafunctional crosslinks (see text). The electron micrograph of an elastic fiber shows crosslinked elastin (dark amorphous material) associated with a bed of microfibrils. In addition to facilitating elastin assembly, microfibrils have numerous signaling and mechanical roles indicated by the red arrows. Elastin also provides information to cells when fragments interact with signaling receptors on cells (green arrow).
The extensive cross-linking found in elastin is important for the protein’s insolubility and contributes to its longevity. Shapiro et al.[27] estimated the life span of elastin using aspartic acid racemization and 14C turnover to be ~80 years in humans. Studies using sensitive immunological techniques to measure elastin peptides in the blood or desmosine cross-links excreted in the urine suggest that less than 1% of the total body elastin pool turns over in a year [28]. Elastin expression in most tissues, including the lung, occurs over a narrow window of development, beginning in mid-gestation and continuing at high levels through the postnatal period [4, 29–31]. Both protein and gene array analysis show that there is minimal elastin synthesis in any tissue in the adult animal [32–35]. This explains why damage to elastic fibers during the adult period is so detrimental and why the elastin protein must have a long half-life.
Another factor contributing to the longevity of mature elastin is its relative resistance to proteolysis. Because there are few lysine or arginine residues in the fully cross-linked protein, and few amino acids with large aromatic side chains, elastin is not degraded by trypsin- or chymotrypsin-like proteases. Proteases that do degrade elastin are those that prefer amino acids with small hydrophobic side chains, such as alanine, valine, glycine, and leucine. These proteases, generally termed elastases [36], are predominantly serine proteases, although cathepsins will degrade elastin under appropriate circumstances [37, 38]. Elastases are produced by interstitial and inflammatory cells, and some of the most potent elastases are produced by bacteria [39–42]. Several matrix metalloproteinases (MMPs) secreted by mammalian cells also have elastolytic activity. These include MMP-2, -3, -7, -9, -10, and -12 [43, 44]. While elastin is clearly a substrate for the elastase class of protease, elastases are not specific for elastin. In fact, elastases—particularly the serine elastases like porcine pancreatic elastase and neutrophil elastase—are relatively nonspecific and have a broad range of substrates, including many, if not most, extracellular matrix proteins [36]. It is important to note that most of these proteases degrade microfibrillar components as well as elastin.
3. THE ELASTIC FIBER
3.1 Elastic fiber assembly
Elastin is a component of a complex fiber structure consisting of, in addition to elastin, fibrillin-containing microfibrils (see electron micrograph in Figure 1). Microfibrils are 10–15 nm fibrils found throughout the ECM that facilitate tropoelastin polymerization and serve as molecular bridges connecting elastic fibers to adjacent cells [45–47]. The association of elastin with microfibrils occurs extracellularly and gene knockout studies confirm that microfibrils are required for elastin polymerization and fiber formation [48]. The process by which tropoelastin monomers are secreted and how they associate with microfibrils is still not completely understood. Numerous studies now support a stepwise model for elastic fiber assembly that involves a number of molecules that assist in the assembly process [49–51]. Tropoelastin is synthesized on membrane-bound polysomes, transported through the Golgi apparatus and packaged into secretory vesicles. At this point, elastin secretion may differ from other ECM proteins by trafficking through an acidic compartment (perhaps a sorting endosome) [50, 52]. There is also evidence that tropoelastin is secreted as a complex with a 67 kDa molecular chaperone that targets the tropoelastin molecule to assembly sites on the cell surface [53]. Electron microscopy and dynamic imaging studies show that tropoelastin is assembled into small globular aggregates in the secretory pathway or on the cell surface that begin the initial stages of cross-linking (a process called microassembly) [54–57]. Cross-linking is not required for tropoelastin’s interaction with microfibrils, however [58, 59]
3.2 Microfibril structure and function
The protein fibrillin provides the major structural component of the microfibril, and several associated proteins interact with fibrillin to modify microfibril function [60–62]. The ancestral fibrillin arose early in evolution (Cnidaria) [63, 64] and has remained relatively unchanged except for late gene duplication events that gave rise to fibrillin-2 &-3 [65]. Fibrillin monomers can self-assemble into individual microfibrils that then associate to form large microfibril bundles [66]. Interestingly, physiological studies show that microfibrillar bundles provide limited elasticity to invertebrate tissues that lack elastin [67–69], suggesting that microfibrils were among the earliest elastic structures in the ECM.
In addition to serving as a scaffold for elastin assembly, microfibrils are important modulators of growth factor localization and function [70]. Fibrillin can interact directly with bone morphogenic proteins (BMPs) as well as with latent forms of TGFβ [71, 72]. All three TGFβs are secreted as an inactive latent dimer bound to a member of the latent TGFβ-binding protein (LTBP) family [73]. This large latent complex (TGFβ-LLC) binds covalently to fibrillin molecules in microfibrils [74], thereby creating a latent growth factor reserve in the ECM that can be activated and mobilized, when needed, through interactions with proteases, αvβ6 or αvβ8 integrins, or other factors [73]. Mutations in fibrillin are proposed to compromise its ability to retain the TGFβ-LLC complex in the matrix, resulting in inappropriate growth factor release, activation, and increased TGFβ activity. Numerous studies suggest that elevated TGFβ activity is the pathogenic mechanism leading to emphysema and aortic aneurysms in diseases such as Marfan syndrome, which has been linked to mutations in Fbn1 [75–77]. However, other studies suggest that elevated TGFβ levels are protective against tissue damage and act to suppress disease progression [78–81].
With the arrival of chordates and vertebrates came several new fibrillin-associated proteins, including fibulins-4 & -5, MAGP-1 & -2, and, as discussed above, elastin [82]. Fibrillins and MAGPs are likely the only constitutive components of microfibrils in vertebrates [82–84]. Fibulins, elastin and LTBPs can associate with microfibrils, but are not integral components and are not found on all microfibrils. None of the microfibril-associated proteins are required for fibrillin assembly. Instead, throughout evolution, we see changes occur to microfibril function brought about by the appearance of the proteins that partner with fibrillin to bring new and specialized functional properties. The ability to tailor microfibril activity by changing associated proteins provides a dynamic mechanism for meeting specific needs, such as altering growth factor signaling or controlling protein assembly.
4. ELASTIN AND LUNG DEVELOPMENT
Elastin is widely distributed in compartments of the mammalian lung including pleura, septa, large vessels, and elastic cartilage. The highest concentration of elastin is in the respiratory parenchyma where it comprises 20–30% of the crude connective tissue dry weight [85–87]. The elastin content of pulmonary blood vessels is 7–16% and that of airways is 3–5%. Numerous cell types produce elastin in the lung, including mesothelial cells [88, 89], airway epithelial cells [90, 91], vascular and airway smooth muscle cells [92, 93], endothelial cells [94, 95], and interstitial and lipid-laden fibroblasts [96–98].
4.1 The Lung Pleura: Elastin production by pleural mesothelial cells
There have been many studies of elastin expression in late fetal and postnatal stages of lung development [99–101], but expression of elastic fiber proteins in early lung development is less well understood. Among the earliest cells to make elastin are the pleural cells that line the lung surface (Figure 2). The pleural mesothelial cell is mesenchymal in origin but exhibits characteristics typical of epithelial cells, such as tight junctions, epithelial cytokeratins, and abundant microvilli on the apical surface. Pleural cells are attached basally to a basement membrane, beneath which is an extracellular matrix layer containing elastic fibers organized into an “elastic lamina” and dense bundles of fibrillar collagen. Ultrastructural analysis suggests that the collagen and elastin in the connective tissue layer are produced by the mesothelial cell, although resident fibroblasts may also contribute. Indeed, mesothelial cells have been shown to produce both collagen and elastin under tissue culture conditions [88, 89]. There is evidence suggesting that a population of progenitor-like mesothelial cells can differentiate into multiple cell types and represent a common lineage to fibroblasts, smooth muscle cells, and vasculature [102, 103]. Lung mesothelial cells also regulate epithelial airway branching and organ size through a FGF9 signaling pathway [104].
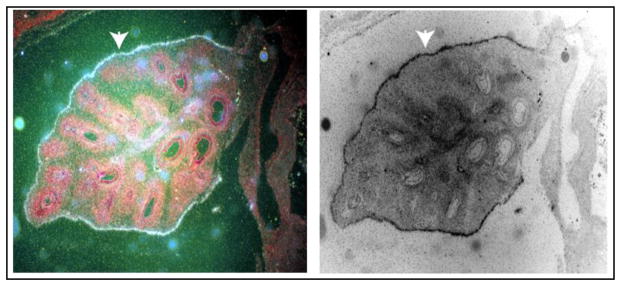
Figure 2.
In situ hybridization showing elastin gene expression in a ~7-week bovine embryonic lung. Tissue sections were hybridized with a 35S-labeled riboprobe specific for elastin mRNA and exposed to photographic emulsion. Developed slides were counterstained with hematoxylin-eosin, and white silver grains over areas of hybridization were visualized with dark-field microscopy. At this stage in development, tropoelastin showed highest expression in the visceral pleura (arrowhead) with detectable expression in vessels associated with large branching airways in the parenchyma. The image on the right is a reversal of dark-field only, where the hybridization signal shows up black.
The visceral pleura contributes to the mechanical properties of the lung and is in mechanical linkage with the lung parenchyma. This structural arrangement helps distribute mechanical stresses within the lung that facilitate patency of the alveoli and respiratory bronchioles. Without an elastic covering, the vertebrate lung would not be able to expand during inhalation or have the elastic recoil required for exhalation. The visceral pleura of the human and large animals tends to be thick, whereas the pleura in smaller animals, including the mouse, tends to be thin. The variable thickness is due to the submesothelial layer containing the connective tissue components, blood vessels, and lymphatics. The blood supply of the visceral pleura is considered to be of pulmonary origin in small mammals (such as mice) and of bronchial origin in larger mammals (including humans), although difficulties in assigning vascular origins make this generalization somewhat debatable (reviewed in [105]). In addition to contributing to lung elasticity, the pleural connective tissue helps prevent leakage of air from alveoli near the lung surface. Alterations in this barrier function may explain why inherited disorders of elastic fiber proteins, such as mutations in fibrillin associated with Marfan syndrome, permit the development of pneumothorax [77].
4.2 Expression of elastic fiber components in the pulmonary vasculature and bronchi
Among the earliest cells to synthesize elastin in the lung are the vascular cells associated with airways that invade the lung mesenchyme during early stages of lung development. The lungs begin as a ventral outpouching on the foregut. By successive dichotomous divisions, the airway tree increases in complexity as it invades the surrounding mesenchyme. Differentiation of the endodermally-derived epithelial tubules is strongly influenced by the surrounding mesoderm [104, 106, 107]. Development of pulmonary arteries and veins before birth is closely related to that of the bronchial tree [100] and the pre-acinar vascular branching pattern (proximal to the respiratory region of the lung) is present by the 20th week of fetal life in the human lung [108]. The intra-acinar arteries, in contrast, form during late fetal life and about half are formed after birth as alveolar ducts and alveoli form.
There is a double arterial and double venous supply in the adult lung, with the pulmonary arteries supplying the respiratory units. The bronchial vessels, which supply oxygenated blood from the systemic arteries, supply the airway walls and lung hilum, including pleura and large blood vessel walls. Precursors of the major bronchial vessels initiate by an angiogenic process in which endothelial cells comprising the vestiges of the brachial arches and segmental arteries migrate into the mesenchyme surrounding the early lung buds. In peripheral regions of the developing lung, small vessels form directly through a vasculogenic process in undifferentiated mesenchyme surrounding the growing airway buds. These vessels form a vascular plexus that links up with larger vessels that sprouted and migrated from more differentiated arteries and veins in proximal regions of the lung. Branching of the airways and arteries is contemporaneous, although branching of the airways occurs in advance of the arteries. Pulmonary veins develop later than the arteries and tend to run in the mesenchyme that demarcates the boundary between segments and subsegments of the lung [100].
In developing systemic arteries, expression of the elastin gene occurs coincident with the accumulation and condensation of mesenchymal cells around endothelial tubes and after expression of smooth muscle cell markers [109–112]. When expression of elastin and other elastic fiber proteins was examined in developing lung, unique and divergent expression patterns were found, suggesting that the function of elastic fiber proteins is more complex than initially anticipated.
Figure 3 shows ~10 week bovine lung hybridized with in situ probes that detect mRNA for elastin, fibrillin-1 and fibrillin-2. At this stage of development (pseudoglandular period), hilar regions of the lung show large, well-differentiated airways, arteries and veins that branch within the enclosing mesenchyme. Regions of the lung around the large airways (lower left region in each figure) are more differentiated than areas where branching is still occurring (upper right region).
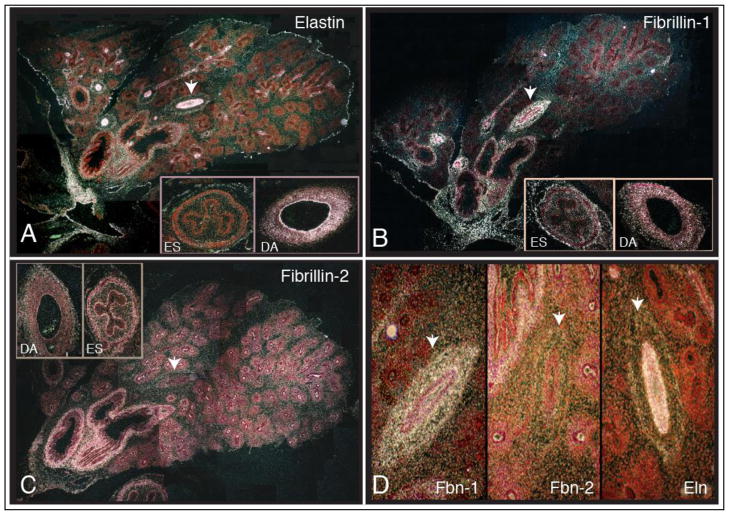
Figure 3.
In situ hybridization of ~10-week bovine embryonic lung with RNA probes for elastin (A), fibrillin-1(B), and fibrillin-2 (C). Panel 3D is a composite of gene expression in the pulmonary artery indicated by the arrowhead in panels A–C. Samples were prepared as described in Figure 2. See text for interpretation and details.
4.2.1 Elastin
Elastin gene expression (Figure 3A) continues in the visceral pleura (see Figure 2) as well as in the airway smooth muscle layer around the large, well-developed airways in the hilar region of the lung. In blood vessels, tropoelastin expression was detected in endothelial cells in both large and small vessels. SMCs in the medial layer of larger arteries also expressed elastin, although at lower levels than endothelial cells. Little tropoelastin expression was detected in the adventitia except for the outermost rim of cells (Figure 3D). Interestingly, tropoelastin was the only probe of the three that clearly hybridized with the small, less-differentiated vessels in peripheral regions of the lung. Probes for tropoelastin gene expression did not hybridize with all cells in the medial layer of the wall (not shown), suggesting heterogeneity in cellular phenotypes, at least in terms of ECM production. Cellular diversity in terms of the ECM gene expression is consistent with our previous observations of elastin and collagen expression in vessels from adult animals [113] and with studies using smooth muscle cell cytoskeletal markers to identify multiple phenotypically distinct SMC populations in pulmonary arteries [114–116]. There was no tropoelastin expression by epithelial cells in any region of the lung at this stage of development, or in the esophagus (ES). High levels of expression were found in the descending aorta (DA), especially by endothelial cells.
4.2.2 Fibrillin-1
Fibrillin-1 was expressed in the loose connective tissue that suspends the hilar region of the lung and, to a lesser extent, in the peripheral mesenchyme separating the branching segments (Figure 3B). Expression was also evident in well-differentiated blood vessels with high expression throughout the wall (Figure 3D). There was no detectable expression in the condensed mesenchyme around the branching epithelium or in the epithelium itself. In contrast to elastin, fibrillin-1 was not detected in the forming blood vessels in less-differentiated, peripheral regions of the lung. It is interesting to note that fibrillin-1 first appears in the large arteries at about the same time that it appears in the airways associated with those arteries, supporting co-differentiation of cells within arteries and airways.
4.2.3 Fibrillin-2
Fibrillin-2 expression overlapped with elastin and fibrillin-1 in the bronchial smooth muscle of large airways but was the only protein of the three that was expressed by epithelial cells in the distal regions of the lung (Figure 3C). No signal was detected in the regions separating the branching segments, but low expression levels were evident in the condensed mesenchyme around the branching epithelium. Interestingly, fibrillin-2 expression was not detected in either large or small pulmonary vessels (Figure 3D).
4.3 Elastin in the respiratory units of the lung
The basic structure of the gas exchange alveolar-capillary units becomes established during the canalicular phase of lung development, roughly from 16 to 26 weeks in humans. Capillary sprouts from larger vessels interact with and fuse to the basal lamina of epithelial cells in what will become the alveolar ducts and alveoli. These capillaries attach to the epithelial basement membrane and form a network at the surface between neighboring air sacs. The mesenchymal cells within the interstitium of the acini differentiate to produce collagen and elastin fibers, with elastic fibers in the walls of the alveolar ducts, saccule, and alveoli confined essentially to areas immediately surrounding the mouths of the alveoli [3, 117, 118]. These elastic fibers are part of an elastic fiber network that forms an elastic “line element” [119], or cable-membrane architecture [117], that originates in the central bronchi and inserts into the distal airspaces and visceral pleura. The cables from a looping system that encircle the alveolar duct and terminal air spaces and ensures that applied forces will be transmitted equally to all parts of the lung [85, 120].
Numerous studies show that elastic fiber formation in the respiratory region of the lung is tightly linked with alveolar formation [99, 121]. As large amounts of elastin are added and alveolar walls thin in the early postnatal period, lung recoil increases and stress relaxation becomes rapid [122]. In humans, alveolarization begins in the late fetal period and continues during the first eight to ten years of life [121]. In contrast to humans, true alveoli do not exist in the mouse or rat lung at birth, but form between postnatal days 3–15 [122–124]. Within the first 2–3 days of mouse postnatal life narrow ridges appear in the walls of the primary saccule. These “secondary crests” always contain elastin fibers and rapidly elongate to divide the primary saccule into many smaller units of alveoli—a process that continues until about postnatal day 14 [123]. Apically situated elastic and collagen fibers appear to be an essential component of all secondary crests, and the initiation of crest formation is associated with the deposition of these fibers.
Electron microscopy of postnatal lungs identified two types of interstitial cells in the alveolar wall [96]. Cells that appear at the tip of developing septa are associated with the surrounding elastic fibers and have ultrastructural features characteristic of cells engaged in protein synthesis and secretion. Fibroblasts at the base of developing septa are markedly different, having fewer secretory organelles and extensive accumulations of intracellular lipid [125]. Recently, multiple lung fibroblast subsets have been identified (reviewed in [97, 126, 127]) using markers associated with the PDGF signaling pathway [118, 128, 129], FGF signaling [130, 131] and Gli1-expression [132, 133]. How each of these unique cell populations relate to extracellular matrix production, especially elastin, is under investigation.
The importance of elastin to alveolar formation was shown by the absence of alveoli in Pdgf-a−/− mice that fail to produce elastin in the lung parenchyma [134]. Likewise, mice in which the elastin gene has been inactivated (Eln−/−) show abnormal terminal airway branches and distal air sacs that are dilated with attenuated tissue septae. Additional studies have documented that disrupting elastin fiber assembly in neonatal mice by inhibiting the crosslinking enzyme, lysyl oxidase, through copper-deficiency or treatment with lathrogens such as β–aminopropionitrile (BAPN) resulted in impaired alveolar septal formation [135, 136]. In contrast to PDGF-A-null mice where ablation of alveolar elastin synthesis is not associated with an aberrant phenotype before P4 (this is true for lathrogen treatment as well), inactivation of the elastin gene in Eln−/− mice results in lungs that have large air-filled cavities at birth. These differences suggest that disruption of elastin expression in regions other than the alveoli contribute to the pulmonary phenotype when elastin production is ablated in the embryo [137].
5. ELASTIN INTEGRITY AND LUNG DISEASE
5.1 Emphysema
5.1.1 Elastin degradation, inflammation, and emphysema
The discovery of an association between emphysema and α1- antitrypsin deficiency [138], in concert with the use of animal models that develop emphysema after intrapulmonary instillation of elastolytic enzymes [139, 140], lead to the recognition that destruction of elastin is central to disease pathogenesis (i.e., the elastase: anti-elastase hypothesis) [141]. The best-known source of proteases in the lung is inflammatory cells recruited to the airspaces by stimulators of inflammation, such as cigarette smoke (reviewed in [141, 142]). Analysis of the cell profile in alveoli and small airways of smokers shows an increase in all of the cell types implicated in chronic obstructive pulmonary disease (COPD), including macrophages, T lymphocytes, B lymphocytes, and neutrophils.
For many years, neutrophils were thought to be the cell type most responsible for tissue destruction in emphysema through the release of neutrophil elastase [143, 144]. Studies in knockout mice, however, found that the macrophage also plays a significant role in the pathophysiology of COPD and can account for many of the known features of the disease [145–147]. Both neutrophils and macrophages secrete potent elastases that degrade elastin and other matrix proteins [148]. Alveolar macrophages from patients with COPD secrete more inflammatory proteins and have a greater elastolytic activity at baseline than those from normal smokers and this is further increased by exposure to cigarette smoke [38]. Elastin fragments liberated during elastic fiber degradation recruit more inflammatory cells to the lung, leading to enhanced lung tissue destruction by placing more macrophages, and thus more elastin-degrading enzymes, within close proximity of the airspace [149–151].
The instillation of elastase into the air spaces of experimental animals produces lesions with the anatomic and physiologic features of emphysema [152]. After the initial destruction of elastin, connective tissue synthesis is stimulated and lung elastin content returns to normal. However, the resynthesized elastic fibers are disorganized and morphologically defective, and the normal elastic network is not restored [153]. The result is progressive airway loss and worsening lung function. Elastic fibers are among the most difficult matrix structures to repair because of their size, molecular complexity, and the requirement for numerous helper proteins to facilitate fiber assembly [154].
5.1.2 Elastin and disease susceptibility
Targeting elastic fiber genes with loss of function mutations illustrates the importance of elastin to lung development and supports the idea that loss of elastin is a major factor in emphysema and destructive lung diseases. As mentioned, elastin-deficient mice (Eln−/−) die within 48h of birth with abnormal lung development. Interestingly, mice heterozygous for the elastin mutation (Eln+/−) with elastin levels about half those of wild-type animals have normal lung morphology [137]. However, on exposure to cigarette smoke (representing chronic pulmonary injury), Eln+/− mice develop more severe airspace enlargement than wild-type (WT) littermate control mice (Figure 4) [155]. These findings suggest that while lower than normal levels of elastin can support regular lung development, elastin insufficient mice are more prone to develop severe lung disease when exposed to injurious environmental stimuli. Thus, the quantity of elastin in the lung may be an important factor in determining susceptibility to lung injury. While 50% elastin can support normal lung development, mice with less than 50% normal elastin levels exhibit airway abnormalities [156]. For example, mice that express only 30% of normal elastin levels have enlarged thoracic cavities occupied by highly distended lungs that are larger than both WT and Eln+/− lungs at identical inflation pressures [155]. Thus, when elastin levels drop below ~50% of normal, lung development is compromised.
Figure 4.
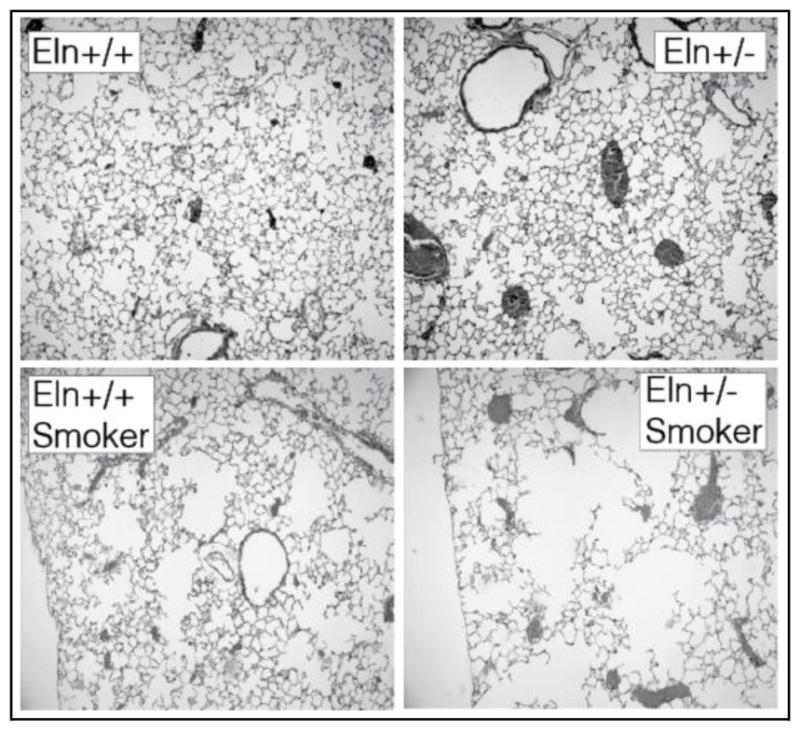
Histological sections of lungs from adult wild-type (Eln+/+) and elastin haploinsufficient (Eln+/−) mice. Eln+/− animals exhibit normal alveolar structure (top panels) but develop worse emphysema than normal mice following cigarette smoke exposure (bottom panels).
5.2 Elastin Gain-of-Function Mutations and Autosomal Dominant Cutis Laxa
Elastin gain-of-function mutations that modify key assembly domains in elastin can also lead to lung disease by altering the ability of elastin to assemble into the functional polymer. Autosomal dominant cutis laxa (ADCL, OMIM #123700) is an inherited disease that produces frameshift mutations near the 3′ end of the gene. The result is missense sequence through a critical assembly domain in exon 36 [157, 158]. Several studies have documented severe COPD in individuals with ADCL, especially those who smoke [159, 160]. ADCL mutations alter the ability of the protein from the mutant allele to assemble properly. There is also the likely possibility that the mutant product acts in a dominant–negative fashion to alter the assembly of the protein from the normal allele. A humanized mouse model of ADCL expressing the human elastin gene with a common ADCL mutation showed that alternative splicing of down-stream exons generates multiple transcripts with different lengths of missense sequence and different stabilities [10]. Analysis of tissues expressing the human ADCL transgene found that the mutant protein is incorporated into elastic fibers in skin and lung with adverse effects, but low levels of incorporation were observed in the aorta, which explains why the vasculature is less affected in this disease as a consequence of this mutation [10]. These results suggest that the process of elastin assembly may be different in the aorta compared with lung and skin, which challenges the current thinking of a common fiber assembly mechanism in all tissues.
In addition to causality mutations like those in ADCL, the genetics of COPD susceptibility has been studied extensively [161–163]. A novel variant in the terminal exon of human elastin (c.2318 G A) resulting in an amino acid substitution of glycine 773 to aspartate (G773D), for example, was identified in a pedigree with severe early onset chronic obstructive pulmonary disease (COPD) [164]. Evidence of airflow limitation was most severe among those mutation carriers who smoked, suggesting a gene-by-environment interaction. These results suggest that the G773D variant confers structural and functional consequences relevant to the pathogenesis of COPD and that individual carriers of this polymorphism could be at increased risk for developing lung disease. Similarly, mutations in most of the known elastic fiber genes, including fibulin-4 [165, 166], fibulin-5 [167–169], fibrillin-1[77, 170], latent TGFβ binding proteins [171, 172], and lysyl oxidase-like 1[173, 174], lead to lung disease in humans and mice.
5.3 Elastin as a biomarker for disease
Because of elastin’s low turnover rate, elastin fragments detected in body fluids can be used diagnostically as markers for elastin-degrading diseases, such as emphysema (reviewed in [175]). The cross-linking amino acids desmosine and isodesmosine exist only in insoluble elastin and their presence in blood, urine or sputum can serve as a marker for elastic fiber degradation by elastases associated with inflammation. Desmosine and isodesmosine levels in sputum reflect the state of elastin degradation in the lung specifically [175]. Assays that can accurately measure elastin cross-links or elastin peptide fragments [176] hold great promise for determining disease activity, severity and responses to therapy.
6. CONCLUSIONS
The ECM is a complex integrated system of interacting molecules required for the normal functioning of the lung. Elastic fibers are a major component of this ECM and are essential for lung development and for ensuring that mechanical forces are transmitted equally to all parts of the lung. Understanding the critical role for elastin in lung development and disease will facilitate the discovery of therapies aimed at promoting pulmonary regeneration through alveolation and lung growth in an effort to improve the outcomes of patients afflicted with emphysema and other destructive lung diseases.
Highlights.
The appearance of elastin in vertebrates was important for the advent of a closed circulatory system and the elastic lung.
In the developing lung, elastin is expressed in the pleura, the blood vascular system, and in the bronchi and respiratory units.
Elastin is required for normal lung development and elastic fiber production in the respiratory region of the lung is tightly linked with alveolar formation.
Destruction of elastin, or abnormalities in elastic fiber assembly, are major factors in emphysema and destructive lung diseases.
Acknowledgments
Original work described in this review was supported by the National Institutes of Health grants R01-HL53325 and R01-HL105314. The author wishes to thank past and present lab members, for their contributions to our understanding of elastic fiber biology. Special thanks to Drs. Jennifer Hausladen and Sean McLean whose work provided the data in Figures 2 & 3 and Dr. Adrian Shifren for data in Figure 4.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hsia CC, Schmitz A, Lambertz M, Perry SF, Maina JN. Evolution of air breathing: oxygen homeostasis and the transitions from water to land and sky. Compr Physiol. 2013;3(2):849–915. doi: 10.1002/cphy.c120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liem KF. Form and function of lungs: The evolution of air breathing mechanisms. Amer Zool. 1988;28(2):739–759. [Google Scholar]
- 3.Loosli C, Potter E. Pre- and postnatal development of the respiratory portion of the human lung with special reference to the elastic fibers. Am Rev Respir Dis. 1959;80(1 Part 2):5–23. doi: 10.1164/arrd.1959.80.1P2.5. [DOI] [PubMed] [Google Scholar]
- 4.Mariani TJ, Reed JJ, Shapiro SD. Expression profiling of the developing mouse lung: insights into the establishment of the extracellular matrix. Am J Respir Cell Mol Biol. 2002;26(5):541–548. doi: 10.1165/ajrcmb.26.5.2001-00080c. [DOI] [PubMed] [Google Scholar]
- 5.Mariani TJ, Sandeful S, Pierce RA. Elastin in lung development. Exp Lung Res. 1997;23:131–145. doi: 10.3109/01902149709074026. [DOI] [PubMed] [Google Scholar]
- 6.Keeley FW. The Evolution of Elastin. In: Keeley FW, Mecham RP, editors. Evolution of Extracellular Matrix. Springer-Verlag; Berlin Heidelberg: 2013. pp. 73–119. [Google Scholar]
- 7.Sage H, Gray WR. Studies on the evolution of elastin-III. The ancestral protein. Comp Biochem Physiol. 1981;68B:473–480. [Google Scholar]
- 8.Sage H, Gray WR. Studies on the evolution of elastin-II. Histology. Comp Biochem Physiol B. 1980;66B:13–22. doi: 10.1016/0305-0491(79)90277-3. [DOI] [PubMed] [Google Scholar]
- 9.Sage H, Gray W. Studies on the evolution of elastin--I. Phylogenetic distribution. Comp Biochem Physiol B. 1979;64(4):313–327. doi: 10.1016/0305-0491(79)90277-3. [DOI] [PubMed] [Google Scholar]
- 10.Sugitani H, Hirano E, Knutsen R, Shifren A, Wagenseil J, Ciliberto C, Kozel B, Urban Z, Davis E, Broekelmann T, Mecham R. Alternative Splicing and Tissue-specific Elastin Misassembly Act as Biological Modifiers of Human Elastin Gene Frameshift Mutations Associated with Dominant Cutis Laxa. J Biol Chem. 2012;287(26):22055–67. doi: 10.1074/jbc.M111.327940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce RA, Alatawi A, Deak SB, Boyd CD. Elements of the rat tropoelastin gene associated with alternative splicing. Genomics. 1992;12(4):651–658. doi: 10.1016/0888-7543(92)90289-5. [DOI] [PubMed] [Google Scholar]
- 12.Yeh H, Anderson N, Ornstein-Goldstein N, Bashir MM, Rosenbloom JC, Abrams W, Indik Z, Yoon K, Parks W, Mecham R, Rosenbloom J. Structure of the bovine elastin gene and S1 nuclease analysis of alternative splicing of elastin mRNA in the bovine nuchal ligament. Biochemistry. 1989;28:2365–2370. doi: 10.1021/bi00432a003. [DOI] [PubMed] [Google Scholar]
- 13.Barrineau LL, Rich CB, Foster JA. The biosynthesis of tropoelastin in chick and pig tissues. Connect Tiss Res. 1981;8:189–491. doi: 10.3109/03008208109152373. [DOI] [PubMed] [Google Scholar]
- 14.Partridge SM. Elastin. Adv Prot Chem. 1962;17:227–302. [Google Scholar]
- 15.Kozel BA, mecham RP, Rosenbloom J. Elastin. In: Mecham RP, editor. Biology of Extracellular Matrix. Springer-Verlag; Berlin-Heidelberg: 2011. pp. 267–301. [Google Scholar]
- 16.Kagan HM, Ryvkin F. Lysyl Oxidase and Lysyl Oxidase-like Enzymes. In: Mecham RP, editor. The Extracellular Matrix: an Overview. Springer-Verlag; Berlin Heidelberg: 2011. pp. 303–335. [Google Scholar]
- 17.Franzblau C, Faris B, Papaioannou R. Lysinonorleucine. A new amino acid from hydrolysates of elastin. Biochemistry. 1969;8(7):2833–7. doi: 10.1021/bi00835a021. [DOI] [PubMed] [Google Scholar]
- 18.Franzblau C, Baris B, Lent RW, Salcedo LL, Smith B, Jaffe R, Crombie G. Chemistry and biosynthesis of crosslinks in elastin. In: Balazs EA, editor. Chemistry and Molecular Biology of the Intracellular Matrix. Vol. 1. Academic Press; New York: 1969. pp. 617–639. [Google Scholar]
- 19.Lent R, Franzblau C. Studies on the reduction of bovine elastin: evidence for the presence of delta-6,7-dehydrolysinonorleucine. Biochem Biophys Res Commun. 1967;26(1):43–50. doi: 10.1016/0006-291x(67)90250-1. [DOI] [PubMed] [Google Scholar]
- 20.Akagawa M, Suyama K. Mechanism of formation of elastin crosslinks. Connect Tissue Res. 2000;41(2):131–141. doi: 10.3109/03008200009067665. [DOI] [PubMed] [Google Scholar]
- 21.Partridge SM, Elsden DF, Thomas J. Constitution of the cross-linkages in elastin. Nature (Lond) 1963;197:1297–1298. doi: 10.1038/1971297a0. [DOI] [PubMed] [Google Scholar]
- 22.Francis G, John R, Thomas J. Biosynthetic pathway of desmosines in elastin. Biochem J. 1973;136(1):45–55. doi: 10.1042/bj1360045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starcher BC, Partridge SM, Elsden DF. Isolation and partial characterization of a new amino acid from reduced elastin. Biochemistry. 1967;6(8):2425–32. doi: 10.1021/bi00860a019. [DOI] [PubMed] [Google Scholar]
- 24.Akagawa M, Yamazaki K, Suyama K. Cyclopentenosine, major trifunctional crosslinking amino acid isolated from acid hydrolysate of elastin. Arch Biochem Biophys. 1999;372(1):112–120. doi: 10.1006/abbi.1999.1462. [DOI] [PubMed] [Google Scholar]
- 25.Umeda H, Takeuchi M, Suyama K. Two new elastin cross-links having pyridine skeleton. Implication of ammonia in elastin cross-linking in vivo. J Biol Chem. 2001;276(16):12579–12587. doi: 10.1074/jbc.M009744200. [DOI] [PubMed] [Google Scholar]
- 26.Umeda H, Nakamura F, Suyama K. Oxodesmosine and isooxodesmosine, candidates of oxidative metabolic intermediates of pyridinium cross-links in elastin. Arch Biochem Biophys. 2001;385(1):209–219. doi: 10.1006/abbi.2000.2145. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 1991;87:1828–1834. doi: 10.1172/JCI115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starcher B. Elastin and the lung. Thorax. 1986;41(8):577–85. doi: 10.1136/thx.41.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holzenberger M, Lievre C, Robert L. Tropoelastin gene expression in the developing vascular system of the chicken: an in situ hybridization study. Anat Embryol (Berl) 1993;188(5):481–92. doi: 10.1007/BF00190142. [DOI] [PubMed] [Google Scholar]
- 30.Keeley F. The synthesis of soluble and insoluble elastin in chicken aorta as a function of development and age. Effect of a high cholesterol diet. Can J Biochem. 1979;57(11):1273–1280. doi: 10.1139/o79-169. [DOI] [PubMed] [Google Scholar]
- 31.Berry CL, Looker T, Germain J. The growth and development of the rat aorta. I. Morphological aspects. JAnat. 1972;113:1–16. [PMC free article] [PubMed] [Google Scholar]
- 32.Bendeck M, Langille B. Rapid accumulation of elastin and collagen in the aortas of sheep in the immediate perinatal period. Circ Res. 1991;69(4):1165–1169. doi: 10.1161/01.res.69.4.1165. [DOI] [PubMed] [Google Scholar]
- 33.Dubick M, Rucker R, Cross C, Last J. Elastin metabolism in rodent lung. Biochim Biophys Acta. 1981;672(3):303–306. doi: 10.1016/0304-4165(81)90297-x. [DOI] [PubMed] [Google Scholar]
- 34.Cleary E, Sandberg L, Jackson D. The changes in chemical composition during development of the bovine nuchal ligament. J Cell Biol. 1967;33(3):469–479. doi: 10.1083/jcb.33.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagenseil J, Mecham R. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89(3):957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bieth JG. Elastases: Catalytic and biological properties. In: Mecham RP, editor. Regulation of matrix accumulation. Academic Press; New York: 1986. pp. 217–320. [Google Scholar]
- 37.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56(4):515–48. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 38.Russell RE, Thorley A, Culpitt SV, Dodd S, Donnelly LE, Demattos C, Fitzgerald M, Barnes PJ. Alveolar macrophage-mediated elastolysis: roles of matrix metalloproteinases, cysteine, and serine proteases. Am J Physiol Lung Cell Mol Physiol. 2002;283(4):L867–73. doi: 10.1152/ajplung.00020.2002. [DOI] [PubMed] [Google Scholar]
- 39.Hajjar E, Broemstrup T, Kantari C, Witko-Sarsat V, Reuter N. Structures of human proteinase 3 and neutrophil elastase--so similar yet so different. FEBS J. 2010;277(10):2238–54. doi: 10.1111/j.1742-4658.2010.07659.x. [DOI] [PubMed] [Google Scholar]
- 40.Hase CC, Finkelstein RA. Bacterial extracellular zinc-containing metalloproteases. Microbiol Rev. 1993;57:823–837. doi: 10.1128/mr.57.4.823-837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morihara K, Tsuzuki H. Elastolytic properties of various proteinases from microbial orgin. Arch Biochem Biophys. 1967;120:68–78. doi: 10.1016/0003-9861(67)90599-1. [DOI] [PubMed] [Google Scholar]
- 42.Mecham RP, Broekelmann T, Fliszar CJ, Shapiro SD, Welgus HG, Senior RM. Elastin degradation by matrix metalloproteinases: Cleavage site specificity and mechanisms of elastolysis. J Biol Chem. 1997;272:18071–18076. doi: 10.1074/jbc.272.29.18071. [DOI] [PubMed] [Google Scholar]
- 43.Houghton AM, Mouded M, Shapiro SD. Consequences of Elastollysis. In: Parks WC, Mecham RP, editors. Extracellular Matrix Degradation. Springer-Verlag; Berlin Heidelberg: 2011. pp. 217–249. [Google Scholar]
- 44.Houghton AM. Matrix metalloproteinases in destructive lung disease. Matrix Biol. 2015;44–46:167–74. doi: 10.1016/j.matbio.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Cleary E, Fanning J, Prosser I. Possible roles of microfibrils in elastogenesis. Connect Tissue Res. 1981;8(3–4):161–166. doi: 10.3109/03008208109152367. [DOI] [PubMed] [Google Scholar]
- 46.Cleary EG, Gibson MA. Elastin-associated microfibrils and microfibrillar proteins. Int Rev Connect Tiss Res. 1983;10:97–209. doi: 10.1016/b978-0-12-363710-9.50009-5. [DOI] [PubMed] [Google Scholar]
- 47.Davis EC. Smooth muscle cell to elastic lamina connections in the developing mouse arota: Role in aortic medial organization. Lab Invest. 1993;68:89–99. [PubMed] [Google Scholar]
- 48.Carta L, Pereira L, Arteaga-Soli E, Lee-Arteaga SY, Lenart B, Starcher B, Merkel CA, Sukoyan M, Kerkis A, Hazeki N, Keene DR, Sakai LY, Ramirez F. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J Biol Chem. 2006;281:8016–8023. doi: 10.1074/jbc.M511599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagenseil J, Mecham R. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today. 2007;81(4):229–40. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- 50.Davis E, Mecham R. Intracellular trafficking of tropoelastin. Matrix Biol. 1998;17(4):245–254. doi: 10.1016/s0945-053x(98)90078-6. [DOI] [PubMed] [Google Scholar]
- 51.Choudhury R, McGovern A, Ridley C, Cain S, Baldwin A, Wang M, Guo C, Mironov A, Jnr, Drymoussi Z, Trump D, Shuttleworth A, Baldock C, Kielty C. Differential regulation of elastic fiber formation by fibulins -4 AND -5. J Biol Chem. 2009;284(36):24553–24567. doi: 10.1074/jbc.M109.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis E, Mecham R. Selective degradation of accumulated secretory proteins in the endoplasmic reticulum. A possible clearance pathway for abnormal tropoelastin. J Biol Chem. 1996;271(7):3787–3794. [PubMed] [Google Scholar]
- 53.Privitera S, Prody CA, Callahan JW, Hinek A. The 67-kDa enzymatically inactive alternatively spliced variant of β-galactosidase is identical to the elastin/laminin binding protein. J Biol Chem. 1998;273:6319–6326. doi: 10.1074/jbc.273.11.6319. [DOI] [PubMed] [Google Scholar]
- 54.Kozel BA, Rongish BJ, Czirok A, Zach J, Little CD, Davis EC, Knutsen RH, Wagenseil JE, Levy MA, Mecham RP. Elastic fiber formation: A dynamic view of extracellular matrix assembly using timer reporters. J Cell Physiol. 2006;207(1):87–96. doi: 10.1002/jcp.20546. [DOI] [PubMed] [Google Scholar]
- 55.Czirok A, Zach J, Kozel BA, Mecham RP, Davis EC, Rongish BJ. Elastic fiber macro-assembly is a hierarchical, cell motion-mediated process. J Cell Physiol. 2006;207:97–106. doi: 10.1002/jcp.20573. [DOI] [PubMed] [Google Scholar]
- 56.Clarke AW, Arnspang EC, Mithieux SM, Korkmaz E, Braet F, Weiss AS. Tropoelastin massively associates during coacervation to form quantized protein spheres. Biochemistry. 2006;45(33):9989–9996. doi: 10.1021/bi0610092. [DOI] [PubMed] [Google Scholar]
- 57.Pasquali-Ronchetti I, Fornieri C, Baccarani-Contri M, Volpin D. The ultrastructure of elastin revealed by freeze-fracture electron microscopy. Micron. 1979;10:89–99. [Google Scholar]
- 58.Ronchetti I, Contri M, Fornieri C, Quaglino DJ, Mori G. Alterations of elastin fibrogenesis by inhibition of the formation of desmosine crosslinks. Comparison between the effect of beta-aminopropionitrile (beta-APN) and penicillamine. Connect Tissue Res. 1985;14(2):159–67. doi: 10.3109/03008208509015021. [DOI] [PubMed] [Google Scholar]
- 59.Kozel BA, Ciliberto CH, Mecham RP. Deposition of tropoelastin into the extracellular matrix requires a competent elastic fiber scaffold but not live cells. Matrix Biol. 2004;23:23–34. doi: 10.1016/j.matbio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Corson GM, Charbonneau NL, Keene DR, Sakai LY. Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics. 2004;83(3):461–472. doi: 10.1016/j.ygeno.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 61.Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986;103:2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H, Hu W, Ramirez F. Developmental expression of fibrillin genes suggests heterogeneity of extracellular microfibrils. J Cell Biol. 1995;129:1165–1176. doi: 10.1083/jcb.129.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robertson I, Jensen S, Handford P. TB domain proteins: evolutionary insights into the multifaceted roles of fibrillins and LTBPs. Biochem J. 2011;433(2):263–76. doi: 10.1042/BJ20101320. [DOI] [PubMed] [Google Scholar]
- 64.Reber-Muller S, Spissinger T, Schuchert P, Spring J, Schmid V. An extracellular matrix protein of jellyfish homologous to mammalian fibrillins forms different fibrils depending on the life stage of the animal. Dev Biol. 1995;169(2):662–672. doi: 10.1006/dbio.1995.1177. [DOI] [PubMed] [Google Scholar]
- 65.Piha-Gossack A, Sossin W, Reinhardt D. The evolution of extracellular fibrillins and their functional domains. PLoS One. 2012;7(3):e33560. doi: 10.1371/journal.pone.0033560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weber C, Schmid V. The fibrous system in the extracellular matrix of hydromedusae. Tissue Cell. 1985;17(6):811–22. doi: 10.1016/0040-8166(85)90038-2. [DOI] [PubMed] [Google Scholar]
- 67.Megill W, Gosline J, Blake R. The modulus of elasticity of fibrillin-containing elastic fibres in the mesoglea of the hydromedusa Polyorchis penicillatus. J Exp Biol. 2005;208(Pt 20):3819–3834. doi: 10.1242/jeb.01765. [DOI] [PubMed] [Google Scholar]
- 68.Davison I, Wright G, DeMont M. The structure and physical properties of invertebrate and primitive vertebrate arteries. J Exp Biol. 1995;198(Pt 10):2185–9216. doi: 10.1242/jeb.198.10.2185. [DOI] [PubMed] [Google Scholar]
- 69.McConnell C, Wright G, DeMont M. The modulus of elasticity of lobster aorta microfibrils. Experientia. 1996;52(9):918–21. doi: 10.1007/BF01938880. [DOI] [PubMed] [Google Scholar]
- 70.Hynes R. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wohl A, Troilo H, Collins R, Baldock C, Sengle G. Extracellular Regulation of Bone Morphogenetic Protein Activity by the Microfibril Component Fibrillin-1. J Biol Chem. 2016;291(24):12732–46. doi: 10.1074/jbc.M115.704734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sengle G, Sakai L. The fibrillin microfibril scaffold: A niche for growth factors and mechanosensation. Matrix Biol. 2015 doi: 10.1016/j.matbio.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Robertson I, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin D. Latent TGF-β-binding proteins. Matrix Biol. 2015;47:44–53. doi: 10.1016/j.matbio.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karonen T, Jeskanen L, Keski-Oja J. Transforming growth factor beta 1 and its latent form binding protein-1 associate with elastic fibres in human dermis: accumulation in actinic damage and absence in anetoderma. Br J Dermatol. 1997;137(1):51–58. [PubMed] [Google Scholar]
- 75.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312(5770):117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lindsay M, Dietz H. The genetic basis of aortic aneurysm. Cold Spring Harb Perspect Med. 2014;4(9):a015909. doi: 10.1101/cshperspect.a015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33(3):407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 78.Li W, Li Q, Jiao Y, Qin L, Ali R, Zhou J, Ferruzzi J, Kim R, Geirsson A, Dietz H, Offermanns S, Humphrey J, Tellides G. Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis. J Clin Invest. 2014;124(2):755–67. doi: 10.1172/JCI69942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu J, Wei H, Jaffe M, Airhart N, Du L, Angelov S, Yan J, Allen J, Kang I, Wight T, Fox K, Smith A, Enstrom R, Dichek D. Postnatal Deletion of the Type II Transforming Growth Factor-β Receptor in Smooth Muscle Cells Causes Severe Aortopathy in Mice. Arterioscler Thromb Vasc Biol. 2015;35(12):2647–56. doi: 10.1161/ATVBAHA.115.306573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oller J, Mendez-Barbero N, Ruiz EJ, Villahoz S, Renard M, Canelas LI, Briones AM, Alberca R, Lozano-Vidal N, Hurle MA, Milewicz D, Evangelista A, Salaices M, Nistal JF, Jimenez-Borreguero LJ, De Backer J, Campanero MR, Redondo JM. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat Med. 2017;23(2):200–212. doi: 10.1038/nm.4266. [DOI] [PubMed] [Google Scholar]
- 81.Mallat Z, Ait-Oufella A, Tedgui A. The pathogenic transforming growth factor-beta overdrive hypothesis in aortic aneurysms and dissections. A mirage? CircRes. 2017;120:1718–1720. doi: 10.1161/CIRCRESAHA.116.310371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Segade F. Functional evolution of the microfibril-associated glycoproteins. Gene. 2009;439(1–2):43–54. doi: 10.1016/j.gene.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 83.Gibson MA, Hatzinikolas G, Kumaratilake JS, Sandberg LB, Nicholl JK, Sutherland GR, Cleary EG. Further characterization of proteins associated with elastic fiber microfibrils including the molecular cloning of MAGP-2 (MP25) J Biol Chem. 1996;271:1096–1103. doi: 10.1074/jbc.271.2.1096. [DOI] [PubMed] [Google Scholar]
- 84.Henderson M, Polewski R, Fanning JC, Gibson MA. Microfibril-associated glycoprotein-1 (MAGP-1) is specifically located on the beads of the beaded-filament structure of fibrillin-containing microfibrils as visualized by the rotary shadowing technique. J Histochem Cytochem. 1996;44:1389–1397. doi: 10.1177/44.12.8985131. [DOI] [PubMed] [Google Scholar]
- 85.Pierce JA, Ebert RV. Fibrosis network of the lung and its change with age. Thorax. 1965;20:469–476. [Google Scholar]
- 86.Chrzanowski P, Keller S, Cerreta J, Mandl I, Turino GM. Elastin content of normal and emphysematous lung parenchyma. Am J Med. 1980;69(3):351–359. doi: 10.1016/0002-9343(80)90004-2. [DOI] [PubMed] [Google Scholar]
- 87.Starcher BC. Determination of the elastin content of tissues by measuring desmosine and isodesmosine. Anal Biochem. 1977;79(1–2):11–5. doi: 10.1016/0003-2697(77)90372-4. [DOI] [PubMed] [Google Scholar]
- 88.Cantor JO, Willhite M, Bray BA, Keller S, Mandl I, Turino GM. Synthesis of crosslinked elastin by a mesothelial cell culture. Proc Soc Exp Biol Med. 1986;181(3):387–91. doi: 10.3181/00379727-181-42269. [DOI] [PubMed] [Google Scholar]
- 89.Rennard SI, Jaurand MC, Bignon J, Kawanami O, Ferrans VJ, Davidson J, Crystal RG. Role of pleural mesothelial cells in the production of the submesothelial connective tissue matrix of lung. Am Rev Respir Dis. 1984;130(2):267–74. doi: 10.1164/arrd.1984.130.2.267. [DOI] [PubMed] [Google Scholar]
- 90.Dabovic B, Chen Y, Choi J, Vassallo M, Dietz H, Ramirez F, von Melchner H, Davis E, Rifkin D. Dual functions for LTBP in lung development: LTBP-4 independently modulates elastogenesis and TGF-beta activity. J Cell Physiol. 2009;219(1):14–22. doi: 10.1002/jcp.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dabovic B, Robertson I, Zilberberg L, Vassallo M, Davis E, Rifkin D. Function of Latent TGFbeta Binding Protein 4 and Fibulin 5 in Elastogenesis and Lung Development. J Cell Physiol. 2015;230(1):226–36. doi: 10.1002/jcp.24704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Noguchi A, Samaha H, deMello D. Tropoelastin gene expression in the rat pulmonary vasculature: a developmental study. Pediatr Res. 1992;31(3):280–5. doi: 10.1203/00006450-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 93.Stenmark KR, Dempsey EC, Badesch DB, Frid M, Mecham RP, Parks WC. Regulation of pulmonary vascular wall cell growth: Developmental and site-specific heterogeneity. Eur Respir Rev. 1993;3:629–637. (review #16) [Google Scholar]
- 94.Cantor J, Keller S, Parshley M, Darnule T, Darnule A, Cerreta J, Turino G, Mandl I. Synthesis of crosslinked elastin by an endothelial cell culture. Biochem Biophys Res Commun. 1980;95(4):1381–6. doi: 10.1016/s0006-291x(80)80050-7. [DOI] [PubMed] [Google Scholar]
- 95.Mecham R, Madaras J, McDonald J, Ryan U. Elastin production by cultured calf pulmonary artery endothelial cells. J Cell Physiol. 1983;116(3):282–288. doi: 10.1002/jcp.1041160304. [DOI] [PubMed] [Google Scholar]
- 96.Vaccaro C, Brody J. Ultrastructure of developing alveoli. I. The role of the interstitial fibroblast. Anat Rec. 1978;192(4):467–79. doi: 10.1002/ar.1091920402. [DOI] [PubMed] [Google Scholar]
- 97.McGowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol. 1997;59:43–62. doi: 10.1146/annurev.physiol.59.1.43. [DOI] [PubMed] [Google Scholar]
- 98.Burri PH. Structural aspects of prenatal and postnatal development and growth of the lung. In: McDonald JA, editor. Lung Growth and Development. Marcel Dekker, Inc; New York: 1997. pp. 1–35. [Google Scholar]
- 99.Burri PH, Weibel ER. Ultrastructure and morphometry of the developing lung. In: Hodson WA, editor. Development of the Lung. Marcel Dekker, Inc; New York: 1977. pp. 215–268. [Google Scholar]
- 100.Hislop A, Reid LM. Formation of the pulmonary vasculature. In: Hodson WA, editor. Development of the Lung. Marcel Dekker, Inc; New York: 1977. pp. 37–86. [Google Scholar]
- 101.O’Rahilly R. The early prenatal development of the human respiratory system. In: Nelson GE, editor. Pulmonary Development. Marcel Dekker, Inc; New York: 1985. pp. 3–18. [Google Scholar]
- 102.Rinkevich Y, Mori T, Sahoo D, Xu P, Bermingham J, Weissman I. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol. 2012;14(12):1251–60. doi: 10.1038/ncb2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Batra H, Antony VB. Pleural mesothelial cells in pleural and lung diseases. J Thorac Dis. 2015;7(6):964–80. doi: 10.3978/j.issn.2072-1439.2015.02.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Colvin J, White A, Pratt S, Ornitz D. Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development. 2001;128(11):2095–2106. doi: 10.1242/dev.128.11.2095. [DOI] [PubMed] [Google Scholar]
- 105.Bernaudin J-F, Fleury J. Anatomy of the blood and lymphatic circulation of the pleural serosa. In: Crétien J, Bignon J, Hirsch A, editors. The Pleura in Health and Disease. Marcel Dekker, Inc; New York - Basel: 1985. pp. 101–124. [Google Scholar]
- 106.Spooner BS, Wessells NK. Mammalian lung development: interactions in primordium formation and bronchial morphogenesis. J Exp Zool. 1970;175(4):445–54. doi: 10.1002/jez.1401750404. [DOI] [PubMed] [Google Scholar]
- 107.Wessells NK. Mammalian lung development: Interactions in formation and morphogenesis of tracheal buds. J Exp Zool. 1970;175:455–466. doi: 10.1002/jez.1401750405. [DOI] [PubMed] [Google Scholar]
- 108.Hislop A, Muir DC, Jacobsen M, Simon G, Reid L. Postnatal growth and function of the pre-acinar airways. Thorax. 1972;27(3):265–74. doi: 10.1136/thx.27.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Selmin O, Volpin D, Bressan GM. Changes of cellular expression of mRNA for tropelastin in the intraembryonic arterial vessels of developing chick revealed by in situ hybridization. Matrix. 1991;11:347–358. doi: 10.1016/s0934-8832(11)80206-4. [DOI] [PubMed] [Google Scholar]
- 110.Bergwerff M, DeRuiter M, Poelmann R, Gittenberger-de Groot A. Onset of elastogenesis and downregulation of smooth muscle actin as distinguishing phenomena in artery differentiation in the chick embryo. Anat Embryol (Berl) 1996;194(6):545–57. doi: 10.1007/BF00187468. [DOI] [PubMed] [Google Scholar]
- 111.Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Dev Biol. 2004;62:153–188. doi: 10.1016/S0070-2153(04)62006-0. [DOI] [PubMed] [Google Scholar]
- 112.McLean SE, Mecham BH, Kelleher CM, Mariani TJ, Mecham RP. Extracellular matrix gene expression in developing mouse aorta. In: Miner JH, editor. Extracellular Matrices and Development. Elsevier; New York: 2005. pp. 82–128. [Google Scholar]
- 113.Prosser IW, Stenmark KR, Suthar M, Mecham RP, Parks WC. Regional heterogeneity of elastin and collagen gene expression in intralobar arteries in response to hypoxic pulmonary hypertension as demonstrated by in situ hybridization. Am J Pathol. 1989;135:1073–1088. [PMC free article] [PubMed] [Google Scholar]
- 114.Hungerford JE, Little CD. Developmental biology of the vascular smooth muscle cell: building a multilayered vessel wall. J Vasc Res. 1999;36(1):2–27. doi: 10.1159/000025622. [DOI] [PubMed] [Google Scholar]
- 115.Frid MG, Moiseeva EP, Stenmark KR. Multiple phenotypically distinct smooth muscle cell populations exist in the adult and developing bovine pulmonary arterial media in vivo. Circ Res. 1994;75:669–681. doi: 10.1161/01.res.75.4.669. [DOI] [PubMed] [Google Scholar]
- 116.Haworth SG. Development of the normal and hypertensive pulmonary vasculature. Exp Physiol. 1995;80(5):843–53. doi: 10.1113/expphysiol.1995.sp003892. [DOI] [PubMed] [Google Scholar]
- 117.Kuhn C, 3rd, Oldmixon EH. The interstitium of the lung. In: Schraufnagel DE, editor. Electon Microscopy of the Lung. Marcel Dekker, Inc; New York: 1990. pp. 177–214. [Google Scholar]
- 118.Branchfield K, Li R, Lungova V, Verheyden JM, McCulley D, Sun X. A three-dimensional study of alveologenesis in mouse lung. Dev Biol. 2016;409(2):429–41. doi: 10.1016/j.ydbio.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wagner W, Bennett RD, Ackermann M, Ysasi AB, Belle J, Valenzuela CD, Pabst A, Tsuda A, Konerding MA, Mentzer SJ. Elastin Cables Define the Axial Connective Tissue System in the Murine Lung. Anat Rec (Hoboken) 2015;298(11):1960–8. doi: 10.1002/ar.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weibel ER. Lung morphometry: the link between structure and function. Cell Tissue Res. 2017;367(3):413–426. doi: 10.1007/s00441-016-2541-4. [DOI] [PubMed] [Google Scholar]
- 121.Thurlbeck WM. Postnatal growth and development of the lung. Am Rev Respir Dis. 1975;111(6):803–44. doi: 10.1164/arrd.1975.111.6.803. [DOI] [PubMed] [Google Scholar]
- 122.Nardell EA, Brody JS. Determinants of mechanical properties of rat lung during postnatal development. J Appl Physiol Respir Environ Exerc Physiol. 1982;53(1):140–8. doi: 10.1152/jappl.1982.53.1.140. [DOI] [PubMed] [Google Scholar]
- 123.Amy RW, Bowes D, Burri PH, Haines J, Thurlbeck WM. Postnatal growth of the mouse lung. J Anat. 1977;124(Pt 1):131–51. [PMC free article] [PubMed] [Google Scholar]
- 124.Burri P, Dbaly J, Weibel E. The postnatal growth of the rat lung. 1. Morphometry. Anat Rec. 1974;178(4):711–30. doi: 10.1002/ar.1091780405. [DOI] [PubMed] [Google Scholar]
- 125.Kaplan NB, Grant MM, Brody JS. The lipid interstitial cell of the pulmonary alveolus. Age and species differences. Am Rev Respir Dis. 1985;132(6):1307–12. doi: 10.1164/arrd.1985.132.6.1307. [DOI] [PubMed] [Google Scholar]
- 126.Ruiz-Camp J, Morty RE. Divergent fibroblast growth factor signaling pathways in lung fibroblast subsets: where do we go from here? Am J Physiol Lung Cell Mol Physiol. 2015;309(8):L751–5. doi: 10.1152/ajplung.00298.2015. [DOI] [PubMed] [Google Scholar]
- 127.Moiseenko A, Kheirollahi V, Chao CM, Ahmadvand N, Quantius J, Wilhelm J, Herold S, Ahlbrecht K, Morty RE, Rizvanov AA, Minoo P, El Agha E, Bellusci S. Origin and characterization of alpha smooth muscle actin-positive cells during murine lung development. Stem Cells. 2017;35(6):1566–1578. doi: 10.1002/stem.2615. [DOI] [PubMed] [Google Scholar]
- 128.McGowan SE, McCoy DM. Platelet-derived growth factor-A and sonic hedgehog signaling direct lung fibroblast precursors during alveolar septal formation. Am J Physiol Lung Cell Mol Physiol. 2013;305(3):L229–39. doi: 10.1152/ajplung.00011.2013. [DOI] [PubMed] [Google Scholar]
- 129.Green J, Endale M, Auer H, Perl AK. Diversity of Interstitial Lung Fibroblasts Is Regulated by Platelet-Derived Growth Factor Receptor alpha Kinase Activity. Am J Respir Cell Mol Biol. 2016;54(4):532–45. doi: 10.1165/rcmb.2015-0095OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Al Alam D, El Agha E, Sakurai R, Kheirollahi V, Moiseenko A, Danopoulos S, Shrestha A, Schmoldt C, Quantius J, Herold S, Chao CM, Tiozzo C, De Langhe S, Plikus MV, Thornton M, Grubbs B, Minoo P, Rehan VK, Bellusci S. Evidence for the involvement of fibroblast growth factor 10 in lipofibroblast formation during embryonic lung development. Development. 2015;142(23):4139–50. doi: 10.1242/dev.109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.El Agha E, Herold S, Al Alam D, Quantius J, MacKenzie B, Carraro G, Moiseenko A, Chao CM, Minoo P, Seeger W, Bellusci S. Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development. 2014;141(2):296–306. doi: 10.1242/dev.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li C, Li M, Li S, Xing Y, Yang CY, Li A, Borok Z, De Langhe S, Minoo P. Progenitors of secondary crest myofibroblasts are developmentally committed in early lung mesoderm. Stem Cells. 2015;33(3):999–1012. doi: 10.1002/stem.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson K, Cardoso W. The molecular basis of lung morphogenesis. Mech Dev. 2000;92(1):55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 134.Lindahl P, Karlsson L, Hellstrom M, Gebre-Medhin S, Willetts K, Heath JK, Betsholtz C. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997;124(20):3943–3953. doi: 10.1242/dev.124.20.3943. [DOI] [PubMed] [Google Scholar]
- 135.O’Dell B, Kilburn K, McKenzie W, Thurston R. The lung of the copper-deficient rat. A model for developmental pulmonary emphysema. Am J Pathol. 1978;91(3):413–32. [PMC free article] [PubMed] [Google Scholar]
- 136.Kida K, Thurlbeck W. The effects of beta-aminopropionitrile on the growing rat lung. Am J Pathol. 1980;101(3):693–710. [PMC free article] [PubMed] [Google Scholar]
- 137.Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol. 2000;23(3):320–326. doi: 10.1165/ajrcmb.23.3.3906. [DOI] [PubMed] [Google Scholar]
- 138.Eriksson S. Alpha 1-antitrypsin deficiency: lessons learned from the bedside to the gene and back again. Historic perspectives. Chest. 1989;95(1):181–9. doi: 10.1378/chest.95.1.181. [DOI] [PubMed] [Google Scholar]
- 139.Gross P, Pfitzer EA, Tolker E, Babyak MA, Kaschak M. Experimental Emphysema: Its Production with Papain in Normal and Silicotic Rats. Arch Environ Health. 1965;11:50–8. doi: 10.1080/00039896.1965.10664169. [DOI] [PubMed] [Google Scholar]
- 140.Kuhn C, Senior RM. The role of elastases in the development of emphysema. Lung. 1978;155(1):185–197. doi: 10.1007/BF02730693. [DOI] [PubMed] [Google Scholar]
- 141.Snider GL. Emphysema: the first two centuries--and beyond. A historical overview, with suggestions for future research: Part 2. Am Rev Respir Dis. 1992;146(6):1615–22. doi: 10.1164/ajrccm/146.6.1615. [DOI] [PubMed] [Google Scholar]
- 142.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22(4):672–88. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 143.Janoff A, White R, Carp H, Harel S, Dearing R, Lee D. Lung injury induced by leukocytic proteases. Am J Pathol. 1979;97(1):111–36. [PMC free article] [PubMed] [Google Scholar]
- 144.Snider GL, Ciccolella DE, Morris SM, Stone PJ, Lucey EC. Putative role of neutrophil elastase in the pathogenesis of emphysema. Ann NY Acad Sci. 1991;624:45–59. doi: 10.1111/j.1749-6632.1991.tb17005.x. [DOI] [PubMed] [Google Scholar]
- 145.Hunninghake GM, Cho MH, Tesfaigzi Y, Soto-Quiros ME, Avila L, Lasky-Su J, Stidley C, Melen E, Soderhall C, Hallberg J, Kull I, Kere J, Svartengren M, Pershagen G, Wickman M, Lange C, Demeo DL, Hersh CP, Klanderman BJ, Raby BA, Sparrow D, Shapiro SD, Silverman EK, Litonjua AA, Weiss ST, Celedon JC. MMP12, lung function, and COPD in high-risk populations. N Engl J Med. 2009;361(27):2599–608. doi: 10.1056/NEJMoa0904006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol. 2003;163(6):2329–2335. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hautamaki R, Kobayashi D, Senior R, Shapiro S. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277(5334):2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 148.Senior RM, Bielefeld DR, Starcher BC. Comparison of the elastolytic effects of human leukocyte elastase and procine pancreatic elastase. Biochem Biophys Res Commun. 1976;72:1327–1334. doi: 10.1016/s0006-291x(76)80160-x. [DOI] [PubMed] [Google Scholar]
- 149.Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980;66:859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hunninghake GW, Davidson JM, Rennard S, Szapiel S, Gadek JE, Crystal RG. Elastin fragments attract macrophage precursors to diseased sites in pulmonary emphysema. Science. 1981;212(4497):925–7. doi: 10.1126/science.7233186. [DOI] [PubMed] [Google Scholar]
- 151.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116(3):753–9. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Karlinsky JB, Snider GL. Animal models of emphysema. Am Rev Respir Dis. 1978;117(6):1109–33. doi: 10.1164/arrd.1978.117.6.1109. [DOI] [PubMed] [Google Scholar]
- 153.Kuhn C, Yu SY, Chraplyvy M, Linder HE, Senior RM. The induction of emphysema with elastase. II. Changes in connective tissue. Lab Invest. 1976;34(4):372–80. [PubMed] [Google Scholar]
- 154.Shifren A, Mecham RP. The stumbling block in lung repair of emphysema: elastic fiber assembly. Proc Am Thorac Soc. 2006;3(5):428–433. doi: 10.1513/pats.200601-009AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shifren A, Durmowicz AG, Knutsen RH, Hirano E, Mecham RP. Elastin Protein Levels are a Vital Modifier Affecting Normal Lung Development and Susceptibility to Emphysema. Am J Physiol Lung Cell Mol Physiol. 2006;292:L778–787. doi: 10.1152/ajplung.00352.2006. [DOI] [PubMed] [Google Scholar]
- 156.Hirano E, Knutsen RH, Sugitani H, Ciliberto CH, Mecham RP. Functional Rescue of elastin insufficiency in mice by the human elastin gene: Implications for mouse models of human disease. Circ Res. 2007;101(5):523–531. doi: 10.1161/CIRCRESAHA.107.153510. [DOI] [PubMed] [Google Scholar]
- 157.Zhang MC, He L, Giro M, Yong SL, Tiller GE, Davidson JM. Cutis laxa arising from frameshift mutations in exon 30 of the elastin gene (ELN) J Biol Chem. 1999;274:981–986. doi: 10.1074/jbc.274.2.981. [DOI] [PubMed] [Google Scholar]
- 158.Tassabehji M, Metcalfe K, Hurst J, Ashcroft GS, Kielty C, Wilmot C, Donnai D, Read AP, Jones CJP. An elastin gene mutation producing abnormal tropoelastin and abnormal elastic fibres in a patient with autosomal dominant cutis laxa. Hum Molec Gen. 1998;7:1021–1028. doi: 10.1093/hmg/7.6.1021. [DOI] [PubMed] [Google Scholar]
- 159.Urban Z, Gao J, Pope FM, Davis EC. Autosomal dominant cutis laxa with severe lung disease: synthesis and matrix deposition of mutant tropoelastin. J Invest Dermatol. 2005;124:1193–1199. doi: 10.1111/j.0022-202X.2005.23758.x. [DOI] [PubMed] [Google Scholar]
- 160.Callewaert B, Renard M, Hucthagowder V, Albrecht B, Hausser I, Blair E, Dias C, Albino A, Wachi H, Sato F, Mecham R, Loeys B, Coucke P, De Paepe A, Urban Z. New insights into the pathogenesis of autosomal-dominant cutis laxa with report of five ELN mutations. Hum Mutat. 2011;32(4):445–55. doi: 10.1002/humu.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wain LV, Shrine N, Artigas MS, Erzurumluoglu AM, Noyvert B, Bossini-Castillo L, Obeidat M, Henry AP, Portelli MA, Hall RJ, Billington CK, Rimington TL, Fenech AG, John C, Blake T, Jackson VE, Allen RJ, Prins BP, Campbell A, Porteous DJ, Jarvelin MR, Wielscher M, James AL, Hui J, Wareham NJ, Zhao JH, Wilson JF, Joshi PK, Stubbe B, Rawal R, Schulz H, Imboden M, Probst-Hensch NM, Karrasch S, Gieger C, Deary IJ, Harris SE, Marten J, Rudan I, Enroth S, Gyllensten U, Kerr SM, Polasek O, Kahonen M, Surakka I, Vitart V, Hayward C, Lehtimaki T, Raitakari OT, Evans DM, Henderson AJ, Pennell CE, Wang CA, Sly PD, Wan ES, Busch R, Hobbs BD, Litonjua AA, Sparrow DW, Gulsvik A, Bakke PS, Crapo JD, Beaty TH, Hansel NN, Mathias RA, Ruczinski I, Barnes KC, Bosse Y, Joubert P, van den Berge M, Brandsma CA, Pare PD, Sin DD, Nickle DC, Hao K, Gottesman O, Dewey FE, Bruse SE, Carey DJ, Kirchner HL, Jonsson S, Thorleifsson G, Jonsdottir I, Gislason T, Stefansson K, Schurmann C, Nadkarni G, Bottinger EP, Loos RJ, Walters RG, Chen Z, Millwood IY, Vaucher J, Kurmi OP, Li L, Hansell AL, Brightling C, Zeggini E, Cho MH, Silverman EK, Sayers I, Trynka G, Morris AP, Strachan DP, Hall IP, Tobin MD. Genome-wide association analyses for lung function and chronic obstructive pulmonary disease identify new loci and potential druggable targets. Nat Genet. 2017;49(3):416–425. doi: 10.1038/ng.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Busch R, Hobbs BD, Zhou J, Castaldi PJ, McGeachie MJ, Hardin ME, Hawrylkiewicz I, Sliwinski P, Yim JJ, Kim WJ, Kim DK, Agusti A, Make BJ, Crapo JD, Calverley PM, Donner CF, Lomas DA, Wouters EF, Vestbo J, Tal-Singer R, Bakke P, Gulsvik A, Litonjua AA, Sparrow D, Pare PD, Levy RD, Rennard SI, Beaty TH, Hokanson J, Silverman EK, Cho MH G. National Emphysema Treatment Trial, C.L.t.I.P.S.E.-P. Evaluation of, C.G.N. International, C.O. Investigators. Genetic Association and Risk Scores in a Chronic Obstructive Pulmonary Disease Meta-analysis of 16,707 Subjects. Am J Respir Cell Mol Biol. 2017;57(1):35–46. doi: 10.1165/rcmb.2016-0331OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Boueiz A, Lutz SM, Cho MH, Hersh CP, Bowler RP, Washko GR, Halper-Stromberg E, Bakke P, Gulsvik A, Laird NM, Beaty TH, Coxson HO, Crapo JD, Silverman EK, Castaldi PJ, DeMeo DL Copdgene, E. Investigators. Genome-Wide Association Study of the Genetic Determinants of Emphysema Distribution. Am J Respir Crit Care Med. 2017;195(6):757–771. doi: 10.1164/rccm.201605-0997OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kelleher CM, Silverman EK, Broekelmann T, Litonjua AA, Hernandez M, Sylvia JS, Stoler J, Reilly JJ, Chapman HA, Speizer FE, Weiss ST, Mecham RP, Raby BA. A functional mutation in the terminal exon of elastin segregates with severe, early onset chronic obstructive pulmonary disease. Am J Resp Cell Molec Biol. 2005;33:355–362. doi: 10.1165/rcmb.2005-0206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ramnath N, van de Luijtgaarden K, van der Pluijm I, van Nimwegen M, van Heijningen P, Swagemakers S, van Thiel B, Ridwan R, van Vliet N, Vermeij M, Hawinkels L, de Munck A, Dzyubachyk O, Meijering E, van der Spek P, Rottier R, Yanagisawa H, Hendriks R, Kanaar R, Rouwet E, Kleinjan A, Essers J. Extracellular matrix defects in aneurysmal Fibulin-4 mice predispose to lung emphysema. PLoS One. 2014;9(9):e106054. doi: 10.1371/journal.pone.0106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hucthagowder V, Sausgruber N, Kim K, Angle B, Marmorstein L, Urban Z. Fibulin-4: a novel gene for an autosomal recessive cutis laxa syndrome. Am J Hum Genet. 2006;78(6):1075–1080. doi: 10.1086/504304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yanagisawa H, Davis E, Starcher B, Ouchi T, Yanagisawa M, Richardson J, Olson E. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415(6868):168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 168.Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415(6868):171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 169.Brandsma CA, van den Berge M, Postma D, Timens W. Fibulin-5 as a potential therapeutic target in COPD. Expert Opin Ther Targets. 2016;20(9):1031–3. doi: 10.1517/14728222.2016.1164696. [DOI] [PubMed] [Google Scholar]
- 170.Barisic-Dujmovic T, Boban I, Adams D, Clark S. Marfan-like skeletal phenotype in the tight skin (Tsk) mouse. Calcif Tissue Int. 2007;81(4):305–315. doi: 10.1007/s00223-007-9059-4. [DOI] [PubMed] [Google Scholar]
- 171.Dabovic B, Chen Y, Choi J, Davis E, Sakai L, Todorovic V, Vassallo M, Zilberberg L, Singh A, Rifkin D. Control of lung development by latent TGF-beta binding proteins. J Cell Physiol. 2011;226(6):1499–509. doi: 10.1002/jcp.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hersh C, Demeo D, Lazarus R, Celedon J, Raby B, Benditt J, Criner G, Make B, Martinez F, Scanlon P, Sciurba F, Utz J, Reilly J, Silverman E. Genetic association analysis of functional impairment in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(9):977–84. doi: 10.1164/rccm.200509-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36(2):178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 174.Lee V, Halabi C, Hoffman E, Carmichael N, Leshchiner I, Lian C, Bierhals A, Vuzman D, Brigham GM, Mecham R, Frank N, Stitziel N. Loss of function mutation in LOX causes thoracic aortic aneurysm and dissection in humans. Proc Natl Acad Sci U S A. 2016;113(31):8759–64. doi: 10.1073/pnas.1601442113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Turino GM, Ma S, Lin YY, Cantor JO, Luisetti M. Matrix elastin: a promising biomarker for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184(6):637–41. doi: 10.1164/rccm.201103-0450PP. [DOI] [PubMed] [Google Scholar]
- 176.Price LS, Roos PJ, Shively VP, Sandberg LB Understanding Society Scientific G, Geisinger-Regeneron Discov EHRC. Valyl-alanyl-prolyl-glycine (VAPG) serves as a quantitative marker for human elastins. Matrix. 1993;13(4):307–311. doi: 10.1016/s0934-8832(11)80026-0. [DOI] [PubMed] [Google Scholar]